Abstract
Background:
Currently, one of the most used strategies for the treatment of newly diagnosed patients with breast cancer is neoadjuvant chemotherapy based on the application of taxanes and anthracyclines. However, despite the high number of patients who develop a complete pathological clinical response, resistance and relapse following this therapy continue to be a clinical challenge. As a component of the innate immune system, the cytotoxic function of Natural Killer (NK) cells plays an important role in the elimination of tumor cells. However, the role of NK cells in resistance to systemic therapy in breast cancer remains unclear. The present project aims to evaluate the gene expression profile of human NK cells in breast cancer tissue resistant to treatment with taxanes–anthracyclines.
Methods:
Biopsies from tumor tissues were obtained from patients with breast cancer without prior treatment. Histopathological analysis and ex vivo exposure to antineoplastic chemotherapeutics were carried out. Alamar blue and lactate dehydrogenase release assays were performed for quantitative analysis of tumor viability. Gene expression profiles from tumor tissues without prior exposure to therapeutic drugs were analyzed by gene expression microarrays and verified by polymerase chain reaction.
Results:
A significant decrease in gene expression of cell-surface receptors related to NK cells was observed in tumor samples resistant to antineoplastic treatment compared with those that were sensitive to treatment.
Conclusion:
A decrease in NK cell infiltration into tumor tissue might be a predictive marker for failure of chemotherapeutic treatment in breast cancer.
Keywords: breast cancer, chemotherapeutic treatment, NK cells, tumor infiltrating lymphocytes, NK cell receptors
Background
Locally advanced breast cancer is a heterogeneous disease characterized by increased local recurrence and decreased survival. It is classified into stages IIB to IIIC by the American Joint Committee on Cancer,1-3 and the prognosis of patients with this disease remains highly variable. Multimodal therapy, including chemotherapy, surgery, and radiation, is the treatment of choice that has shown the highest percentage survival in such patients with breast cancer.4,5 Particularly, chemotherapeutic schemes based on anthracycline and taxanes are widely accepted as first alternatives in the treatment of breast cancer6-8; these therapeutic strategies provide a benefit in recurrence-free survival as well as overall survival.9,10
Management in clinical practice, using a neoadjuvant chemotherapy regimen in either a concomitant or sequential manner, is preferred in locally advanced breast cancer. The main objectives of the neoadjuvant chemotherapy regimen are to eliminate distant micrometastases that exist at the time of diagnosis, decrease tumor size allowing conservative surgeries, and to assess the in vivo response of the tumor to chemotherapy.1,2 Moreover, it has been shown that when neoadjuvant chemotherapy leads to a complete pathological response (pCR), patients enjoy prolonged disease-free survival and have a better clinical outcome. Consequently, pCR has been considered as one of the best markers of survival.1,2,4,5
Predictive factors associated with greater probability of achieving a pCR using neoadjuvant chemotherapy regimen are tumor size, histological type (ductal–lobular carcinoma), intrinsic subtype tumor (basal–luminal), hormone receptor status (negative–positive estrogen receptor), expression of human epidermal growth factor receptor 2 (HER2), Ki-67, and the Scarff-Bloom-Richardson grade.11,12 In this sense, large numbers of studies have focused on specific characteristics of phenotype, molecular patterns, and growth rates of tumor cells after chemotherapy. For instance, there is now sufficient evidence that chemotherapy regimens employing anthracyclines and taxanes lead to higher pCR rates in triple-negative tumors compared to estrogen and progesterone receptor-positive subtypes of breast cancer. Regrettably, only a fraction of the patients categorized in a specific subtype of breast cancer respond to neoadjuvant chemotherapy and achieve a pCR showing a better prognosis. In fact, despite the benefits of using modern chemotherapy regimens, multidrug resistance continues to be a clinical challenge, and the need for biological markers that can predict the response to chemotherapy is evident.
Distinguishing responsive from nonresponder patients can significantly improve therapeutic decisions. Therefore, much effort has been focused on the identification of specific features of the tumor microenvironment including biological and molecular markers that could help to tailor or develop new therapies. Recently, the presence of tumor infiltrating lymphocytes (TILs) has been correlated with clinical outcomes in many types of cancer.13,14 Patients with breast cancer with prominent lymphocyte infiltration, before any treatment, show stronger responses to neoadjuvant chemotherapy.15,16 Moreover, high lymphocyte infiltration correlates with higher pCR rates as well as with better patient prognosis. Because of this, it has been suggested that infiltration of tumor-associated lymphocytes may represent a new independent predictive factor of response to neoadjuvant chemotherapy in breast cancer.17,18
The composition of tumor infiltrating immune cells can vary according to cancer type. Moreover, different types of infiltrating immune cells involved in both innate and adaptive immunity have diverse effects on tumor behavior with either pro-tumoral or antitumoral consequences.14,16 In breast cancer, it has been shown that diverse patterns of gene expression in the tumoral stroma are related to good prognosis. Interestingly, overexpression of a group of genes related to the immune response, including T cells and NK (Natural Killer) cell markers, indicating a TH1-like response (granzyme A, CD52, CD247 and CD8A), are indicators of good prognosis.19 In addition, it has been shown that tumor infiltration by T and B cells is associated with high response rates to neoadjuvant chemotherapy as well as a high rate of pCR.18,20,21
To date, there is no clear evidence to establish an association between NK cell infiltration and clinical outcome in patients with breast cancer. Therefore, the present study aims to evaluate the expression of NK cell surface receptors in breast cancer tissues and their association with the pathological response to neoadjuvant chemotherapy.
Methods
Obtention of Tumor Explants
Infiltrating carcinoma specimens were collected from patients with breast cancer during surgery at the Hospital of Gynecology and Obstetrics of the Mexican Institute of Social Security (IMSS). Immediately after surgery, to confirm its tumorous nature and to avoid contamination, the specimen was dissected and evaluated by a pathologist. One small piece of tissue was collected in cold serum-free Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) medium (Invitrogen, Grand Island, New York) and transported at 4°C to the organotypic culture laboratory for its immediate processing; another piece of tissue was stored in RNAlater RNA Stabilization Reagent (Qiagen, Hilden, Germany) at −80°C.
Evaluation of TILs in formalin-fixed, paraffin-embedded Specimens
Clinical and histopathological information were obtained from patients’ files archived at the Gynecology Hospital and Specialty Hospital of the Western National Medical Center (CMNO) of the IMSS, and the percentage of TILs was evaluated from formalin-fixed, paraffin-embedded (FFPE) specimens of invasive breast ductal carcinoma (n = 90) that were retrospectively obtained.
Tumor Infiltrating Lymphocytes
The distribution pattern of tumor cells and the presence of inflammation that accompanies the tumor was determined by observation under light microscopy using the 5× objective. Following this, at 10× magnification, the proportion of tumor to inflammatory cells was assessed according to Supplemental Figure 1. Finally, after analysis of different representative areas of the tissue, a mean percentage was calculated.
Treatment of Tumor Explants With Antineoplastic Drugs
From representative tumor samples, small tumor explants (4-5 mm in diameter and 250-300 μm in thickness) were prepared using the Krumdieck slicing system (Alabama Research and Development Corporation, Birmingham, Alabama) as described previously.22 Tumor explants were placed in 6-well microplates containing DMEM/F12 culture medium–supplemented with 10% fetal bovine serum, 5 μg/mL bovine insulin, 100 μg/mL gentamicin, insulin–transferrin–selenium, and 25 mM glucose (DMEM/F12-supplemented medium). Plates were preincubated for 1 hour at 37°C, 5% CO2, 95% air, and agitated at 35 rpm. Time from harvest to slicing was kept to an absolute minimum (<2 hours). The entire process was performed under aseptic conditions. The treatment of tumor explants with antineoplastic compounds was performed after 1 h of preincubation. The tumor explants were transferred to 24-well microplates and treated with 20 μg/mL paclitaxol (TX). The control group (100% viability) consisted of untreated explants, which were incubated only with culture medium.
Alamar Blue Viability Assay
The effect of treatments on the viability of the tumor explants was assessed by the alamar Blue (AB) assay. After 48 hours of incubation with compounds, as well as with cell culture medium (control), the explants were incubated for an additional 4 hours with 10% AB in 500 μL DMEM/F12-supplemented medium at 37°C in the conditions described above. Afterward, 100 μL was collected from each sample and transferred to a 96-well microplate. Fluorescence values were read using a multimode microplate reader (Synergy BioTek HT, Winooski, Vermont) at 530 nm excitation/590 nm emission wavelengths.
Histological Analysis
Explant tissue sections prior to treatment were fixed in 10% neutral formalin and then embedded in paraffin using the conventional histological technique. Formalin-fixed, paraffin-embedded specimens of invasive breast ductal carcinoma were obtained from the Pathology Department of the CMNO, IMSS. From all samples, tissue sections of 4 μm were prepared on a microtome and mounted on glass slides, sections were deparaffinized and hematoxylin-eosin (H&E) stained. The preparations were then evaluated by a pathologist.
RNA Isolation and Gene Expression Microarrays
Biopsies stored at −80°C in RNAlater were used for the isolation of total RNA with the RNeasy Plus Mini kit (Qiagen) according to the manufacturer’s instructions. RNA was quantified by absorbance at 260/280 nm, and quality was evaluated using an Agilent 2100 Bioanalyzer and the RNA 6000 NanoChip kit (Agilent Technologies, Santa Clara, California). Double-stranded complementary DNA (ds-cDNA) was generated from 5 µg of RNA using the cDNA Synthesis Kit System (Roche Applied Science, Indianapolis, Indiana) and purified with the GenElute polymerase chain reaction (PCR) Clean-up Kit (Sigma-Aldrich Quimica, Toluca, Mexico). Afterward, the ds-cDNA was labeled with Cy3 and hybridized in a Human Gene Expression Array 12 × 135 k (Roche Applied Science). Finally, the microarrays were scanned on an MS200 Scanner (Roche Applied Science), and the data obtained were processed using the DEVA 1.2 software (Roche Applied Science). Fluorescence intensities were normalized using the RMA algorithm, and all data obtained were subsequently analyzed using the CLC Main WorkBench version 7.0.3 software (Qiagen). Intensities of sensitive versus resistant samples were compared and the genes were considered differentially expressed when the intensities showed a difference of >1.5 and a P value <.05.
In Silico Analysis
To further explore the molecular pathways in which differentially expressed genes are involved, we performed an analysis using the WEB-based-Gene-Set-Analysis toolkit (WebGestalt, http://www.webgestalt.org/). Additionally, genes associated with NK cell-mediated cytotoxicity were further introduced into the cBio Cancer Genomics Portal (cBioportal, http://www.cbioportal.org/); the breast invasive carcinoma (TCGA, provisional) was selected and the messenger RNA (mRNA) expression data with a z score threshold of ± 2.0 was used. This platform is a resource for interactive exploration of cancer genomics data sets that allows us to access molecular profiles and clinical attributes from large-scale cancer genomics studies.
Real-Time Polymerase Chain Reaction and PCR Evaluation
Complementary DNA synthesis was performed from 5 μg of total RNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science) primed with Oligo dT. The cDNA obtained was employed in quantitative polymerase chain reactions to evaluate gene expression levels using the LightCycler FastStart DNA Master PLUS SYBR Green I Kit with 2.0 LightCycler technology (Roche Applied Science) under conditions specified by the manufacturer. Primer pairs were designed using Oligo Primer Analysis version 7.0 software (Molecular Biology Insights, Inc, Colorado Springs, Colorado) from sequences obtained at the Entrez Nucleotide Database of the National Center of Biotechnology Information (Table 1). Polymerase chain reaction products were resolved by agarose (2%) gel electrophoresis.
Table 1.
Primer Pairs Designed for Cell Surface Receptors Mainly Related to Natural Killer Cells.
| Gene Symbol | Gene Description | Primer Sequence | |
|---|---|---|---|
| Sense | Antisense | ||
| RPL32 | Ribosomal protein L32 | ||
| KLRC1 | Killer cell lectin-like receptor subfamily C, member 1 | GAGGCAGCAACGAAAACCTA | GCCATTAAGATAAGACAGAT |
| KLRC2 | Killer cell lectin-like receptor subfamily C, member 2 | TTTCCCCGAATACAAGAACG | AGCCAAACCATTTATTGTCA |
| KLRC3 | Killer cell lectin-like receptor subfamily C, member 3 | TTTCTGGCCAGCATTTTACC | CAGTAATCCCAGCAACTTGG |
| KLRC4 | Killer cell lectin-like receptor subfamily C, member 4 | CGGATCATCAAGGGAATGAC | GATCAGAGTTCTTCGAAGCA |
| NCR1 | Natural cytotoxicity triggering receptor 1 | TTC ATC CTG GAC CCG AAG TG | GCA AGG CTG GTG TTC TCA ATG |
Quantification of PCR bands
Densitometric analysis of PCR bands was carried out by using the ImageJ software version 1.51j8 (https://imagej.nih.gov/ij/). Densitometric values of RPL32 in each sample were used as reference.
Statistical Analysis
Statistical analysis was performed with SPSS version 22.0 software (IBM Corporation, Armonk, New York). Quantitative data were expressed as mean and standard error of the mean. Differences between treatments of tumor slices were analyzed by Student t test and the significance between groups was analyzed by the nonparametric Mann-Whitney test. A P value of < .01 was considered to be statistically significant.
Results
Response of Tumor Slices to Chemotherapeutic Treatment
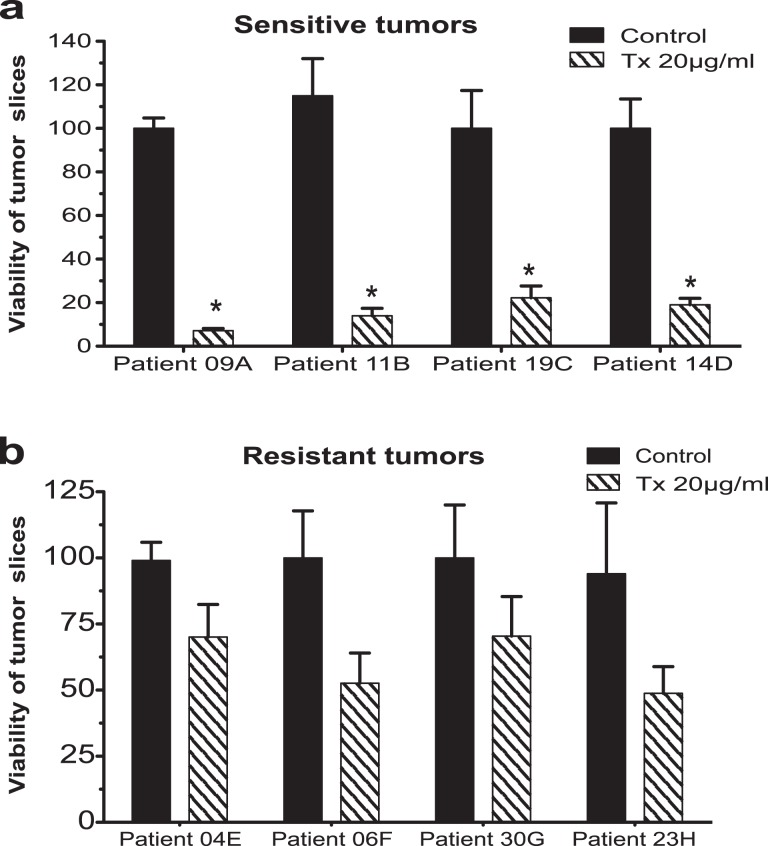
Once confirmed that breast cancer slices remained viable and actively proliferating during the ex vivo culture, tumor slices from 36 patients were incubated in the presence of TX. Afterward, the viability was analyzed by AB assay. Results showed that tumor slices presented different sensitivity patterns to TX, and considering this fact, we grouped the samples as resistant or sensitive, taking into account the percentage viability after treatment (samples with viability <25% were designated as sensitive and those with viability of ≥50% were considered resistant). Four tumor samples met the criteria to be considered sensitive (Figure 1A: 9A, 11B, 14C, and 19D) and 4 were resistant to TX treatment (Figure 1B: 4E, 6F, 23G, and 30H). The clinical and histopathological data of these patients are described in Table 2. Viability values of sensitive tumors were statistically significant (P < .01), whereas in tumor slices cultured without treatment (control), no significant loss in metabolic activity was observed during the incubation period. Histopathological analysis of breast slice cultures incubated with TX confirmed cell death in the majority of cancer cells from sensitive tumors (results not shown). Conversely, cells retaining their viability were observed in resistant tumors treated with TX.
Figure 1.
Percentage viability of tumor slice samples after incubation with paclitaxol (TX). Samples with viability <25% were designated as sensitive. Data are expressed as mean ± standard of the mean (SEM) *P < .01.
Table 2.
Clinical and Histopathological Data of Patients With Breast Cancer From Whom Tumor Slice Samples Were Obtained.
| Patient | Age | Histologic Type | Clinical Stage | Tumor Size | Laterality | Body Mass Index | Hormonal Receptors | Molecular Classification | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | ER | HER Status | ||||||||
| 4E | 81 | Lobulillar infiltrating | T3N4M0 | 5 × 7 cm | Left | 37.4 | + | + | − | Luminal A |
| 6F | 60 | Canalicular infiltrating | T4bN2M0 | 5 × 6 cm | Left | 30.1 | − | − | − | Basal-like |
| 23G | 45 | Canalicular infiltrating | T4N0M0 | 6 × 4 cm | Right | 32.1 | + | + | − | Luminal A |
| 30H | 56 | Ductal infiltrating | T4N0M0 | 5 × 5 cm | Right | 29.6 | + | + | − | Luminal A |
| 9A | 46 | Ductal infiltrating | T3N1M0 | 6 × 6 cm | Left | 27.2 | + | − | + | Luminal B |
| 11B | 63 | Ductal infiltrating | T2N1M0 | 3 × 2 cm | Left | 25.6 | − | − | + | Her2+ |
| 14C | 46 | Canalicular infiltrating | T3N1M0 | 5 × 3 cm | Left | 31.2 | − | − | − | Basal-like |
| 19D | 72 | Lobulillar infiltrating | T4BN0M0 | 3 × 2 cm | Left | 22.8 | + | + | − | Luminal A |
Abbreviations: ER, estrogen receptor; HER, human epidermal growth factor receptor; M, metastasis; N, node; PR, progesterone receptor; T, tumor.
Tumor Infiltrating Lymphocytes Are Decreased in Breast Cancer Tissue Resistant to Chemotherapy
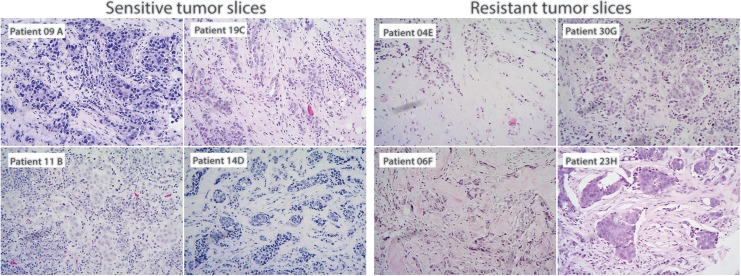
Once tumor slice samples were grouped into sensitive and resistant, histopathological analysis was performed not only on breast slice cultures incubated with TX but also in tumor slices without any treatment. Hematoxylin-eosin staining showed a differential proportion of TILs between tumor slice samples cataloged as sensitive (9A, 11B, 14C, and 19D) compared to slice samples cataloged as resistant (4E, 6F, 23G, and 30H). High-level lymphocyte infiltration was evident in tumor slices sensitive to TX treatment (Figure 2).
Figure 2.
Photomicrographs showing tumor samples from mammary gland samples stained with hematoxylin and eosin (H&E) to qualitatively evaluate the presence of tumor infiltrating lymphocytes. Sensitive tumor slices (A, B, C, and D) and resistant tumor slices (E, F, G, and H) without prior exposure to therapeutic drugs.
Additionally, TILs were evaluated in FFPE specimens of invasive breast ductal carcinoma (n = 90), obtained retrospectively within the last 5 to 10 years.
Evolution and follow-up information of patients with breast cancer was obtained from clinical records, and the specimens were classified as either good or bad prognosis, according to clinical parameters such as histological type, clinical stage, hormonal receptors, and molecular classification, but above all response to chemotherapy (pCR) and the presence of recurrent disease.
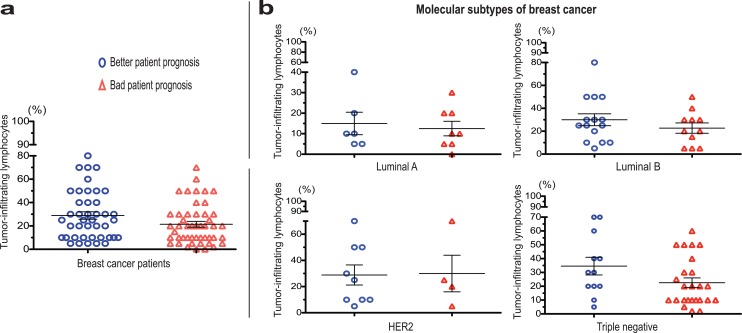
Initially, an analysis of the percentage of TILs was performed in all patients with breast cancer (n = 90) divided into patients with better prognosis (n = 42) and bad prognosis (n = 48). An increased tendency of tumor infiltrate was found in patients with breast cancer with better prognosis (28.9% ± 3.2%) compared to patients with breast cancer with bad prognosis (21.4% ± 2.4%; Figure 3A).
Figure 3.
Percentage of tumor infiltrating lymphocytes in tumor samples of patients with breast cancer. (A) Percentage of tumor infiltrate according to patient prognosis and (B) in relation to the molecular subtype classification.
Since molecular classification in breast tumors has been employed to inform decisions regarding therapeutic treatment, the percentage of TILs was analyzed dividing patients with breast cancer into luminal A (n = 14), luminal B (n = 26), HER2 (n = 13), and triple negative (n = 37) tumors. Overall, an increased tendency for presence of TILs was also seen in patients with better prognosis compared to patients with bad prognosis classified into luminal A (15.0% ± 5.4% vs 12.5% ± 3.5%), luminal B (30% ± 5.2% vs 22.7% ± 4.4%), and triple negative tumors (34.5% ± 6.3% vs 22.56% ± 3.5%; Figure 3B).
Decreased Gene Expression of NK Cell Surface Receptors in Breast Tumors Resistant to Chemotherapy
Once a tumor slice was identified as sensitive or resistant, and the histopathologic analysis by H&E showed a decrease in TILs in either tumor slice samples or FFPE specimens of invasive breast ductal carcinoma, expression microarrays were performed to characterize the molecular profile of sensitive tumor slices compared to resistant tumor slices. Sensitive tumor slices were taken as reference, and variations in the gene expression levels were evaluated with an absolute difference value >1.5 and P < .05. All genes significantly modulated were introduced into WebGestalt pathways database, as described in the Methods section, and the significant KEGG main routes are depicted in Table 3.
Table 3.
Genes Regulated Significantly in Sensitive and Resistant Tumor Slices Associated With KEGG Main Routes.a
| Name | #Genes |
|---|---|
| Olfactory transduction | 55 |
| Natural killer cell–mediated cytotoxicity | 30 |
| Cytokine–cytokine receptor interaction | 40 |
| Pathways in cancer | 44 |
| Focal adhesion | 32 |
| FCM receptor interaction | 19 |
| Chemokine signaling pathway | 27 |
| Antigen processing and presentation | 17 |
| Neuroactive ligand–receptor interaction | 32 |
Abbreviation: FCM, fragment crystallizable M.
aSensitive tumor slices were taken as reference.
NK cell–mediated cytotoxicity was found to be one of the most significant routes, associated with down-modulation of 30 genes in resistant tumor slices compared to sensitive tumor slices, with an absolute difference value of >1.5 (P < .05). Among them, natural cytotoxicity receptor 1 (NCR1/Nkp46), killer cell immunoglobulin-like receptor (KIR; activator and inhibitor receptors), C-type killer cell lectin-like receptors (KLRC; activator and inhibitor receptors), and effector molecules (granzyme B and perforin 1) were found (Table 4).
Table 4.
Gene Expression (Displayed as Difference) of Cell Surface Receptors Mainly Related to Natural Killer Cells. Samples of Sensitive Tumor Slices Were Taken as Reference.a
| Difference | Symbol | Name |
|---|---|---|
| −2.08 | NCR1 | Natural cytotoxicity triggering receptor 1 |
| −2.26 | KIR2DL2 | Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 2 |
| −2.49 | KIR2DL3 | Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 3 |
| −2.14 | KIR2DS5 | Killer cell immunoglobulin-like receptor, two domains, short cytoplasmic tail, 5 |
| −2.08 | KIR3DL1 | Killer cell immunoglobulin-like receptor, three domains, long cytoplasmic tail, 1 |
| −2.11 | KIR2DS1 | Killer cell immunoglobulin-like receptor, two domains, short cytoplasmic tail, 1 |
| −2.07 | KIR2DL5A | Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 5a |
| −1.98 | KIR2DS4 | Killer cell immunoglobulin-like receptor, two domains, short cytoplasmic tail, 4 |
| −1.98 | KIR2DS3 | Killer cell immunoglobulin-like receptor, two domains, short cytoplasmic tail, 3 |
| −1.96 | KIR3DL2 | Killer cell immunoglobulin-like receptor, three domains, long cytoplasmic tail, 2 |
| −1.78 | KIR2DL4 | Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 4 |
| −2.11 | KIR2DL1 | Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 1 |
| −2.21 | KIR2DS2 | Killer cell immunoglobulin-like receptor, two domains, short cytoplasmic tail, 2 |
| −2.21 | GZMB | Granzyme B (cytotoxic T-lymphocyte-associated serine esterase 1) |
| −1.67 | PRF1 | Perforin 1 (pore forming protein) |
| −1.77 | KLRC3 | Killer cell lectin-like receptor subfamily C, member 3 |
| −1.66 | KLRC4 | Killer cell lectin-like receptor subfamily C, member 4 |
| −1.69 | KLRC1 | Killer cell lectin-like receptor subfamily C, member 1 |
| −1.62 | KLRC2 | Killer cell lectin-like receptor subfamily C, member 2 |
aAll genes down-regulated with an absolute difference value >1.5 (P < .05) are shown.
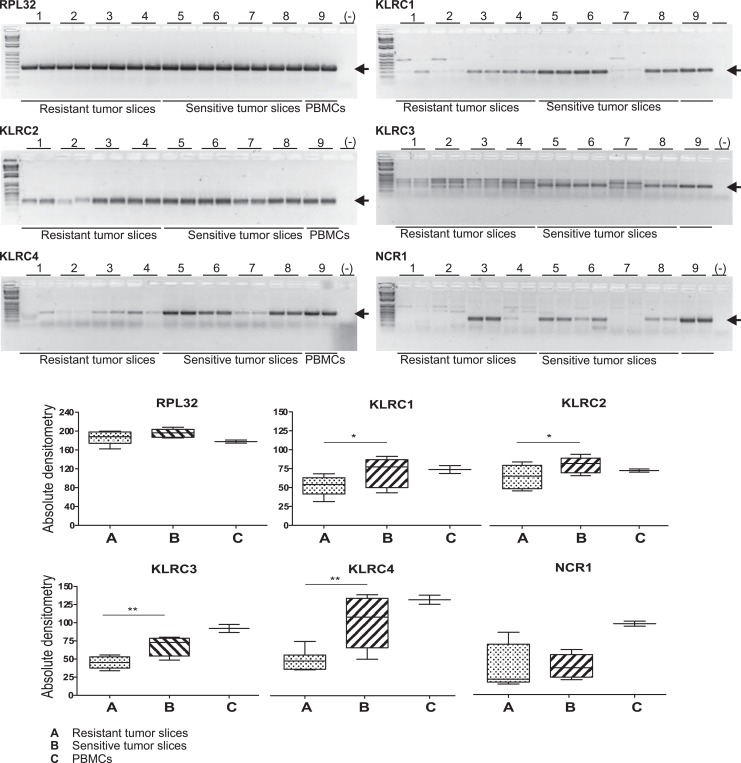
To validate the observations found in the microarray experiments, all differentially expressed genes related to NK cells were evaluated by PCR in both sensitive and resistant tumor slice samples. For these assays, RPL32 was used as reference gene and a peripheral blood mononuclear cell sample was included as a positive control. Down-modulated expression in resistant tumor slice samples of all analyzed genes described in Table 3 was confirmed by PCR. Final PCR products were resolved by agarose (2%) gel electrophoresis to confirm the size and specificity of the product. Image processing and quantitative analysis was performed by ImageJ software; representative results are shown in Figure 4. KLRC1 to 4 were the most significantly down-modulated genes in resistant tumor slices compared to sensitive counterparts. However, all selected genes described in Table 3 showed a downward trend in resistant tumor slices compared to sensitive samples.
Figure 4.
Agarose DNA electrophoresis of polymerase chain reaction (PCR) amplification products from complementary DNA (Cdna) of tumor slices resistant or sensitive to antineoplastic treatment (upper panels), arrows are indicating the expected products. Bands outer the expected size were considered PCR artifacts, and the 1 kb plus DNA ladder was used. Periferal Blood Mononuclear Cells (PBMCs) amplification products were included as positive controls and RPL32 as reference gene. Densitometric comparisons are shown in the lower panels. *Indicates significant differences between groups (P < .05).
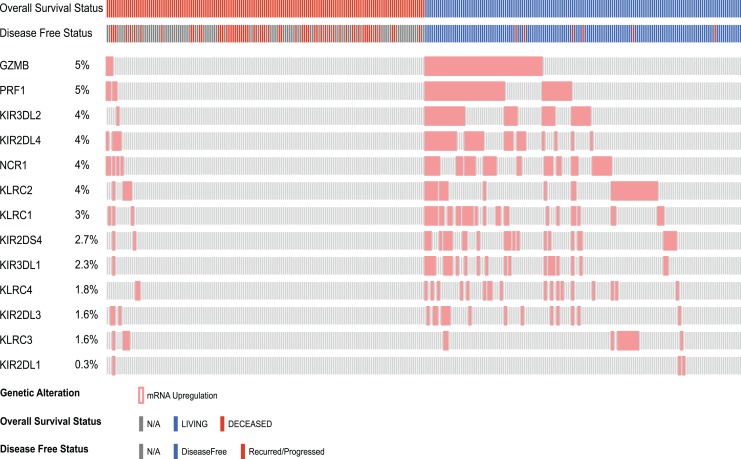
The genes of interest related to NK cells that were found to be significantly modulated in the present study (expression microarrays) were introduced in cBioportal and a breast invasive carcinoma cancer study was selected, which included 1105 samples, and the mRNA expression performed by RNAseq was analyzed. Finally, the differential gene expression patterns of query genes were compared with clinical tracks (overall survival and disease-free status). Messenger RNA upregulation of effector molecules (GZMB and PRF1), natural cytotoxicity receptors (NCR1), KIRs, and KLRCs were associated with overall survival (living) and disease-free status in patients with breast cancer (Figure 5).
Figure 5.
Expression pattern of selected genes of the natural killer cell–mediated cytotoxicity pathway in patients with invasive breast cancer classified according to overall survival status and disease-free status.
Discussion
Identification of new and reliable factors that predict responses to neoadjuvant chemotherapy are urgently needed in clinical practice. Treatment decisions in breast cancer, particularly involving chemotherapy, are principally based on tumor cell phenotype; however, the percentage of patients who achieve pCR is still relatively low.23 It has now been shown that the host immune system not only helps to elucidate cancer prognosis, it can also determine the response to antineoplastic therapies.24 In breast cancer, the clinical relevance of TILs has been clearly established by their correlation with pCR as well as with long-term positive outcomes.15,25 Currently, only limited data regarding particular TILs are available, and their clinical assessment has not yet been fully standardized.
Now, however, measuring the overall extent of TILs is not enough because it does not distinguish between the diversity of innate and adaptive immune cells, thereby limiting the knowledge of their specific contributions in the tumor tissue.26 In this way, the strong association between TILs and the response to neoadjuvant chemotherapy suggests that characterization of the nature and location of the immune infiltrate should be considered.
The composition of the immune infiltrate is influenced by the type of cancer;15 in breast cancer, there is evidence that neoadjuvant chemotherapy is particularly efficient if the patient shows signs of an antitumor immune response both preexisting and induced by therapy.25,27 Regarding neoadjuvant anthracycline-based chemotherapy, a study demonstrated that only tumor size and TILs were independent predictors of anthracycline response in breast cancer.28
In this work, we observed that tumor slice samples sensitive to TX treatment showed high levels of lymphocyte infiltration compared to resistant tumor slices. Furthermore, an analysis of FFPE specimens (biopsies) of invasive breast ductal carcinoma from patients who received taxane and anthracycline-based chemotherapy treatment demonstrated an increased tendency for the presence of TILs in samples classified as good prognosis compared to bad prognosis counterparts. The same behavior was found when dividing breast cancer specimens into molecular subtypes (luminal A, luminal B, and triple negative), demonstrating that TILs may be useful as an independent predictive factor.
Different chemotherapeutic agents have been described to stimulate antitumor immune responses29; among them, cyclophosphamide, anthracyclines, and taxanes have been demonstrated to stimulate T cell proliferation, activate NK cells, deplete circulating regulatory T cells (Treg), and inhibit Treg infiltration into tumor tissues.30 Furthermore, immunogenic cell death induced by chemotherapy releases tumor-associated antigens that can be recognized by antigen-presenting cells, leading to lysis of tumor cells by activated CD8+ cytotoxic T cells.25,31,32 Because interactions between TILs and tumor cells are relevant for clinical outcomes, it is extremely important to understand the preexisting inflammatory infiltrate in the tumor before subjecting it to chemotherapeutic treatment. Evidence is accumulating that high levels of TILs in breast cancer compromising T (CD3+ CD8+ FOXP3+) and B cell populations are significantly associated with pCR and increased disease-free survival, especially if the tumors also lack immunosuppressive CD68+ macrophages.18,25,28,33-35
Due to the variability regarding the characterization of TILs, which may partly reside in the sensitivity of the detection methods used, the controversy continues as to what will be the best method to evaluate specific populations of TILs. This method must be easy to use and to standardize for application in the clinic. Most studies have used immunohistochemical methods to investigate the composition of immune populations infiltrating breast tumors, but a few have identified immune transcripts and gene sets relevant to predicting pCR in breast cancer subtypes.36-38 Chemokine (C-X-C motif) ligand 13 is a metagene signature reflecting not only T-cell infiltration but also intratumoral presence of interferon-γ-producing T cells and has been demonstrated as metagene predicting pCR after neoadjuvant breast cancer chemotherapy.25
Our gene expression analysis, used to compare molecular profiles of sensitive tumor slices with resistant tumor slices, showed that highly significant modulated genes were related to NK cells. Thirty genes were found to be down-modulated in resistant tumor slices, among them Nkp46, KIRs (activator and inhibitor receptors), KLRCs (activator and inhibitor receptors), and effector molecules (granzyme B and perforin 1). Results obtained by gene expression microarray were validated by PCR, and so these results might indicate more than just a regulation in their state of activation, but a deficiency in the infiltration of NK cells in tumor tissue resistant to chemotherapy prior to any treatment.
NK cells are a vital component of the innate immune system that plays an important role in tumor immune surveillance and in the prevention of progressive tumor growth as well as in defense against metastatic progress.39,40 It has been demonstrated that most human tumors show low levels of NK cell infiltration; however, human tumors that present more substantial infiltration of NK cells have been associated with improved prognosis and reduction in tumor recurrence.41-43
A previous report by Verma et al44 showed a significant inhibition of a specific phenotype of circulating NK cells and NK cell cytotoxicity in women with breast cancer. A significant reduction in cytotoxicity mediated by circulating NK cells in tumors that responded poorly to neoadjuvant chemotherapy was found. On the other hand, their study showed a significant increase in NK cells in the peritumoral environment in patients who achieved a good pathological response. However, intratumoral NK cells did not show the same correlation.44 In our study, RNA obtained from the whole tumor sample showed significantly down-modulated expression of NK cell receptor genes (KLRC1 to 4) in resistant tumor slices compared to sensitive counterparts.
Finally, it has been demonstrated that molecular signatures associated with NK cells are predictive markers of relapse-free survival in patients with breast cancer.45 These results are in accordance with our in silico analysis, where mRNA upregulation of effector molecules (GZMB, PRF1), NCR1, KIRs, and KLRCs was associated with overall survival (living) and disease-free status in patients with breast cancer.
However, aspects related to the migration and presence of NK cells in tumor tissue, and their correlation with achievement of a pCR following chemotherapeutic treatment in breast cancer, remain poorly documented in the literature.
Conclusion
A signature of NK cell-related genes in breast cancer might be useful for identification of a group of tumors highly sensitive to neoadjuvant chemotherapy. A diminished inflammatory infiltrate, as well as decreased expression of NK cells receptor genes, may be predictive markers for a poor chemotherapeutic response.
Supplemental Material
Supplementary Figure for Expression of NK Cell Surface Receptors in Breast Cancer Tissue as Predictors of Resistance to Antineoplastic Treatment by Garcia-Chagollan Mariel, Carranza-Torres Irma Edith, Carranza-Rosales Pilar, Guzmán-Delgado Nancy Elena, Ramírez-Montoya Humberto, Martínez-Silva María Guadalupe, Mariscal-Ramirez Ignacio, Barrón-Gallardo Carlos Alfredo, Pereira-Suárez Ana Laura, Aguilar-Lemarroy Adriana, and Jave-Suárez Luis Felipe in Technology in Cancer Research & Treatment
Abbreviations
- AB
alamar Blue
- ds-DNA
double-stranded complementary DNA
- FFPE
formalin-fixed, paraffin-embedded
- H&E
hematoxylin-eosin
- HER2
human epidermal growth factor receptor
- IMSS
Mexican Institute of Social Security
- KIR
killer cell immunoglobulin-like receptor
- KLRC
C-type killer cell lectin-like receptors
- mRNA
messenger RNA
- NCR1
natural cytotoxicity receptor 1
- pCR
complete pathological response
- PCR
polymerase chain reaction
- TILs
tumor infiltrating lymphocytes
- Treg
regulatory T cells
- Tx
paclitaxel
Footnotes
Authors’ Note: The data that support the findings of this study are available at the Gen Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) Series record: GSE99225.
This protocol was approved by the Ethical and Research National Committee of IMSS, with registration numbers: R-2014-785-022 (patient recruitment to test ex vivo sensitivity to taxanes) and R-2013-785-061 (obtaining formalin-fixed, paraffin-embedded [FFPE] biopsies and their clinical information to assess the infiltration of immune cells). Informed consent was obtained from all patients involved in this study. Clinical and personal information from retrospective samples (FFPE biopsies) collected from the Department of Pathology of the CMNO of IMSS were handled confidentially. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Authors’ Contributions: G.-C.M. performed microarray, PCR, and qRT-PCR experiments; she was also involved in the recruitment of patient samples and drafting the manuscript. C.-T.I. E. and C.-R.P. recruited biopsies of patients with breast cancer and evaluated its in vitro sensibility to chemotherapeutic drugs. R.-M.H. and M.-R.I. were involved in the recruitment of patients, collection of clinical information, and analysis and interpretation of data. G.-D.N.E. and M.-S.M.G. performed the pathological analysis; C.-R.P., P.-S.A.L., A.-L.A., and J.-S.L.F. conceived of and designed the theoretical framework of the study, provided scientific guidance throughout the project, and contributed to the writing of the manuscript. All authors approved this final version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by Fondo de Investigación en Salud, IMSS (FIS/IMSS/PROT/PRIO/14/030, to J-SLF).
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Franceschini G, Terribile D, Magno S, et al. Update in the treatment of locally advanced breast cancer: a multidisciplinary approach. Eur Rev Med Pharmacol Sci. 2007;11(5):283–289. [PubMed] [Google Scholar]
- 2. Giordano SH. Update on locally advanced breast cancer. Oncologist. 2003;8(6):521–530. [DOI] [PubMed] [Google Scholar]
- 3. Nicolini A, Carpi A. Advanced breast cancer: an update and controversies on diagnosis and therapy. Biomed Pharmacother. 2003;57(10):439–446. [DOI] [PubMed] [Google Scholar]
- 4. Masood S. Neoadjuvant chemotherapy in breast cancers. Womens Health (Lond). 2016;12(5):480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathew J, Asgeirsson KS, Cheung KL, Chan S, Dahda A, Robertson JF. Neoadjuvant chemotherapy for locally advanced breast cancer: a review of the literature and future directions. Eur J Surg Oncol. 2009;35(2):113–122. [DOI] [PubMed] [Google Scholar]
- 6. Greene J, Hennessy B. The role of anthracyclines in the treatment of early breast cancer. J Oncol Pharm Pract. 2015;21(3):201–212. [DOI] [PubMed] [Google Scholar]
- 7. Munro AF, Cameron DA, Bartlett JM. Targeting anthracyclines in early breast cancer: new candidate predictive biomarkers emerge. Oncogene. 2010;29(38):5231–5240. [DOI] [PubMed] [Google Scholar]
- 8. Steger GG, Bartsch R. Trends and novel approaches in neoadjuvant treatment of breast cancer. Breast Care (Basel). 2011;6(6):427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26(1):44–53. [DOI] [PubMed] [Google Scholar]
- 10. Palmieri C, Krell J, James CR, et al. Rechallenging with anthracyclines and taxanes in metastatic breast cancer. Nat Rev Clin Oncol. 2010;7(10):561–574. [DOI] [PubMed] [Google Scholar]
- 11. Faneyte IF, Schrama JG, Peterse JL, et al. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer. 2003;88(3):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tewari M, Krishnamurthy A, Shukla HS. Predictive markers of response to neoadjuvant chemotherapy in breast cancer. Surg Oncol. 2008;17(4):301–311. [DOI] [PubMed] [Google Scholar]
- 13. Becht E, Giraldo NA, Germain C, et al. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv Immunol. 2016;130:95–190. [DOI] [PubMed] [Google Scholar]
- 14. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. [DOI] [PubMed] [Google Scholar]
- 15. Stoll G, Zitvogel L, Kroemer G. Differences in the composition of the immune infiltrate in breast cancer, colorectal carcinoma, melanoma and non-small cell lung cancer: a microarray-based meta-analysis. Oncoimmunology. 2016;5(2):e1067746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann Oncol. 2015;26(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. [DOI] [PubMed] [Google Scholar]
- 18. Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013;16(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–527. [DOI] [PubMed] [Google Scholar]
- 20. Garcia-Martinez E, Gil GL, Benito AC, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16(6):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Issa-Nummer Y, Loibl S, von Minckwitz G, Denkert C. Tumor-infiltrating lymphocytes in breast cancer: a new predictor for responses to therapy. Oncoimmunology. 2014;3:e27926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carranza-Torres IE, Guzmán-Delgado NE, Coronado-Martínez C, et al. Organotypic culture of breast tumor explants as a multicellular system for the screening of natural compounds with antineoplastic potential. Biomed Res Int. 2015;2015:618021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chollet P, Amat S, Cure H, et al. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002;86(7):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Criscitiello C, Esposito A, Trapani D, Curigliano G. Prognostic and predictive value of tumor infiltrating lymphocytes in early breast cancer. Cancer Treat Rev. 2016;50:205–207. [DOI] [PubMed] [Google Scholar]
- 25. Stoll G, Enot D, Mlecnik B, Galon J, Zitvogel L, Kroemer G. Immune-related gene signatures predict the outcome of neoadjuvant chemotherapy. Oncoimmunology. 2014;3(1):e27884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buisseret L, Garaud S, de Wind A, et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. Oncoimmunology. 2017;6(1):e1257452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andre F, Dieci MV, Dubsky P, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19(1):28–33. [DOI] [PubMed] [Google Scholar]
- 28. West NR, Milne K, Truong PT, et al. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13(6):R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. [DOI] [PubMed] [Google Scholar]
- 30. Zitvogel L, Tesniere A, Apetoh L, Ghiringhelli F, Kroemer G. . Immunological aspects of anticancer chemotherapy [in French]. Bull Acad Natl Med. 2008;192(7):1469–1487; discussion 1487-1489. [PubMed] [Google Scholar]
- 31. Mahmoud S, Lee A, Ellis I, Green A.CD8(+) T lymphocytes infiltrating breast cancer: a promising new prognostic marker? Oncoimmunology. 2012;1(3):364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- 33. Miyashita M, Sasano H, Tamaki K, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res. 2015;17:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma C, Zhang Q, Ye J, et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189(10):5029–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. [DOI] [PubMed] [Google Scholar]
- 36. Ascierto ML, Kmieciak M, Idowu MO, et al. A signature of immune function genes associated with recurrence-free survival in breast cancer patients. Breast Cancer Res Treat. 2012;131(3):871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ignatiadis M, Singhal SK, Desmedt C, et al. Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J Clin Oncol. 2012;30(16):1996–2004. [DOI] [PubMed] [Google Scholar]
- 38. Yau C, Esserman L, Moore DH, Waldman F, Sninsky J, Benz CC. A multigene predictor of metastatic outcome in early stage hormone receptor-negative and triple-negative breast cancer. Breast Cancer Res. 2010;12(5):R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roberti MP, Mordoh J, Levy EM. Biological role of NK cells and immunotherapeutic approaches in breast cancer. Front Immunol. 2012;3:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Platonova S, Cherfils-Vicini J, Damotte D, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71(16):5412–5422. [DOI] [PubMed] [Google Scholar]
- 42. Eckl J, Buchner A, Prinz PU, et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med (Berl). 2012;90(1):55–66. [DOI] [PubMed] [Google Scholar]
- 43. Sznurkowski JJ, Zawrocki A, Biernat W. Subtypes of cytotoxic lymphocytes and natural killer cells infiltrating cancer nests correlate with prognosis in patients with vulvar squamous cell carcinoma. Cancer Immunol Immunother. 2014;63(3):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verma C, Kaewkangsadan V, Eremin JM, et al. Natural killer (NK) cell profiles in blood and tumour in women with large and locally advanced breast cancer (LLABC) and their contribution to a pathological complete response (PCR) in the tumour following neoadjuvant chemotherapy (NAC): differential restoration of blood profiles by NAC and surgery. J Transl Med. 2015;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ascierto ML, Idowu MO, Zhao Y, et al. Molecular signatures mostly associated with NK cells are predictive of relapse free survival in breast cancer patients. J Transl Med. 2013;11:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure for Expression of NK Cell Surface Receptors in Breast Cancer Tissue as Predictors of Resistance to Antineoplastic Treatment by Garcia-Chagollan Mariel, Carranza-Torres Irma Edith, Carranza-Rosales Pilar, Guzmán-Delgado Nancy Elena, Ramírez-Montoya Humberto, Martínez-Silva María Guadalupe, Mariscal-Ramirez Ignacio, Barrón-Gallardo Carlos Alfredo, Pereira-Suárez Ana Laura, Aguilar-Lemarroy Adriana, and Jave-Suárez Luis Felipe in Technology in Cancer Research & Treatment