Abstract
Aim
The purpose of this study was to evaluate the longitudinal changes in brain perfusion in patients with Lyme disease treated with human embryonic stem cells.
Material and methods
The study included 59 (age range 41.68 ± 16.37 years) patients with Lyme disease whose single-photon emission tomography imaging was performed before and after the human embryonic stem cell therapy. Technetium-hexa methyl propylene aminoxime single-photon emission tomography imaging was used to assess the hypoperfused lesions/regions in the brain prior to the therapy, as well as the improvement in perfusion after human embryonic stem cell treatment.
Results
After receiving human embryonic stem cell therapy, single-photon emission tomography imaging reflects a significant (>60%) improvement in 43 patients along with moderate (30–60%) and mild (<30%) improvement in 12 and four patients, respectively. The cerebral perfusion flow improved and the degree of hypoperfusion in the other regions significantly decreased after the human embryonic stem cell therapy. Interpretation of single-photon emission tomography imaging of brain images (before and after therapy) clearly presented the changes in color at various brain regions which represent the improvements in patients.
Conclusion
Single-photon emission tomography imaging could be used as a potential diagnostic tool to assess the response of Lyme disease patients to human embryonic stem cell therapy.
Keywords: Imaging, perfusion, magnetic resonance imaging, single-photon emission tomography imaging, neurological disease, human embryonic stem cell therapy
Introduction
Lyme disease (LD), also known as Lyme borreliosis, is an infectious disease involving a corkscrew-shaped spirochete, Borrelia burgdorferi (Bb) and is transmitted to humans through the bite of the black-legged tick, Ixodesricinus.1 It is also considered as a multi-organ-system infectious disease as its typical symptoms include fever, muscle and joint pain, swollen lymph nodes, headache, fatigue, and erythema migrans (rash with a bull’s eye appearance). If left untreated, it affects joints, heart, eyes, etc.2,3 However, the nervous system, i.e. central nervous system (CNS), the peripheral nervous system (PNS), and the autonomic nervous system (ANS), appear to be its primary targets.4–6
The diagnosis of LD is difficult for two reasons. The first diagnostic dilemma is established due to the variations in clinical presentation of the disease. Second, the symptoms presented by the people affected with LD are similar to the symptoms of other diseases such as influenza, multiple sclerosis, juvenile rheumatoid arthritis, etc. 7 Even, the cognitive decline in the patient may be considered psychological.8
Despite the non-specific nature of LD symptoms, LD is usually diagnosed/confirmed by serological tests, cerebro-spinal fluid (CSF) analysis, etc. However, the affectation associated with the brain can be visualized with both single-photon emission tomography (SPECT) imaging and magnetic resonance imaging (MRI) brain imaging.9,10 MRI represents abnormality by showing some areas of T2 hyper-intense signal. However, SPECT imaging can be considered a better technique, as it highlights the abnormalities related to brain perfusion. It has been observed that hypoperfusion in single region or multiple areas of brain accounts for about 70–75% of patients with LD.11,12 Figure 1 explains the distribution of hypoperfusion in various lobes of brain in patients with LD.
Figure 1.
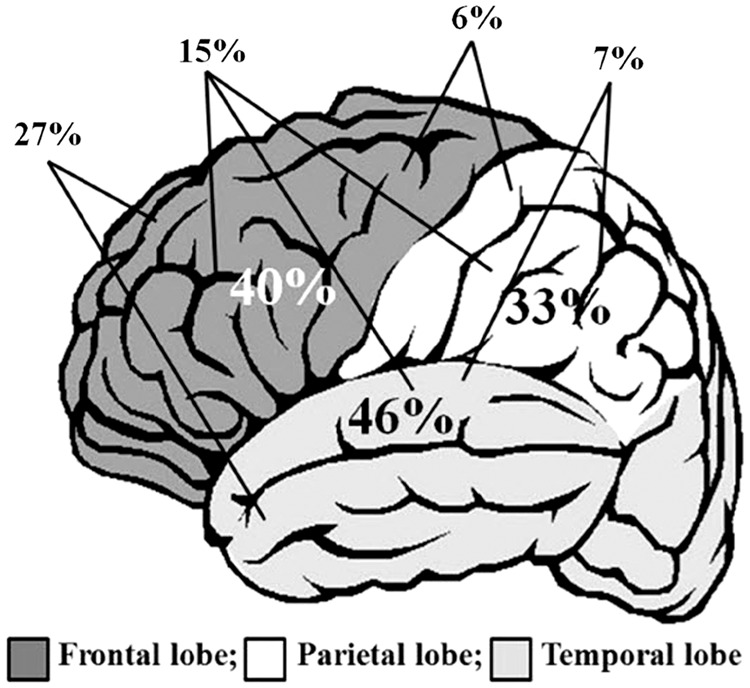
Distribution of hypoperfusion in various lobes of brain in patients with Lyme disease.
SPECT imaging using technetium-hexamethylpropyleneamine oxime (99 m Tc-HMPAO) is a useful tool to assess the LD patients neurologically.13 The level of perfusion depends upon the uptake of tracer, where an absence, reduced and increased tracer uptake is attributed to no perfusion, hypoperfusion and hyperperfusion in brain regions/lobes, respectively.14
Besides the ability of SPECT imaging to diagnose perfusion abnormalities, SPECT scan imaging is used to determine the improvement in patients over time after the treatment.15 The extent of perfusion improvement and regenerative potential of hESCs in patients with LD has been previously reported.1 The present study was conducted to evaluate the longitudinal changes in brain perfusion in patients with LD, treated with human embryonic stem cell (hESC) therapy, as reported previously.1
Materials and methods
Cases
All the patients with previously documented diagnosis of LD were admitted. Pregnant patients were not included in the study. Written informed consent and video consent were obtained from the patients or their guardians. The clinical transplantation of hESCs was approved by the Independent Ethics Committee (IEC) in a Good Clinical Practice (GCP)-compliant center. The study consisted of eight weeks of treatment period where SPECT imaging was performed before (to assess the number/area of hypoperfusion) and after the therapy (to determine the improvement in patients after receiving hESC therapy). The improvement in patients was clinically demonstrated by the efficacy of hESC therapy in treating LD patients, this was assessed by evaluating the change in SPECT scan at the end of treatment.16–18
SPECT imaging
Preparation of 99 m Tc-HMPAO
A HMPAO kit was purchased from Ceretec, Amersham Ltd, Amersham, UK where the fresh 99 m Tc elutes were used for preparation of 99 m Tc-HMPAO. The procedure for preparation has been previously reported.19
SPECT acquisition
The intravenous injection of 99 m Tc-HMPAO was administered into the antecubital vein of the patients in a quiet and dimly lit room. After 20 min, the brain SPECT imaging of patients was performed using a tomographic camera low-energy high resolution collimator on a dual-headed gamma camera. After a period of 15–120 min, the patient was supinely positioned so as to attain vertical positioning of the orbito-meatal line (OML) which was centered in the field of view.19
The SPECT scan was used to determine the abnormalities in brain perfusion prior to the therapy. It also assessed the improvement in perfusion to the brain after receiving the therapy. The SPECT imaging was scheduled to be performed before therapy or within seven days of initiation of hESC therapy and thereafter, the SPECT imaging of the same patients was performed after the treatment period was completed.
Gamma camera imaging
A dual-headed gamma camera of 8.5 mm crystal thickness equipped with a dual Digital Correlated Signal Enhancement (CSE) detector and 55–540 keV energy window, connected to a computer, was used to perform each imaging. Images were clicked in a step-and-shoot mode (128 projections, 35 s/projection) and were obtained on a 128 × 128 matrix. The peak of the photo was centered at 140 keV with a symmetrical 10% window. Reconstruction of images was performed using filtered back projection technique with zero attenuation correction.19
SPECT imaging analysis
The statistical parametric mapping (SPM) (NeuroGam) (The MathWorks, Inc., Natick, Massachusetts, USA) software, a complete package to display and quantify functional brain studies, was applied on the reconstructed data to evaluate brain perfusion. It performed qualitative and quantitative comparison of sequential brain images on the same and/or given patient. The details and working of the software has been described earlier.19
Image processing and interpretation
Interpretation of SPECT imagings was performed by Parul Mohan, having more than 15 years of experience in nuclear medicine. The interpretation of functional imaging has been described in Figure 2. The enhancement in perfusion of blood to the brain was an indication of improvement in LD patients receiving hESC therapy.
Figure 2.
Interpretation of single-photon emission tomography imagings. Grey: normal perfused regions; red, pink and white: above normal perfused regions; green, light/ dark blue, black: below normal perfused regions.
Statistical analysis
Regression analysis was used to evaluate the outcome measures of SPECT scans. An inbuilt database of the patient retains “x%” of the normal brain function. If the reading of a typical brain for the given gender and age is 100; then the reading of the brain function (RBF) can be taken as “100*x” and inherently, the brain function lost (BFL) can be taken as
This RBF, and consequently BFL, are theoretical and constructed variables. However, “x%” was available only for 44 cases. The database of 44 cases had (a) gender; (b) age at admission (ADAGE); (c) first reading of brain function (FRBF); all of these parameters were considered as theoretical and constructed variables. The percentage improvement (y%) over first brain function lost (FBFL) due to hESC therapy was considered as an independent variable (can be considered as last brain function lost (LBFL) or last reading of brain function (LRBF)). A p-value of 0.05 and confidence level of 95% was considered statistically significant.
Results
Cases
A total of 59 patients, with an age range of 41.68 ± 16.37 years, were included in the study, out of which 26 were males and 33 were females. The patients were treated with hESC therapy for around 56 days.
Outcome
The SPECT imaging was performed for all the patients, before and after the treatment. A clear/noticeable improvement was observed and reported after comparing the reports of SPECT imaging before and after the therapy (Table 1). Patients with significant improvement showed more than 60% improvement, whereas, moderate improvement in SPECT imaging was depicted by >30% and <60% change in perfusion, followed by mild improvement (>10% and <30%). Figure 3 represents the level of improvement in perfusion level in patients with LD receiving hESC therapy. It was observed that, out of 59 patients, 43 patients showed significant improvement followed by 12 patients showing moderate change. Only four patients exhibited mild improvement. None of the subjects was found to show deterioration after receiving hESC treatment in SPECT imaging.
Table 1.
Single photon emission computed tomography (SPECT) imaging investigations of patients with Lyme disease before and after human embryonic cell therapy.
| Patients | Before | After |
|---|---|---|
| 1. | A mild hypoperfusion was observed in bilateral temporal cortices, whereas, a moderate hypoperfusion was observed in bilateral cerebellar region. Besides, normal perfusion was observed in rest of the brain. | Minimal hypoperfusion in bilateral temporal cortices along with significant improvement in the degree of cerebral and cerebellar perfusion. |
| 2. | Mildly reduced perfusion was seen in both, frontal and right temporal lobes of the brain. | Normal perfusion was observed in temporal lobe with mildly reduced perfusion in right frontal region. |
| 3. | SPECT imaging revealed severe global cortical hypoperfusion with relative sparing of bilateral occipital regions. | Imaging evidence showed moderate hypoperfusion in right temporal region with moderate to severe hypoperfusion in bilateral cerebellar region. A significant improvement in the degree of cerebral and cerebellar perfusion was also seen. |
| 4. | Moderate hypoperfusion was observed in bilateral fronto-temporal region. A moderate to severe hypoperfusion in bilateral cerebellar region was also observed. | SPECT imaging showed mild hypoperfusion in bilateral fronto- and bilateral cerebellar region. However, no significant interval changes were noted. |
| 5. | Severe hypoperfusion in bilateral fronto temporal region along with moderate hypoperfusion in bilateral cerebellar and bilateral parietal region was observed. | Minimal hypoperfusion in bilateral fronto temporal and bilateral cerebellar region was observed. A significant improvement in degree of perfusion was also seen in cerebral and cerebellar region. |
| 6. | Severe global cortical hypoperfusion with relative sparing of bilateral posterior parietal and occipital cortices was observed. | Mild hypoperfusion was observed in bilateral parietal and temporal regions with moderate decrease in degree of hypoperfusion in cerebral regions. |
| 7. | There was moderate hypoperfusion in bilateral fronto- parieto-temporal region as well as in bilateral cerebellar region. | An improvement was reflected as mild to moderate hypoperfusion was observed in left cerebellar region along with minimal hypoperfusion in right cerebellar region. |
| 8. | There was mild hypoperfusion in right cerebellar, bilateral frontal and temporal region. | There was normal cerebral perfusion flow with no significant interval changes. |
| 9. | Mild to moderate hypoperfusion was seen in bilateral fronto - parieto-temporal region. | Minimal hypoperfusion was seen in left frontal region with normal perfusion in rest of brain was observed. Significant decrease in the degree of hypoperfusion was also seen in the cerebral regions. |
| 10. | Moderate hypoperfusion in bilateral fronto-parieto-temporal region with moderate hypoperfusion in bilateral cerebellar region was observed. | Minimal hypoperfusion in right temporal region was seen with no significant interval changes. |
| 11. | Imaging evidence showed severe hypoperfusion in bilateral frontal cortices; moderate to severe hypoperfusion in bilateral temporal regions, mild hypoperfusion in bilateral parietal cortices, moderate to severe hypoperfusion in left cerebellar region. The rest of the brain maintained normal perfusion. | There was normal cerebral perfusion flow with significant decrease in the degree of hypoperfusion seen in the cerebral regions. |
| 12. | Moderately reduced perfusion was observed in left orbito-frontal region, whereas mild hypoperfusion in right frontal, left temporal and left cerebellar region was also seen. Moreover, reduced perfusion in left basal ganglia was also reported. | Mild hypoperfusion in left temporal, left orbito-frontal, right frontal region and left cerebellum along with normal and symmetric perfusion in bilateral basal ganglia and right cerebellar cortex was reported. |
| 13. | Imaging evidence showed moderate to severe hypoperfusion in bilateral frontal and temporo-parietal region, moderately reduced perfusion in bilateral cerebellar regions. However, normal perfusion was maintained to the rest of brain. | After therapy, the following changes were observed: mild hypoperfusion in right frontal and bilateral temporal regions, minimal hypoperfusion in bilateral cerebellar regions. Others maintained perfusion to the rest of brain. |
| 14. | Global cortical hypoperfusion with relative sparing of bilateral occipital lobes was observed. | Mild to moderate hypoperfusion was observed in left frontal and bilateral temporal regions along with minimal hypoperfusion in bilateral cerebellar regions. Significant improvement in degree of perfusion was seen in cerebral and cerebellar regions. |
| 15. | Moderate to severe hypoperfusion was observed in bilateral temporo-parietal regions with mild hypoperfusion in bilateral thalamic and ganglionic regions. However, normal perfusion was maintained to the rest of brain. | After therapy, the following changes were observed: Moderate hypoperfusion in bilateral temporal regions, mild hypoperfusion in bilateral frontal regions, moderate decrease in hypoperfusion in cerebral region. |
| 16. | The SPECT imaging revealed the following: Moderate to severe hypoperfusion in bilateral fronto-temporal region, moderate hypoperfusion in left occipital region, moderate to severe hypoperfusion in bilateral cerebellar region. However, normal perfusion was maintained to the rest of brain. | After therapy, the following changes were observed: Moderate to severe hypoperfusion in bilateral fronto temporal region, moderate to severe hypoperfusion in bilateral cerebellar region. The degree of perfusion seen in the cerebral and cerebellar regions was mildly improved. |
| 17. | Global cortical hypoperfusion with relative sparing of bilateral occipital lobes was observed. | Only mild hypoperfusion in bilateral frontal and bilateral temporal regions. Comparatively, no significant interval changes notes. |
| 18. | Moderate hypoperfusion in bilateral temporal regions, small area of right frontal region and mild hypoperfusion in bilateral parietal, occipital and cerebellar region was noticed. | Improvement was noticed as the small area of right frontal region, left parietal and left temporal regions were mildly perfused. Minimal hypoperfusion was also noticed in bilateral cerebellar regions. The degree of perfusion in cerebral region was moderately improved. |
| 19. | The imaging findings revealed moderate hypoperfusion in cerebral region. | Normal cerebral perfusion flow was noticed. Moreover, the degree of perfusion in the cerebral region has significantly decreased. |
| 20. | Bilateral fronto temporal regions were moderately to severely hypoperfused. Moreover, moderate hypoperfusion was also observed in bilateral parietal region, bilateral basal ganglia and bilateral cerebellar regions. | There was no change observed in bilateral temporal region, whereas bilateral frontal regions, bilateral basal ganglia and bilateral cerebellar regions were found to be moderately hypoperfused. Moderate improvement in degree of cerebral and cerebellar perfusion was also observed. |
| 21. | The SPECT imaging revealed the following: Moderate to severe hypoperfusion in bilateral fronto-parietal regions; moderate hypoperfusion in bilateral temporal region, mild hypoperfusion in bilateral bilateral cerebellar regions. | A normal cerebral perfusion flow was observed with significant improvement in degree of perfusion in cerebral and cerebellar region. |
| 22. | The SPECT imaging revealed the following: Moderately reduced perfusion in right temporal region; mildly reduced perfusion in right prefrontal and left temporal regions; small area of reduced perfusion in left parietal region, normal perfusion in right basal ganglia and cerebellar cortices. | A normal cerebral perfusion flow was observed with significant improvement in degree of perfusion in cerebral and cerebellar region. |
| 23. | Moderate hypoperfusion in bilateral temporal cortices (left > right); mild to moderate hypoperfusion in bilateral parietal cortices were noticed. Rest of the brain maintained normal perfusion flow. | There was mild hypoperfusion in left temporal region whereas, near to normal cerebral and cerebellar perfusion flow in rest of the regions. |
| 24. | Severe hypoperfusion in bilateral fronto-parieto-temporal region with mild hypoperfusion in bilateral cerebellar region was observed. | Minimal hypoperfusion in left temporal and bilateral cerebellar region was observed. A significant improvement in degree of perfusion was also noticed in cerebral and cerebellar regions. |
| 25. | Mild to moderate hypoperfusion in bilateral parieto-temporal region along with mild hypoperfusion in right cerebellar region was seen in imaging. | Near normal cerebral perfusion flow was a significant improvement noticed after therapy. Significant improvement in the degree of perfusion in cerebral and cerebellar regions was also observed. |
| 26. | Moderate hypoperfusion in bilateral parietal, temporal and cerebellar regions was observed. | Bilateral temporal regions were found mildly hypoperfused whereas, the degree of hypoperfusion in cerebral and cerebellar region significantly decreased. |
| 27. | Mild hypoperfusion in bilateral temporal and cerebellar regions. | The degree of hypoperfusion in cerebral regions has moderately decreased. |
| 28. | Global cortical hypoperfusion was noticed with relative sparing of bilateral occipital cortices. | Normal cerebral perfusion flow along with significantly decreased degree of hypoperfusion in the cerebral and cerebellar regions was observed. |
| 29. | Global cortical hypoperfusion was observed with relative sparing of bilateral posterior parietal and occipital cortices. | The therapy showed following improvements: mild hypoperfusion in the right frontal region; mild to moderate hypoperfusion in bilateral parieto-temporal regions; mild hypoperfusion in bilateral cerebellar regions. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 30. | The imaging showed significant perfusion abnormality in the following areas: right fronto-temporal region (mild to moderately hypoperfused), left frontal region (mildly reduced). | The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 31. | Global cortical hypoperfusion was noticed with relative sparing of left occipital lobe. | The imaging showed mild hypoperfusion in bilateral fronto-temporal regions, whereas, moderate to severe hypoperfusion was observed in left cerebellar region. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 32. | Moderate hypoperfusion was noticed in bilateral cerebellar region. Besides, normal perfusion was maintained to the rest of the brain. | Mild hypoperfusion in bilateral parieto-temporal regions was noticed with no significant interval changes. |
| 33. | Global cortical hypoperfusion was noticed with relative sparing of bilateral posterior parietal and occipital cortices. | Mild hypoperfusion was noticed in bilateral fronto-temporal and left cerebellar regions.The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 34. | Moderate to severe hypoperfusion was noticed in bilateral fronto-temporal regions. Moreover, bilateral parietal regions were moderately hypoperfused. Moderate to severe hypoperfusion in bilateral cerebellar regions was also observed. | Moderate hypoperfusion in bilateral fronto-temporal regions along with minimal hypoperfusion in left cerebellar region was observed. The degree of perfusion in the cerebral and cerebellar regions was significantly improved. |
| 35. | Mild hypoperfusion in bilateral frontal and temporal regions along with minimal hypoperfusion in bilateral cerebellar regions was noticed. | Normal cerebral perfusion flow was observed. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 36. | The imaging evidence showed severe hypoperfusion in bilateral fronto-temporal regions, moderate hypoperfusion in bilateral parietal regions and minimal hypoperfusion in bilateral cerebellar regions. | A mild to moderate hypoperfusion was observed in left frontal and bilateral temporal regions. Left cerebellar region was found minimally hypoperfused. The degree of perfusion in the cerebral region was significantly improved. |
| 37. | The imaging evidence showed moderate hypoperfusion in bilateral temporal regions, mild to moderately reduced perfusion in bilateral frontal and parietal regions, moderate to severe hypoperfusion in bilateral cerebellar regions. | Normal cerebral perfusion flow was observed. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 38. | Global cortical hypoperfusion was noticed with relative sparing of bilateral occipital regions. | There was moderate to severe hypoperfusion involving bilateral fronto-temporal regions and bilateral cerebellar regions. |
| 39. | Global cortical hypoperfusion was noticed with relative sparing of bilateral occipital lobes. | There was minimal hypoperfusion in bilateral temporal regions. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly reduced. |
| 40. | Global cortical hypoperfusion was noticed with relative sparing of bilateral occipital lobes. | There was minimal hypoperfusion in bilateral temporal regions, whereas moderate hypoperfusion was observed in left cerebellar region. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 41. | Global cortical hypoperfusion was noticed with relative sparing of bilateral occipital lobes and cerebellum. | Normal cerebral perfusion flow was observed. The degree of perfusion in the cerebral and cerebellar regions has significantly improved. |
| 42. | Severe hypoperfusion was noticed in bilateral fronto-temporal regions. Moderate to severe hypoperfusion was also noticed in bilateral parietal regions and bilateral cerebellar regions. | The imaging evidence detected mild to moderate hypoperfusion in bilateral fronto-temporal regions; mild hypoperfusion in bilateral parietal regions and bilateral cerebellar regions. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly reduced. |
| 43. | Moderate hypoperfusion was observed in bilateral temporal as well as small area of right frontal region. There was mild hypoperfusion in bilateral parietal, occipital and cerebellar region. | The imaging evidence detected moderate hypoperfusion in bilateral fronto-temporal regions; minimal hypoperfusion in bilateral cerebellar regions. The degree of hypoperfusion in the cerebral and cerebellar regions was moderately decreased. |
| 44. | Moderate hypoperfusion was observed in bilateral fronto-parieto-temporal regions was well as in bilateral cerebellar regions. | There was normal cerebral perfusion flow. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 45. | Moderate hypoperfusion was detected in bilateral frontal, parietal and temporal regions. | There was mild to moderate hypoperfusion in bilateral temporal and left frontal regions and mild hypoperfusion in bilateral cerebellar regions. The degree of hypoperfusion in the cerebral regions was significantly decreased. |
| 46. | Global cortical hypoperfusion was noticed with relative sparing of bilateral posterior parietal and occipital cortices. | After therapy results showed moderate hypoperfusion in bilateral parieto-temporal regions; mild to moderate hypoperfusion in bilateral cerebellar regions. The degree of perfusion in the cerebral and cerebellar regions was significantly improved. |
| 47. | The imaging findings revealed moderate hypoperfusion in cerebellar region. | There was normal cerebral perfusion flow. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 48. | There was reduced perfusion in left basal ganglia and left temporal cortex. Small area of right fronto-parietal cortex was also found with moderately reduced perfusion. | There was normal cerebral perfusion flow. The degree of perfusion in the cerebral region was significantly decreased. |
| 49. | Before therapy, severe hypoperfusion was observed in bilateral fronto-temporal regions along with moderate hypoperfusion in bilateral parietal regions and moderate to severe hypoperfusion in bilateral cerebellar regions. Rest of the brain maintained normal perfusion flow. | After therapy, moderate to severe hypoperfusion was observed in bilateral fronto-temporal regions. Mild to moderate hypoperfusion was also noticed in bilateral cerebellar regions. The degree of perfusion in the cerebral and cerebellar regions was moderately improved. |
| 50. | Global cortical hypoperfusion was noticed with relative sparing of bilateral occipital lobes. | After therapy, the following improvements were observed: moderate hypoperfusion in bilateral fronto-parieto-temporal regions; moderate hypoperfusion in bilateral cerebellar regions. The degree of hypoperfusion in the cerebral and cerebellar regions was moderately decreased. |
| 51. | Before therapy, the following perfusions defects were observed: Moderately reduced perfusion in left temporal and frontal lobes; Mildly reduced perfusion in right temporal and frontal lobes; Small area of mildly reduced perfusion in both parietal lobes; Reduced perfusion in left basal ganglia. | After therapy, the following improvements were observed: minimal hypoperfusion in the left temporal region. Rest of the brain maintained normal perfusion flow. The degree of hypoperfusion in the cerebral region has significantly decreased. |
| 52. | Severe hypoperfusion in bilateral fronto-temporal regions was observed along with moderate hypoperfusion in bilateral parietal regions. Moderate to severe hypoperfusion in bilateral cerebellar regions was also noticed. | After therapy, mild hypoperfusion was noticed in bilateral fronto-temporal regions along with minimal hypoperfusion in bilateral parietal regions. Mild to moderate hypoperfusion was also observed in right cerebellar region. The degree of perfusion in the cerebral and cerebellar regions has significantly improved. |
| 53. | The SPECT imaging results before therapy showed Severe hypoperfusion in bilateral parieto-occipital regions (right > left); mild hypoperfusion in left fronto-parietal region; moderate to severe hypoperfusion in left cerebellar region. | After therapy, bilateral parieto-occipital regions (right > left) were found to be moderate to severely hypoperfused. There was mild hypoperfusion in left fronto-parietal region and left cerebellar region. The degree of hypoperfusion in the cerebral and cerebellar regions was mildly decreased. |
| 54. | The SPECT imaging results before therapy showed moderate to severe hypoperfusion in bilateral fronto-parietal and bilateral temporal regions; Moderate hypoperfusion in bilateral cerebellar regions. | Normal cerebral perfusion was observed. The degree of perfusion in the cerebral and cerebellar regions was significantly improved. |
| 55. | Moderate hypoperfusion in bilateral fronto-temporal regions. | Mild hypoperfusion was noticed in bilateral fronto-temporal regions. The degree of hypoperfusion in the cerebral region was moderate to significantly decreased. |
| 56. | The SPECT imaging results before therapy showed severe hypoperfusion in bilateral temporal regions; moderate hypoperfusion in bilateral parietal regions, minimal hypoperfusion in bilateral cerebellar regions. Normal perfusion was maintained to the rest of the brain. | Normal cerebral perfusion was observed. The degree of perfusion in the cerebral and cerebellar regions was significantly improved. |
| 57. | Moderate to severe hypoperfusion in bilateral parieto-temporal regions along with moderate hypoperfusion in bilateral cerebellar regions was noticed. | Normal cerebral perfusion flow was noticed. The degree of perfusion in the cerebral and cerebellar regions was significantly improved. |
| 58. | Mildly reduced perfusion was noticed in the both the temporal lobes, left basal ganglia, and both fronto-parietal regions. A perfusion defect was also observed in focal area of left cerebellum. | Minimal focal hypoperfusion was noticed in left cerebellum. Normal perfusion was maintained to the rest of the brain. The degree of hypoperfusion in the cerebral and cerebellar regions was significantly decreased. |
| 59. | Global cortical hypoperfusion was noticed with relative sparing of bilateral occipital lobes. | Moderate hypoperfusion was noticed in bilateral frontal and left temporal regions. |
Figure 3.

Improvement in hypoperfusion levels in patients with Lyme disease after receiving human embryonic stem cell therapy.
The clinical improvement was based on the brain perfusion state of patients. After comparing the SPECT imaging results of prior and after therapy, it was observed that the patients showed an improvement. They were observed with severe/moderate/mild brain hypoperfusions prior to therapy. After receiving hESC therapy, their perfusion level upgraded to moderate/mild/minimal and even normal perfusion levels. The regression analysis showed that the variables FRBF and LRBF predominate the course of the linear regression equation. ADAGE has marginal presence in the regression equation. For women, R2 = 0.97 or the overall accuracy of the regression analysis was good (Table 2) and for the men R2 = 0.82 or the overall accuracy of the regression analysis was satisfactory (Table 3). However, the ADAGE for women was −2.256 (p < 0.05) and for men was 0.702 (p > 0.05). The results shown that the ADAGE contributed negatively (in the case of women), insignificantly and inefficiently in both the cases which corresponds to the statement that, the higher age produces higher estimates of LRBF. In women, the correlation between FRBF and LRBF was good but, the correlation with ADAGE was not so good. Whereas, in men the correlation among FRBF and LRBF as well as with ADAGE was good.
Table 2.
Correlation of different factors related to females.
| Parameters | ADAGE | FRBF | LRBF |
|---|---|---|---|
| ADAGE | |||
| Pearson correlation | 1 | 0.248a | 0.163a |
| Significance (two-tailed) | 0.231 | 0.436 | |
| n | 25 | 25 | 25 |
| FRBF | |||
| Pearson correlation | 0.248a | 1 | 0.981a |
| Significance (two-tailed) | 0.231 | 0 | |
| n | 25 | 25 | 25 |
| LRBF | |||
| Pearson correlation | 0.163a | 0.981a | 1 |
| Significance (two-tailed) | 0.436 | 0 | |
| n | 25 | 25 | 25 |
ADAGE: age at admission; FRBF: first reading of brain function; LRBF: last reading of brain function; n: number of subjects.
Correlation is significant at 0.01 level (two-tailed).
Table 3.
Correlation of different factors related to males.
| Parameters | ADAGE | FRBF | LRBF |
|---|---|---|---|
| ADAGE | |||
| Pearson correlation | 1 | 0.651a | 0.648a |
| Significance (two-tailed) | 0.003 | 0.003 | |
| n | 19 | 19 | 19 |
| FRBF | |||
| Pearson correlation | 0.651a | 1 | 0.913a |
| Significance (two-tailed) | 0.003 | 0 | |
| n | 19 | 19 | 19 |
| LRBF | |||
| Pearson correlation | 0.648a | 0.913a | 1 |
| Significance (two-tailed) | 0.003 | 0 | |
| n | 19 | 19 | 19 |
ADAGE: age at admission; FRBF: first reading of brain function; LRBF: last reading of brain function; n: number of subjects.
Correlation is significant at 0.01 level (two-tailed).
Interpretation of SPECT imaging of brain images (before and after therapy) clearly presented the change in color at various brain regions. This represented the improvement in patients. In a patient A (Figure 4a), moderate hypoperfusion was observed in bilateral fronto-parieto-temporal and bilateral cerebellar regions in the before therapy images. After receiving hESC therapy, normal cerebral perfusion flow was observed. Moreover, the degree of hypoperfusion in the cerebral and cerebellar regions also significantly decreased. The prior therapy SPECT-imaging images of patient B demonstrated global cortical hypoperfusion with relative sparing of bilateral occipital cortices. However, the perfusion flow significantly improved to normal in cerebral and cerebellar regions after receiving hESC therapy (Figure 4b).
Figure 4a.
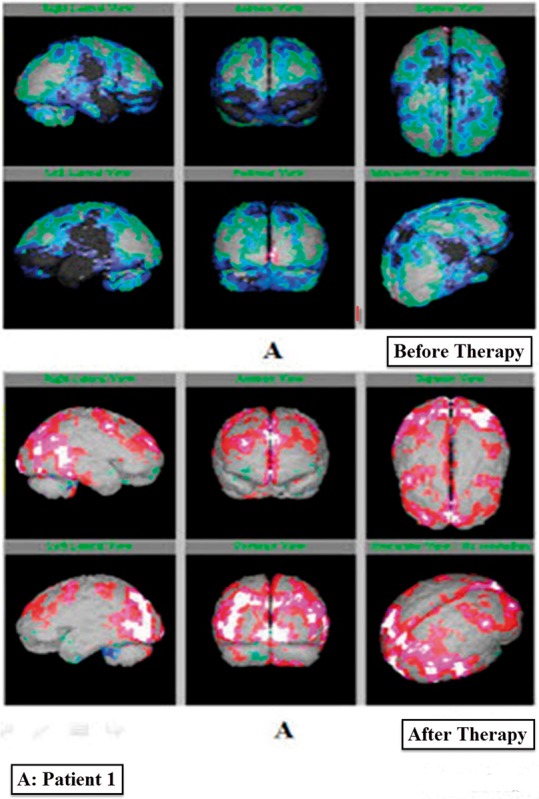
Single-Photon Emission Tomography imaging reports of patient A with lyme disease before and after therapy.
Figure 4b.
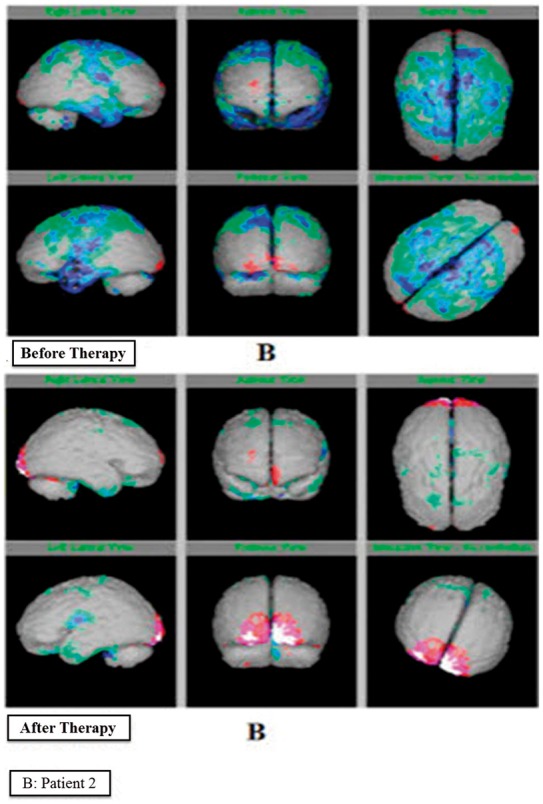
Single-Photon Emission Tomography imaging reports of patient B with lyme disease before and after therapy.
Discussion
LD results from an infection with Bb, affecting multiple organs and organ systems. The nervous system is usually targeted, leading to various manifestations associated with the CNS.4–6 The patients with LD usually suffer from poor circulation in regions affecting memory and cognition, with an overflow in the occipital lobe. This might be the cause of poor concentration, cognitive disabilities and highly sensitive eyes in LD patients.20
The screening of patients with neurological manifestation of LD is generally performed using MRI or SPECT imaging techniques.21 But, SPECT imaging is considered as a better diagnostic tool.22 SPECT imaging has been performed in many previous studies to determine abnormalities of the brain.6,11,15,23
Besides the use of SPECT imaging to diagnose perfusion abnormalities, it is also used to determine the improvement in patients over time due to treatment.15 The present study evaluated SPECT imaging as a diagnostic tool for detection of LD in patients receiving hESC therapy. An improvement detected by SPECT imaging in LD patients showing an increased perfusion flow in brain was the evidence of the beneficial effects of hESC therapy. As Figure 4 reveals, before the hESC therapy, the green, dark blue, light blue and black colored areas depict the hypoperfused regions while, after hESC therapy, the color changed to grey, red white and pink which depicts normal regions. A clear change from red to grey, white and pink is showing an increased perfusion flow in the brain. A reduced uptake of tracer was observed in patients before therapy, portraying the hypoperfused regions of the brain. During and after treatment, the migration of hESCs to the affected area led to an improvement in perfusion which was evident from the SPECT imaging reports. 24 The highest percentage of patients was observed to exhibit significant improvement in perfusion levels, reflecting the efficacy of hESC therapy in treating LD, as well as the diagnostic ability of the SPECT imaging technique to detect the improvement in patients. hESC therapy has also shown beneficial effects in treating incurable/degenerative diseases.22 It has been used for the treatment of various disorders such as spinal cord injury, multiple sclerosis, cerebral palsy, Friedreich’s ataxia, Parkinson’s disease, etc.17,18,25,26 It has also been reported that stem cells have the tendency to replace damaged neurons and repair damaged brain cells.27 The results of the treatment of LD patients have been published where hESC therapy helped patients in regaining their strength to walk and balance along with other significant improvements. An advantage of using hESCs in treating the patients with LD has also been well documented in the same case series demonstrating the use of hESC therapy in treating five patients with LD. The clinical condition of the patient was correlated with the positive changes in the SPECT scan.1
A study conducted by Sumiya et al., has suggested that the brain perfusion SPECT provides useful information in diagnosing the patients with Lyme neuroborreliosis.28 SPECT imaging of the brain of LD patients provides an objective measure/evidence of abnormalities in patients which can assist/support in clinical diagnosis of LD.11 It also helps in the early detection of diseases.19 Its increased imaging sensitivity and ability to depict the brain’s working physiology helps in achieving a reliable depiction (as compared to other imaging techniques such as MRI, positron emission tomography) which makes it easy to distinguish LD from diseases showing similar symptomatic or diagnostic presentations.13,18 Being inexpensive and a useful tool to differentiate various neuro-indications, it is preferred over other neuro-imaging techniques.29 A study conducted by Sumiya and co-authors in 1997 reported SPECT imaging using 99mTc-HMPAO as an efficient tool for differential diagnosis of LD.13 Another study conducted by Donta et al., 2012 screened brains of 183 patients with LD using SPECT imaging technique and other standard nuclear imaging techniques. It was observed that around 75% of patients showed perfusion defects in the frontal, temporal, and parietal lobes of brain which were detected by SPECT imaging, whereas only 14% of patients were diagnosed as LD affected by MRI.11
SPECT uses radioisotopes which are bound to neuro-specific pharmaceuticals to evaluate regional cerebral blood flow (rCBF) as well as metabolic activity. HMPAO and ethylcysteinate dimer (ECD) are two most commonly available radiopharmaceuticals which are FDA-approved. HMPAO was used in the present study as its perfusion is intensive in the cerebellum.29,30 Full, uniform and symmetrical perfusion images are observed in normal SPECT imaging. Therefore, the presence of asymmetry, areas of increased and decreased perfusion represents abnormalities. It is important to keep the patient’s age under consideration due to the change in rCBF over time.31
Though SPECT imaging is highly used as a screening technique for patients with LD, it has many flaws associated with its sensitivity to diagnose a patient with LD.21 The presentation of decreased perfusion as a Swiss cheese pattern also creates confusion.15,29 Another issue associated with SPECT imaging is that it cannot differentiate between the problem arising due to malfunctioning of blood vessels or nerve cells.15 SPECT imaging has been suggested to be used as a supporting tool in the differential diagnosis of LD, and not as a diagnostic imaging technique.18 However, the present study demonstrated SPECT imaging in use as an important tool for monitoring the benefits in patients treated with hESC therapy. The present study has been conducted on a small cohort of patients, which is the major limitation of the study. Therefore, the replication of such a study using a large sample size is warranted.
Conclusion
Brain SPECT imaging might be used as a prognostic and single tool for assessment of improvement in patients (in terms of brain perfusion) treated with hESC therapy. Further studies must be conducted to examine the diagnostic value of SPECT imaging for the detection of LD as well as for evaluation of the efficacy of hESC therapy in LD. SPECT imaging is an effective tool for differential diagnosis and detection of relapsing and persistent symptomatic diseases. Therefore, its screening ability could be utilized to assess the patient with LD before and after hESC therapy.
Acknowledgements
The author wishes to acknowledge Harsh Mahajan and Parul Mohan for contribution in result analysis and all the doctors, staff and patients of the Nutech Mediworld. The author also acknowledges Knowledge Isotopes Pvt Ltd (http://www.knowledgeisotopes.com) for medical writing assistance.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Shroff G. Treatment of Lyme disease with human embryonic stem cells: A case series. J Neuroinfect Dis 2015; 6: 1–6. [Google Scholar]
- 2.Vasudevan B, Chatterjee M. Lyme borreliosis and skin. Indian J Dermatol 2013; 58: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Lyme disease, http://www.cdc.gov/lyme/index.html (2017, accessed 20 February 2017).
- 4.Cadavid D, O’Neill T, Schaefer H, et al. Localization of Borrelia burgdorferi in the nervous system and other organs in a nonhuman primate model of Lyme disease. Lab Invest 2000; 80: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 5.Fallon BA, Nields JA. Lyme disease: A neuropsychiatric illness. Am J Psychiatry 1994; 151: 1571–1583. [DOI] [PubMed] [Google Scholar]
- 6.Donta ST. Late and chronic Lyme disease. Med Clin North Am 2002; 86: 341–349. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. Lyme disease: What you need to know, http://www.cdc.gov/lyme/resources/brochure/lymediseasebrochure.pdf (accessed 20 February 2017).
- 8.Matteson W. Missing the diagnosis: The hidden medical causes of mental disorders, https://www.continuingedcourses.net/active/courses/course067.php (2015, accessed 22 February 2017).
- 9.Henry B, Crabtree A, Roth D, et al. Lyme disease: Knowledge, beliefs, and practices of physicians in a low-endemic area. Can Fam Physician 2012; 58: e289–e295. [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron DJ. Clinical trials validate the severity of persistent Lyme disease symptoms. Med Hypotheses 2009; 72: 153–156. [DOI] [PubMed] [Google Scholar]
- 11.Donta ST, Noto RB, Vento JA. SPECT brain imaging in chronic Lyme disease. Clin Nucl Med 2012; 37: e219–e222. [DOI] [PubMed] [Google Scholar]
- 12. Lyme and Tick-Borne Diseases Research Center, Spinal Fluid and Brain Tests, http://www.columbia-lyme.org/patients/ld_spinal_fluid.html (accessed 1 April 2016).
- 13.Sumiya H, Kobayashi K, Mizukoshi C, et al. Brain perfusion SPECT in Lyme neuroborreliosis. J Nucl Med 1997; 38: 1120. [PubMed] [Google Scholar]
- 14.Catafau AM. Brain SPECT in clinical practice. Part I: perfusion. J Nucl Med 2001; 42: 259–271. [PubMed] [Google Scholar]
- 15.Fallon BA, Das S, Plutchok JJ, et al. Functional brain imaging and neuropsychological testing in Lyme disease. Clin Infect Dis 1997; 25: S57–S63. [DOI] [PubMed] [Google Scholar]
- 16.Claassen L, Uden T, Ettinger M, et al. Influence on therapeutic decision making of SPECT-CT for different regions of the foot and ankle. Biomed Res Int 2014; 2014: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shroff G, Gupta R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Ann Neurosci 2015; 22: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shroff G, Gupta A, Barthakur JK. Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J Transl Med 2014; 12: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shroff G, Barthakur JK, Mohan P, et al. Single photon emission computed tomography scan as a diagnostic tool in children with cerebral palsy treated with human embryonic stem cells. J Nucl Med Radiat Ther 2015; 6: 1–6. [Google Scholar]
- 20.Grier TM. The complexities of Lyme disease. A microbiology tutorial. Lyme Disease Survival Manual 2000. [Google Scholar]
- 21.Bransfield R. The neuropsychiatric assessment of Lyme disease, http://www.mentalhealthandillness.com/tnaold.html (2008, accessed 22 February 2017).
- 22.Shroff G, Barthakur JK. Safety of human embryonic stem cells in patients with terminal/incurable conditions – a retrospective analysis. Ann Neurosci 2015; 22: 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silbersweig DA, Stern E. Symptom localization in neuropsychiatry. A functional neuroimaging approach. Ann N Y Acad Sci 1997; 835: 410–420. [DOI] [PubMed] [Google Scholar]
- 24.Kang SK, Shin IS, Ko MS, et al. Journey of mesenchymal stem cells for homing: Strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int 2012; 2012: 342968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shroff G, Das L. Human embryonic stem cell therapy in cerebral palsy children with cortical visual impairment: A case series of 40 patients. Int J Stem Cell Res Ther 2014; 5: 1. [Google Scholar]
- 26.Shroff G. A novel approach of human embryonic stem cells therapy in treatment of Friedreich’s ataxia. Int J Case Rep Imag 2015; 6: 261–266. [Google Scholar]
- 27.Pabon MM, Borlongan CV. Advances in the cell-based treatment of neonatal hypoxic-ischemic brain injury. Future Neurol 2013; 8: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumiya H, Kobayashi K, Mizukoshi C, et al. Brain perfusion SPECT in Lyme neuroborreliosis. The Journal of Nuclear Medicine 1997; 38: 1120. [PubMed] [Google Scholar]
- 29.Amen DG, Trujillo M, Newberg A, et al. Brain SPECT imaging in complex psychiatric cases: An evidence-based, underutilized tool. Open Neuroimag J 2011; 5: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datz F, Patch G, Arias J, et al. Nuclear medicine: A teaching profile, St Louis, MO: Mosby Yearbook, 1992. [Google Scholar]
- 31.Mena F, Mena I, Álamos F, et al. SPECT Tc99m-HMPAO brain uptake in normal children: A comparison to normal elderly subjects. Alasbimn Journal 1998; 1. [Google Scholar]



