Abstract
Purpose
This study explored the prevalence and characteristics of Enterococcus faecalis biofilm formation by urinary tract infection (UTI) isolates in order to identify virulence factors associated with biofilm formation.
Methodology
A total of 113 E. faecalis isolates were collected from UTI patients in Shenzhen, China. The isolates were subjected to multilocus sequence typing based on housekeeping genes. Biofilms were detected by crystal violet staining and the expression levels of the E. faecalis genes were detected by quantitative real-time PCR.
Results/Key findings
The main sequence types (STs) were ST16 and ST179 with the ST16 isolates more likely to form strong biofilms than the ST179 isolates (P=0.008). Strong biofilm formation was more frequently detected in aggregation substance (agg)-positive (+) isolates than in negative (−) isolates (P=0.033). Biofilm formation was also more common in isolates containing enterococcal surface protein (esp), or cytolysin A (cylA)-positive (+) isolates than in isolates negative (−) for these virulence factors. Multivariate regression analysis indicated that cylA [odds ratio (OR), 7.143, P=0.012] was associated with weak biofilm formation, and that agg (OR, 4.471, P=0.004) was associated with strong biofilm formation. The expression of cylA was increased (8.75- to 23.05-fold) in weak biofilm, and the expression of agg was greatly elevated (11.99- to 439.10-fold) in strong biofilm isolates when compared to biofilm-negative isolates.
Conclusion
ST16 classification was positively associated with strong biofilm formation in E. faecalis as was agg, while cylA was associated with weak biofilm formation.
Keywords: Enterococcus faecalis, biofilm, urinary-tract infections, virulence genes, multilocus sequence typing
Introduction
Urinary tract infections (UTIs) are common in women and are one of the most frequent human bacterial infections [1]. With 150 million cases per year, UTIs are responsible for significant worldwide morbidity and loss of workplace productivity [2]. Although most UTIs (80 %–90 %) are caused by extra-intestinal Escherichia coli strains, recently Enterococcus faecalis strains have more frequently been isolated and have been reported to be causative agents in up to 20 % of all cases [3, 4]. E. faecalis UTIs are of particular concern in that they are intrinsically resistant to first-line antimicrobial agents, especially vancomycin [5]. Another concern is that E. faecalis strains can form biofilms that are difficult to eradicate with one report demonstrating that 100 % [6] and another that 70 % of urinary tract E. faecalis strains can form biofilms [7]. However, in China the prevalence and characteristics of E. faecalis UTI strains and their capacity to form biofilms is unknown.
Phylogenetic relationships of bacterial pathogens can be investigated by multilocus sequence typing (MLST), including Staphylococcus spp. and Enterococcus spp. [8]. The virulence and drug resistance of strains are distinctly different between various sequence types (STs) [9]. Biofilm formation, another important characteristic of bacteria, is also diverse between various ST isolates [10]. For example, Staphylococcus epidermidis ST27 was found to occur preferentially in hospitals and differed from community isolates in that hospital ST27 had the capacity to form biofilms [10]. ST17 and ST19 lineages of group B Streptococcus strains derived from invasive disease were significantly more likely to form weak biofilms than strains recovered from asymptomatic individuals that formed strong biofilms [11]. The relationship of various E. faecalis STs to biofilm formation has not been explored.
There are several virulence factors that are related to E. faecalis biofilm formation. The enterococcal surface protein (esp) has been found to adhere to and colonize abiotic surfaces, playing an important role in E. faecalis biofilm formation [12, 13]. E. faecalis gelatinase (gelE) is an extracellular metalloprotease, which can hydrolyse gelatin, collagen and haemoglobin, and is reported to be involved in bacterial adherence and biofilm formation [14, 15]. In a large collection of E. faecalis isolates no significant relationship among esp, gelE and biofilm formation was found [16–18]. Likewise, E. faecalis aggregating substance (agg) was found to promote biofilm formation by Chuang-Smith et al., but that report was not confirmed by other studies [19]. Thus, the association of virulence factors with E. faecalis biofilm formation is controversial and unresolved.
The aims of this study are to explore the prevalence and characteristics of UTI E. faecalis isolates, to assess their capacity to form biofilms, and to probe STs and virulence factors associated with biofilm formation. To our knowledge this is the first study to simultaneously assess biofilm formation, virulence genes, antimicrobial resistance and MLST for E. faecalis UTI isolates from China. As such, this study will provide a better understanding of E. faecalis and its capacity to form biofilms.
Methods
Bacterial isolates
A total of 113 E. faecalis isolates were obtained from the urine of UTI patients at Shenzhen Nanshan Hospital, Shenzhen University, China between 1 January 2011 and 30 June 2016. Bacterial isolates from patients were identified by the VITEK 2 system (Biomérieux, Marcy l’Etoile, France). E. faecalis ATCC29212 and OG1RF (ATCC47077) were used as reference strains and obtained from the American Type Culture Collection (ATCC).
Antimicrobial susceptibility testing
Isolates' susceptibility to the clinically relevant antimicrobials; penicillin G, ampicillin, ciprofloxacin, teicoplanin, vancomycin, linezolid, high-level gentamicin, erythromycin and tetracycline was assessed with the VITEK 2 system (Biomérieux, Marcy l’Etoile, France). The MIC of ampicillin, vancomycin and linezolid were assessed by the broth macrodilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. The MIC breakpoints were determined based on the guidelines of CLSI for 2016.
Isolation of DNA
The DNA from all isolates was extracted and purified using DNeasy Blood and Tissue Kits (QIAGEN China, Zhangjiang Hi-Tech Park Pudong, Shanghai, PR China) according to the manufacturer’s protocol for Gram-positive bacteria. The microbial DNA was eluted with 100 µl AE buffer (Qiagen) and stored at −20 °C.
Detection of virulence genes by PCR
The extracted DNA served as a template for the amplification of virulence genes. All primer sequences and corresponding references are listed in Table S1 (available in the online version of this article) [20, 21]. PCR amplification was performed in a total volume of 50 µl, containing 2X PCR Master Mix (Tiangen Biotech (Beijing)), 0.5 mM of each of the primers and 1 µl of template DNA. The cycling conditions were as follows: 95 °C for 3 min, followed by 30 cycles of 95 °C for 30 s, 52 °C for 30 s and 72 °C for 60 s, with final extension at 72 °C for 10 min. Each set of PCR reactions included a no-template control and a positive control. The amplification products were analysed by gel electrophoresis using a 1.0 % agarose gel.
E. faecalis MLST and clonal complex (CC) testing
MLST of E. faecalis isolates was performed according to the reference method [22]. Briefly, the seven housekeeping genes; gdh, gyd, pstS, gki, aroE, xpt and yqiL were PCR amplified and sequenced. All primer sequences and corresponding references are listed in Table S2. The PCR reaction system and cycling conditions were as described above, except for the primers. For each locus, a distinct allele number was assigned to each sequence, in accordance with the E. faecalis MLST database (http://efaecalis.mlst.net/). Allelic profile or STs were assigned in the order; gdh, gyd, pstS, gki, aroE, xpt and yqiL to a total of seven integers corresponding to the allele numbers at the seven loci. STs were assigned to isolates in such a way that the same ST names were consistent with the same isolates analysed. E. faecalis CCs were analysed by the eBURST v 3.0 program [23]. CCs were defined as groups of two or more independent isolates that shared identical alleles at six or more loci; each complex was named after the putative founder ST.
Biofilm assay
Biofilm formation by E. faecalis isolates was done according to the reference method with modifications [24]. Briefly, the E. faecalis isolates were cultivated overnight in Tryptic Soy Broth (TSB) (OXOID; Basingstoke, Hampshire, UK) at 37 °C in ambient air for 10–12 h and shaken at 220 r min−1. Overnight cultures were diluted 1 : 100 in 200 µl of TSBG (TSB with 0.25 % glucose) (1.0–3.0×107 c.f.u. ml−1) and inoculated into polystyrene microtitre plates (Costar3599, Corning). After 24 h of static incubation at 37 °C, the supernatant was discarded, and plates were washed thrice with deionized water to remove unattached cells, fixed with methanol for 30 min, stained with 1 % crystal violet (CV) for 30 min, and rinsed with distilled water. The optical density at 570 nm (OD570) was determined. Each assay was performed in triplicate at least three times.
Quantitative real-time PCR
The expression levels of esp, agg and cylA genes of E. faecalis clinical isolates were detected by quantitative real-time (qRT)-PCR. The primers used for qRT-PCR are listed in Table S3. The RNA extraction was conducted using the reference method [25] with modification. Briefly, the E. faecalis isolates were cultured at 37 °C until the OD600 reached 0.6, the cell pellets were washed with ice-cold normal saline and then homogenized using 0.1 mm zirconia-silica beads in a Mini-BeadBeater (Biospec, Bartlesville, USA) at a speed of 4000 r.p.m. for 1 min, followed by cooling on ice for 1 min. This homogenization and cooling cycle was repeated five times. The samples were then centrifuged at 15 000 r.p.m. and the bacterial RNA in the supernatant was purified using RNeasy Mini Kits (Qiagen) and quantified using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA). RNA samples, which had a 260/280 ratio between 2.0 and 2.2 were reverse transcribed with the PrimeScript RT Reagent Kit (TaKaRa). Finally, qRT-PCR was performed with SYBR Premix Ex Taq II Kits (TaKaRa) and the Mastercycler ep realplex system (Eppendorf), with an initial incubation at 95 °C for 2 min, followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C. Each sample was analysed in triplicate.
For all samples, the internal control gene recA was used to normalize the expression of esp, agg and cylA [26]. The threshold cycle (Ct) numbers were determined by the detection system software and the data were analysed based on the 2−△△Ct method.
Statistical analysis
The prevalence of E. faecalis biofilms is shown as percentages and compared using the chi-square test. The virulence factors associated with biofilm formation were analysed with a multivariate logistic regression model. The logistic regression model was constructed by a backward selection approach based on the Wald statistic. P-values<0.05 were regarded as statistically significant. All data were analysed using the statistical software SPSS (version 14.0, Chicago, IL, USA).
Results
Biofilm formation by E. faecalis isolates from UTIs
OD570 microplate readings after CV staining ranged from 0.05 to 3.5 and were blanked with the non-inoculated reference well. Isolates with a biofilm phenotype were categorized as described previously [24], as strong (OD570≥1) or weak (OD570>0.5 and <1). The median OD570 values for controls was 0.55 for strain OG1RF (weak biofilm [27]) and 0.16 for strain ATCC29212 (negative biofilm).
The prevalence of E. faecalis biofilms from urinary tracts was 50.4 % (57/113) and the distribution characteristics of the biofilm phenotype were as follows: strong biofilms were 26.5 % (30/113) and weak biofilms were 23.9 % (27/113). Among the biofilm-forming isolates, more than half had a strong biofilm phenotype 52.6 % (30/57). There were 29 E. faecalis isolates from catheter-related UTIs in this study, and the prevalence of biofilm formation was similar between those catheter-related UTIs (14/29, 48.3 %) and catheter-unrelated UTIs (43/84, 51.2 %). The strong biofilm phenotype of E. faecalis was also similar between those catheter-related UTIs (7/29, 24.1 %) and catheter-unrelated UTIs (23/84, 27.4 %).
Correlation between biofilm formation and virulence factors
The E. faecalis virulence factors were PCR amplified and analysed. As shown in Table 1, esp-positive isolates had more prevalent biofilm formation than esp-negative isolates (59.7 vs 30.6 %, P=0.004). Similarly, biofilm formation was more frequently detected in asa1 (surface aggregating protein) and cylA (cytolysin A)-positive isolates than in negative isolates (respectively, 56.3 vs 17.6 %, P=0.003 and 58.3 vs 27.6 %, P=0.004). Strong biofilm formation was more frequently detected in agg-positive isolates than in negative isolates (38.1 vs 19.7 %, P=0.033). However, gelE-negative isolates had greater biofilm formation than gelE-positive isolates (68.4 vs 41.3 %, P=0.007). This was particularly true for those strong biofilm-forming isolates (Table 1, see the footnote).
Table 1. Relationship between biofilm-forming capacity and virulence factors.
| Virulence factors (no. of isolates tested) | No. (%) of isolates with biofilm phenotype | Pa | ||
|---|---|---|---|---|
| Weak | Strong | All positive | ||
| esp+ (77) | 21 (27.3) | 25 (32.5) | 46 (59.7) | 0.004 |
| esp- (36) | 6 (16.7) | 5 (13.9) | 11 (30.6) | |
| gelE+ (75) | 16 (21.3) | 15 (20.0) | 31 (41.3) | 0.007 |
| gelE- (38) | 11 (28.9) | 15 (39.5)b | 26 (68.4) | |
| asa1+ (96) | 25 (26.0) | 29 (30.2) | 54 (56.3) | 0.003 |
| asa1- (17) | 2 (11.8) | 1 (5.9) | 3 (17.6) | |
| cylA+ (84) | 25 (29.8) | 24 (28.6) | 49 (58.3) | 0.004 |
| cylA- (29) | 2 (6.9) | 6 (20.6) | 8 (27.6) | |
| hyl+ (25) | 5 (20.0) | 7 (28.0) | 12 (48.0) | 0.782 |
| hyl- (88) | 22 (25.0) | 23 (26.1) | 45 (51.1) | |
| agg+ (42) | 7 (16.7) | 16 (38.1)c | 23 (54.8) | 0.480 |
| agg- (71) | 20 (28.2) | 14 (19.7) | 34 (47.9) | |
a, Biofilm formation: + versus −; b, strong biofilm: gelE- versus gelE+, P=0.027; c, strong or medium biofilm: agg+ versus agg-, P=0.033.
The association between biofilm formation and MLST
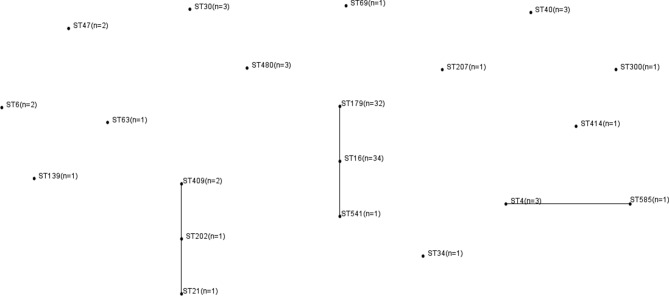
Of the 113 E. faecalis clinical isolates, 98 were identified successfully. The MLST of the remaining 15 E. faecalis isolates could not be determined due to the poor sequencing results despite multiple attempts. Overall, 23 different STs were identified and 21 of those were represented by four or fewer isolates. CC16 was predominant (Fig. 1). The biofilm characteristics and number of isolates assigned to each ST are reported in Table S4.
Fig. 1.
Distribution of MLST and clonal complex (CC) among 98 E. faecalis isolates. Connecting lines indicate CCs.
The main MLSTs of this study were ST16 and ST179, and these two STs accounted for 67.3 % (66/98) of the isolates. The relationship between E. faecalis biofilm formation and ST16 and ST179 was analysed with strong biofilm formation more frequently detected in ST16 isolates than in ST179 isolates (32.4 vs 6.3 %, P=0.008), Table 2. Total biofilm formation was also greater in ST16 isolates than in ST179 isolates (64.8 vs 31.3 %, P=0.007). E. faecalis virulence factors of ST16 and ST179 were analysed and agg-positive isolates were more common in ST16 isolates than in ST179 isolates (58.8 vs 15.6 %, P<0.001), Table 3. However, gelE-positive isolates were more prevalent in ST179 isolates than in ST16 isolates (87.5 vs 20.6 %, P<0.001).
Table 2. Biofilm-forming capacity for ST16 and ST179 isolates.
| Biofilm phenotype | No. (%) with phenotype | P | |
|---|---|---|---|
| ST16(n=34) | ST179(n=32) | ||
| Weak | 11 (32.4) | 8 (25.0) | 0.510 |
| Strong | 11 (32.4) | 2 (6.3) | 0.008 |
| All positive | 22 (64.8) | 10 (31.3) | 0.007 |
ST, sequence type.
Table 3. Virulence factor traits for ST16 and ST179.
| STs (n) | No. (%) of isolates with virulence factors | |||||
|---|---|---|---|---|---|---|
| esp+ | gelE+ | asa1+ | cylA+ | hyl+ | agg+ | |
| ST16 (n=34) | 27 (79.4) | 7 (20.6) | 32 (94.1) | 31 (91.2) | 3 (8.8) | 20 (58.8)a |
| ST179 (n=32) | 27 (84.4) | 28 (87.5)b | 31 (96.9) | 30 (93.8) | 5 (15.6) | 5 (15.6) |
ST, sequence type; +, positive.
a, agg+: ST16 versus ST179, P<0.001; b, gelE+: ST179 versus ST16, P<0.001.
Association of biofilm formation with antimicrobial susceptibility
The MICs of ampicillin, vancomycin and linezolid were tested by the broth macrodilution method, and the drug resistance was analysed based on the guidelines of CLSI for 2016. A total of 18 isolates were insensitive to linezolid (MIC>2 µl ml−1), four isolates were resistant to ampicillin, and none of the isolates were resistant to vancomycin or teicoplanin. Biofilm formation was not associated with antimicrobial sensitive or insensitive isolates for ciprofloxacin, tetracycline or high-level gentamicin, Table S5.
Multivariate regression analysis of virulence factors associated with biofilm formation
In order to determine the independent contribution of each virulence factor to biofilm formation by E. faecalis, multiple logistic regression analysis was performed. Virulence factors, esp, gelE, asa1, cylA, hyl (hyaluronidase) and agg, were used as independent variables for weak biofilm formation, with strong biofilm formation used as a dependent variable in the multivariate model. The logistic regression model was constructed by a backward selection approach based on the Wald statistic. P-values<0.05 were regarded as statistically significant.
As Table 4 shows, for weak biofilm formation only cylA (OR, 7.143, 95 % CI, 1.539–33.140; P=0.012) was an independent risk factor. Other virulence factors, esp, gelE, asa1 and agg, were not risk factors for E. faecalis weak biofilm formation. Virulence factor agg (OR, 4.471, 95 % CI, 1.593–12.552; P=0.004) was independently associated with strong biofilm formation. It is of interest that gelE-positive isolates (OR, 0.215, 95 % CI, 0.076–0.608; P=0.004) showed diminished strong biofilm formation.
Table 4. Multivariate regression analysis of virulence factors associated with E. faecalis biofilm formation.
| Isolate biofilm phenotype | Factor | OR (95 % CI) | P |
|---|---|---|---|
| Weak biofilm formation | |||
| cylA | 7.143 (1.539–33.140) | 0.012 | |
| Strong biofilm formation | |||
| gelE | 0.215 (0.076–0.608) | 0.004 | |
| agg | 4.471 (1.593–12.552) | 0.004 |
OR, odds ratio; CI, confidence interval.
High expressions of agg and cylA in E. faecalis biofilm positive isolates
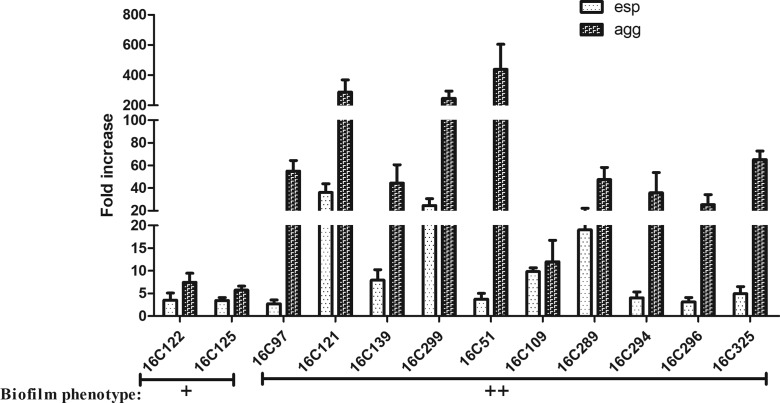
In order to verify the roles of esp, agg and cylA genes in biofilm formation, expression levels were determined by qRT-PCR. For the detection of esp and agg, 13 clinical E. faecalis isolates positive for esp and agg were selected. 16C99 was used as a negative biofilm reference strain (esp expression=1 and agg expression=1). As shown in Fig. 2, agg expression was markedly elevated (11.99- to 439.10-fold) in strong biofilm formation, compared to weak biofilm formation (16C125, 5.73-fold and 16C122, 7.45-fold). However, esp expression was mildly elevated (2.13- to 41.55- fold) in strong biofilm formation. These results suggest that agg plays a more important role than esp in strong biofilm formation by E. faecalis isolates from UTIs, which is consistent with previous findings.
Fig. 2.
Expression levels of esp and agg in 12 clinical E. faecalis isolates. The expression levels of esp and agg were determined by qRT-PCR, with the clinical E. faecalis isolate 16C99 as the reference strain (expression=1, biofilm negative). Biofilm phenotype: +, weak; ++, strong. In the strong biofilm isolates, agg was overexpressed (11.99- to 439.10-fold).
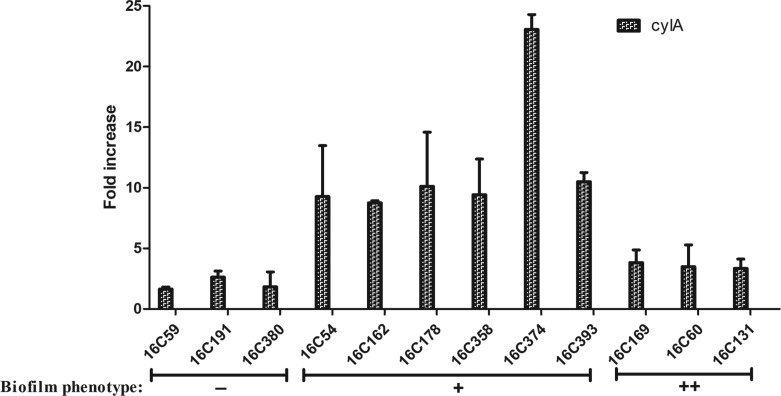
In total, 13 clinical cylA positive E. faecalis isolates were assessed for biofilm formation with the 16C76 strain used as a negative biofilm reference strain (cylA expression=1). As shown in Fig. 3, cylA gene expression was higher (8.75- to 23.05- fold) in those isolates that formed weak biofilms, compared with negative biofilm isolates (16C59, 1.64-fold; 16C191, 2.62-fold; 16C380, 1.83-fold), or strong biofilm isolates (16C169, 3.82-fold; 16C60, 3.49-fold; 16C131, 3.34-fold). These data demonstrate cylA to be associated with weak biofilm formation and not with strong biofilm formation by these E. faecalis UTI isolates.
Fig. 3.
Expression levels of cylA in 12 clinical E. faecalis isolates. The expression levels of cylA were determined by qRT-PCR, with the clinical E. faecalis isolate 16C76 as the reference strain (expression=1, biofilm negative). Biofilm phenotype: −, negative; +, weak; ++, strong. In the weak biofilm isolates, cylA was overexpressed (8.75- to 23.05-fold).
Discussion
E. faecalis has become a common UTI pathogen. The drug resistance of E. faecalis is less than that of E. faecium but the prevalence of E. faecalis biofilm formation is higher than that for E. faecium [28] even though differences in incidence vary from one study to another. For example, 100 % of urinary tract E. faecalis isolates in Japan formed biofilms, while in India the prevalence was less than 80 % [6, 7]. In this study 50.4 % formed biofilms, significantly lower than those studies. Differences may be due to differing STs. The prevalence of catheter-related UTIs in this study was 25.7 %, which was similar to previous research (30.4 %) [6]. This study found E. faecalis from catheter-related UTIs was not associated with increased biofilm formation, and this is consistent with a previous study [6].
In China, the role of different E. faecalis STs in biofilm formation and the relationship of these to MLST was previously unknown. This study found 23 different STs, with ST16 and ST179 predominant. This result is similar to a previous report from Malaysia that found ST6, ST16, ST28, ST179 and ST399 in E. faecalis isolates [29]. Herein biofilm formation by ST16 and ST179 was significantly different even though these two STs belong to the same CC group. ST16 isolates produced more biofilms with stronger biofilm formation than ST179 isolates. This difference may be due to the expression of more agg and less gelE by ST16 isolates than ST179 isolates. Previously, ST16 isolates have been associated with hospital-adapted isolates [22]. Bacterial biofilms are more resistant to adverse environmental conditions and are more resistant to antimicrobials and disinfectants than planktonic bacteria [30]. Hospital environments rather than community environments have more antimicrobials and disinfectants, thus biofilm formation among hospital-adapted isolates is more likely than community-acquired isolates. ST16 of E. faecalis, like ST27 of Staphylococcus epidermidis, may have arisen in nosocomial environments, where there is a higher prevalence of STs that form biofilms [10].
Virulence factors of E. faecalis that contribute to biofilm formation are controversial. Previous studies have associated esp with E. faecalis biofilm formation [12, 13] and esp was shown to enhance E. faecalis biofilm formation [31]. As mentioned above, a previous Japanese study found E. faecalis with esp to form biofilms at a significantly higher rate than those without the gene [6]. Another study from India found both medium and strong biofilm isolates to be esp-positive [7]. In contrast, a study of clinical and laboratory E. faecalis isolates found no significant relationship between esp and E. faecalis biofilm formation [16–18]. In this study no association of esp with either weak or strong biofilm formation was found. Clarification of the virulence factors involved in E. faecalis biofilm formation requires further investigation.
Previous studies have found gelE, which hydrolyses gelatin, collagen and haemoglobin, to be associated with the development of E. faecalis biofilms [14, 15, 32]. However, in a large collection of E. faecalis isolates no significant relationship was found between gelE and biofilm formation [17, 18]. This study found biofilm formation to be more common in gelE-negative isolates than in gelE-positive isolates. This was particularly true for those isolates with the capacity to form strong biofilms. It may be that the gene sequence types of E. faecalis isolates in this study differ from those previous studies, or that unknown factors contribute to E. faecalis strong biofilm formation.
In addition to esp and gelE, previous studies have reported that aggregating substance promotes E. faecalis biofilm formation [19]. It is a plasmid-encoded bacterial adhesin that mediates efficient contact between donor and recipient bacteria, facilitating plasmid exchange [33]. In addition to its adhesive function during bacterial conjugation, agg mediates adhesion of E. faecalis to a variety of eukaryotic cells in vitro. This study confirmed that agg plays an important role in strong biofilm formation, but has no effect on weak biofilm formation. The role of agg in E. faecalis biofilm formation is under reported and requires further investigation.
This study has shown that E. faecalis isolates from UTIs easily form biofilms. The MLSTs of E. faecalis in this study were diverse, with ST16 and ST179 predominant. ST16 isolates displayed a higher biofilm-forming capacity than ST179 isolates. Further, the cylA gene was associated with weak biofilm formation, while agg was associated with strong biofilm formation by E. faecalis isolates from UTIs.
Funding information
This work was supported by grants from the National Natural Science Foundation of China (No. 81170370), Sanming Project of Medicine in Shenzhen (SMGC201705029), the Shenzhen Scientific Research Program (Nos. JCYJ20170412143551332, JCYJ20170307153714512, JCYJ20170307153425389 and JCYJ20170307153919735), the Scientific Research Project of the Shenzhen Health and Family Planning System (No. 201601058) and the Shenzhen Nanshan District Scientific Research Program of the People’s Republic of China (No. 2016010).
Acknowledgements
The authors would like to thank Yang Wu (Key Laboratory of Medical Molecular Virology of Ministries of Education and Health, School of Basic Medical Science and Institutes of Biomedical Sciences, Shanghai Medical College of Fudan University, China) for his excellent technical support and suggestions.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
All procedures involving human participants were in accordance with the ethical standards of Shenzhen University and with the 1964 Helsinki declaration and its later amendments and comparable ethical standards. For this type of study formal consent is not required.
Supplementary Data
Footnotes
Abbreviations: agg, aggregation substance; asa1, surface aggregating protein; cylA, cytolysin A; eDNA, extracellular DNA; esp, enterococcal surface protein; gelE, gelatinase; hyl, hyaluronidase; MLST, multilocus sequence typing; ST, sequence type; UTI, urinary tract infection.
Five supplementary tables are available with the online version of this article.
References
- 1.Jakobsen L, Garneau P, Bruant G, Harel J, Olsen SS, et al. Is Escherichia coli urinary tract infection a zoonosis? Proof of direct link with production animals and meat. Eur J Clin Microbiol Infect Dis. 2012;31:1121–1129. doi: 10.1007/s10096-011-1417-5. [DOI] [PubMed] [Google Scholar]
- 2.Abat C, Huart M, Garcia V, Dubourg G, Raoult D. Enterococcus faecalis urinary-tract infections: do they have a zoonotic origin? J Infect. 2016;73:305–313. doi: 10.1016/j.jinf.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–474. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 4.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113:14S–19S. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 5.O'Driscoll T, Crank CW. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist. 2015;8:217–230. doi: 10.2147/IDR.S54125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seno Y, Kariyama R, Mitsuhata R, Monden K, Kumon H. Clinical implications of biofilm formation by Enterococcus faecalis in the urinary tract. Acta Med Okayama. 2005;59:79–87. doi: 10.18926/AMO/31979. [DOI] [PubMed] [Google Scholar]
- 7.Garg S, Mohan B, Taneja N. Biofilm formation capability of enterococcal strains causing urinary tract infection vis-a-vis colonisation and correlation with enterococcal surface protein gene. Indian J Med Microbiol. 2017;35:48–52. doi: 10.4103/ijmm.IJMM_16_102. [DOI] [PubMed] [Google Scholar]
- 8.Urwin R, Maiden MC. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 2003;11:479–487. doi: 10.1016/j.tim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Manning SD, Springman AC, Lehotzky E, Lewis MA, Whittam TS, et al. Multilocus sequence types associated with neonatal group B streptococcal sepsis and meningitis in Canada. J Clin Microbiol. 2009;47:1143–1148. doi: 10.1128/JCM.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozitskaya S, Olson ME, Fey PD, Witte W, Ohlsen K, et al. Clonal analysis of Staphylococcus epidermidis isolates carrying or lacking biofilm-mediating genes by multilocus sequence typing. J Clin Microbiol. 2005;43:4751–4757. doi: 10.1128/JCM.43.9.4751-4757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker RE, Laut C, Gaddy JA, Zadoks RN, Davies HD, et al. Association between genotypic diversity and biofilm production in group B Streptococcus. BMC Microbiol. 2016;16:86. doi: 10.1186/s12866-016-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toledo-Arana A, Valle J, Solano C, Arrizubieta MJ, Cucarella C, et al. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl Environ Microbiol. 2001;67:4538–4545. doi: 10.1128/AEM.67.10.4538-4545.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paganelli FL, Willems RJ, Leavis HL. Optimizing future treatment of enterococcal infections: attacking the biofilm? Trends Microbiol. 2012;20:40–49. doi: 10.1016/j.tim.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Kayaoglu G, Orstavik D. Virulencefactorsof Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med. 2004;15:308–320. doi: 10.1177/154411130401500506. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Kim KM, Lee JH, Seo SJ, Lee IH. Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum. Infect Immun. 2007;75:1861–1869. doi: 10.1128/IAI.01473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristich CJ, Li YH, Cvitkovitch DG, Dunny GM. Esp-independent biofilm formation by Enterococcus faecalis. J Bacteriol. 2004;186:154–163. doi: 10.1128/JB.186.1.154-163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed JA, Murray BE. Lack of correlation of gelatinase production and biofilm formation in a large collection of Enterococcus faecalis isolates. J Clin Microbiol. 2005;43:5405–5407. doi: 10.1128/JCM.43.10.5405-5407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson AC, Jonas D, Huber I, Karygianni L, Wölber J, et al. Enterococcus faecalis from food, clinical specimens, and oral sites: prevalence of virulence factors in association with biofilm formation. Front Microbiol. 2016;6:1534. doi: 10.3389/fmicb.2015.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang-Smith ON, Wells CL, Henry-Stanley MJ, Dunny GM. Acceleration of Enterococcus faecalis biofilm formation by aggregation substance expression in an ex vivo model of cardiac valve colonization. PLoS One. 2010;5:e15798. doi: 10.1371/journal.pone.0015798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vankerckhoven V, van Autgaerden T, Vael C, Lammens C, Chapelle S, et al. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J Clin Microbiol. 2004;42:4473–4479. doi: 10.1128/JCM.42.10.4473-4479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duprè I, Zanetti S, Schito AM, Fadda G, Sechi LA. Incidence of virulence determinants in clinical Enterococcus faecium and Enterococcus faecalis isolates collected in Sardinia (Italy) J Med Microbiol. 2003;52:491–498. doi: 10.1099/jmm.0.05038-0. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Garbajosa P, Bonten MJ, Robinson DA, Top J, Nallapareddy SR, et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol. 2006;44:2220–2228. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect Immun. 2004;72:3658–3663. doi: 10.1128/IAI.72.6.3658-3663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu T, Wu Y, Lin Z, Bertram R, Götz F, et al. Identification of genes controlled by the essential YycFG two-component system reveals a role for biofilm modulation in Staphylococcus epidermidis. Front Microbiol. 2017;8:724. doi: 10.3389/fmicb.2017.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz-Cruz S, Espinosa M, Goldmann O, Bravo A. Global regulation of gene expression by the mafr protein of Enterococcus faecalis. Front Microbiol. 2015;6:1521. doi: 10.3389/fmicb.2015.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montealegre MC, La Rosa SL, Roh JH, Harvey BR, Murray BE. The Enterococcus faecalis EbpA pilus protein: attenuation of expression, biofilm formation, and adherence to fibrinogen start with the rare initiation codon ATT. MBio. 2015;6:e00467-15. doi: 10.1128/mBio.00467-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandoe JA, Witherden IR, Cove JH, Heritage J, Wilcox MH. Correlation between enterococcal biofilm formation in vitro and medical-device-related infection potential in vivo. J Med Microbiol. 2003;52:547–550. doi: 10.1099/jmm.0.05201-0. [DOI] [PubMed] [Google Scholar]
- 29.Weng PL, Ramli R, Shamsudin MN, Cheah YK, Hamat RA. High genetic diversity of Enterococcus faecium and Enterococcus faecalis clinical isolates by pulsed-field gel electrophoresis and multilocus sequence typing from a hospital in Malaysia. Biomed Res Int. 2013;2013:1–6. doi: 10.1155/2013/938937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014;78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tendolkar PM, Baghdayan AS, Gilmore MS, Shankar N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun. 2004;72:6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas VC, Thurlow LR, Boyle D, Hancock LE. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol. 2008;190:5690–5698. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kayaoglu G, Ørstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med. 2004;15:308–320. doi: 10.1177/154411130401500506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.