Abstract
The Partitiviridae is a family of small, isometric, non-enveloped viruses with bisegmented double-stranded (ds) RNA genomes of 3–4.8 kbp. The two genome segments are individually encapsidated. The family has five genera, with characteristic hosts for members of each genus: either plants or fungi for genera Alphapartitivirus and Betapartitivirus, fungi for genus Gammapartitivirus, plants for genus Deltapartitivirus and protozoa for genus Cryspovirus. Partitiviruses are transmitted intracellularly via seeds (plants), oocysts (protozoa) or hyphal anastomosis, cell division and sporogenesis (fungi); there are no known natural vectors. This is a summary of the International Committee on Taxonomy of Viruses (ICTV) Report on the taxonomy of the Partitiviridae, which is available at www.ictv.global/report/partitiviridae.
Keywords: Partitiviridae, ICTV, taxonomy, Alphapartitivirus, Betapartitivirus, Deltapartitivirus, Gammapartitivirus, Cryspovirus
Virion
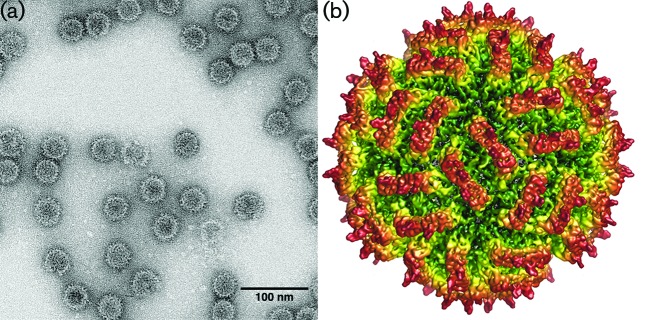
Virus particles are isometric, non-enveloped, and 25–43 nm in diameter (Table 1, Fig. 1a, b). Each capsid is composed of 120 copies of a single protein arranged as 60 dimers with T=1 icosahedral symmetry [1]. Dimeric surface protrusions are frequently observed on viral capsids. One or two molecules of RNA-dependent RNA polymerase (RdRP) are packaged inside each particle [2].
Table 1. Characteristics of the family Partitiviridae.
| Typical member: | Atkinsonella hypoxylon virus, 2H (RNA1, L39125; RNA2, L39126), species Atkinsonella hypoxylon virus, genus Betapartitivirus |
|---|---|
| Genome | 3–4.8 kbp of linear bisegmented dsRNA |
| Virion | Isometric, non-enveloped, 25–43 nm in diameter; dsRNA1 and dsRNA2 are separately encapsidated |
| Replication | Cytoplasmic. Genomic RNA acts as a template for mRNA synthesis within the virus particle; transcription occurs by a semiconservative mechanism |
| Translation | From monocistronic positive-sense transcripts of both genomic dsRNAs |
| Host range | Plants, fungi and protozoa |
| Taxonomy | Five genera, including >40 species, and 15 species unassigned to a genus |
Fig. 1.
(a) Transmission electron micrograph of negatively-stained purified particles of Penicillium stoloniferum virus S, a representative member of the genus Gammapartitivirus. (b) Cryo-EM reconstructions of Penicillium stoloniferum virus S at 0.45 nm resolution, and rendered with radial colour mapping.
Replication
Each dsRNA is monocistronic. The RdRP is believed to function as both a transcriptase and a replicase and catalyzes in vitro end-to-end transcription of each dsRNA to produce mRNA by a semi-conservative mechanism. Virions accumulate in the cytoplasm.
Genome
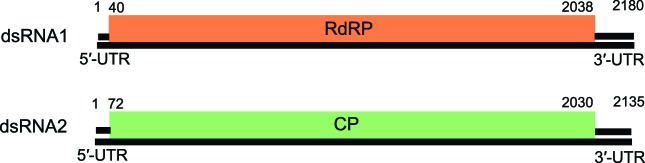
Members of all five genera possess two essential genome segments, dsRNA1 and dsRNA2, each containing one large ORF on the positive-strand RNA molecule (Fig. 2). The smaller of the two dsRNA genome segments usually encodes the coat protein (CP) and the larger usually encodes the virion-associated RNA polymerase. The linear dsRNA segments are separately encapsidated. Additional dsRNA segments (satellite or defective) may also be present.
Fig. 2.
Atkinsonella hypoxylon virus [10], an isolate of the type species of the genus Betapartitivirus, has a bipartite genome consisting of dsRNA1 and dsRNA2.
Taxonomy
Alphapartitivirus
Members of the genus Alphapartitivirus infect either plants, or ascomycetous or basidiomycetous fungi. The two essential dsRNA genome segments are individually about 1.9–2.0 kbp (dsRNA1) and 1.7–1.9 kbp (dsRNA2), typically containing a poly(A) tract near the plus-strand 3′-terminus. There is a single major CP with predicted Mr of 51–57 kDa. Plant alphapartitiviruses cause persistent infections, whereas some fungal alphapartitiviruses cause host effects, such as hypovirulence or a reduced growth rate [3, 4].
Betapartitivirus
Members of the genus Betapartitivirus infect either plants, or ascomycetous or basidiomycetous fungi. The two essential dsRNA genome segments are about 2.2–2.4 kbp (dsRNA1) and 2.1–2.4 kbp (dsRNA2), typically containing a poly(A) tract near the plus-strand 3′-terminus. There is a single major CP with predicted Mr of 71–77 kDa. Plant betapartitiviruses cause persistent infections [5, 6]. Some fungal betapartitiviruses cause reduced host virulence and changes in colony morphology [7].
Gammapartitivirus
All known members of the genus Gammapartitivirus infect ascomycetous fungi. The two essential dsRNA segments are about 1.6–1.8 kbp (dsRNA1) and 1.4–1.6 kbp (dsRNA2). There is a single major CP with predicted Mr of 44–47 kDa. Most gammapartitiviruses seem to induce latent infections. Aspergillus fumigatus partitivirus 1, a related, unclassified virus, has been associated with host effects.
Deltapartitivirus
All known members of the genus Deltapartitivirus induce persistent infections in plants [8]. They are transmitted by ovule and pollen to the seed embryo. The two essential dsRNA segments are individually 1.6–1.7 kbp (dsRNA1) and 1.4–1.6 kbp (dsRNA2). There is a single major CP with predicted Mr of 38–49 kDa.
Cryspovirus
Members of the genus Cryspovirus infect apicomplexan protozoa of the genus Cryptosporidium [9]. The viral genome comprises two dsRNA segments, which are individually 1.5 and 1.8 kbp. There is a single major CP with predicted Mr of 37 kDa. Virions are disseminated within Cryptosporidium oocysts. Infections of the Cryptosporidium host cells appear to be latent.
Resources
Full ICTV Online (10th) Report: www.ictv.global/report/partitiviridae.
Funding information
Production of this summary, the online chapter, and associated resources was funded by a grant from the Wellcome Trust (WT108418AIA).
Acknowledgements
Members of the ICTV Report Consortium are Elliot J. Lefkowitz, Andrew J. Davison, Stuart G. Siddell, Sead Sabanadzovic, Donald B. Smith, Richard J. Orton and Peter Simmonds.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CP, coat protein; RdRP, RNA-dependent RNA polymerase.
References
- 1.Nibert ML, Tang J, Xie J, Collier AM, Ghabrial SA, et al. 3D structures of fungal partitiviruses. Adv Virus Res. 2013;86:59–85. doi: 10.1016/B978-0-12-394315-6.00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nibert ML, Ghabrial SA, Maiss E, Lesker T, Vainio EJ, et al. Taxonomic reorganization of family Partitiviridae and other recent progress in partitivirus research. Virus Res. 2014;188:128–141. doi: 10.1016/j.virusres.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Chiba S, Lin YH, Kondo H, Kanematsu S, Suzuki N. Effects of defective interfering RNA on symptom induction by, and replication of, a novel partitivirus from a phytopathogenic fungus, Rosellinia necatrix. J Virol. 2013;87:2330–2341. doi: 10.1128/JVI.02835-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vainio EJ, Hantula J. Taxonomy, biogeography and importance of Heterobasidion viruses. Virus Res. 2016;219:2–10. doi: 10.1016/j.virusres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Lesker T, Rabenstein F, Maiss E. Molecular characterization of five betacryptoviruses infecting four clover species and dill. Arch Virol. 2013;158:1943–1952. doi: 10.1007/s00705-013-1691-x. [DOI] [PubMed] [Google Scholar]
- 6.Roossinck MJ. Lifestyles of plant viruses. Philos Trans R Soc Lond B Biol Sci. 2010;365:1899–1905. doi: 10.1098/rstb.2010.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao X, Cheng J, Tang J, Fu Y, Jiang D, et al. A novel partitivirus that confers hypovirulence on plant pathogenic fungi. J Virol. 2014;88:10120–10133. doi: 10.1128/JVI.01036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabanadzovic S, Valverde RA. Properties and detection of two cryptoviruses from pepper (Capsicum annuum) Virus Genes. 2011;43:307–312. doi: 10.1007/s11262-011-0634-4. [DOI] [PubMed] [Google Scholar]
- 9.Nibert ML, Woods KM, Upton SJ, Ghabrial SA. Cryspovirus: a new genus of protozoan viruses in the family Partitiviridae. Arch Virol. 2009;154:1959–1965. doi: 10.1007/s00705-009-0513-7. [DOI] [PubMed] [Google Scholar]
- 10.Oh CS, Hillman BI. Genome organization of a partitivirus from the filamentous ascomycete Atkinsonella hypoxylon. J Gen Virol. 1995;76:1461–1470. doi: 10.1099/0022-1317-76-6-1461. [DOI] [PubMed] [Google Scholar]