Abstract
Background
Around the world, disabilities due to musculoskeletal disorders have increased and are a major health problem worldwide. In recent years, stem cells have been considered to be powerful tools for musculoskeletal tissue engineering. Human adipose-derived stem cells (hADSCs) and amniotic fluid-derived stem cells (hAFSCs) undergo typical differentiation process into cells of mesodermal origin and can be used to treat muscular system diseases. The aim of the present study was to compare the biological characteristic of stem cells isolated from different human tissues (adipose tissue and amniotic fluid) with respect to myogenic capacity and skeletal and smooth muscle differentiation under the same conditions.
Material/Methods
hAFSCs and hADSCs were isolated during standard medical procedures and widely characterized by specific markers expression and differentiation potential. Both cell types were induced toward smooth and striated muscles differentiation, which was assessed with the use of molecular techniques.
Results
For phenotypic characterization, both stem cell types were assessed for the expression of OCT-4, SOX2, CD34, CD44, CD45, and CD90. Muscle-specific markers appeared in both stem cell types, but the proportion of positive cells showed differences depending on the experimental conditions used and the source from which the stem cells were isolated.
Conclusions
In this study, we demonstrated that hADSCs and hAFSCs have different capability of differentiation toward both muscle types. However, hADSCs seem to be a better source for myogenic protocols and can promote skeletal and smooth muscle regeneration through either direct muscle differentiation or by paracrine mechanism.
MeSH Keywords: Adipose Tissue, Amniotic Fluid, Cell Dedifferentiation, Muscle Cells, Stem Cells
Background
Great interest has been focused on stem cell therapy in regenerative medicine. Among the various cell types investigated, mesenchymal stem cells (MSCs) are an attractive stem cell source in clinical protocols and in muscle regeneration. This cell population can be isolated from different sources (e.g., bone marrow, umbilical cord blood, adipose tissue, and amniotic fluid) and are cultured to be expandable for therapeutic application. MSCs derived from different sources have been well characterized with respect to, inter alia, the isolation and culture procedure, cell number, and aging process. However, although phenotypically similar, these culture-expanded cells exhibit cell source-related heterogeneity (e.g., in differentiation potential).
Adipose-derived stem cells are a stem cell source easily accessible from liposuction in large numbers without ethical and political issues [1]. These 2 main advantages, also with self-renewal property and multipotential differentiation, make ADSCs a more acceptable solution for regenerative medicine. Adipose tissue is part of the mesodermal layer and is composed of adipocytes and a stromal vascular fraction (SVF), which is a set of heterogeneous cells, including ADSCs [2,3]. Differentiation of ADSCs was initially considered to be limited; however, more recent studies have revealed that these stem cells have a variety of differentiation pathways. As mentioned above, adipose tissue contains various cell types. However, ADSCs are distinguished from other cells by morphology and immunophenotype. Adipose-derived stem cells strongly express CD13, CD29, CD49d, CD73, CD90, and CD133 but they do not express CD106, which is commonly expressed in other mesenchymal stem cells types (e.g., isolated from bone marrow). It is also well known that differences in cell number or even immunophenotype are caused by donor characteristics, such as sex, age, ethnicity, BMI, and disease history, as well as the type of fat tissue (yellow/brown), location (subcutaneous/visceral fat), and the tissue collection method or culture conditions [4,5], which is why a proper approach and a cost-effective isolation method are essential for further applications.
Amniotic fluid is widely used in diagnostic fields and comprises multiple cell types derived from the developing fetus. It is also a rich source of stem cells. Different types of stem cells have been isolated and characterized from amniotic fluid, and within this heterogenous population, cells can give rise to various differentiated cells, including adipose, osteoblasts, muscle, bone, and neuronal lineages. These include cells found in mid-gestation, expressing the hematopoietic marker CD34, as well as cells with mesenchymal features [6–12]. Human amniotic fluid stem cells possess many characteristics that may be identical to human ESCs and they appear to be safer and more pluripotent than stem cells derived from bone marrow. They also do not form tumors or teratoma in vivo. A low risk of tumorigenicity would be advantageous for future therapeutic applications. Thus, hAFSCs represent a new class of stem cells with properties of plasticity intermediate between embryogenic and adult stem cell types. However, one of the most interesting issues is the plasticity of stem cells [13]. Amniotic fluid-derived stem cells undergo typical differentiation process into cells of mesodermal origin: osteocytes, adipocytes, and chondrocytes [14–18]. It was also shown that they can differentiate into myocytes and endothelial cells in vitro, as well as non-mesodermal cell lines, such as hepatocytes, the insulin-producing cells, keratinocytes, intestinal epithelial cells, and neuronal cells [14,18,19]. Differentiation of amniotic fluid stem cells (AFSCs) in vitro requires the use of specific growth factors or chemical compounds with differentiating properties.
Molecular mechanisms underlying the differentiation of adult stem cells remain largely unknown. Little is also known about the differentiation of the cells in vivo, as the most commonly used in vitro agents are absent in humans and animals. However, in vitro cell culture offers great opportunities for exploring the potential of mesenchymal stem cells.
The aim of the present study was to compare the biological characteristic of stem cells isolated from human adipose tissue (hADSCs) and amniotic fluid (hAFSCs) with respect to myogenic capacity and skeletal and smooth muscle differentiation under the same conditions. The myogenic commitment of stem cells derived from various tissues may be helpful for selecting a suitable source for a specified musculoskeletal clinical application.
Material and Methods
Our stem cells sources were adipose tissue and amniotic fluid. To reduce individual variability among the recruited population, homogenous in sex, age, and, where necessary, in the sampling site, stem cell samples from 20 donors were isolated. All patients gave written informed consent and were informed about the procedure carried out according to the protocol of this study, which was approved by the Local University Ethics Committee (KB 239/2011 and KB 287/2011).
Human adipose-derived stem cells
Adipose tissue was harvested using lipoaspirate obtained during power-assisted liposuction from 20 healthy women. hADSCs isolation was initiated by washing adipose tissue with sterile PBS (phosphate-buffered saline, Sigma-Aldrich, Germany) containing 5 μg/ml amphotericin B, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, Germany) to eliminate blood cells, saline, and anesthetics used during tumescent liposuction. The washed adipose lipoaspirate underwent enzymatic digestion with type I collagenase at a final concentration of 0.075% (Sigma-Aldrich, Germany) at 37°C for 30 min. The digestion was interrupted with the addition of an equal volume of complete culture medium DMEM/Ham’s F12 (Dulbecco’s Modified Essential Medium, Sigma-Aldrich, Germany) supplemented with 10% FBS, 5 μg/ml amphotericin B, 100 μg/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, Germany). Then, samples were centrifuged twice at 170×g for 5 min at room temperature, and the SVF pellet was resuspended in complete DMEM/Ham’s F12 medium. Suspended cells were then passed through a 100-μm cell strainer (BD Bioscience, US AP) to separate the undigested tissue fragments, and once again centrifuged. The SVF pellet was suspended in complete culture medium and isolated cells were plated at an equivalent of ~15 g lipoaspirate per T25 flask. The cells were cultured at 37ºC in 5% CO2. The medium was changed every second day until the cells reached 80–90% confluence.
Human amniotic fluid-derived stem cells
Amniotic fluid samples were obtained from routine amniocentesis performed during the 14th to 27th weeks of gestation from 20 healthy pregnant women between the ages of 18 to 46 years. Isolation of hAFSCs was performed using a method described by Kim et al. (2007) with minor modification [20]. Briefly, amniotic fluid was centrifuged for 10 min at 350×g. Subsequently, the cell pellet was resuspended in growth medium DMEM/Ham’s F12 (PAA, Austria) supplemented with 20% FBS (PAA, Austria), 10 ng/ml bFGF (Sigma, Germany), 5 μg/ml of amphotericin B (PAA, Austria), 100 μg/ml penicillin/streptomycin (PAA, Austria), and L – glutamine, and incubated at 37°C with 5% humidified CO2.
Biological characteristic of hADSCs and hAFSCs
Colony-forming efficiency assay
hADSCs and hAFSCs after the 3rd passage were seeded in 6-well culture plates (BD Biosciences) with 1×103/well and 5×103/well, respectively. After 14 days of incubation, colonies were stained with the use of rhodamine B (Sigma, Germany).
Multipotential differentiation
Differentiation capacity into the adipogenic, osteogenic, and chondrogenic lineage was performed as described previously [21].
Phenotype analysis by real-time PCR
Stem cells phenotype was confirmed by analyzing the expression of OCT4, SOX2, CD34, CD44, CD45, and CD90 markers by real-time PCR. Briefly, total RNA from undifferentiated cells was isolated by the Chomczyński method [22] using TRI Reagent (Sigma, Germany). The reverse transcription was carried out using the Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Thermo Scientific) according to manufacturer’s protocol. For 1 reaction, 1 μg of RNA was incubated with reverse transcriptase in reaction mixture for 10 min at 25°C followed by 15 min at 50°C. The reaction was stopped by incubation of the sample at 85°C for 5 min. Real-time PCR reactions were performed with a LightCycler 480 Instrument (Roche, Switzerland) using the Real-time Ready Custom Panel 96 (Roche, Switzerland) according to the manufacturer’s protocol. For the reaction, the following program was used: 1) pre-incubation at 95°C for 10 min; 2) amplification (45 cycles) with denaturation stage at 95°C for 10 s, hybridization stage at 60°C for 30 s, and elongation stage at 72°C for 1 s; and 3) cooling at 40°C for 30 s. The relative expression of analyzed genes was calculated by the 2−ΔΔCt method with LightCycler 480 software. Data for analyzed genes were normalized using the mean result of β-actin and GAPDH as reference genes.
Phenotype analysis by flow cytometry
Stem cells were additionally analyzed for the presence of the specific surface markers CD34, CD44, CD45, and CD90 by flow cytometry according to the protocol previously described [21].
hADSCs and hAFSCs differentiation toward muscle lineage
Stem cells were analyzed for their capacity to differentiate into skeletal and smooth muscle cells. Cells were plated at a density of 2×104/1 cm2 and incubated for 24 h in standard medium and standard conditions. After pre-incubation, medium was changed for differentiation medium into skeletal and smooth muscle cells using conditioned medium or medium supplemented with TGF-β1, respectively. Cells were cultured in differentiation conditions for 14 days.
Preparation of conditioned medium
Conditioned medium for the differentiation into skeletal muscle cells was prepared with the use of fetal human skeletal muscle cells (HSkMCs) from the European Collection of Cell Cultures (ECACC, UK). These cells are isolated from the limbal skeletal muscle and can undergo differentiation to exhibit actin and myosin myofilaments. Cells were cultured according the manufacturer’s protocol in Skeletal Muscle Cell Growth Medium (ECACC, UK). Next, cells were transferred in a density of 1×104/1 cm2 to the culture plates covered with collagen solution (Sigma, Germany) in a volume of 1 ml/10 cm2. The medium was changed every day until the cells reached 80% confluence. After that, the differentiation process of HSkMCs (toward multinucleated myotubes) was initiated by the use of the Skeletal Muscle Differentiation Medium (Sigma, Germany). Cells were cultured until the multinucleated myotubes were formed (at about 1 week). During the differentiation process, the conditioned medium was collected every 48 h until the 6th passage. Then, the medium was filtered and stored at −80°C until use.
Preparation of smooth muscle differentiation medium
For smooth muscle differentiation, stem cells were cultured in DMEM/Ham ‘ s F12 (3: 1) medium supplemented with 20% FBS, 10 ng bFGF, 5 μg/ml amphotericin B, 100 μg/ml penicillin/streptomycin, and 1 ng/ml TGF-β1 (all reagents were purchased from Sigma, Germany).
Evaluation of hADSCs and hAFSCs differentiation toward muscle cells
Cells after myogenic differentiation were analyzed by qPCR for the expression of specific markers: DES (desmin), CNN1(calponin-1), MYH11 (myosin 11), and TAGLN (transgelin) for smooth muscle cells and DES (desmin), MYOG (myogenin), and ACTA1(α-actin) for skeletal muscle cells. All analysis was performed with the use of the Real-time Ready Custom Panel 96 (Roche, Switzerland).
Results
Growth and culture characteristics of hADSCs and hAFSCs
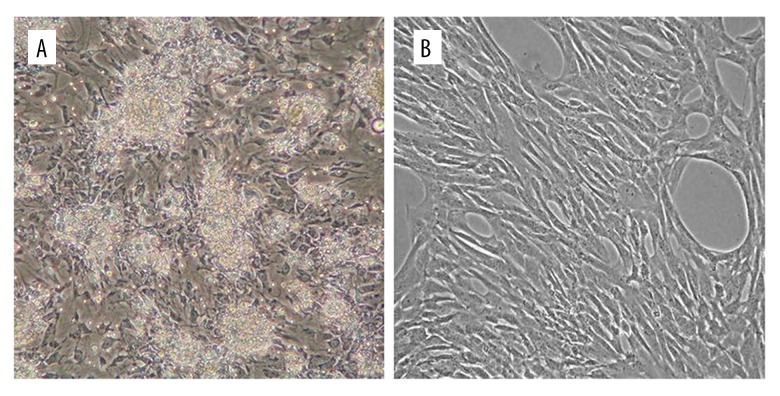
During primary culture, both stem cells types adhered to the culture flasks. After 48 h of culture, non-adherent floating cells were removed and the adhered cell population was washed with PBS. After 3–4 days of incubation, the cells grew into small colonies with fibroblastic-like morphology, which increased in numbers (Figure 1A, 1B).
Figure 1.
Isolation and in vitro culture of hADSCs (A) and hAFSCs (B) 7 days after isolation, homologous population with fibroblastic morphology was observed.
The clonogenicity of both stem cell types was evaluated after 14 days with rhodamine B staining. hADSCs seeded at 1×103/well formed on average 24±4 colonies (Figure 2A), and hAFSCs seeded at 5×103/well formed on average 9.6±0.9 colonies (Figure 2B).
Figure 2.
Clonogenicity of hADSCs and hAFSCs. Colony-forming potential; staining with rhodamine B after 14 days of hADSCs (A) and hAFSCs (B) culture.
Multipotential differentiation
The full protocol of the differentiation process and ability of both stem cell types to differentiate into 3 lineages was presented in detail in a previously study [21]. Briefly, the potential of hADSCs and hAFSCs to differentiate into adipocytes was assessed on the basis of fat droplet presence in the cytoplasm. Changes in morphology from the spindle shaped cells to round appeared after an average 7 days in differentiation medium toward adipocytes. Fat droplets were observed in the cytoplasm of both stem cell types and the differentiation was confirmed by staining with Oil Red O solution. The chondrogenic potential was evaluated by culture of micropellets in differentiating medium. After 14 days of culture, immunocytochemical analysis was performed and all tested cells showed expression of collagen type II. Also, the induction of osteogenesis resulted in the change of hADSCs and hAFSCs morphology after an average 6 days of culture in the presence of differentiation factors. After 2 weeks, osteogenesis potential was confirmed by staining of extracellular matrix calcification [data not shown].
Phenotype confirmation of hADSCs and hAFSCs
For phenotypic characterization, hADSCs and hAFSCs were assessed for the expression of CD34, CD44, CD45, and CD90 (Figure 3). Both stem cell types, analyzed after the 2nd passage, showed high expression of CD90 and lower expression of CD34 and CD44. The presence of CD45 was not detected. Moreover, the expression of OCT-4 and SOX2 – pluripotent stem cells markers was also analyzed. Only hADSC exhibited low expression of only 1 of the 2 markers investigated (i.e., OCT-4).
Figure 3.
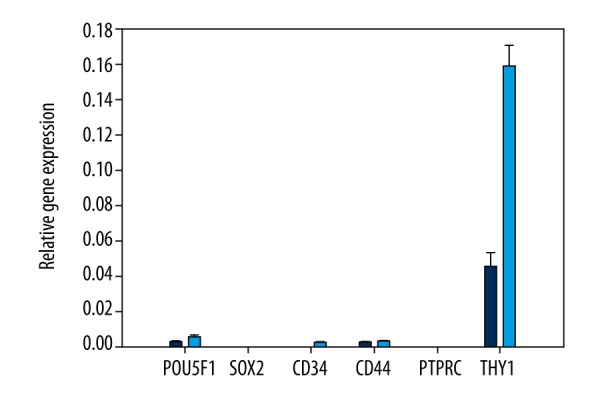
The relative expression of specific markers in hADSCs (blue color) and hAFSCs (light blue color).
In our previously studies, we also analyzed CD34, CD44, CD45, and CD90 in both stem cell types with the use of flow cytometry, in the following passages. All tested hADSCs were characterized by strong and stable expression of CD90 and CD44, which are markers presented in stem cells population [data not shown]. We also noticed the expression of CD34, which decreased in the following passages [21]. A similar trend was also observed in hAFSCs. The only difference was in case of CD34 expression, which was low at all tested passages [data not shown]. These results are also well described in our previous study [23].
Effect of environmental conditions on differentiation of hAFSCs toward muscle cells
The effect of conditioned medium and TGF-β1 on desmin, myogenin, calponin, transgelin, and α-actin expression was analyzed by qPCR. We observed differences in expression of specific smooth muscle markers comparing these 2 different stem cell types. After differentiation, hAFSCs showed the expression of only 2 markers (calponin-1 and transgelin), while in hADSCs-derived culture we observed expression of all tested specific smooth muscle cells markers. However, higher levels were determined for desmin and calponin-1 (Figure 4).
Figure 4.
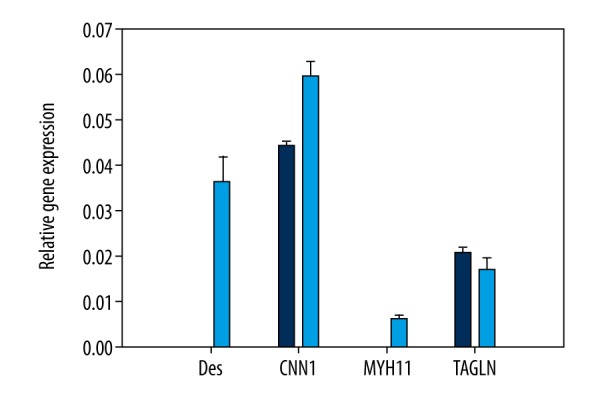
The relative expression level of smooth muscle markers in hAFSCs (blue color) and hADSCs (light blue color) differentiated with TGF-β1 factor.
Examining the influence of HSkMCs conditioned medium on differentiation induction toward skeletal muscle cells, we analyzed the expression of 3 markers commonly found in skeletal muscle cells: desmin, myogenin, and α-actin. After 14 days of culture, we also noticed differences in expression of specific markers in cultures derived from hAFSCs and hADSCs (Figure 5). In hAFSCs cultures after differentiation, we observed the low expression of only 1 marker: α-actin. However, hADSCs differentiated into skeletal muscles, as expressed in all 3 analyzed markers.
Figure 5.
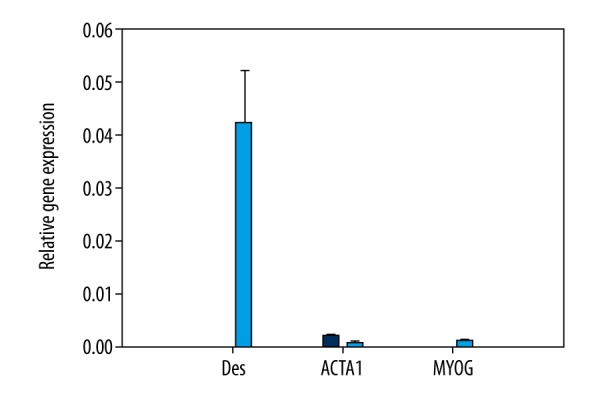
The relative expression level of skeletal muscle markers in hAFSCs (blue color) and hADSCs (light blue color) differentiated with the conditioned medium harvested from HSkMCs culture.
Discussion
Complete regeneration of muscles and recovery of their functional capacity still remains a big challenge. Involvement of stem cells in muscle regeneration raises a huge hope for novel therapies, which can be used in the case of reduction or depletion of endogenous progenitor cells population (e.g., as the result of X-ray irradiation) [10]. The ability of skeletal muscle to regenerate is achieved due to the presence of a mononuclear population of progenitor cells called satellite cells, which show the characteristics of stem cells [21,25]. Although these cells have regenerative potential, the total repair of the muscle with their participation is difficult (e.g., due to small and difficult to obtain biopsies) [26–28]. Therefore, additional sources of myogenic stem cells have been explored, and cell therapy techniques based on the use of adult stem cells appear to be an attractive strategy for the treatment of muscle damage. It is safer and more ethically acceptable to use adult multipotent stem cells, such as mesenchymal stem cells (MSCs). Many researchers demonstrated that MSCs isolated from bone marrow have the ability to differentiate into the muscle cells and are involved in muscle the healing process [29–31]. Another MSC type is adipose-derived stem cells, which can differentiate into multiple mesenchymal tissues, including myocytes. Moreover, they have a paracrine function as they release growth factors and cytokines. Also, another source of adult stem cells is a very attractive tool in regeneration approaches. The use of fetal stem cells opens new possibilities in transplantation medicine, and include amniotic fluid-derived stem cells (AFSCs). These cells could potentially be used for treatment of many diseases due to their characteristic properties resulting from the expression of both embryonic stem cells (ESCs) and adult stem cells (ASCs) markers, long telomeres, and normal karyotype through multiple cycles of replication, and the possibility of differentiation into cells of all 3 germ layers [17,32].
ADSCs differentiation toward a myogenic lineage has been described by several authors [33–35]. Myogenic differentiation media induces the changes in ADSCs morphology and expression of muscle differentiation markers. However, the efficacy of cell differentiation is low and the percentage of differentiated cells is small. Moreover, the process of differentiation is characterized by low reproducibility [36]. A similar trend is observed during differentiation of hAFSCs into muscle cells. Despite the many different methods used (e.g., co-culture, growth factors, chemical and mechanical factors), the protocol efficiency is often low [34,37–39]. In the absence of optimization methods for the differentiation of hADSCs and hAFSCs, standardization is essential.
In our study, the induction of hADSCs and hAFSCs differentiation process toward skeletal muscle cells was achieved by cell culture in conditioned medium obtained from human skeletal muscle cells (HSkMC). Expression of specific markers was evaluated with the use of qPCR. We observed the presence of all 3 skeletal muscle markers in differentiated hADSCs (alpha-actin, desmin, and myogenin), while in hAFSCs we observed only 1 (alpha-actin). Although the expression of all 3 markers was confirmed in hADSCs, 2 of them (alpha-actin and myogenin) were at very low levels. Also, the expression of alpha-actin in hAFSCs was very poor. The ability of adipose-derived stem cells to differentiate in vitro toward a myogenic lineage has been reported by several research groups. These cells, cultured with the use of myogenic differentiation media, show an elongated morphology and expression of early and late markers of muscle [40]. However, many studies also show variable differentiation efficiencies, which probably is the result of using different inductive media. It is also worth noting that in vivo studies on the transplantation of ADSCs for muscle regeneration present many contradictions [33,41], which is why it is often suggested that muscle regeneration is achieved by a paracrine mechanism rather than by a direct differentiation of ADSCs. Bossolasco et al. conducted hAFSCs differentiation using a commercially available growth medium for skeletal muscle cells and medium containing 5′azacitidine, demonstrating that hAFSCs did not differentiate into multinucleated muscle cells and did not show the expression of MyoD, myogenin, or desmin [42]. In the studies presented by Gekas et al., human AFSCs from the second trimester of pregnancy, which express the CD117 marker, demonstrated the muscle cells phenotype under in vitro differentiation on polystyrene plates coated with Matrigel in the presence of 5-aza-2′-deoxycytidine as a differentiating factor [43]. These cells also showed the expression of desmin and myogenin. However, the same cells injected in undifferentiated state into undamaged muscles of SCID mice did not shown the phenotype of muscle cells. Ma et al. demonstrated that hAFSCs, without CD117 selection, are able to differentiate into skeletal muscles in both in vivo and in vitro conditions [44]. This result may indicate the existence of different populations of progenitor cells in amniotic fluid. Cells exposed to 5-aza-2′-deoxycytidine or co-cultured with C2C12 cell line (a mouse myoblast cell line) differentiated into skeletal muscle and expressed the specific markers: desmin, troponin I, and α-actinin. In vivo these cells have been differentiated into muscle precursor cells with the expression of desmin, laminin, and MYF5. Piccoli et al., using an in vivo mice model with symptoms of human muscular dystrophy, demonstrated that injection of hAFSCs in skeletal muscles increased their strength and proper distribution of dystrophin in muscles [45]. Transplanted cells were also characterized by the expression of PAX7 and integrin α-7. Chun et al. attempted to differentiate hAFSCs toward muscle progenitor cells with the co-culture system; co-culture involved the skeletal muscle cells and conditioned medium harvested from skeletal muscle culture [46]. They revealed that conditioned medium inhibited the hAFSCs proliferation in favor of differentiation toward muscle cells. The differentiated cells showed the expression of specific muscle markers: MYF5, myogenin, and desmin [46]. Nevertheless, all these in vitro studies with both types of stem cells indicate that different stimuli can promote differentiation toward myogenic lineage, ranging from hormones and growth factors present in media to cell-cell contacts or even additional factors such as plating surfaces [28].
Stem cells capable of differentiation toward a smooth muscle phenotype may have a key role in both vascular and hollow organ tissue engineering, holding promise for regenerative medicine applications. There have been many attempts to derive functional smooth muscle cells using ESCs, adult stem cells, or induced pluripotent stem cells (iPSCs) [47]. According to the available literature on somatic stem cells, TGF-β1, PDGF, and ascorbic or retinoic acid can initiate the differentiation process into smooth muscle lineage [48–50]. In the present study, the differentiation potential of hADSCs and hAFSCs toward smooth muscle cells was also analyzed. Both cell types were cultured in medium supplemented with TGF-β1 factor, which is secreted at the site of tissue damage by platelets and is responsible for the regulation of cellular processes, among which cell adhesion, proliferation, differentiation, apoptosis, and extracellular matrix synthesis can be distinguished [51]. After 14 days of differentiation, we observed expression of desmin, calponin-1, myosin-11, and transgelin in hADSCs, while in hAFSCs we found only calponin-1 and transgelin. In the literature, similar studies can be found with the use of the same factor. The results concerning hADSCs are also similar. Expression of calponin, caldesmon, and myosin is confirmed on both gene and protein level in differentiated hADSCs [52]. However, there is also evidence that more than 1 differentiation factor is necessary for an effective differentiation process [53].
To date, there have been no attempts at hAFSCs differentiation toward the smooth muscle cells with TGF-β1 factor. For the first time, in 2013, Ghionzoli et al. showed that it is possible to obtain functional smooth muscle cells from hAFSCs; however, they supplemented differentiating medium with additional growth factors [47]. Narita et al. demonstrated that low level of TGF-β1 (1ng/ml) compared to the high level (10ng/ml) does not affect the expression profile of specific markers of smooth muscle [54]. The same researchers also indicated that low level of serum (5% and 10%) compared with high level (20%) in differentiating medium supplemented with the TGF-β1 factor does not influence the expression profile specific for smooth muscle cells. Treuger et al. confirmed the aforementioned results, since the calponin and α-actin are defined as markers of early stages of smooth muscle cells differentiation [55]. However, in our study, the expression of desmin and myosin 11 was not observed. Previous studies suggest that desmin and α-actin are not specific markers, only for smooth muscle cells [56,57]. Lack of MYH11, the myosin heavy chain marker (unique for smooth muscle cells), may result from the early stage of hAFSCs differentiation. In addition, obtaining the fully differentiated and functional cells in vitro seems to be unclear [58]. However, transgelin and calponin are also specific markers undergoing expression in smooth muscle cells [32,59]. It has recently been reported that the differentiation of stem cells toward the smooth muscle lineage relies mostly on the activation of specific intracellular pathways (e.g., Notch signaling) [60]. Due to the limited data concerning hAFSCs differentiation toward smooth muscle cells, the obtained results were mostly in bone marrow mesenchymal stem cells differentiation. Wang et al. proved the influence of intracellular interactions on differentiation process of rat bone marrow MSCs toward smooth muscle cells [53]. Cells subjected to direct co-culture with smooth muscle cells showed expression of calponin and α-actin. The results obtained in our study suggest that in further research on hAFSCs differentiation, the direct co-culture system and in vivo animal model with the smooth muscle injury should be used.
Our results suggest that hADSCs and hAFSCs can be reprogrammed to myogenic lineage in vitro. However, the efficiency of this conversion is low. In this study, we also clearly demonstrated that there are differences in differentiation potential toward muscle cells between both types of adult stem cells. It seems that adipose-derived stem cells are more prone to differentiation factors and myogenic differentiation. However, for successful regenerative therapies it is necessary that transplanted stem cells not only engraft in muscles but also result in a positive functional output. In conclusion, our study has shown interesting data on the role of hADSCs and hAFSCs in muscle regeneration. Nevertheless, new protocols and tools that allow manipulation of stem cells differentiation, which could promote muscle regeneration, are needed. Also, it seems that a universal media composition for myogenic differentiation of adult stem cells is essential.
Conclusions
We demonstrated that hADSCs and hAFSCs have different capabilities of differentiation toward both muscle types. However, hADSCs seem to be a better source for myogenic protocols and may promote skeletal and smooth muscle regeneration through either direct muscle differentiation or by paracrine mechanism.
Abbreviations
- hADSCs
human adipose-derived stem cells
- hAFSCs
human amniotic fluid stem cells
- SVF
stromal vascular fraction
- ESCs
embryonic stem cells
- ASCs
adult stem cells
- MSCs
mesenchymal stem cells
- DES
desmin
- CNN1
calponin-1
- MYOG
myogenin
- ACTA1
alpha-actin
- MYH11
myosin 11
- TAGLN
transgelin
- ACTB
beta actin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HSkMCs
human skeletal muscle cells
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Dai R, Wang Z, Samanipour R, et al. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016;2016:6737345. doi: 10.1155/2016/6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization and heterogenity. Stem Cells Int. 2012;2012:812693. doi: 10.1155/2012/812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olkowska-Truchanowicz J. Izolacja i charakterystyka komórek progenitorowych tkanki tłuszczowej. Post Biol Kom. 2008;35:517–26. [in Polish] [Google Scholar]
- 6.Roubelakis MG, Pappa KI, Bitsika V, et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: Comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:931–52. doi: 10.1089/scd.2007.0036. [DOI] [PubMed] [Google Scholar]
- 7.In’t Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogenous multilineage differentiation potential. Haematologica. 2003;88:845–52. [PubMed] [Google Scholar]
- 8.Sessarego N, Parodi A, Podesta M, et al. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica. 2008;93:339–46. doi: 10.3324/haematol.11869. [DOI] [PubMed] [Google Scholar]
- 9.Da Sacco S, Sedrakyanm S, Boldrin F, et al. Human Amniotic Fluid as a potential new source of organ specific precirsor cells for future regenerative medicine applications. J Urol. 2010;183:1193–200. doi: 10.1016/j.juro.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaviani A, Perry TE, Dzakovic A, et al. The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg. 2001;36:1662–65. doi: 10.1053/jpsu.2001.27945. [DOI] [PubMed] [Google Scholar]
- 11.In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–49. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 12.Nadri S, Soleimani M. Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy. 2007;9:729–37. doi: 10.1080/14653240701656061. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz EM. Stem cell plasticity: The growing potential of cellular therapy. Arch Med Res. 2003;34:600–6. doi: 10.1016/j.arcmed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 14.De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 15.Kolambkar YM, Peister A, Soker S, et al. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007;38:405–13. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- 16.Phermthai T, Odglun Y, Julavijitphong S, et al. A novel method to derive amniotic fluid stem cells for therapeutic purposes. BMC Cell Biol. 2010;11:79. doi: 10.1186/1471-2121-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Coppi P, Callegari A, Chiavegato A, et al. Amniotic fluid abd bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol. 2007;177:369–76. doi: 10.1016/j.juro.2006.09.103. [DOI] [PubMed] [Google Scholar]
- 18.Atala A. Engineering organs. Curr Opin Biotechnol. 2009;20:575–92. doi: 10.1016/j.copbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Carraro G, Perin L, Sedrakyan S, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902–11. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Lee Y, Kim H, Hwang KJ, et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajek A, Gurtowska N, Olkowska J, et al. Does the harvesting technique affect the properties of adipose-derived stem cells? – the comparative biological characterization. J Cell Biochem. 2017;118(5):1097–107. doi: 10.1002/jcb.25724. [DOI] [PubMed] [Google Scholar]
- 22.Mackey K, Chomczynski P. Long-term stability of RNA isolation reagents. J NIH Res. 1996;8:72. [Google Scholar]
- 23.Bajek A, Olkowska J, Walentowicz-Sadłecka M, et al. High quality independent from a donor: Human Amniotic Fluid Derived Stem Cells – a practical analysis based on 165 clinical cases. J Cell Biochem. 2017;118(1):116–26. doi: 10.1002/jcb.25618. [DOI] [PubMed] [Google Scholar]
- 24.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–95. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JE. The satellite cell as a companion in skeletal muscle plasticity: Currency, conveyance, clue, connector and colander. J Exp Biol. 2016;209:2276–92. doi: 10.1242/jeb.02088. [DOI] [PubMed] [Google Scholar]
- 26.Jarvinen TA, Jarvinen TL, Kaarjainen M, et al. Muscle injuries: Biology and treatment. Am J Sports Med. 2005;33:745–64. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 27.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:345–53. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 28.Forcales SV. Potential of adipose-derived stem cells in muscular regerative therapies. Front Aging Neurosci. 2015;7:123. doi: 10.3389/fnagi.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matziolis G, Winkler T, Schaser K, et al. Autologous bone marrow-derived cells enhance muscle strength following skeletal muscle crush injury in rats. Tissue Eng. 2006;12:361–67. doi: 10.1089/ten.2006.12.361. [DOI] [PubMed] [Google Scholar]
- 30.Dezawa M, Ishikawa H, Itokazu Y, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–17. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 31.Tamama K, Sen CK, Wells A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 2008;17:897–908. doi: 10.1089/scd.2007.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Gu Y, Zhou C, et al. Expression of Calponin-1 and Transgelin in human uterine smooth muscles in non-labor and labor situation. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:1073–79. doi: 10.3969/j.issn.1672-7347.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Pecanha R, Bagno LL, Ribeiro MB, et al. Adipose-derived stem cell treatment of skeletal muscle injury. J Bone Joint Surg Am. 2012;94:609–17. doi: 10.2106/JBJS.K.00351. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XH, Zeng ZP, Li H, et al. Expression of renin-angiotensin-aldosterone system in human adipose tissues. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:766–69. [PubMed] [Google Scholar]
- 35.Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882–88. doi: 10.1016/j.bbrc.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 36.Tholpady SS, Llull R, Ogle RC, et al. Adipose tissue: Stem cells and beyond. Clin Plast Surg. 2006;33:55–62. doi: 10.1016/j.cps.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Gekas J, Walther G, Skuk D, et al. In vitro and in vivo study of human amniotic fluid-derived stem cell differentiation into myogenic lineage. Clin Exp Med. 2010;10:1–6. doi: 10.1007/s10238-009-0060-2. [DOI] [PubMed] [Google Scholar]
- 38.Yeh YC, Wei HJ, Lee WY, et al. Cellular cardiomyoplasty with human amniotic fluid stem cells: In vitro and in vivo studies. Tissue Eng Part A. 2010;16:1925–36. doi: 10.1089/ten.TEA.2009.0728. [DOI] [PubMed] [Google Scholar]
- 39.Bollini S, Pozzobon M, Nobles M, et al. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev. 2011;7:364–80. doi: 10.1007/s12015-010-9200-z. [DOI] [PubMed] [Google Scholar]
- 40.Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12:1891–901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 41.Bacou F, el Andalousi RB, Daussin PA, et al. Transplantation of adipose tissue-derived stromal cells increases mass and functional capacity of damaged skeletal muscle. Cell Transplant. 2004;13:103–11. [PubMed] [Google Scholar]
- 42.Bossolasco P, Montemurro T, Cova L, et al. Molecular and phenotypic characetrization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–36. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- 43.Joo S, Ko IK, Atala A, et al. Amniotic fluid-derived stem cells in regenerative medicine research. Arch Pharm Res. 2012;35:271–80. doi: 10.1007/s12272-012-0207-7. [DOI] [PubMed] [Google Scholar]
- 44.Ma X, Zhang S, Zhou J, Chen B, Shang Y, Gao T, Wang X, Xie H, Chen F. Clone-derived human AF-amniotic fluid stem cells are capable of skeletal myogenic differentiation in vitro and in vivo. J Tissue Eng Regen Med. 2012;6:598–613. doi: 10.1002/term.462. [DOI] [PubMed] [Google Scholar]
- 45.Piccoli M, Franzin C, Bertin E, et al. Amniotic fluid stem cells restore the muscle cell niche in a HAS-Cre, Smn (F7/F7) mouse model. Stem Cells. 2012;30:1675–84. doi: 10.1002/stem.1134. [DOI] [PubMed] [Google Scholar]
- 46.Chun SY, Cho DH, Chae SY, et al. Human amniotic fluid stem cell-derived muscle progenitor cell therapy for stress urinary incontinence. J Korean Med Sci. 2012;27:1300–7. doi: 10.3346/jkms.2012.27.11.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghionzoli M, Repele A, Sartiani L, et al. Human amniotic fluid stem cell differentiation along smooth muscle lineage. FASEB J. 2013;27:4853–65. doi: 10.1096/fj.12-218578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majack RA. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. Ather Thrombo Res. 1987;1:465–71. doi: 10.1083/jcb.105.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miano JM, Berk BC. Retinoids: Versatile biological response modifiers of vascular smooth muscle phenotype. Circ Res. 2000;87:355–62. doi: 10.1161/01.res.87.5.355. [DOI] [PubMed] [Google Scholar]
- 50.Cananzi M, De Coppi PL. CD117+ amniotic fluid stem cells: State of the art. And future perspectives. Organogenesis. 2012;8:77–88. doi: 10.4161/org.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bajek A, Olkowska J, Gurtowska N, et al. Human amniotic-fluid-derived stem cells: A unique source for regenerative medicine. Expert Opin Biol Ther. 2014;14:831–39. doi: 10.1517/14712598.2014.898749. [DOI] [PubMed] [Google Scholar]
- 52.Myula B, Abrahamse H. Differentiation potential of adipose-derived stem cells when cocultured with smooth muscle cells, and the role of low-intensity laser irradiation. Photomed Laser Surg. 2016;34(11):509–15. doi: 10.1089/pho.2015.3978. [DOI] [PubMed] [Google Scholar]
- 53.Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109:74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 54.Narita Y, Yamawaki A, Kagami H, et al. Effects of transforming growth cator-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 2008;333:449–59. doi: 10.1007/s00441-008-0654-0. [DOI] [PubMed] [Google Scholar]
- 55.Treuger K, Naye F, Thiebaud P, et al. Smooth muscle cell differentiation from human bone marrow: Variations in cell type specific markers and Id gene expression in a new model of cell culture. Cell Biol Int. 2009;33:621–31. doi: 10.1016/j.cellbi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Graves DC, Yablonka-Reuveni Z. Vascular smooth muscle cells spontaneously adopt a skeletal muscle phenotype: A unique Myf5-/MyoD+ myogenic program. J Histochem Cytochem. 2000;48:1173–93. doi: 10.1177/002215540004800902. [DOI] [PubMed] [Google Scholar]
- 57.Hirschi KK, Lai L, Belaguli NS, et al. Transforming growth factor-beta induction of smooth muscle cell phenotype requires transcriptional and post-transcriptional control of serum response factor. J Biol Chem. 2002;77:6287–95. doi: 10.1074/jbc.M106649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635–48. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi K, Saga H, Chimori Y, et al. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J Biol Chem. 1998;273:28860–67. doi: 10.1074/jbc.273.44.28860. [DOI] [PubMed] [Google Scholar]
- 60.Kurpinski K, Lam H, Chu J, et al. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;8:734–42. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]