Abstract
Backgrounds
Hepatocellular carcinoma (HCC) accounts for one of the most prevalent cancer types in the world. The ubiquitin specific protease 7 (USP7), a kind of deubiquitylating enzyme, has been reported to play multifaceted roles in different tumor types. The aim of this study was to investigate the expression and function of USP7 in HCC.
Material/Methods
Immunohistochemical staining and quantitative PCR were performed to explore the expression of USP7 in both HCC tissues and adjacent normal liver tissues. Chi-square test, univariate analysis, and multivariate analysis were conducted to statistically evaluate the clinical significance of USP7 in HCC. Proliferation, migration, and invasion capacities of HCC cells were assessed after overexpressing or silencing USP7.
Results
Both the RNA and protein levels of USP7 were upregulated in HCC tissues compared to normal liver tissues. High expression of USP7 was correlated with advanced tumor stage and poor overall survival. Moreover, USP7 was identified as a novel independent prognostic factor for HCC patients. Cellular studies showed that USP7 could enhance the proliferation, migration, and invasion capacities of HCC cells, thereby promoting tumor progression.
Conclusions
High expression of USP7 is frequent in HCC tissues, which promotes tumor proliferation and invasion, and is correlated with a poor overall survival. Targeting USP7 may be a novel direction for the drug development of HCC therapy.
MeSH Keywords: Carcinoma, Hepatocellular; Neoplasm Invasiveness; Prognosis
Background
Liver cancer is the fifth most common malignant cancer worldwide and the third leading cause of cancer-related death worldwide [1]. Hepatocellular carcinoma (HCC) accounts for more than 90% of all liver cancers [2,3]. Although long-term survival of HCC has been reported in some case reports [4,5], the general clinical outcome remains dismal due to early metastasis and frequency of recurrence [6,7]. Till now, surgery resection has been the best treatment for HCC, but most patients are diagnosed at advanced tumor stages, characterized by multifocal progression, lymph node metastasis, and portal vein invasion, therefore most cases are not appropriate for curative R0 resection (resection for cure or complete remission). Development and progression of HCC is a multistep process which involves numerous gene and protein dysfunctions. The biological and clinical behavior of HCC shows great differences, thus predicting prognosis is not easy for each specific patient. A better understanding of the cellular and molecular mechanisms underlying HCC progression is therefore necessary to identify novel predictive biomarkers and develop targeted therapies.
Besides protein expression level, protein modification is attracting more and more attention in molecular studies. Ubiquitination is one of the most important protein modification, which is reversible and has been revealed to participate in cell differentiation and tissue development [8]. Ubiquitination balance is reciprocally regulated by ubiquitin ligases and deubiquitinases (DUBs), the latter functions by directly hydrolyzing the ubiquitin residues, thereby controlling the signaling or abundance of certain substrates [9,10]. Ubiquitin specific proteases (USPs) is the largest subfamily of DUBs, and several USPs have been reported to participate in tumor progression. For example, high USP4 can improve the prognosis of esophageal cancer by inhibiting cell migration [11,12]. Low USP-11 expression was independently correlated with a better survival of breast cancer patients [13]. USP33 can also inhibit tumor progression of colorectal cancer by targeting Slit-Robo signaling pathway or β-arrestin signaling [14,15]. However, not all USPs are anti-tumor proteins, for example USP22 can promote human colorectal cancer progression and predict therapy failure [16]. Another known tumor-promoting USPs is USP7, which has been reported to be upregulated in ovarian cancer [17], breast cancer [18], and lung cancer [19]. However, the clinical role of USP7 in HCC has not been well established.
In this study, we explored the RNA and protein expression levels of USP7 in HCC tissues and adjacent normal liver tissues, which revealed an elevated expression in tumor tissues. Chi-square test and univariate and multivariate analyses were performed to determine clinical significance. Furthermore, our cellular experiments showed that USP7 can directly enhance the proliferation and invasion of HCC cells, demonstrating its potential as a drug target.
Material and Methods
Patients and samples
This study was approved by the Ethics Committee of East Hospital Affiliated to Tongji University in Shanghai; it was performed in accordance with Declaration of Helsinki. Written informed consent was obtained from all patients. A total of 119 HCC patients diagnosed and surgical treated in our hospital were enrolled in this study. All diagnoses were based on pathological examination. None of the patients received any cancer treatment therapy before surgery resection. During the follow-up, we obtained patients’ information included gender, age, tumor number, tumor size, and TNM stage. An additional 27 pairs of HCC tissues and adjacent liver samples were frozen in liquid nitrogen immediately after resection until experimental use.
RNA extraction, reverse transcription and real-time quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from clinical resected tissues using Total RNA mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. The concentration and purity of total RNA was measured by Nanodrop spectrophotometry. RNA was then reverse transcribe into cDNA using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative-PCR was performed using a quantitative RT-PCR Detection Kit (GeneCopoeia, Rockville, MD) according to the manufacturer’s instructions. The primers were listed below:
Immunohistochemical (IHC) staining
Immunohistochemical (IHC) staining was performed for paraffin-embedded tissue samples [22]. Briefly, tissues were cut into 4-μm sections and then mounted onto glass slides. After dried and dewaxed in xylol, the slides were rehydrated with an alcohol gradient. Then the endogenous peroxidase was blocked by treated with 3% hydrogen peroxide. Antigen retrieval was performed by incubating in 10 mM citrate buffer. After blocked with goat serum for 30 min, slides were incubated with primary USP7 antibody (ab4080, Abcam, USA) at 4 C overnight. PBS was used as a negative control. Slides were then incubated with secondary antibody for 30 min, and the immunoreactivity was finally detected by 3,3′-diaminobenzidine tetrahydrochloride (DAB) staining and counterstained with hematoxylin.
IHC evaluation
The expression of USP7 in tissue samples were assessed based on both the staining intensity and percentage of positive stained cells. For the staining intensity, we scored as followed: 1 (negative staining), 2 (lightly yellow), 3 (deep yellow), and 4 (yellow brown). For the percentage of positive stained cells, we classified as score 1 (0–25% positive tumor cells), 2 (25–50% positive tumor cells), 3 (50–75% positive tumor cells), and 4 (75–100% positive tumor cells). The final IHC score was calculated by multiplying the two scores above (ranging 1–16). All the results were analyzed by 2 independent pathologists.
Cell and transfection
Human HCC cell lines Hep3B and Huh7 were obtained from the American Type Culture Collection (Shanghai, China). All cells were maintained in DMEM media supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, Inc.) and cultured in a 37°C incubator with 5% CO2. Both cell lines were transfected with USP7 construct or USP7 siRNA. The USP7 DNA construct was purchased from Addgene (https://www.addgene.org/16655/). And USP7 siRNA was obtained from Sigma with the following sequence: 5′-ACCCUUGGACAAUAUUCCU-3′ [23].
Transfection was conducted using Lipofectamine (Invitrogen; Thermo Fisher Scientific) according to the manufacturer’s instructions. After 24 hours, transfected cells were expanded and subjected to functional assays
Cell proliferation assay
MTT assay were conducted to assess cell proliferation [24]. Briefly, 1000 cells in 200 μL FBS-containing DMEM were seeded into each well of 96-well plates and cultured for several days. Three wells from each group were measured every day. For the proliferation measurement, 100 μL fresh medium containing MTT (5 mg/mL) was added into each well and incubated at 37°C for 4 hours, the medium was then replaced by 150 μL DMSO and shaken at room temperature for 10 mins to resolve crystals. The absorbance was measured at 490 nm wavelength. Each experiment was repeated 3 times, and results were presented as means ±SD.
Wound healing assay
Transfected cells were cultured to full confluence in 6-well plates and subsequently scratched using 200 μL sterilized pipette tips. After scratching, wells were carefully washed with DMEM to remove detached cell. At designated time points, scratched cells were photographed under an inverted microscope. Migration ability of transfected cells was evaluated by measuring the width of the scratched area from three independent experiments.
Invasion assay
Invasion assays were performed by using Transwells with 8.0 μm membrane coated with Matrigel. Transfected Hep3B and Huh7 cells were seeded in a separate culture well and allowed for invasion in 37°C incubator for 48 hours. The cells were cultured in 10% FBS in the upper chamber, while the lower was supplied with DMEM containing 20% FBS. After 48-hour culture, cells in the lower surface of membrane were fixed and stained with crystal violate. For each well, 10 random fields were counted, and the average number of cells was determined. The invasion results were normalized by the total cell numbers to minimize the effect of proliferation/viability. Each experiment was performed in triplicate and repeated at least 3 times.
Statistics
Overall survival (OS) was defined as the time from diagnosis of HCC to the death of the patient or last date of follow-up. The SPSS software package (version 16.0, IBM, USA) and GraphPad Prism (version 5.0, GraphPad Software Inc, USA) were used for the statistical analysis. The associations between USP7 expression and clinicopathologic parameters were evaluated by chi-square tests.
Kaplan-Meier method was used to generate survival curves, and the differences were compared by log-rank test. A Cox multivariate proportional hazard regression model was introduced to determine the independent effect on OS. Data for the cellular experiments were presented with mean ±SD, and differences between groups were determined by Student’s t-test or one-way ANOVA test. Differences were considered statistically significant with P<0.05.
Results
Patient information
This retrospective study included 119 HCC patients that underwent curative resections in East Hospital Affiliated to Tongji University (Shanghai, China). The mean age at the time of diagnosis is 51.0 years old. Most of the patients were male (92 out of 119, 77.3%), only 27 were females. Seventy-five patients (63.0%) had single tumor lesions, and the other 44 patients (37.0%) had multiple HCC lesions. Patients were also grouped into 2 subgroups according to the tumor size, 50 patients (42.0%) with the largest tumor diameter less than 5.0 cm, and the other 69 patients (58.0%) with the largest tumor diameter greater than 5.0 cm (Table 1).
Table 1.
Clinical characteristics of enrolled HCC patients.
| Variable | Cases (n=119) | USP7 protein level | P value | |
|---|---|---|---|---|
| High (n=64) | Low (n=55) | |||
| Age (years) | 0.720 | |||
| ≤51.0 | 67 | 37 | 30 | |
| >51.0 | 52 | 27 | 25 | |
| Gender | 0.504 | |||
| Female | 27 | 13 | 14 | |
| Male | 92 | 51 | 41 | |
| Tumor number | 0.001* | |||
| Single | 75 | 32 | 43 | |
| Multiple | 44 | 22 | 12 | |
| Tumor diameter | 0.001* | |||
| ≤5.0 | 50 | 18 | 32 | |
| >5.0 | 69 | 46 | 23 | |
| TNM stage | 0.001* | |||
| I–II | 46 | 16 | 30 | |
| III–IV | 73 | 48 | 25 | |
Indicates P<0.05 by Chi-square test.
USP7 is highly expressed in HCC tissues
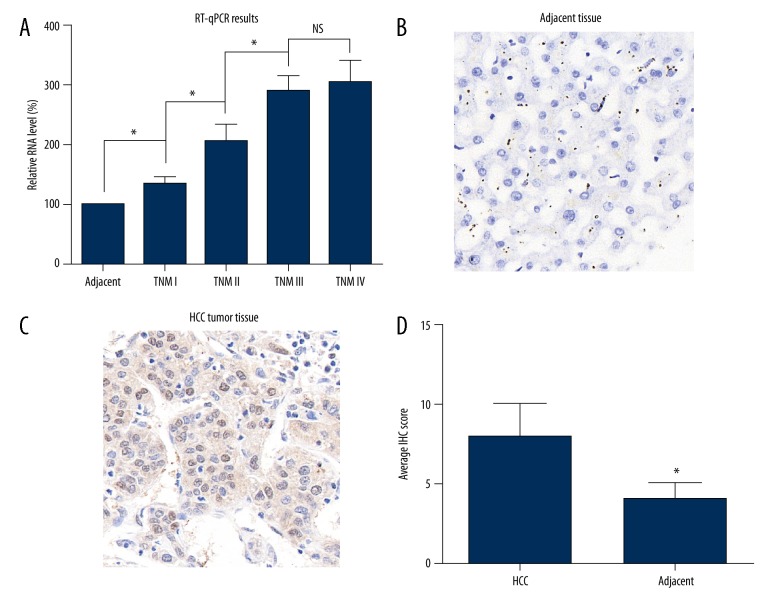
We first tested the RNA levels of USP7 in 27 pairs of HCC tissues and adjacent normal liver tissues. TNM stages of the 27 patients included stage I (4 cases), stage II (12 cases), stage III (8 cases), and stage IV (3 cases). By normalizing with the adjacent tissues, we found that the RNA levels of USP7 showed an elevated prevalence as the stage increased (Figure 1A). The protein expression and localization of USP7 was later identified by IHC strategy. Adjacent liver tissues showed negative staining or slight positive staining (Figure 1B), while the HCC cells possessed a large amount of USP7 proteins located predominantly in the nucleus (Figure 1C). Consistent with the RT-PCR results, statistical analyses of the IHC scores indicated that USP7 protein was significantly upregulated in HCC tissues (Figure 1D).
Figure 1.
USP7 is upregulated in HCC tumor tissues. (A) RT-PCR results showed that the RNA level of USP7 was increased in HCC tumor tissues than that in adjacent normal liver tissues, and was positively correlated with the TNM stage. (B) Representative IHC result showed the negative protein expression of USP7 in normal liver tissues. (C) Representative IHC result showed the high protein level of USP7 in HCC tissues, which predominately located in the nucleus. (D) Statistical analysis of IHC scores in normal liver tissues and HCC tissues showed a significantly higher USP7 protein level in tumor tissues. Magnification: ×400. Data was presented as mean ±SD. * P<0.05 by Student’s t-test.
Correlations between USP7 and clinicopathological characteristics
Although USP7 showed a generally increased level in HCC tissue, we classified patients into a low-USP7 group and a high-USP7 group based on the IHC scores to better investigate its clinical effects (Table 1). According to the chi-square tests, high USP7 level was associated with multiple tumor lesions (P=0.001), larger tumor size (P=0.001), and advanced TNM stage (P=0.001). The correlations between high USP7 level with unfavorable clinicopathological characteristics indicated that USP7 may promote tumor progression of HCC.
Prognostic effect of USP7 for HCC patients
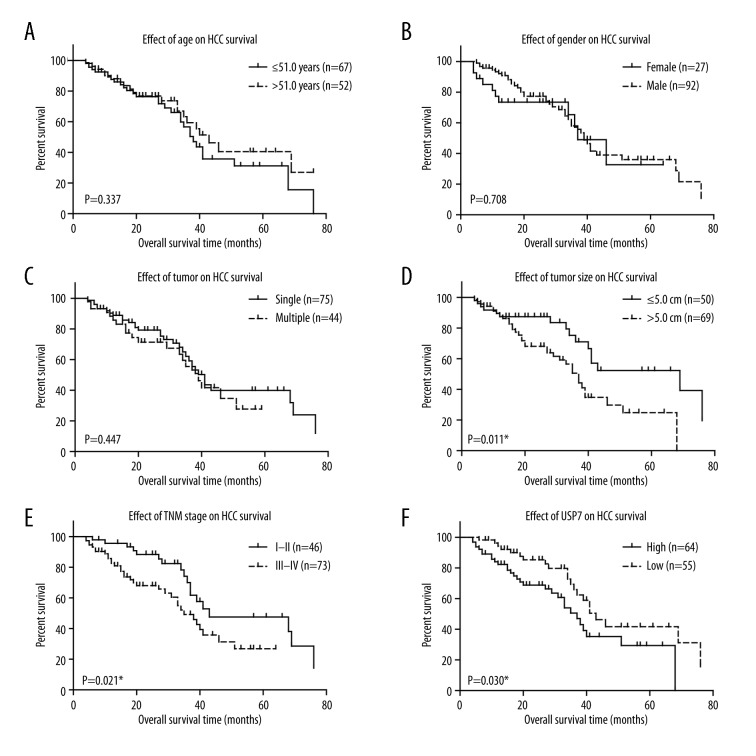
We next plotted the survival curves using Kaplan-Meier methods based on different variables (Figure 2, Table 2). The overall survival of HCC patients was not statistically correlated with patients’ age (Figure 2A, P=0.337), gender (Figure 2B, P=0.708), or tumor number (Figure 2C, P=0.447). Patients with larger tumor size or advanced TNM stages showed poorer overall survival (Figure 2D, 2E; P=0.011 and P=0.021, respectively). Importantly, high USP7 level was correlated with unfavorable clinical outcomes of HCC patients (Figure 2F). The mean overall survival time of patients in low-USP7 group was 49.40±4.10 months, while only 38.28±3.55 months in the patients with higher USP7 levels (Table 2, P=0.030).
Figure 2.
Kaplan-Meir survival curves of HCC patients. Overall survival analyses were conducted by Kaplan-Meir method and compared by log-rank test according to patients’ age (A), gender (B), tumor number (C), tumor size (D), TNM stage (E), and USP7 protein level (F). * P<0.05 by log-rank test.
Table 2.
Kaplan-Meier overall survival of enrolled HCC patients.
| Variable | Cases (n=119) | OS (months) Mean ±S.D. | 5-year OS | P value |
|---|---|---|---|---|
| Age (years) | 0.337 | |||
| ≤51.0 | 67 | 41.96±3.76 | 31.2% | |
| >51.0 | 52 | 41.59±4.31 | 40.5% | |
| Gender | 0.708 | |||
| Female | 27 | 39.15±5.12 | 32.6% | |
| Male | 92 | 44.64±3.17 | 36.0% | |
| Tumor number | 0.447 | |||
| Single | 75 | 45.81±3.67 | 40.0% | |
| Multiple | 44 | 55.4±5.7 | 27.8% | |
| Tumor diameter | 0.011* | |||
| ≤5.0 | 50 | 52.01±4.51 | 50.1% | |
| >5.0 | 69 | 37.60±3.18 | 24.8% | |
| TNM stage | 0.021* | |||
| I–II | 46 | 51.30±4.29 | 47.6% | |
| III–IV | 73 | 36.62±2.96 | 26.8% | |
| USP7 expression | 0.030* | |||
| High | 64 | 38.28±3.55 | 29.4% | |
| Low | 55 | 49.40±4.10 | 41.7% |
Indicates P<0.05 by log-rank test.
Multivariate analysis was performed to further identify independent prognostic factors of HCC. The tumor size showed no significantly independent effect (Table 3, P=0.072). However, advanced TNM stages were independently correlated with a poor overall survival (HR=3.667, 95% CI 1.768–7.594, P=0.016). Furthermore, low USP7 expression was an independent protective factor for the clinical outcomes (HR=0.442, 95% CI 0.210–0.883, P=0.037).
Table 3.
Cox multivariate analysis of enrolled HCC patients.
| Variable | P value | HR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Tumor diameter (vs. ≤5.0cm) | 0.072 | 1.440 | 0.949 | 3.395 |
| TNM stage (vs. I–II) | 0.016* | 3.667 | 1.768 | 7.594 |
| USP7 expression (vs. high) | 0.037* | 0.442 | 0.210 | 0.883 |
Indicates P<0.05 by Cox regression test.
HR – hazard ratio; 95% CI – 95% confidence interval.
USP7 enhances tumor phonotype of HCC cells
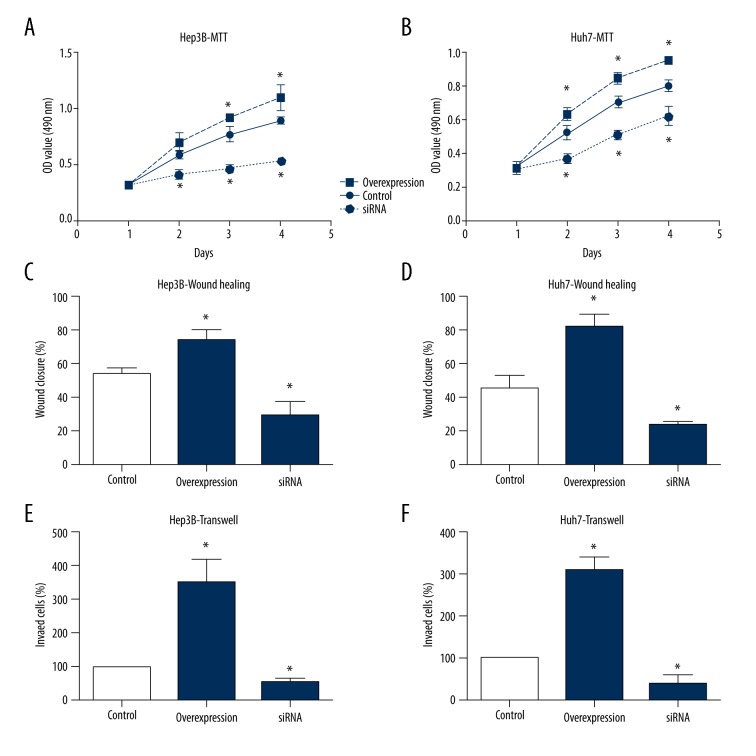
We also conducted cellular experiments using 2 human HCC cell lines, the Hep3B cells and Huh7 cells. HCC cells showed an elevated proliferation capacity upon USP7 overexpression (Figure 3A, 3B), which was consistent with the clinical finding that high USP7 level was associated with larger tumor size. In contrast, silencing USP7 significantly inhibit the cell proliferation process. The migration capacity of HCC cells was also tested by wound-healing assay, revealing that USP7 can promote tumor cell migration (Figure 3C, 3D). Similarly, overexpression USP7 enhanced the invasion ability of HCC cells as showed by the Matrigel-Transwell assay, while USP7-knockdown attenuated the invasion process (Figure 3E, 3F). The effects of USP7 on HCC migration and invasion can at least partially explained why USP7 was clinically correlated with multiple tumor locations and advanced TNM stages.
Figure 3.
USP7 enhances tumor cell proliferation, migration, and invasion. The proliferation curves of HCC cells were plotted by MTT assays, which showed that USP7 can enhance the cell viability in both Hep3B (A) and Huh7 (B) cells. Wound-healing assays were performed to evaluate the migration capacity of HCC cells, indicating a promoting role of USP7 on cell migration (C, D). The invasion process of tumor cells was monitored by Matrigel-transwell assay, demonstrating that USP7-overexpression enhanced cell invasion, while USP7-knockdown suppressed tumor invasion (E, F). Data was presented as mean ±SD. * P<0.05 by Student’s t-test.
Discussion
Ubiquitination modifications can regulate almost all physiological or pathological processes. Deubiquitylating enzyme USP7, also known as herpes virus-associated ubiquitin-specific protease (HAUSP), has been reported to regulate DNA damage repair [25], DNA replication [26], cell mitosis [27], virus infection [28], and immune response [29] by targeting different protein substrates. Dysregulation of USP7 is correlated with certain pathological changes including inflammation [30] and neurodegenerative diseases [31]. Recently, USP7 was also reported to be involved in tumor progression. Here we initiated to investigate the expression and function of USP7 in HCC.
One of the most well-known substrate of USP7 is p53, and deubiquitination of p53 by USP7 is critical for its stabilization [32], indicating its function in regulating tumor development. Another important substrate of USP7 is the PTEN (Phosphatase and tensin homolog) tumor suppressor [33], whose deubiquitination will prevent protein degradation [34]. However, the clinical significance of USP7 seems distinct among various tumor types. For example, high expression of USP7 was correlated with a poor prognosis in lung squamous cell carcinoma and large cell carcinoma [35]. Database analysis of neuroblastoma patients showed that high expression of USP7 predicted poor outcomes [36]. Studies on ovarian cancer patients also indicated the role of USP7 as an oncoprotein [17]. On the other hand, a lower expression of USP7 was observed in colon cancer tissues [37]. Similarly, upregulation of USP7 can inhibit the proliferation of esophagus cancer cells, indicating its tumor-suppressing roles [38]. Therefore, the specific function of USP7 seems to depend on its downstream substrates, which may be expressed distinctively in different tissues. Besides its downstream effectors, USP7 has been reported to be modulated by its upstream molecule microRNA-205, which also showed tumor regulating potential [39]. However, the role of USP7 on HCC remains elusive.
Our study showed that USP7 was upregulated in HCC tumor tissues compared normal liver tissues. The high expression of USP7 was associated with tumor growth and tumor metastasis according to clinical data. Moreover, univariate and multivariate analyses identified USP7 as an independent prognostic factor in HCC. Accordingly, we conducted cellular experiments using 2 human HCC cell lines; both showed that high expression of USP7 can promote tumor cell proliferation and invasion. In contrast, USP7 knockdown significantly inhibited tumor progression, revealing its potential as an anti-tumor drug target. In fact, several inhibitors of USP7 have been reported to suppress tumor phenotypes. For example, the USP7 inhibitor P22077 can inhibit neuroblastoma growth [36]. P5091, another small molecule inhibitor targeting USP7, can induce apoptosis in multiple myeloma cells [40]. Selective dual inhibitors targeting both USP7 and USP47 have also been reported, showing remarkable potential in cancer treatment [41]. Since our data provided evidence that USP7 plays a role in promoting HCC, it is worthwhile conducting future studies to test whether USP7 inhibitors can suppress HCC progression.
Conclusions
USP7 is upregulated in HCC tissues, which can serve as an independent prognostic predictor. USP7 promotes HCC progression by directly enhance tumor cell proliferation and invasion.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W-Q, Zheng R-S, Zhang S-W. Liver cancer incidence and mortality in China, 2009. Chin J Cancer. 2013;32(4):162–69. doi: 10.5732/cjc.013.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun XJ, Xu GL. Overexpression of Acyl-CoA Ligase 4 (ACSL4) in patients with hepatocellular carcinoma and its prognosis. Med Sci Monit. 2017;23:4343–50. doi: 10.12659/MSM.906639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon RT-P, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–82. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Zhang M, Peng Y, He J. Ubiquitin associated protein 2-like (UBAP2L) overexpression in patients with hepatocellular carcinoma and its clinical significance. Med Sci Monit. 2017;23:4779–88. doi: 10.12659/MSM.907071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouza C, López-Cuadrado T, Alcázar R, et al. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009;9(1):31. doi: 10.1186/1471-230X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu S, Chen X, Hu C, et al. Up-regulated maternal embryonic leucine zipper kinase predicts poor prognosis of hepatocellular carcinoma patients in a Chinese Han population. Med Sci Monit. 2017;23:5705–13. doi: 10.12659/MSM.907600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strikoudis A, Guillamot M, Aifantis I. Regulation of stem cell function by protein ubiquitylation. EMBO Rep. 2014;15(4):365–82. doi: 10.1002/embr.201338373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138(2):389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Ann Rev Biochem. 2009;78:363–97. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao N, Li H, Luo J, et al. Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration. Biochem J. 2012;441(3):979–87. doi: 10.1042/BJ20111358. [DOI] [PubMed] [Google Scholar]
- 12.Yao R, Pu J, Fan R, et al. Ubiquitin-specific protease 4 improves the prognosis of the patients in esophageal cancer. Cancer Biomark. 2017;20(3):317–23. doi: 10.3233/CBM-170308. [DOI] [PubMed] [Google Scholar]
- 13.Bayraktar S, Barrera AMG, Liu D, et al. USP-11 as a predictive and prognostic factor following neoadjuvant therapy in women with breast cancer. Cancer J. 2013;19(1):10–17. doi: 10.1097/PPO.0b013e3182801b3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: New insights into β-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–40. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Wen P, Kong R, et al. USP33 mediates Slit-Robo signaling in inhibiting colorectal cancer cell migration. Int J Cancer. 2015;136(8):1792–802. doi: 10.1002/ijc.29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YL, Yang YM, Xu H, Dong XS. Increased expression of ubiquitin-specific protease 22 can promote cancer progression and predict therapy failure in human colorectal cancer. J Gastroenterol Hepatol. 2010;25(11):1800–5. doi: 10.1111/j.1440-1746.2010.06352.x. [DOI] [PubMed] [Google Scholar]
- 17.Ma M, Yu N. Ubiquitin-specific protease 7 expression is a prognostic factor in epithelial ovarian cancer and correlates with lymph node metastasis. Onco Target Ther. 2016;9:1559–69. doi: 10.2147/OTT.S100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-Pérez S, Cabrera E, Salido E, et al. DUB3 and USP7 de-ubiquitinating enzymes control replication inhibitor Geminin: Molecular characterization and associations with breast cancer. Oncogene. 2017;36(33):4817. doi: 10.1038/onc.2017.220. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Cao L, Sheng X, et al. WDR79 promotes the proliferation of non-small cell lung cancer cells via USP7-mediated regulation of the Mdm2-p53 pathway. Cell Death Dis. 2017;8(4):e2743. doi: 10.1038/cddis.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori S, Ito G, Usami N, et al. p53 apoptotic pathway molecules are frequently and simultaneously altered in nonsmall cell lung carcinoma. Cancer. 2004;100(8):1673–82. doi: 10.1002/cncr.20164. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–90. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Liu Z, Li K, et al. TBL1XR1 predicts isolated tumor cells and micrometastasis in patients with TNM stage I/II colorectal cancer. J Gastroenterol Hepatol. 2017;32(9):1570–80. doi: 10.1111/jgh.13749. [DOI] [PubMed] [Google Scholar]
- 23.He J, Zhu Q, Wani G, et al. Ubiquitin-specific protease 7 regulates nucleotide excision repair through deubiquitinating XPC protein and preventing XPC protein from undergoing ultraviolet light-induced and VCP/p97 protein-regulated proteolysis. J Biol Chem. 2014;289(39):27278–89. doi: 10.1074/jbc.M114.589812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Yuan L, Liu D, et al. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol Res. 2014;84:32–44. doi: 10.1016/j.phrs.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Schwertman P, Lagarou A, Dekkers DH, et al. UV-sensitive syndrome protein UVSSA recruits USP7 to regulate transcription-coupled repair. Nat Genet. 2012;44(5):598–602. doi: 10.1038/ng.2230. [DOI] [PubMed] [Google Scholar]
- 26.Lecona E, Rodriguez-Acebes S, Specks J, et al. USP7 is a SUMO deubiquitinase essential for DNA replication. Nat Struct Mol Biol. 2016;23(4):270–77. doi: 10.1038/nsmb.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yim H, Shin S, Woo S, et al. Plk1-mediated stabilization of 53BP1 through USP7 regulates centrosome positioning to maintain bipolarity. Oncogene. 2017;36(7):966–78. doi: 10.1038/onc.2016.263. [DOI] [PubMed] [Google Scholar]
- 28.Lee H-R, Choi W-C, Lee S, et al. Bilateral inhibition of HAUSP deubiquitinase by a viral interferon regulatory factor protein. Nat Struct Mol Biol. 2011;18(12):1336–44. doi: 10.1038/nsmb.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Kumar S, Dahiya S, et al. Ubiquitin-specific protease-7 inhibition impairs Tip60-dependent Foxp3+ T-regulatory cell function and promotes antitumor immunity. EBioMedicine. 2016;13:99–112. doi: 10.1016/j.ebiom.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Loosdregt J, Fleskens V, Fu J, et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity. 2013;39(2):259–71. doi: 10.1016/j.immuni.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao Y-H, Fountain MD, Tacer KF, et al. USP7 acts as a molecular rheostat to promote WASH-dependent endosomal protein recycling and is mutated in a human neurodevelopmental disorder. Mol Cell. 2015;59(6):956–69. doi: 10.1016/j.molcel.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Chen D, Shiloh A, et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416(6881):648–53. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 33.Song MS, Salmena L, Carracedo A, et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008;455(7214):813–17. doi: 10.1038/nature07290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trotman LC, Wang X, Alimonti A, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128(1):141–56. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao G-Y, Lin Z-W, Lu C-L, et al. USP7 overexpression predicts a poor prognosis in lung squamous cell carcinoma and large cell carcinoma. Tumor Biol. 2015;36(3):1721–29. doi: 10.1007/s13277-014-2773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan Y, Cheng J, Vasudevan S, et al. USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis. 2013;4(10):e867. doi: 10.1038/cddis.2013.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhi Y, ShouJun H, Yuanzhou S, et al. STAT3 repressed USP7 expression is crucial for colon cancer development. FEBS Lett. 2012;586(19):3013–17. doi: 10.1016/j.febslet.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Lu S. Metformin inhibits esophagus cancer proliferation through upregulation of USP7. Cell Physiol Biochem. 2013;32(5):1178–86. doi: 10.1159/000354517. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L, Liu R, Zhang W, et al. MicroRNA-205 regulates ubiquitin specific peptidase 7 protein expression in hepatocellular carcinoma cells. Mol Med Rep. 2015;12(3):4652–56. doi: 10.3892/mmr.2015.3998. [DOI] [PubMed] [Google Scholar]
- 40.Chauhan D, Tian Z, Nicholson B, et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22(3):345–58. doi: 10.1016/j.ccr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinstock J, Wu J, Cao P, et al. Selective dual inhibitors of the cancer-related deubiquitylating proteases USP7 and USP47. ACS Med Chem Lett. 2012;3(10):789–92. doi: 10.1021/ml200276j. [DOI] [PMC free article] [PubMed] [Google Scholar]