Abstract
Introduction
Lenalidomide is an immunomodulatory drug approved by the US Food and Drug Administration in 2006 for the treatment of multiple myeloma. In 2012, the Food and Drug Administration issued a statement warning physicians of the increased risk with lenalidomide treatment of the following secondary primary malignancies: Acute myelogenous leukemia, myelodysplastic syndromes, and Hodgkin lymphoma. The statement did not mention glioblastoma multiforme, a Grade 4 astrocytoma, or other high-grade astrocytomas that have been reported on rare occasions in the setting of multiple myeloma.
Case Presentation
A 72-year-old man, who had been in complete remission from multiple myeloma for 1 year after treatment that included lenalidomide, presented with confusion, headache, nausea and vomiting, and recurrent falls. A magnetic resonance image of his brain revealed a mass that on stereotactic biopsy was found to be glioblastoma multiforme.
Discussion
We present the seventh reported case of high-grade astrocytoma as a second primary malignancy in multiple myeloma and the first reported occurrence of glioblastoma multiforme after the use of lenalidomide in multiple myeloma. This report adds to the pool of cases that reveal associations between use of lenalidomide and increased risk of developing secondary primary high-grade astrocytomas in multiple myeloma.
INTRODUCTION
Multiple myeloma (MM) is a neoplastic proliferation of plasma cells that usually is treated with various chemotherapy regimens, including immunomodulatory drugs.1 Lenalidomide (Revlimid, Celgene Corporation, Summit, NJ), an immunomodulatory agent approved by the US Food and Drug Administration (FDA) in 2006 for treatment of MM,2,3 is associated with the development of secondary cancers.4 The FDA issued a statement in 2012 about the increased risk of secondary primary malignancies (SPMs) in patients with newly diagnosed MM who received lenalidomide.5 However, the FDA statement did not include glioblastoma multiforme (GBM), a Grade 4 astrocytoma with a poor prognosis, or other high-grade astrocytomas. Here we present a rare case of a patient with MM who was treated with lenalidomide and in whom GBM developed as an SPM. We also present a literature review of GBM and high-grade astrocytomas occurring as the SPM in MM.
CASE PRESENTATION
Presenting Concerns
A 72-year-old man presented to our hospital with 6-weeks of confusion, headache, nausea and vomiting, and recurrent falls. His medical history was remarkable for λ-light-chain MM diagnosed 4 years earlier (immunoglobulin G predominant with 60% plasma cells on bone marrow; Durie-Salmon Stage II, Group A; International Staging System Stage I). After experiencing symptoms related to MM, our patient underwent 4 cycles of bortezomib (Velcade, Takeda Pharmaceuticals, Cambridge, MA), lenalidomide (Revlimid), and low-dose dexamethasone, followed by autologous stem cell transplantation, and then lenalidomide maintenance therapy for 2 years. He had been in complete remission for 1 year before presentation. Other medical conditions included hyperlipidemia, hypertension, and insulin-dependent diabetes mellitus. He denied use of tobacco, alcohol, or illicit drugs. His family history was notable for diabetes mellitus and hypertension.
On presentation, his vital signs were normal. The patient was alert but not oriented. He had expressive aphasia, unsteady gait, and masked facies.
Biochemical and hematologic investigations yielded normal results apart from the following values: Glucose 108 mg/dL (reference range = 70–99 mg/dL), total protein 5.4 mg/dL (6.4–8.3 mg/dL), albumin 3.0 mg/dL (3.5–5.2 mg/dL), mean corpuscular volume 104.6 fL (80–100 fL), platelets 83 × 109/L (150–450 × 109/L), and vitamin B12 above 1500 pg/mL (211–911 pg/mL).
Magnetic resonance imaging of the brain with and without contrast enhancement revealed a heterogenous mass (4.9 × 4.5 × 6 cm3) in the left temporal lobe extending into the uncus and surrounded by vasogenic edema, which caused a mass effect on the midbrain (Figure 1). Stereotactic biopsy of the mass revealed GBM, a World Health Organization Grade 4 fibrillary astrocytoma. Immunohistochemical staining was negative for isocitrate dehydrogenase 1 but positive for glial fibrillary acidic protein and p53 (Figure 2).
Figure 1.
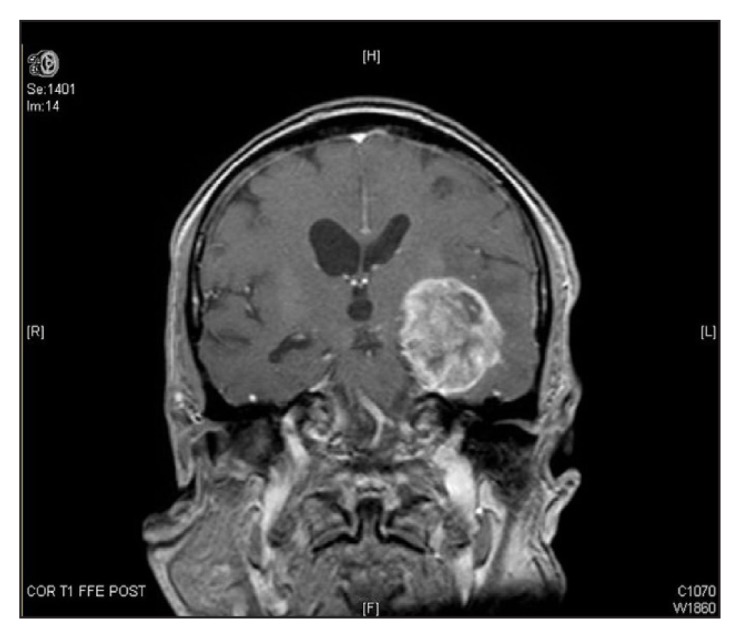
T1-weighted magnetic resonance image showing a left temporal mass, surrounded by vasogenic edema, extending to the uncus and causing a mild mass effect on the midbrain.
Figure 2.
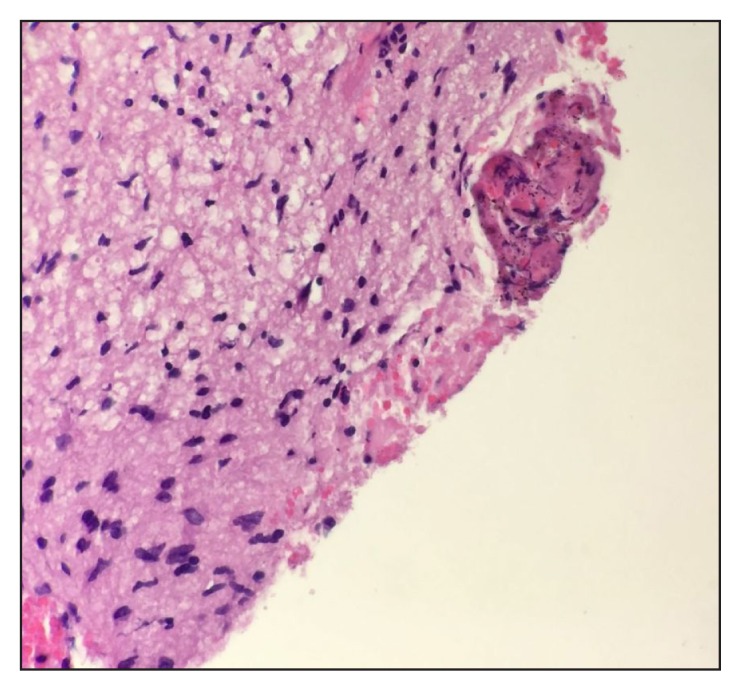
Touch preparation of biopsy specimen and hematoxylin-eosin slides at high-power (magnification ×40) show atypical astrocytes, endothelial proliferation, and focal necrosis.
Therapeutic Intervention and Treatment
Dexamethasone and levetiracetam were given to decrease the vasogenic edema and for seizure prophylaxis, respectively. We sought the opinion of a neurosurgeon, who advised forgoing complete tumor resection because the location in the dominant temporal lobe and the presence of aphasia made the procedure too risky. Repeated brain imaging after biopsy showed development of a communicating hydrocephalus, for which a ventriculostomy shunt was placed. Within 48 hours of shunt placement, the patient’s mental status improved and his symptoms of headache and nausea/vomiting resolved.
During the next 4 weeks, the patient received 10 sessions of palliative whole-brain radiation therapy at 30 Gy to 40 Gy.
Follow-up and Outcomes
Our patient’s clinical status deteriorated, and his power of attorney opted for hospice care. He died four months later because of complications secondary to hospital-associated pneumonia. Table 1 shows a timeline of the case.
Table 1.
Case timeline
| Date | Presenting symptoms | Work-up (findings) | Treatment | Outcomes |
|---|---|---|---|---|
| 09/2011 | None | Diagnosed (asymptomatic) λ-light-chain multiple myeloma (DS Stage II, Group A; ISS, Stage 1) | Conservative | Became symptomatic |
| 09/2013 | Symptomatic disease | Re-evaluation by Medical Oncology | Induction therapy with 4 cycles of bortezomib, lenalidomide, and low-dose dexamethasone; Autologous stem cell transplant; maintenance therapy with lenalidomide | Symptoms improved, disease stabilized |
| 09/2014 | None (Medical Oncology re-evaluation) | Clinical remission | Maintenance therapy with lenalidomide | Asymptomatic |
| 7/2015 | Symptoms: Confusion, headache, nausea/vomiting, and recurrent falls | none | Conservative | Symptoms progressed |
| 09/2015–10/2015 | Worsening of symptoms, hematologic abnormalities | MRI of head (large mass involving left temporal lobe with edema and midline shift) | Admission to hospital for dexamethasone and stereotactic biopsy (Grade IV fibrillary astrocytoma: Glioblastoma multiforme); ventriculostomy shunt from neurosurgery; whole-brain palliative radiation therapy (10 fractions of 30 Gy-40 Gy) from radiation oncology | Symptoms improved after shunt placement and radiation therapy |
| 10/2015 | Clinical deterioration with dyspnea | Chest X-ray (hospital-associated pneumonia) | Power of attorney opted for hospice (discontinuation of radiation therapy) | Patient died |
DS = Durie-Salmon Staging System; ISS = International Staging System; MRI = magnetic resonance image.
DISCUSSION
Multiple myeloma is a neoplastic proliferation of plasma cells producing a monoclonal gammopathy with related organ or tissue impairment.1 Newly diagnosed patients undergo induction therapy with corticosteroids plus proteasome inhibitors bortezomib or carfilzomib, thalidomide or immunomodulatory drugs lenalidomide or pomalidomide, and/or alkylating agents (eg, cyclophosphamide or melphalan), followed by autologous stem cell transplant if eligible and maintenance with thalidomide or lenalidomide and/or corticosteroids until disease progression or intolerance to treatment develops.1
Lenalidomide, a derivative of thalidomide, is an immunomodulatory drug approved by the FDA for treatment of MM in 2006.2,3 In 2012, Yang et al4 compared three Phase 3 trials examining maintenance therapy with lenalidomide vs placebo and found a nearly three-fold increase in invasive malignancies, especially hematologic malignancies, in patients with MM receiving lenalidomide. That same year, the FDA issued a warning statement about hematologic SPMs, including acute myelogenous leukemia, myelodysplastic syndromes, and Hodgkin lymphoma, after MM maintenance therapy with lenalidomide.5
One uncommon nonhematologic SPM is GBM, a Grade 4 astrocytoma. It is a solid tumor involving glial cells of the brain and is the most common primary and malignant brain tumor, in addition to the most aggressive.6 High-grade astrocytomas are extremely rare in the setting of MM and have been reported previously in 6 studies, all of which are summarized in Table 2.7–12 Our patient is the 7th reported case. The exact etiology in this setting is unclear, and researchers have postulated it to be multifactorial, involving the treatment, MM itself, patient characteristics, and factors in the environment and human behavior.13 To our knowledge, no other cases of GBM arising after MM treatment with lenalidomide have been reported.
Table 2.
Reported cases of high-grade astrocytoma as secondary primary tumor in patients with multiple myeloma
| Source, year | Patient sex, age in years | Multiple myeloma characteristics | Therapy for multiple myeloma (duration) | Astrocytoma (WHO grade) | Interval from diagnosis of multiple myeloma to astrocytoma (mo) |
|---|---|---|---|---|---|
| Han et al,7 2013 | Male, 49 | λ-light-chain predominant, 69.5% plasma cells on bone marrow biopsy | Bortezomib, cyclophosphamide, and dexamethasone (3 mo) followed by autologous stem cell transplant, then thalidomide (22 mo) | Anaplastic (3) | 26 |
| Kato et al,8 1989 | Male, 76 | 15% plasma cells on bone marrow biopsy | Melphalan, prednisone, and cyclophosphamide (2 mo) | Diffuse gemistocytic (2) | 2 |
| Gisserot et al,9 1997 | Male, 73 | IgG predominant | Melphalan and prednisone (35 cycles, 1991–1997); interferon-α and pamidronate (1993) | Glioblastoma multiforme (4) | 144 |
| González Silva,10 1993 | Male, 55 | IgD predominant, 52% plasma cells on bone marrow biopsy | Vincristine, melphalan, prednisone, and cyclophosphamide; vincristine, carmustine, doxorubicin, and prednisone (9 cycles for 6 months), then vincristine, doxorubicin, dexamethasone, and interferon (3 cycles) | Glioblastoma multiforme (4) | 24 |
| Sonoda et al,11 1998 | Male, 53 | IgG predominant, 17.8% plasma cells on bone marrow biopsy | Melphalan and prednisone (14 d) | Glioblastoma multiforme (4) | NA (coexistence, diagnosed 14 d later) |
| Treto Rosal et al,12 2005 | Male, 70 | 80% plasma cells on bone marrow biopsy | Melphalan and prednisone (19 mo) | Glioblastoma multiforme (4) | 19 |
Ig = immunoglobulin; NA = not applicable; WHO = World Health Organization.
Our case report reiterates the risk of developing SPMs in the setting of MM. Our case also highlights the potential association with lenalidomide and increased risk of tumors such as high-grade astrocytomas, including GBM, because it is not uncommon for patients with one malignancy to have another develop.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 May 19;127(20):2375–90. doi: 10.1182/blood-2016-01-643569. DOI: https://doi.org/10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KC. Lenalidomide and thalidomide: Mechanisms of action—similarities and differences. Semin Hematol. 2005 Oct;42(4 Suppl 4):S3–8. doi: 10.1053/j.seminhematol.2005.10.001. DOI: https://doi.org/10.1053/j.seminhematol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Revlimid (lenalidomide) capsules [prescribing information] Summit, NJ: Celene Corporation; 2015. Feb, [Google Scholar]
- 4.Yang J, Terebelo HR, Zonder JA. Secondary primary malignancies in multiple myeloma: An old NEMESIS revisited. Adv Hematol. 2012;2012:801495. doi: 10.1155/2012/801495. DOI: https://doi.org/10.1155/2012/801495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FDA drug safety communication: Safety review update of cancer drug Revlimid (lenalidomide) and risk of developing new types of malignancies [Internet] Washington, DC: US Food and Drug Administration; 2012. May 7, [updated 2016 Mar 7; cited 2017 Jan 30]. Available from: www.fda.gov/Drugs/DrugSafety/ucm302939.htm. [Google Scholar]
- 6.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA. 2013 Nov 6;310(17):1842–50. doi: 10.1001/jama.2013.280319. DOI: https://doi.org/10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 7.Han X, Jin D, Zheng G, Luo Y, Cai Z. Astrocytoma development following complete multiple myeloma remission in a 49-year-old patient: A case report. Exp Ther Med. 2013 Aug;6(2):509–12. doi: 10.3892/etm.2013.1179. DOI: https://doi.org/10.3892/etm.2013.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato I, Kinouchi H, Imaizumi S, Katakura R, Yoshimoto T. [An autopsy case of cerebral astrocytoma associated with multiple myeloma]. No Shinkei Geka. 1989 Sep;17(9):877–81. [Article in Japanese] [PubMed] [Google Scholar]
- 9.Gisserot O, de Jaureguiberry JP, Ribeil JA, Villemagne B, Jaubert D. [Cerebral glioblastoma complicating the course of myeloma]. Presse Med. 1997 Sep 6;26(25):1197. [Article in French] [PubMed] [Google Scholar]
- 10.González Silva M. [Second neoplasm in a patient diagnosed with IgD myeloma. Presentation of a case and review of the literature]. Sangre (Barc) 1993 Feb;38(1):47–9. [Article in Spanish] [PubMed] [Google Scholar]
- 11.Sonoda Y, Kumabe T, Umezawa K, et al. [Rapid growth of glioblastoma during therapy for multiple myeloma: Case report]. No Shinkei Geka. 1998 Aug;26(8):737–41. [Article in Japanese] [PubMed] [Google Scholar]
- 12.Treto Rosal JA, Gonzalez Aleman I, Gutierrez Ronquillo J, Machado I, Rodriguez Rodriguez L. Glioblastoma multiforme arising in the setting of multiple myeloma. Haema. 2005;8(4):704–6. [Google Scholar]
- 13.Thomas A, Mailankody S, Korde N, Kristinsson SY, Turesson I, Landgren O. Second malignancies after multiple myeloma: From 1960s to 2010s. Blood. 2012 Mar 22;119(12):2731–7. doi: 10.1182/blood-2011-12-381426. DOI: https://doi.org/10.1182/blood-2011-12-381426. [DOI] [PMC free article] [PubMed] [Google Scholar]


