Abstract
Introduction
Acute pulmonary embolism is the third leading cause of cardiovascular death. Management options include anticoagulation with or without thrombolysis. Concurrent persistent hypoxemia should be a clue to the existence of an intracardiac shunt.
Case Presentation
A 46-year-old man experienced acute hypoxemic respiratory failure requiring mechanical ventilation after anesthesia induction for elective hip arthroplasty. He was found to have submassive bilateral pulmonary emboli with acute right ventricular dysfunction and a coexisting patent foramen ovale with right-to-left shunt. He remained profoundly hypoxemic despite catheter-directed thrombolysis. He underwent surgical embolectomy with partial endarterectomy, resulting in clinical improvement.
Discussion
The management of acute submassive pulmonary embolism is undertaken on an individualized basis because of the wide spectrum of clinical presentations. In this report we review the literature and discuss the evidence behind the management of cases of acute pulmonary embolism complicated by hypoxemia from a patent foramen ovale. In a case of acute pulmonary embolism complicated by refractory hypoxemia from an intracardiac shunt, adjunctive therapies in addition to anticoagulation and thrombolysis must be considered.
INTRODUCTION
Acute pulmonary embolism (PE) is the third leading cause of cardiovascular death.1 PE can be stratified by prognosis to guide treatment decisions. Submassive or intermediate-risk PE is diagnosed on the basis of normal blood pressure in conjunction with evidence of cardiac dysfunction, shown either on cardiothoracic imaging or with elevated serum cardiac biomarkers. Acute management options for PE include anticoagulation alone or in combination with systemic thrombolytic therapy or catheter-directed thrombolysis, although guideline recommendations reserve thrombolytic therapy for cases of PE associated with hypotension (massive or high-risk PE). Persistent hypoxemia in cases of acute PE should alert clinicians to the possibility of an intracardiac shunt, such as a patent foramen ovale (PFO).
CASE PRESENTATION
Presenting Concerns
Acute hypoxemic respiratory failure developed in a 46-year-old white man during anesthesia induction for elective left total hip arthroplasty. Four days before the surgery, the patient and his wife had noticed that he was having episodes of palpitations and dyspnea. He had been largely sedentary because of chronic hip pain. His medical history included morbid obesity, pseudotumor cerebri maintained on a regimen of acetazolamide, obstructive sleep apnea, and stable schizoaffective disorder. His prior cardiac workup, which was performed in the setting of chest pain and was ultimately deemed nonanginal, included a normal transthoracic echocardiogram and a coronary angiogram demonstrating nonobstructive coronary arterial disease. His social history was unremarkable, although his family history contained a Factor V Leiden mutation.
During induction of anesthesia, he remained hemodynamically stable but had progressively worsening hypoxemia demonstrated on serial arterial blood gas values. He was sedated with propofol, paralyzed initially with succinylcholine and maintained with rocuronium, and received mechanical ventilation with volume assist/control with a 100% fraction of inspired oxygen (FiO2) and positive end-expiratory pressure (PEEP) of 10 cm H2O. An arterial blood gas test on this setting showed a pH of 7.29, PCO2 of 50 mmHg, and PO2 of 65 mmHg. A transesophageal echocardiogram demonstrated right-sided heart dysfunction with a large right ventricle (RV) to left ventricle (LV) ratio and was positive for McConnell sign (contraction of the right ventricular apex with akinesis of the free wall).
Therapeutic Intervention and Treatment
Acute PE was suspected, and an unfractionated heparin infusion was started. The patient’s scheduled elective hip arthroplasty was aborted because of the degree of hypoxemia. The patient was transferred to the intensive care unit.
On arrival to the intensive care unit, he was afebrile, with a heart rate of 91 beats/min and blood pressure of 121/61 mmHg by arterial line. Laboratory findings included leukocytosis (15.2 × 109/L) with 3% band forms. Lactate level was 1.39 mmol/L (within the normal range). Troponin I level was 0.05 ng/mL (normal range, 0–0.4 ng/mL) and did not change on serial testing.
A computed tomography angiogram of the chest showed bilateral large pulmonary emboli, nearly occluding the right and left pulmonary arteries, with extension into the upper and lower lobes as well as into the right middle lobe (Figures 1 and 2). Because of his hypoxemia while receiving an FiO2 of 100%, we were concerned he had an intracardiac shunt. A transthoracic echocardiogram with agitated saline confirmed the presence of a PFO with right-to-left shunt. The estimated shunt fraction was approximately 40%. Because the patient had severe hypoxemia secondary to the acute PE, which was causing elevated RV pressures and right-to-left cardiac shunting, we performed catheter-directed thrombolysis with infusion of alteplase through bilateral femoral lysis catheters at 1 mg/h for 5 hours, and then 0.5 mg/h for 48 hours. The presence of a PFO was also confirmed during this procedure.
Figure 1.
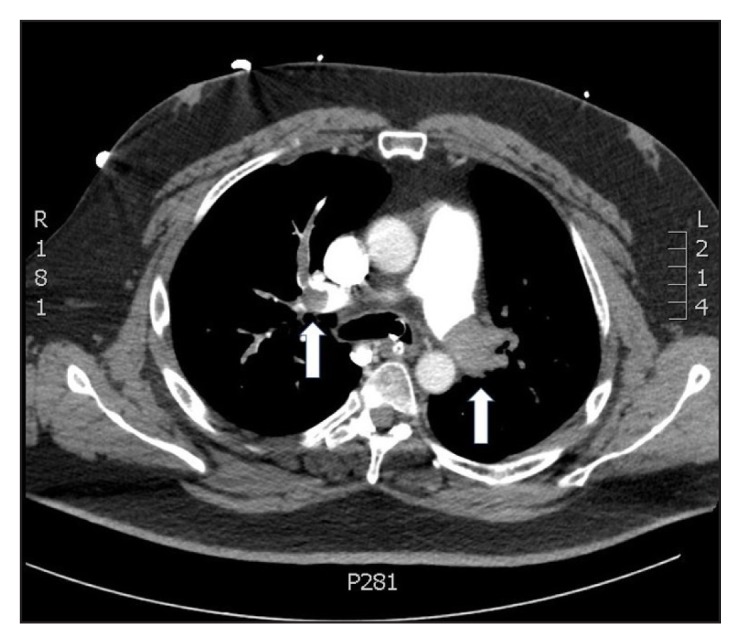
Computed tomography angiogram of the chest demonstrating bilateral large pulmonary emboli (arrows), nearly occluding the right and left pulmonary arteries.
Figure 2.
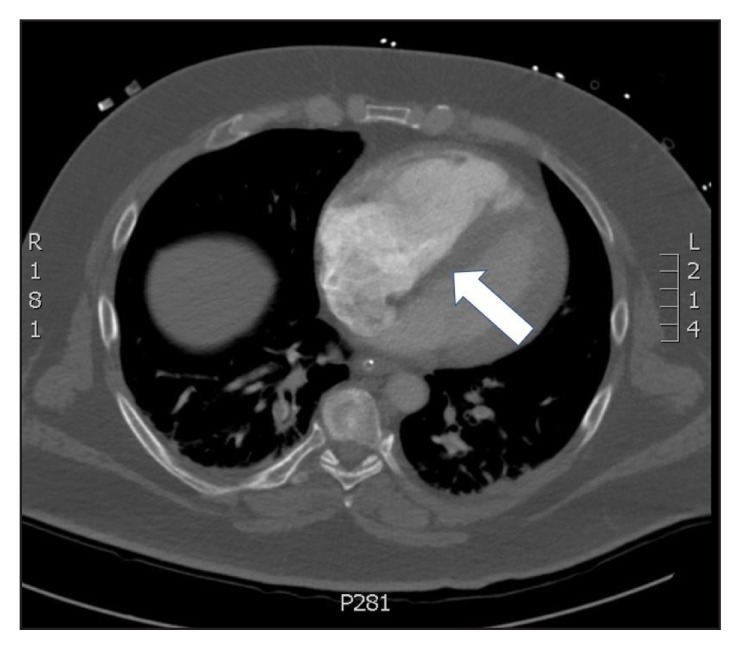
Computed tomography angiogram of the chest demonstrating dilation of the right ventricle (arrow).
The patient continued to have intermittent desaturations despite oxygenation with 100% FiO2. The PEEP was lowered to 5 cm H2O, and inhaled nitric oxide therapy was started in an attempt to reduce the shunt fraction. Cardiothoracic surgery was consulted for possible embolectomy in light of unsuccessful directed thrombolysis. However, our patient’s hypoxemia stabilized with the administration of 100% FiO2 and inhaled nitric oxide on 30 ppm, with arterial partial pressure of oxygen maintained above 60 mmHg. He was unable to wean off inhaled nitric oxide despite 48 hours of thrombolytic therapy, so we made the decision to pursue surgical intervention. Surgical embolectomy (Figure 3), partial endarterectomy, and closure of the PFO were performed on hospital day 4. The patient was successfully extubated on hospital day 6.
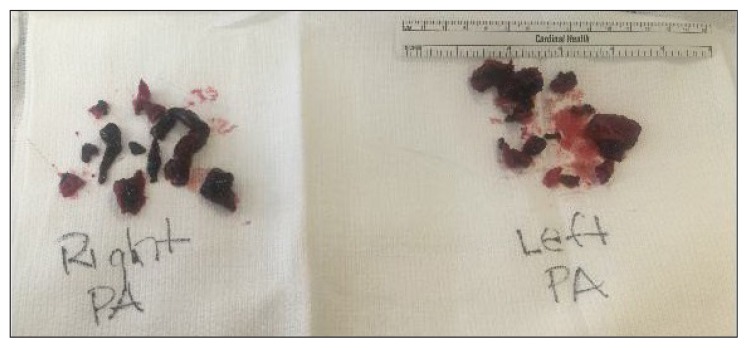
Figure 3.
Thrombi removed from the pulmonary arteries during surgical embolectomy.
PA = pulmonary artery.
The hospital course was complicated by right-sided hemiparesis that was discovered on sedation hold that was performed a few hours before embolectomy. A noncontrast-enhanced computed tomography scan of the head demonstrated multiple small embolic strokes affecting the left premotor cortex. These were probably paradoxical emboli in the setting of the PFO, without evidence of hemorrhagic conversion. The patient was discharged to a skilled nursing facility on hospital day 11. Table 1 shows a timeline of the case.
Table 1.
Case timeline
| Milestone | Events/Interventions/Outcomes |
|---|---|
| Hospital day 1 | Hypoxemia on anesthesia induction for elective hip repair |
| Transthoracic echocardiogram: Increased RV to LV ratio; positive McConnell sign; PFO seen | |
| Computed tomography angiogram of the chest: Pulmonary emboli in both pulmonary arteries | |
| Catheter-directed thrombolysis administered | |
| Hospital day 2 | Persistent hypoxemia |
| Inhaled nitric oxide administered | |
| Hospital day 4 | Computed tomography of the head: Left premotor cortex embolic strokes |
| Surgical embolectomy with PFO closure | |
| Hospital day 6 | Extubation |
| Hospital day 11 | Discharge to rehabilitation facility |
| 4-week outpatient follow-up | No residual neurologic deficits or hypoxia |
LV = left ventricle; PFO = patent foramen ovale; RV = right ventricle.
Follow-up and Outcomes
On follow-up with his primary care physician four weeks after hospital discharge, our patient was noted to be well-appearing and without residual deficits from his stroke. As of this writing, he remains on an anticoagulation regimen of rivaroxaban. He was evaluated for an underlying hypercoagulable state but did not have Factor V Leiden mutation or antiphospholipid antibodies.
Written informed consent was obtained from the patient. Institutional review board approval was waived by the Veterans Affairs Portland Health Care System in OR because the patient’s demographic information was deidentified in this case report.
DISCUSSION
The all-cause mortality for patients in the US and Europe with acute PE ranges from 9% to 17%.1 Acute PE is categorized into massive, submassive, and nonmassive types. Massive, or high-risk, PE occurs in the setting of persistent hypotension with systolic blood pressure less than 90 mmHg for 15 minutes or greater. Normotensive individuals with evidence of right-sided heart dysfunction, whether on imaging such as computed tomography or echocardiography, or by elevated cardiac biomarkers such as troponin or creatine kinase-myocardial band, are classified as having submassive, or intermediate-risk, PE.2,3 PE may also be stratified by the PE Severity Index, which is a validated scoring system based on independent predictors of mortality.3 We presented a case of a patient with a submassive PE and right ventricular dysfunction. He had an intermediate-risk PE Severity Index score (Class III), which gave him a 3% to 7% risk of 30-day mortality.
Acute PE is often considered when hypoxemia develops. However, PE alone rarely causes hypoxemia to the degree seen in our patient, and it may be useful in such cases to perform an evaluation for an intracardiac shunt. On the basis of autopsy studies, approximately 35% of the general population have a probe-patent PFO, and 5% to 10% have a flow-patent PFO, which is determined with the application and sudden removal of PEEP.4 In acute PE, the PFO acts as a “pop-off valve” such that the right atrial pressure increases, causing a right-to-left shunt. The shunt permits LV filling and near-normal cardiac output, thus preventing hypotension. However, hypoxemia ensues because of the intracardiac mixing of deoxygenated with oxygenated blood.4–8 The presence of PFO was associated with a significantly lower arterial partial pressure of oxygen among 85 patients with a hemodynamically significant PE, in whom there was a 39% prevalence of PFO.4 Unlike many other causes of severe hypoxemia, such as in acute respiratory distress syndrome, the administration of PEEP often worsens the hypoxemia, as it did in our case.9,10
We conducted a PubMed search using the search terms [pulmonary embolism OR embolus] AND [hypoxemia OR hypoxemic] AND [patent foramen ovale] for articles published between 1990 and 2016. Individual cases, presented in either case reports or case series, were selected. Further relevant case reports were extracted from the bibliography of articles gathered from the search. In total, there were 9 articles with 12 individual cases reported of acute PE occurring in the setting of PFO and resulting in persistent hypoxemia (Table 2).4,6–13 Submassive PE was present in 45% of patients, and of these, survival was 100%. In those with massive PE, survival was 20%. The overall survival was 45%. Two patients without hypotension received systemic thrombolytics, 27% of the total.7,8 In one case report describing a patient without hypotension who received thrombolysis, the authors advocated for systemic thrombolysis because of the size of the emboli and the impending risk of hemodynamic collapse.8 Surgical intervention was reported in 18% of patients. No patients received catheter-directed thrombolysis.
Table 2.
Literature review of reported cases of acute pulmonary embolus in the setting of patent foramen ovale and intracardiac shunting
| Source | Age, years/sex | Presentation | Diagnostics | Hemodynamic classification | Mechanical ventilation | Management | Survival |
|---|---|---|---|---|---|---|---|
| Brydon et al, 19934 | 64/M | Found unresponsive | RHC: PFO, bilateral PE | Submassive | Yes | Surgical closure of PFO; surgical embolectomy | Yes |
| Estagnasié et al, 19966 | 74/F | Acute respiratory failure | TEE: PFO, atrial septal aneurysm; angiography: PE | Massive | Yes | Unfractionated heparin; inhaled nitric oxide | No |
| 68/F | Subacute dyspnea leading to respiratory failure and shock | TEE: PFO, atrial septal aneurysm | Massive | Yes | NR | No | |
| 77/F | Respiratory failure | TEE: PFO, atrial septal aneurysm; angiography: Bilateral PE | Massive | Yes | NR | No | |
| Mirarchi et al, 200011 | 74/M | Acute dyspnea and chest pain with hypotension and respiratory failure | TEE: PFO and right atrial thrombus | Massive | Yes | Unfractionated heparin alone | No |
| Slebos et al, 20007 | 33/F | Respiratory failure | Angiography: PE; presumptive diagnosis of PFO | Submassive | No | Systemic thrombolytics | Yes |
| Rajan, 20079 | 89/M | Intraoperative respiratory failure during open reduction and internal fixation of hip | CT: Multiple bilateral PEs; TTE: Dilated RV and PFO | Submassive | Yes; worsening oxygenation with increasing PEEP | NR | NR |
| Moua et al, 20088 | 80/F | Acute dyspnea and presyncope | CT angiography: Bilateral PE; TTE: Dilated RV and PFO | Submassive | No | Systemic thrombolytics | Yes |
| 75/M | Acute dyspnea and dizziness | CT angiography: Bilateral PE; TTE: McConnell sign and PFO | Submassive | Yes | Unfractionated heparin, with addition of systemic thrombolytics | Yes | |
| Weig et al, 201112 | 34/F | Respiratory failure 4 weeks after biventricular assist device placement for postpartum dilated cardiomyopathy | TEE: PFO | Massive | Yes | Venovenous ECMO; surgical embolectomy; inhaled pulmonary vasodilator; percutaneous transcatheter closure of PFO with PFO occludera | Yes |
| Vaid et al, 201113 | 62/M | Respiratory failure 6 hours after vitrectomy to treat retinal detachment | CT angiography: Limited examination; TTE: McConnell sign; autopsy: PE, PFO present | NR | Yes; high PEEP and high FiO2 used | Systemic thrombolytics | No |
| Granati, 201610 | 40/M | Acute dyspnea | CT angiography: PE with right-sided heart strain; TTE: PFO and atrial septal aneurysm; duplex ultrasonography: Left lower extremity DVT | NR | Yes; increasing PEEP caused worsening SaO2; improvement with switch to airway pressure release ventilation | NR | Yes |
PFO occluder manufactured by Amplatzer, St Jude Medical, St Paul, MN.
CT = computed tomography; DVT = deep vein thrombosis; ECMO = extracorporeal membrane oxygenation; F = female; FiO2 = fraction of inspired oxygen; M = male; NR = not reported; PE = pulmonary embolus; PEEP = positive end-expiratory pressure; PFO = patent foramen ovale; RHC = right-sided heart catherization; RV = right ventricle; SaO2 = arterial oxygen saturation; TEE = transesophageal echocardiogram; TTE = transthoracic echocardiogram.
Among 1006 patients with submassive PE in the PEITHO (Pulmonary Embolism Thrombolysis) randomized controlled trial, systemic thrombolysis with tenecteplase resulted in significant improvement in the combined primary outcome of all-cause mortality or hemodynamic compromise within 7 days over the administration of unfractionated heparin alone (2.6% vs 5.6%, odds ratio [OR] = 0.44, 95% confidence interval [CI] = 0.23–0.87, p = 0.02). However, there was no overall mortality benefit, and there were more patients with major extracranial hemorrhages (6.3% vs 1.2%, OR = 5.55, 95% CI = 2.3–13.39, p < 0.001) and strokes (2.4% vs 0.2%, OR = 12.10, 95% CI = 1.57–93.39, p = 0.003) within 7 days.14
The ULTIMA (Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism) trial demonstrated that ultrasound-assisted, catheter-directed thrombolytic therapy in patients with submassive PE resulted in benefit in terms of change in RV-to-LV ratio from baseline to 24 hours, over unfractionated heparin alone (0.30 ± 0.20 vs 0.03 ± 0.16, p < 0.001). There were no instances of major bleeding in this study. Patients with a right-to-left heart shunt, such as a PFO, were excluded from the study population.15
Two recent meta-analyses,16,17 both including PEITHO, found that systemic thrombolysis decreases overall mortality while increasing major bleeding for acute PEs of both high and intermediate risk. The meta-analysis by Marti et al16 found that 30-day mortality was significantly decreased with systemic thrombolysis (2.3% vs 3.9%; OR = 0.59, 95% CI = 0.36–0.96, p = 0.034) while there were significantly increased rates of major hemorrhage (9.9% vs 3.6%; OR = 2.91, 95% CI = 1.95–4.36, p < 0.0001) and fatal or intracranial hemorrhage (1.7% vs 0.3%; OR = 3.18, 95% CI = 1.25–8.11, p = 0.008). However, 30-day overall mortality was not significantly different when the analysis included only intermediate risk PE. The analysis also did not include ULTIMA.16 The meta-analysis by Chatterjee et al17 found a similar significant benefit in the 30-day all-cause mortality rate for all acute PEs with a number needed to treat of 59, which was counterbalanced by a significant increase in the rates of major bleeding (number needed to harm of 18) and intracranial hemorrhage (number needed to harm of 78). Additionally, a systematic review of 35 trials demonstrated a pooled survival of 87% among patients with massive PE treated with catheter-directed interventions; 60% to 67% of these individuals also had received systemic thrombolytics.18 We could not find analyses from these trials that separately reported outcomes for patients with significant intracardiac right-to-left shunts.
The 2016 revision of the American College of Chest Physicians guidelines for venous thromboembolism,2 as well as the 2014 European Society of Cardiology guidelines for acute PE,3 recommend systemic thrombolysis for acute massive PE (a Grade 1B recommendation in the American College of Chest Physicians guidelines). Both sets of guidelines recommend against routine systemic thrombolysis in individuals with PE but who do not have shock or hypotension, unless they have clinical deterioration (Grade 1B recommendation).2,3 We elected to treat our patient’s submassive PE with thrombolysis because we did not expect his severe hypoxemia to improve otherwise.
Although there are no comparative trials of catheter-directed vs systemic thrombolytics,19 we pursued catheter-directed thrombolysis because of the possible lower risk of bleeding and our institutional experience with this procedure (Grade 2C recommendation per American College of Chest Physicians guidelines).2 In one systematic review and meta-analysis, the risk of major bleeding from catheter-directed thrombolysis was about 10% from 24 studies performed in both massive and submassive PE20; in another systematic review stratified by PE classification, the risk of major bleeding was 3.9 per 100 cases of catheter-directed thrombolysis in hemodynamically stable PE.21 In our literature review of similar patients, none of the reported cases used catheter-directed interventions; however, only 1 of these cases was published after ULTIMA.
Another negative consequence of PFO is the risk of paradoxical emboli resulting in strokes. Such patients may hypothetically be at increased risk of intracranial hemorrhage from hemorrhagic conversion of these paradoxical strokes after undergoing systemic or catheter-directed thrombolysis. The coexistence of a PFO was associated with the increased incidence of ischemic stroke among patients with acute PE in 1 observational study, with a 13% rate of stroke in 48 patients who were found to have a PFO, and 2.2% stroke rate among 91 patients who did not have a PFO. However, there was no between-group difference in the proportion of patients receiving systemic thrombolysis.22 Although our patient was found to have strokes from paradoxical emboli, the use of catheter-directed thrombolysis in his case did not result in intracranial hemorrhage.
Other management considerations in patients with PE besides thrombolytics or anticoagulation therapy include mechanical or medical support of RV failure and surgical embolectomy. Pulmonary vasodilators may also be used to decrease pulmonary vascular resistance in situations of acute RV failure, such as acute PE. Inhaled nitric oxide has a rapid onset and short half-life, making it easily titratable.23 Inhaled pulmonary vasodilators were used in 2 cases in our literature review.6,13 Extracorporeal membrane oxygenation (ECMO) can be used as another bridge to definitive therapy for persistent shock (venoarterial ECMO) or hypoxemia alone (venovenous). Venovenous ECMO was used in 1 case in our literature review as a bridge to surgery; that patient had concurrent cardiac failure and was reliant on a biventricular assist device.13 Surgical embolectomy is the definitive management for cases in which thrombolytics have failed or are contraindicated. The 1-year survival rate for surgical embolectomy in 1 series was 80% for submassive PE, compared with 66% for massive PE.24 Surgical intervention was successful in resolving our patient’s hypoxemia.
CONCLUSION
The management of acute submassive PE is undertaken on an individualized basis, given the spectrum of clinical presentations of such cases. The use of systemic thrombolysis should be weighed against the risk of severe bleeding. The outcomes of catheter-directed interventions compared with systemic thrombolysis are yet unknown. In acute PE with refractory hypoxemia in which an intracardiac shunt is the cause, there is even less evidence to guide decision making, because many of the large trials exclude patients with PFO or do not specifically identify these patients. When deciding between systemic or catheter-directed thrombolysis or anticoagulation therapy alone, the clinician should consider individual patient factors and the potential increased risk of intracranial hemorrhage because of a PFO. Finally, if there is a failure of systemic thrombolysis to decrease pulmonary arterial pressure and the intracardiac shunt, then bridging therapies such as ECMO or surgical embolectomy must be considered.
Acknowledgment
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012 May;379(9828):1835–46. doi: 10.1016/S0140-6736(11)61904-1. DOI: https://doi.org/10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 2.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016 Feb;149(2):315–52. doi: 10.1016/j.chest.2015.11.026. DOI: https://doi.org/10.1016/j.chest.2015.11.026. Erratum in: Chest 2016 Oct;150(4):988. DOI: https://doi.org/10.1016/j.chest.2016.08.1442. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinides S, Torbicki A, Agnelli G, et al. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014 Nov 14;35(43):3033–73. doi: 10.1093/eurheartj/ehu283. DOI: https://doi.org/10.1093/eurheartj/ehu283. Erratum in: Eur Heart J 2015 Oct 14;36(39):2666. DOI: https://doi.org/10.1093/eurheartj/ehv131. Erratum in: Eur Heart J 2015 Oct 14;36(39):2642. DOI: https://doi.org/10.1093/eurheartj/ehu479. [DOI] [PubMed] [Google Scholar]
- 4.Brydon C, Fawcett WJ, Treasure T, Clarke JT. Pulmonary embolus and patent foramen ovale: A rare cause of refractory hypoxaemia. Br J Anaesth. 1993 Aug;71(2):298–300. doi: 10.1093/bja/71.2.298. DOI: https://doi.org/10.1093/bja/71.2.298. [DOI] [PubMed] [Google Scholar]
- 5.Kasper W, Geibel A, Tiede N, Just H. Patent foramen ovale in patients with haemodynamically significant pulmonary embolism. Lancet. 1992 Sep 5;340(8819):561–4. doi: 10.1016/0140-6736(92)92102-l. DOI: https://doi.org/10.1016/0140-6736(92)92102-l. [DOI] [PubMed] [Google Scholar]
- 6.Estagnasié P, Djedaïni K, Le Bourdellès G, Coste F, Dreyfuss D. Atrial septal aneurysm plus a patent foramen ovale. A predisposing factor for paradoxical embolism and refractory hypoxemia during pulmonary embolism. Chest. 1996 Sep;110(3):846–8. doi: 10.1378/chest.110.3.846. DOI: https://doi.org/10.1378/chest.110.3.846. [DOI] [PubMed] [Google Scholar]
- 7.Slebos DJ, Tulleken JE, Ligtenberg JJ, Zijlstra JG, van der Werf TS. A narrow escape: Surviving massive pulmonary thromboembolism due to a persistently patent foramen ovale. Intensive Care Med. 2000 Sep;26(9):1400. doi: 10.1007/s001340000584. DOI: https://doi.org/10.1007/s001340000584. [DOI] [PubMed] [Google Scholar]
- 8.Moua T, Wood KE, Atwater BD, Runo JR. Major pulmonary embolism and hemodynamic stability from shunting through a patent foramen ovale. South Med J. 2008 Sep;101(9):955–8. doi: 10.1097/SMJ.0b013e3181809ed8. DOI: https://doi.org/10.1097/smj.0b013e3181809ed8. [DOI] [PubMed] [Google Scholar]
- 9.Rajan GR. Intractable intraoperative hypoxemia secondary to pulmonary embolism in the presence of undiagnosed patent foramen ovale. J Clin Anesth. 2007 Aug;19(5):374–7. doi: 10.1016/j.jclinane.2006.09.011. DOI: https://doi.org/10.1016/j.jclinane.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Granati GT, Teressa G. Worsening hypoxemia in the face of increasing PEEP: A case of large pulmonary embolism in the setting of intracardiac shunt. Am J Case Rep. 2016 Jul 5;17:454–8. doi: 10.12659/AJCR.898521. DOI: https://doi.org/10.12659/ajcr.898521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirarchi FL, Hecker J, Kramer CM. Pulmonary embolism complicated by patent foramen ovale and paradoxical embolization. J Emerg Med. 2000 Jul;19(1):27–30. doi: 10.1016/s0736-4679(00)00177-3. DOI: https://doi.org/10.1016/s0736-4679(00)00177-3. [DOI] [PubMed] [Google Scholar]
- 12.Weig T, Dolch ME, Frey L, et al. Delayed intracardial shunting and hypoxemia after massive pulmonary embolism in a patient with a biventricular assist device. J Cardiothorac Surg. 2011 Oct 11;6:133. doi: 10.1186/1749-8090-6-133. DOI: https://doi.org/10.1186/1749-8090-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaid U, Baram M, Marik PE. Thrombolytic therapy in a patient with suspected pulmonary embolism despite a negative computed tomography pulmonary angiogram. Respir Care. 2011 Mar;56(3):336–8. doi: 10.4187/respcare.00738. DOI: https://doi.org/10.4187/respcare.00738. [DOI] [PubMed] [Google Scholar]
- 14.Meyer G, Vicaut E, Danays T, et al. PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. New Engl J Med. 2014 Apr 10;370(15):1402–11. doi: 10.1056/NEJMoa1302097. DOI: https://doi.org/10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 15.Kucher N, Boekstegers P, Müller O, et al. Randomized controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014 Jan 28;129(4):479–86. doi: 10.1161/CIRCULATIONAHA.113.005544. DOI: https://doi.org/10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 16.Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. Eur Heart J. 2015 Mar 7;36(10):605–14. doi: 10.1093/eurheartj/ehu218. DOI: https://doi.org/10.1093/eurheartj/ehu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: A meta-analysis. JAMA. 2014 Jun 18;311(23):2414–21. doi: 10.1001/jama.2014.5990. DOI: https://doi.org/10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 18.Avgerinos ED, Chaer RA. Catheter-directed interventions for acute pulmonary embolism. J Vasc Surg. 2015 Feb;61(2):559–65. doi: 10.1016/j.jvs.2014.10.036. DOI: https://doi.org/10.1016/j.jvs.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Meyer G, Planquette B, Sanchez O. Fibrinolysis for acute care of pulmonary embolism in the intermediate risk patient. Curr Atheroscler Rep. 2015 Dec;17(12):68. doi: 10.1007/s11883-015-0546-1. DOI: https://doi.org/10.1007/s11883-015-0546-1. [DOI] [PubMed] [Google Scholar]
- 20.Tafur AJ, Shamoun FE, Patel SI, Tafur D, Donna F, Murad MH. Catheter-directed treatment of pulmonary embolism: A systematic review and meta-analysis of modern literature. Clin Appl Thromb Hemost. 2017 Oct;23(7):821–9. doi: 10.1177/1076029616661414. DOI: https://doi.org/10.1177/1076029616661414. [DOI] [PubMed] [Google Scholar]
- 21.Bajaj NS, Kalra R, Arora P, et al. Catheter-directed treatment for acute pulmonary embolism: Systematic review and single-arm meta-analyses. Int J Cardiol. 2016 Dec 15;225:128–39. doi: 10.1016/j.ijcard.2016.09.036. DOI: https://doi.org/10.1016/j.ijcard.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Konstantinides S, Geibel A, Kasper W, Olschewski M, Blümel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation. 1998 May 19;97(19):1946–51. doi: 10.1161/01.cir.97.19.1946. DOI: https://doi.org/10.1161/01.cir.97.19.1946. [DOI] [PubMed] [Google Scholar]
- 23.Summerfield DT, Desai H, Levitov A, Grooms DA, Marik PE. Inhaled nitric oxide as salvage therapy in massive pulmonary embolism: A case series. Respir Care. 2012 Mar;57(3):444–8. doi: 10.4187/respcare.01373. DOI: https://doi.org/10.4187/respcare.01373. [DOI] [PubMed] [Google Scholar]
- 24.Neely RC, Byrne JG, Gosev I, et al. Surgical embolectomy for acute massive and submassive pulmonary embolism in a series of 115 patients. Ann Thorac Surg. 2015 Oct;100(4):1245–51. doi: 10.1016/j.athoracsur.2015.03.111. DOI: https://doi.org/10.1016/j.athoracsur.2015.03.111. [DOI] [PubMed] [Google Scholar]