Abstract
Introduction
We investigated the effect of inflammatory biomarkers (neutrophil, platelet, and lymphocyte counts) on risk of progression in patients with metastatic melanoma treated with an immune checkpoint inhibitor targeting programmed cell death protein-1 (PD-1).
Methods
This retrospective cohort study included 108 patients with malignant melanoma treated with an anti-PD-1 checkpoint inhibitor from August 2014 through December 2015. The outcome was disease progression noted on imaging or clinical examination. Follow-up began on the date of initiation of anti-PD-1 therapy and ended on the date of progression, disenrollment, death of causes other than malignant melanoma, or the end of the study in February 2017.
Results
The median time from initiating therapy with an anti-PD-1 checkpoint inhibitor (nivolumab or pembrolizumab) to the end of follow-up was 118 days. After adjustment, baseline neutrophil and platelet counts were associated with progression. The hazard ratio (HR) for neutrophil counts ≥ 5501/μL vs ≤ 3900/μL was 2.3 (95% confidence interval [CI] = 1.2–4.6, p < 0.05). For platelet counts ≥ 304,000 vs ≤ 215,000/μL, the HR was 2.0 (CI = 1.0–3.9, p < 0.05). For lymphocyte counts ≥ 1716/μL vs ≤ 1120/μL, the HR was 0.5 (CI = 0.2–1.0, p = 0.05).
Conclusion
For patients with metastatic melanoma treated with nivolumab or pembrolizumab, higher neutrophil or platelet counts, or lower lymphocyte counts, are associated with higher risk of progression. For these patients, we recommend more frequent assessment for progression and closer follow-up, especially for patients with substantial comorbidities or poor physical performance.
INTRODUCTION
Nivolumab and pembrolizumab are immune checkpoint inhibitors targeting the programmed cell death protein-1 (PD-1) and have been approved for the treatment of melanoma and several other malignancies. As single agents, both drugs produce a response rate of approximately 30% to 40% in ipilimumab-naive patients with metastatic melanoma.1,2 In ipilimumab-refractory patients, both drugs show superior activity over chemotherapy.3,4 When nivolumab and ipilimumab were combined in previously untreated patients with metastatic melanoma, the progression-free survival (PFS) was better than either drug alone only in patients with a PD-1-ligand-negative tumor.5 Despite these excellent results, many patients fail to benefit from these drugs and experience serious toxicities. Identifying valid markers for predicting treatment benefits from these drugs has been an intense subject of investigation.6
Neutrophil count, platelet count, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) reflect the patient’s systemic inflammatory response and can be readily obtained from serum. Multiple studies have shown that elevated NLR and PLR are associated with decreased overall survival, decreased disease-free survival, increased postoperative complications, and poor response to chemotherapy or tyrosine kinase inhibitors.7 Tumor-associated neutrophils have been shown to promote tumor progression, angiogenesis, and metastasis by producing proinflammatory cytokines, metalloproteinases, and angiogenic factors that can alter tumor microenvironment and suppress immune response.8,9 There is also evidence that platelets can promote progression and immune evasion of cancer by interacting with other factors in the tumor microenvironment.10
We hypothesized that the baseline state of the patient’s systemic inflammatory response may be associated with the response to immune checkpoint inhibitors. We aimed to determine whether three systemic inflammatory biomarkers—serum neutrophil, platelet, and lymphocyte counts measured at baseline—were associated with progression in patients with metastatic melanoma treated with nivolumab or pembrolizumab.
METHODS
The study was approved by the Kaiser Permanente Northern California (KPNC) institutional review board.
The retrospective cohort study included adult KPNC patients with metastatic melanoma who received an anti-PD-1 checkpoint inhibitor, either nivolumab or pembrolizumab, as a single agent during the study period, August 2014 to December 2015. Nivolumab dosage was 3 mg/kg every 2 weeks, and pembrolizumab dosage was 2 mg/kg every 3 weeks by intravenous infusion according to the manufacturer’s instructions. The study was restricted to patients who were enrolled in the Health Plan during the 12 months preceding the date of starting anti-PD-1 checkpoint inhibitor. We further required a baseline complete blood cell (CBC) count during the 4 weeks before treatment initiation or 1 week after the initiation of treatment. All patients had a baseline imaging study obtained before the initiation of treatment, including a computed tomography (CT) scan, positron emission tomography (PET)/CT scan, or magnetic resonance image.
Data were obtained from the electronic health record. The date of progression was based on the treating oncologist’s assessment. For most patients, this was guided by imaging studies. However, for patients who progressed quickly (within one to three cycles of first treatment), before an imaging study was performed, the progression date was based on clinical symptoms. Most patients had a PET/CT scan or CT scan for assessment of response within three or four months after the initiation of treatment. For patients who responded or achieved stable disease as of the initial assessment, a PET/CT or CT scan was obtained every two to four months thereafter.
Inflammatory markers were obtained from the CBC count and included absolute neutrophil, platelet, and lymphocyte counts. Most CBC counts were obtained within the two weeks preceding treatment initiation. In past studies of this question, investigators have combined inflammatory markers into ratios for NLR and PLR. However, Pearson11 noted that spurious correlations can arise when indexes used to measure human physiology are combined into ratios instead of being analyzed as separate variables, and during preliminary analysis of the ratios (PLR, NLR), we observed these spurious correlations. We therefore analyzed each of the three inflammatory markers as separate exposure variables. Potential confounders included patient age, sex, and race/ethnicity; Eastern Cooperative Oncology Group (ECOG) performance status; Charlson comorbidity index; BRAF mutation status of the primary tumor; and the anti-PD-1 agent.
Follow-up for observation of outcomes began on the date of initiation of treatment of metastatic melanoma with an anti-PD-1 checkpoint inhibitor. Follow-up ended on the earliest date of progression noted on results of imaging or clinical examination, disenrollment from the Health Plan, death from causes other than malignant melanoma, or the end of the study on February 18, 2017. Patients whose disease did not progress were censored on the date that their follow-up ended.
During preliminary analysis, we examined the correlation of each biomarker with the others. We then grouped each of the biomarkers into tertiles. We assessed the association of each tertile of each biomarker with PFS using Kaplan-Meier plots, and we compared the statistical significance of differences in survival probability using the log-rank test. We cross-tabulated the data to assess the association of biomarker levels with the patients’ clinicopathologic features. Covariates that were associated with biomarker levels or that were clinically relevant were identified for inclusion in the Cox multivariable proportional hazards model.
RESULTS
From August 2014 to December 2015, a total of 112 patients with metastatic malignant melanoma received nivolumab or pembrolizumab as a single agent. None of these patients were treated in a clinical trial protocol. Four of these cases were excluded: 2 without a CBC count at baseline, 1 who was referred for treatment outside the Health Plan, and 1 who was initially diagnosed with ocular melanoma. Thus, 108 patients were included in the final analysis.
After treatment initiation, 3 of the 108 patients did not complete follow-up. One patient died of aortic dissection after a single cycle of therapy and was censored at that time. Another patient, who did not undergo scheduled imaging within 3 months after treatment initiation, was censored at 3 months. One patient was censored at disenrollment from the Health Plan at 9 months. The 3 patients who were censored contributed observation time to their date of censoring, but the 105 cases that had complete follow-up were observed to the date of progression or to the end of the study on February 18, 2017.
Overall, the median age at the start of treatment was 65 years (range = 26–91 years; Table 1). Sixty-two percent of cohort members were men, and 92% were white. Among the 86% of patients with ECOG performance status recorded, about half were scored 1 or greater. The Charlson comorbidity index was 1 or higher for 63%, and 27% of the cohort was BRAF positive. About two-thirds started a pembrolizumab regimen and one-third began a nivolumab regimen, and 43% received anti-PD-1 inhibitor as a single agent during first-line therapy (Table 2), whereas 40% received the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor (ipilimumab) and 14% received a BRAF inhibitor as first-line therapy.
Table 1.
Baseline characteristics of patients with malignant melanoma (N = 108) in relation to median counts of neutrophils, platelets, and lymphocytes, August 2014 to December 2015
| Characteristic | Percentage | Median counts, μL | ||
|---|---|---|---|---|
| Neutrophils | Platelets, 000s | Lymphocytes | ||
| Age at start of treatment, years | ||||
| ≤ 56 | 24 | 4212 | 246 | 1440 |
| 57–64 | 26 | 5220 | 312 | 1336 |
| 65–72 | 24 | 4381 | 252 | 1428 |
| ≥ 73 | 27 | 4712 | 234 | 1425 |
| Sex | ||||
| Men | 62 | 4712 | 239 | 1316 |
| Women | 38 | 4026 | 269a | 1690a |
| Race/ethnicity | ||||
| White | 92 | 4422 | 252 | 1440 |
| Asian | 6 | 4712 | 257 | 1110 |
| Black | 2 | 3109 | 290 | 988 |
| Hispanic | 6 | 4965 | 331 | 900 |
| ECOG performance status | ||||
| Missing | 14 | |||
| 0 | 43 | 4092 | 240 | 1456 |
| ≥ 1 | 43 | 4980 | 275 | 1316 |
| Charlson comorbidity index | ||||
| 0 | 37 | 4719 | 244 | 1482 |
| ≥ 1 | 63 | 4281 | 255 | 1379 |
| BRAF mutation type | ||||
| Negative | 73 | 4340 | 254 | 1404 |
| Positive | 27 | 4422 | 243 | 1462 |
| Anti-PD-1 agent | ||||
| Nivolumab | 35 | 4719 | 257 | 1294 |
| Pembrolizumab | 65 | 4380 | 249 | 1443 |
p < 0.05.
ECOG = Eastern Cooperative Oncology Group; PD-1 = programmed cell death protein-1.
Table 2.
Line of therapy used for patients with malignant melanoma (N = 108), August 2014 to December 2015, no. (%)
| Line of therapy | Anti-PD-1 checkpoint inhibitor (pembrolizumab or nivolumab) | CTLA-4 inhibitor (ipilimumab) | BRAF inhibitora | Chemotherapyb |
|---|---|---|---|---|
| First | 47 (43) | 43 (40) | 15 (14) | 3 (3) |
| Second | 43 (40) | 8 (8) | 8 (8) | 6 (6) |
| Third | 15 (14) | 1 (1) | 1 (1) | 2 (2) |
| Fourth | 3 (3) | 0 (0) | 0 (0) | 0 (0) |
Vemurafenib, dabrafenib, or trametinib.
Carboplatin and paclitaxel, or temozolomide.
CTLA-4 = cytotoxic T-lymphocyte-associated protein-4; PD-1 = programmed cell death protein-1.
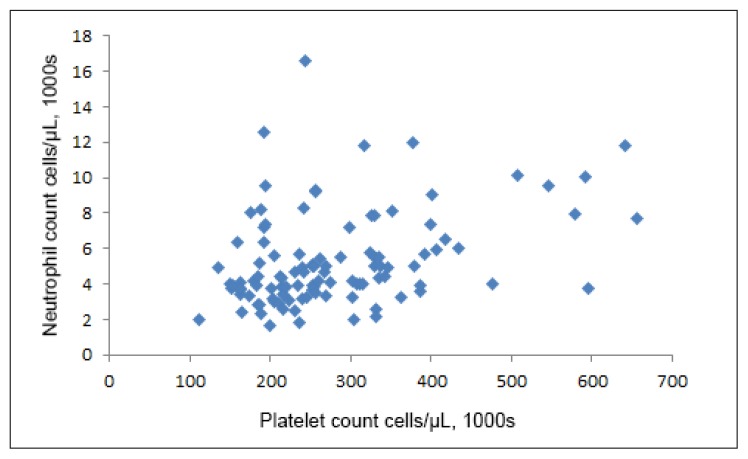
We found no important correlation of the lymphocyte count with either the neutrophil count (Pearson ρ, −0.09, p = 0.38) or the platelet count (ρ, 0.04, p = 0.69). However, the neutrophil count was correlated with the platelet count (ρ = 0.37, p < 0.001; Figure 1).
Figure 1.
Scatterplot of neutrophil count vs platelet count (/μL), Kaiser Permanente Northern California, August 2014 to December 2015 (ρ = 0.37, p < 0.001).
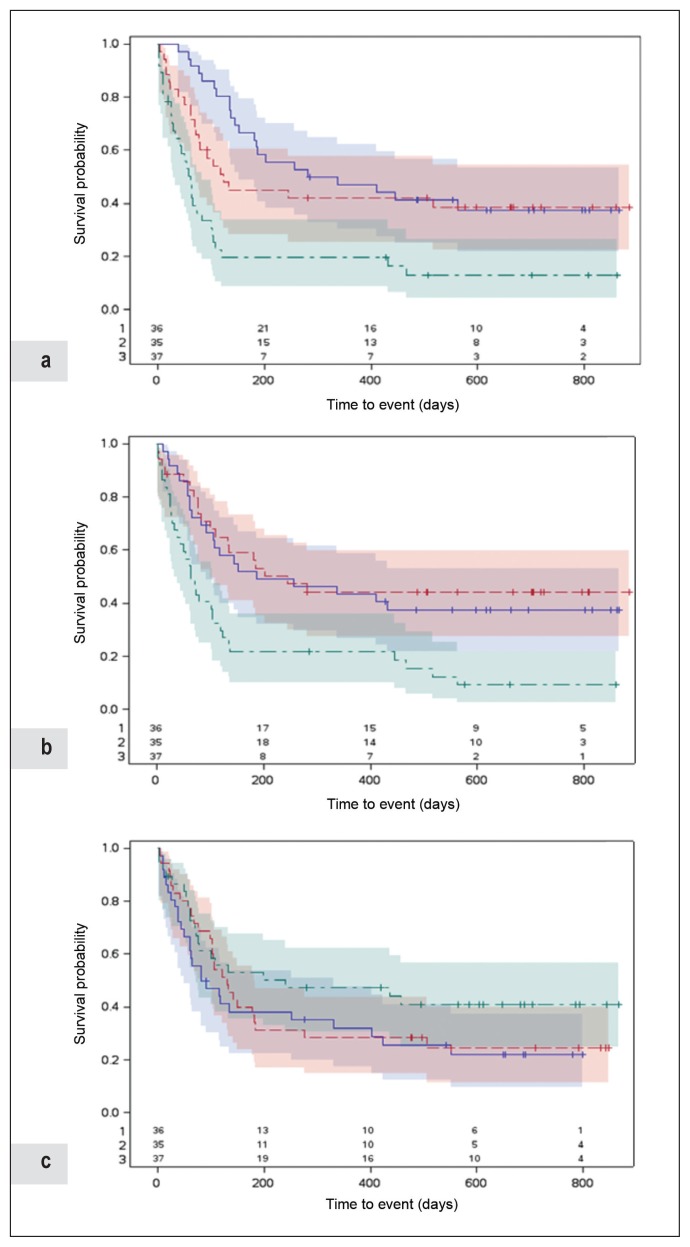
The median time from initiating anti-PD-1 inhibitor to the end of follow-up was 118 days (range = 2–884 days). During follow-up, 31 (29%) had stable disease or a response to therapy and 74 (70%) had progressive disease, of whom 60 (56%) died of melanoma and 3 (3%) were lost to follow-up. At 180 days after initiation, 60 (57%) of the 105 patients not lost to follow-up had progressed with a median time to progression of 64 days. We used the Kaplan-Meier method to plot PFS in relation to tertiles of each biomarker (Figure 2), although this method does not readily allow for consideration of potential confounding factors. We observed the neutrophil count (log-rank test, p < 0.0001) and the platelet count (log-rank test, p < 0.001) to be associated with PFS to a statistically significant degree, whereas the lymphocyte count was not.
Figure 2.
Progression-free survival according to counts of a) neutrophils, b) platelets, and c) lymphocytes (/μL), Kaiser Permanente Northern California, August 2014 to December 2015.
Follow-up began on the date of initiation of treatment of metastatic melanoma with anti-PD-1 checkpoint inhibitor. Follow-up ended on the earliest date of progression noted on results of imaging or clinical examination (event); or disenrollment from the Health Plan, death of causes other than malignant melanoma, or end of study on February 18, 2017 (censor). We determined the date of progression using the treating oncologist’s assessment guided by imaging studies or clinical symptoms in patients who progressed quickly (within 1–3 cycles of first treatment) before imaging could be performed. Numbers shown at bottom of figures are numbers of patients at risk of progression at each time point based on different range of cell count as indicated (1, blue; 2, red; and 3, green).
PD-1 = programmed cell death protein-1.
After adjustment for age, Charlson comorbidity index, ECOG performance status, and BRAF mutation status, baseline neutrophil and platelet counts were associated with progression, whereas the association of lymphocyte count was of borderline statistical significance (Table 3). When we compared neutrophil counts ≥ 5501/μL vs ≤ 3900/μL, the hazard ratio was 2.3 with a 95% confidence interval (CI) of 1.2 to 4.6 (p < 0.05). For platelet counts ≥ 304,000/μL vs ≤ 215,000/μL, the hazard ratio was 2.0 (CI = 1.0–3.9, p < 0.05). For lymphocyte counts ≥ 1716/μL vs ≤ 1120/μL, the hazard ratio was 0.5 (CI = 0.2–1.0, p = 0.05).
Table 3.
Adjusted hazard ratios and 95% confidence intervals for association of baseline characteristics with disease progression for 108 patients with malignant melanoma, August 2014 to December 2015a
| Characteristic | At follow-up (180 days)b | At full follow-up (884 days)b | ||||
|---|---|---|---|---|---|---|
| No. patients progressed at 180 days | Probability of progression by 180 days, % | 95% CI | Hazard ratio | 95% CI | p value | |
| Age group, years | ||||||
| ≤ 56 | 17/25 | 68 | 50–85 | 1.0c | — | |
| 57–64 | 20/28 | 71 | 54–86 | 1.3 | 0.6–3.0 | 0.68 |
| 65–72 | 11/26 | 44 | 27–65 | 0.8 | 0.4–1.9 | 0.27 |
| ≥ 73 | 12/29 | 42 | 27–62 | 0.6 | 0.3–1.4 | 0.78 |
| Sex | ||||||
| Women | 20/41 | 50 | 36–66 | 1.0c | — | |
| Men | 40/67 | 60 | 49–72 | 1.0 | 0.6–2.0 | 0.78 |
| ECOG performance status | ||||||
| 0 | 18/46 | 39 | 27–55 | 1.0c | — | |
| ≥ 1 | 33/37 | 71 | 58–83 | 2.2 | 1.1–4.2 | 0.01 |
| Charlson comorbidity index | ||||||
| 0 | 21/40 | 53 | 39–69 | 1.0c | — | |
| ≥ 1 | 39/68 | 58 | 47–70 | 0.8 | 0.4–1.5 | 0.51 |
| BRAF | ||||||
| Negative | 38/79 | 49 | 38–60 | 1.0c | — | |
| Positive | 22/29 | 76 | 59–89 | 1.5 | 0.8–3.0 | 0.25 |
| Neutrophil count, μL | ||||||
| ≤ 3900 | 12/36 | 33 | 20–51 | 1.0c | — | |
| 3901–5500 | 19/35 | 55 | 39–72 | 1.3 | 0.6–2.8 | 0.4 |
| ≥ 5501 | 29/37 | 80 | 66–91 | 2.3 | 1.2–4.6 | 0.01 |
| Platelet count, 1000 cells/μL | ||||||
| ≤ 215 | 17/36 | 48 | 33–65 | 1.0c | — | |
| 216–303 | 14/35 | 41 | 27–60 | 1.2 | 0.6–2.6 | 0.57 |
| ≥ 304 | 29/37 | 78 | 64–90 | 2.0 | 1.0–3.9 | 0.04 |
| Lymphocyte count, μL | ||||||
| ≤ 1120 | 22/36 | 62 | 47–77 | 1.0c | — | |
| 1121–1715 | 21/35 | 60 | 44–76 | 0.9 | 0.5–1.8 | 0.75 |
| ≥ 1716 | 17/37 | 47 | 32–64 | 0.5 | 0.2–1.0 | 0.05 |
All variables shown in the Table were evaluated as potential confounding factors. The final statistical model included age, Charlson comorbidity index, ECOG performance status, and BRAF mutation.
Follow-up began at treatment initiation and ended on the earliest of: Date of progression (event) (n = 74); death from an unrelated cause (n = 1); “loss to follow-up” (n = 2); or end of the study, on February 18, 2017 (n = 31). The median time from initiating anti-PD-1 checkpoint inhibitor therapy to the end of follow-up was 118 days (range, 2–884 days).
Reference group.
CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; PD-1 = programmed cell death protein-1.
DISCUSSION
Immune checkpoint inhibitors have improved the outcome of many malignancies, although with limited efficacy. The response rate for refractory Hodgkin lymphoma is as high as 80% and is approximately 30% to 40% for metastatic melanoma and 20% for most other malignancies,1,2,12–18 indicating that tumor biology is a key factor for treatment response. Factors that have an impact on the tumor microenvironment, including the presence of tumor-infiltrating lymphocytes, high antigen load, and expression of PD-1 ligand are associated with higher response rate.19 Malignancies of diverse histologic types harboring deficiency of mismatched repair proteins were found to show impressive response rates of around 40%, but these malignancies often contain dense immune cell infiltrates.20,21 However, not all patients with tumor-infiltrating lymphocytes respond to immune checkpoint inhibitors, whereas patients with tumors negative for PD-1 ligand expression occasionally do respond,22 indicating that other mechanisms are also involved in determining the response.
Studies exploring the effect of systemic factors on the patient’s response to immune checkpoint inhibitors have been limited. Martens et al23 examined the baseline peripheral blood biomarkers and identified a signature of markers, including low lactate dehydrogenase (LDH) level, low absolute monocyte count, low myeloid-derived suppressor cells, high absolute eosinophil count, high regulatory T cells (Tregs), and relative lymphocyte count to be associated with favorable outcome after therapy with ipilimumab in patients with metastatic melanoma. The correlation of high Tregs with favorable response to ipilimumab was interesting. Tregs express high CTLA-4 and can be directly activated by CTLA-4 targeting, in contrast to study findings that showed an inverse relationship between PFS and Treg function in the peripheral blood as well as an increase in tumor Tregs.24,25 In a follow-up study, Martens et al26 showed that an increase in absolute lymphocyte count and the delayed increase of CD4+/CD8+ T cells 2 to 8 weeks after ipilimumab therapy were correlated with improved outcome. In a study by Diem et al,27 elevated LDH level, ECOG performance status above 0, and involvement of multiple organs were independent adverse factors for overall survival after ipilimumab therapy.
Weide et al28 studied the correlation of baseline biomarkers with the outcome of patients in metastatic melanoma treated with pembrolizumab and showed that high relative eosinophil count, relative lymphocyte count, low LDH level, and absence of visceral metastasis were associated with favorable overall survival. Several other peripheral blood markers, including absolute neutrophil count, were associated with outcome but only by univariate analysis.28 In a small study of 66 patients conducted by Diem et al,29 elevated baseline LDH level and its increase after treatment with pembrolizumab or nivolumab were correlated with poorer overall survival. Baseline LDH level was found to be associated with overall survival in a separate study.30 In a Phase 1a trial with the anti-PD-1 ligand antibody atezolizumab in patients with metastatic renal cell carcinoma, the exploration of serum markers showed that low baseline levels of acute serum reactants including α-antitrypsin and fibrinogen and the decrease of ferritin level after treatment were associated with longer overall survival.31
In our cohort study of a well-described, community-based population, we focused on 3 readily available peripheral blood cell counts and observed that absolute neutrophil count and platelet count were significantly associated with PFS in patients with metastatic melanoma receiving pembrolizumab or nivolumab. Patients whose baseline absolute neutrophil count was greater than 5508/μL were 2.3 times (95% CI = 1.2–4.6) more likely to have disease progression compared with patients whose baseline absolute neutrophil count was 3900/μL or lower. Patients whose platelet count was 304,000/μL or higher were 2.0 times (95% CI = 1.0–3.9) more likely to have disease progression compared with patients whose platelet count was 215,000/μL or lower. The association of absolute lymphocyte count with PFS had borderline statistical significance; patients whose absolute lymphocyte count was 1716/μL or higher had a 50% (95% CI = 0.2–1.0) lower rate of progression compared with patients whose absolute lymphocyte count was 1120/μL or lower. Approximately half of our cohort was ipilimumab-naive and half was refractory to treatment. Nivolumab and pembrolizumab retain substantial activity in ipilimumab-refractory patients, with response rates exceeding 30%.3,4 Our data therefore are applicable to both ipilimumab-naive and ipilimumab-refractory patients. We excluded a few patients who were treated with the combination of ipilimumab plus either nivolumab or pembrolizumab because the combination of 2 checkpoint inhibitors exerts a very different effect on a patient’s immune response and possibly has different correlation of biomarker profiles and should be studied separately.
The ECOG physical performance was associated with PFS. Patients whose ECOG performance status exceeded 0 had a 2.2 times higher risk of disease progression, consistent with the study findings by Nakamura et al.30 Intriguingly, patients who were age 73 years or older appeared to show a lower risk of progression compared with patients age 55 years or younger. The neutrophil, platelet, and lymphocyte counts did not appear to differ significantly among the age groups, although the platelet count was significantly higher in patients whose ECOG performance status exceeded 0. The impact of age on progression is surprising because controversy exists regarding a possible association between age and prognosis; some elderly patients with metastatic melanoma have been found to follow an indolent course, whereas others have a quicker progression.32
Limitations of this observational study included its setting in a single institution, its relatively short follow-up, and its small sample size. Also, we could not look at the other inflammatory markers such as LDH and C-reactive protein levels. The study has several strengths. We had complete follow-up for 97% of patients, as well as highly complete information to assess response to treatment, serum markers, and comorbidities. In addition, the study quantifies the risk of disease progression with 2 readily available serum markers that can be immediately applied to daily clinical practice. Our results suggested that these markers were both predictive and prognostic of PFS in these patients with metastatic melanoma treated with immune checkpoint inhibitors. The elevated neutrophil and platelet counts likely reflect, in part, a more suppressive tumor microenvironment dominated by Tregs over cytotoxic T cells, leading to the failure of nivolumab and pembrolizumab to adequately mobilize and activate cytotoxic T cells.
CONCLUSION
Our study findings suggest that for patients with metastatic melanoma with absolute neutrophil counts greater than 5508/μL and/or platelet counts greater than 304,000/μL, the risk of disease progression with nivolumab or pembrolizumab therapy may be higher. For these patients, we recommend more frequent assessment for progression and closer follow-up, especially for patients with substantial comorbidities or poor physical performance. Because of its retrospective nature, this work should be considered hypothesis generating, and further studies must be done to understand the exact relationship between elevated inflammatory biomarkers at baseline and patient outcomes and whether these markers are prognostic or predictive, or both, for anti-PD-1 immunotherapy in melanoma. Future research should also examine the correlation of these biomarkers with tumor-infiltrating lymphocytes and the tumor’s gene signature.
Acknowledgments
We thank Jennie Yee, PharmD; Laura Asakura, PharmD; Wenli Li, PhD, MS; and Xian Ning, MS, for assistance with data collection.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
This study was supported by The Permanente Medical Group Inc in Oakland, CA. The funder played no role in the data analysis or writing of this manuscript. The author(s) have no conflicts of interest to disclose.
References
- 1.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015 Jan 22;372(4):320–30. doi: 10.1056/NEJMoa1412082. DOI: https://doi.org/10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Schachter J, Long GV, et al. KEYNOTE-006 Investigators. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015 Jun 25;372(26):2521–32. doi: 10.1056/NEJMoa1503093. DOI: https://doi.org/10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 3.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015 Apr;16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8. DOI: https://doi.org/10.1016/s1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 4.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015 Aug;16(8):908–18. doi: 10.1016/S1470-2045(15)00083-2. DOI: https://doi.org/10.1016/s1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015 Jul 2;373(1):23–34. doi: 10.1056/NEJMoa1504030. DOI: https://doi.org/10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014 May 29;106(6):dju124. doi: 10.1093/jnci/dju124. DOI: https://doi.org/10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 8.Tazzyman S, Niaz H, Murdoch C. Neutrophil-mediated tumour angiogenesis: Subversion of immune responses to promote tumour growth. Semin Cancer Biol. 2013 Jun;23(3):149–58. doi: 10.1016/j.semcancer.2013.02.003. DOI: https://doi.org/10.1016/j.semcancer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013 Jun;23(3):141–8. doi: 10.1016/j.semcancer.2013.02.005. DOI: https://doi.org/10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: A casual or causal relationship: Revisited. Cancer Metastasis Rev. 2014 Mar;33(1):231–69. doi: 10.1007/s10555-014-9498-0. DOI: https://doi.org/10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson K. Mathematical contributions to the theory of evolution. On a form of spurious correlation which may arise when indices are used in the measurement of organs. Proc R Soc Lond. 1897;60(1):489–98. DOI: https://doi.org/10.1098/rspl.1896.0076. [Google Scholar]
- 12.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016 Sep;17(9):1283–94. doi: 10.1016/S1470-2045(16)30167-X. DOI: https://doi.org/10.1016/s1470-2045(16)30167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015 Jan 22;372(4):311–9. doi: 10.1056/NEJMoa1411087. DOI: https://doi.org/10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016 Nov 1;34(31):3733–9. doi: 10.1200/JCO.2016.67.3467. DOI: https://doi.org/10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015 Oct 22;373(17):1627–39. doi: 10.1056/NEJMoa1507643. DOI: https://doi.org/10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Escudier B, McDermott DF, et al. CheckMate 025 Investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015 Nov 5;373(19):1803–13. doi: 10.1056/NEJMoa1510665. DOI: https://doi.org/10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017 Mar;18(3):312–22. doi: 10.1016/S1470-2045(17)30065-7. DOI: https://doi.org/10.1016/s1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 18.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016 Nov 10;375(19):1856–67. doi: 10.1056/NEJMoa1602252. DOI: https://doi.org/10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016 Dec;34(34):4102–9. doi: 10.1200/JCO.2016.67.2477. DOI: https://doi.org/10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015 Jun 25;372(26):2509–20. doi: 10.1056/NEJMoa1500596. DOI: https://doi.org/10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999 Jun;154(6):1805–13. doi: 10.1016/S0002-9440(10)65436-3. DOI: https://doi.org/10.1016/s0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016 Nov 15;22(22):5461–71. doi: 10.1158/1078-0432.CCR-15-2839. DOI: https://doi.org/10.1158/1078-0432.CCR-15-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martens A, Wistuba-Hamprecht K, Geukes Foppen M, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016 Jun 15;22(12):2908–18. doi: 10.1158/1078-0432.CCR-15-2412. DOI: https://doi.org/10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Retseck J, VanderWeele R, Lin HM, Lin Y, Butterfield LH, Tarhini AA. Phenotypic and functional testing of circulating regulatory T cells in advanced melanoma patients treated with neoadjuvant ipilimumab. J Immunother Cancer. 2016 Jun 21;4:38. doi: 10.1186/s40425-016-0141-1. DOI: https://doi.org/10.1186/s40425-016-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One. 2014 Feb 3;9(2):e87705. doi: 10.1371/journal.pone.0087705. DOI: https://doi.org/10.1371/journal.pone.0087705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martens A, Wistuba-Hamprecht K, Yuan J, et al. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin Cancer Res. 2016 Oct 1;22(19):4848–58. doi: 10.1158/1078-0432.CCR-16-0249. DOI: https://doi.org/10.1158/1078-0432.ccr-16-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diem S, Kasenda B, Martin-Liberal J, et al. Prognostic score for patients with advanced melanoma treated with ipilimumab. Eur J Cancer. 2015 Dec;51(18):2785–91. doi: 10.1016/j.ejca.2015.09.007. DOI: https://doi.org/10.1016/j.ejca.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016 Nov 15;22(22):5487–96. doi: 10.1158/1078-0432.CCR-16-0127. DOI: https://doi.org/10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016 Feb 2;114(3):256–61. doi: 10.1038/bjc.2015.467. DOI: https://doi.org/10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura Y, Kitano S, Takahashi A, et al. Nivolumab for advanced melanoma: Pretreatment prognostic factors and early outcome markers during therapy. Oncotarget. 2016 Nov 22;7(47):77404–15. doi: 10.18632/oncotarget.12677. DOI: https://doi.org/10.18632/oncotarget.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016 Mar 10;34(8):833–42. doi: 10.1200/JCO.2015.63.7421. DOI: https://doi.org/10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 32.Hegde UP, Chakraborty N, Kerr P, Grant-Kels JM. Melanoma in the elderly patient: Relevance of the aging immune system. Clin Dermatol. 2009 Nov-Dec;27(6):537–44. doi: 10.1016/j.clindermatol.2008.09.012. DOI: https://doi.org/10.1016/j.clindermatol.2008.09.012. [DOI] [PubMed] [Google Scholar]