Abstract
Purpose
Non-invasive early accurate detection of malignant breast cancer is paramount to the clinical management of the life-threatening disease. Here we aim to test a small peptide targeted MRI contrast agent, ZD2-Gd(HP-DO3A), specific to an oncoprotein, extradomain-B fibronectin (EDB-FN), in the tumor microenvironment for MR molecular imaging of breast cancer.
Method
EDB-FN expression in 4T1 and MDA-MB-231 cancers was analyzed with quantitative real-time PCR (qRT-PCR) and western blot. Primary and metastatic triple negative breast cancer mouse models were developed using 4T1 and MDA-MB-231 cells. Contrast enhanced MRI was performed to evaluate utility of ZD2-Gd(HP-DO3A) in detecting 4T1 and MDA-MB-231 primary and metastatic tumors.
Results
EDB-FN was abundantly expressed in the extracellular matrix (ECM) of both the primary and metastatic TNBC tumors. In T1-weighted MRI, ZD2-Gd(HP-DO3A) generated superior contrast enhancement in primary TNBC tumors than a non-specific clinical agent Gd(HP-DO3A), during 30 min after contrast injection. ZD2-Gd(HP-DO3A) also produced a significant increase in contrast-to-noise ratio (CNR) of TNBC metastases, enabling sensitive localization and delineation of metastases that occulted in non-contrast-enhanced or Gd(HP-DO3A)-enhanced MRI.
Conclusions
These findings potentiate the use of ZD2-Gd(HP-DO3A) for MR molecular imaging of malignant breast cancers to improve the healthcare of breast cancer patients.
Keywords: MRI, extradomain-B fibronectin, breast cancer, metastasis, tumor microenvironment
Introduction
Metastatic breast cancer is resistant to the standard treatments and primarily responsible for cancer mortality (1–3). Accurate early detection and characterization of malignant and metastatic breast cancer is critical to tailor effective treatments for this life-threatening disease. Non-invasive sensitive imaging of both primary and metastatic tumors will also provide timely assessment of therapeutic efficacy and image guidance for precise therapy and interventions. Mammography and ultrasonography are the commonly used imaging modalities for clinical management of breast cancer. However, these modalities lack the sensitivity and specificity for early detection and risk-stratification of aggressive breast cancer with metastatic potential (4). Magnetic resonance imaging (MRI) provides superior resolution of soft tissues and have demonstrated better sensitivity in detecting breast tumors than mammography and ultrasound (5). Gd(III) based contrast agents are routinely used in MR imaging of breast cancer. However, currently available contrast agents are not specific to tumors and unable to provide accurate early detection of aggressive breast cancer and metastases. There is an unmet clinical need for novel MRI contrast agents capable of early detection of aggressive breast cancers and their micrometastases.
Development of targeted MRI contrast agents for specific imaging of cancer is challenging because of the low sensitivity of MRI and low concentration of molecular targets on cancer cells, especially for highly aggressive triple negative breast cancer (TNBC), which is characterized by the loss of cell markers commonly used for cancer targeting (6,7). TNBCs are also heterogeneous in nature, making it difficult to find a common cell marker to image all cancers (8). In order to overcome the challenges of targeting cell biomarkers for molecular MRI, we have been focused on targeting the abundant oncoproteins in the tumor microenvironment in the past decade (9–12). Aggressive tumors have a unique microenvironment that facilitates cancer cell proliferation and invasion. Design and development targeted contrast agents specific to the abundant oncoproteins in tumor microenvironment have a great potential to improve the effectiveness of molecular MRI for early accurate detection of aggressive breast cancer, non-invasively monitoring tumor progression and metastases, and timely assessing of tumor response to therapies.
Fibronectin is one of the abundant extracellular matrix (ECM) proteins. Fibronectin serves as a central organizer of a variety of ECM components and modulate ECM remodeling during cancer progression (13). Previously, we have shown that a targeted contrast agent, CREKA-Tris(Gd-DOTA)3, specific to fibronectin-fibrin clots was able to produce significant contrast enhancement for detecting breast micrometastases smaller than 0.5 mm in diameter with high-resolution MRI (14). Recently, we have designed and developed a peptide targeted small molecular contrast agent, ZD2-Gd(HP-DO3A), specific to extradomain-B fibronectin (EDB-FN), an oncofetal isoform of fibronectin generated by alternative splicing of fibronectin pre-mRNA (15). EDB-FN contains an extra type III domain insertion between III7 and III8 domain, leading to conformational changes related to fibronectin matrix assembly(16). EDB-FN overexpression is a marker of epithelial-to-mesenchymal transition (EMT) (17), a biological process associated with cancer metastasis and drug resistance (18). It is highly expressed in the ECM of aggressive prostate tumors of high metastatic potential, low in low-risk prostate tumors of low metastatic potential (15). ZD2 was discovered by screening short peptides affinitive to EDB using phage display technique (19). We have shown that ZD2-Gd(HP-DO3A) is able to detect aggressive prostate cancer with high metastatic potential and to differentiate aggressive tumors from slow-growing prostate tumors with low metastatic potential in MRI (15).
In this study, we investigated the potential of molecular MRI with ZD2-Gd(HP-DO3A) for detection of aggressive breast cancer and metastases. We hypothesize that EDB-FN is abundant in the ECM of malignant breast tumors and allows easy access and specific binding of a sufficient amount of the targeted MRI contrast agent for effective MR molecular imaging. Murine 4T1 TNBC cells (20,21) and human MDA-MB-231 TNBC cells were used to develop primary and metastatic TNBC mouse models. The elevated expression of EDB-FN was determined in the cancer cells and tumor tissues. The effectiveness of molecular MRI with ZD2-Gd(HP-DO3A) in delineating primary TNBC tumors and distant metastases was investigated with tumor models.
Methods
Materials and cell culture
All reagents used were of the highest grade commercially available. Reagents for chemical synthesis were purchased from Sigma-Aldrich (Saint Louis, MO, USA) unless otherwise stated. The fluorescence imaging probe, ZD2-Cy5.5, and MRI contrast agent, ZD2-Gd(HP-DO3A), were synthesized and characterized as previously described (15). The firefly luciferase-expressing cell line, 4T1-GFP-Luc, breast cancer cells was acquired from PerkinElmer (Waltham, MA, USA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and 1% Penicillin-Streptomycin (5,000 U/mL, Thermofisher Scientific, Waltham, MA, USA). MDA-MB-231 cells were acquired from American Type Culture Collection (ATCC, Rockville, MD, USA). To stably express firefly luciferase, MDA-MB-231 cells were transfected with pNifty-CMV-luciferase, followed by selection with Zeocin (500 μg/mL). 4T1-GFP-Luc and MDA-MB-231-Luc cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% Penicillin-Streptomycin (5,000 U/mL, Thermo fisher Scientific). Cells were cultured in an incubator maintained at 37 °C and 5% CO2.
Quantitative real-time PCR
MDA-MB-231-luc or 4T1-GFP-luc cells were seeded onto 6-well plates overnight. TGFβ1 (5 ng/mL, Abcam, Cambridge, MA, USA) was used to treat the 4T1-GFP-Luc and MDA-MB-231-luc cells for 3 days. The total RNA was isolated using the RNeasy Plus Kit (Qiagen, Valencia, CA, USA). Reverse transcription was performed using the High Capacity cDNA Transcription Kit (Applied Biosystems, Waltham, MA, USA). Quantitative real-time PCR was conducted with the SYBR Green Master Mix (Applied Biosystems, San Diego, CA, USA) and the Master ep realplex2 (Eppendorf, Hamburg, Germany) according to manufacturer’s recommendations. Quantification of the relative expression of EDB mRNA was performed using the 2−ΔΔCt method and the level of GAPDH mRNA as internal control. Primers used in this study included: 5′-CCTGGAGTACAATGTCAGTG -3′ (forward) and 5′-GGTGGAGCCCAGGTGACA -3′ (reverse) for human EDB; 5′-CCTGGAGTACAATGTCAGTG -3′ (forward) and 5′-GGTGGAGCCCAGGTGACA-3′ (reverse) for mouse EDB; 5′-ACCCAGAAGACTGTGGATGG-3′ (forward) and 5′-TCTAGACGGCAGGTCAGGTC -3′ (reverse) for human GAPDH; 5′-TCCATGACAACTTTGGTATTCGT-3′ (forward) and 5′-AGTAGAGGCAGGGATGATGTT -3′ (reverse) for mouse GAPDH.
Western Blot
The 4T1 primary and metastatic tumor tissues (30–100 mg) were collected from orthotopic 4T1 tumor models constructed as described previously (14). Tumors were homogenized in 200–500 μL T-PER buffer (Thermo Fisher Scientific) supplemented with the protease inhibitor cocktail (Sigma-Aldrich) and PMSF (Phenylmethanesulfonyl fluoride) (Sigma-Aldrich). After centrifugation at 10,000 g for 10 min at 4 °C, the supernatant of the lysates was collected and the protein concentration quantified using BCA assay (Biorad, Hercules, CA, USA). The 4T1 tumor lysates have been used in a previous study (14). Proteins of 25 μg were resolved using SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen, Carlsbad, CA, USA), and incubated with the anti-EDB-FN antibody (BC-1, Abcam, Hercules, CA). After washing with TBST, fluorescein-conjugated anti-mouse secondary antibody was applied. For visualization of β-actin, the fluorescein-conjugated anti-β-actin antibody was used. The Typhoon trio scanner (GE healthcare) was used for visualization of EDB-FN and β-actin bands using the channel for fluorescein.
3D culture and peptide binding study
The 3D culture of 4T1 and MDA-MB-231 cells was used to evaluate binding of the EDB-targeting probe to breast cancer cells within a tumor microenvironment. The 3D matrigel culture was prepared as described previously (22). Briefly, matrigel (3.6–4.4 mg/mL) diluted with PBS was pipetted onto a glass bottom plate and allowed to gel at 37 °C for 30 min. A thick matrigel layer was formed. Cells were then seeded into the plate. After 5 days, fluorescence dye-labeled peptides (200 nM) were added to the medium and incubated with the 3D spheres for 1 h. Confocal laser scanning microscopy (Olympus FV1000, Japan), was used to image the 3D spheres. Intensive shaking was avoided during peptide binding and imaging.
Primary and metastatic breast cancer models
All animal studies were carried out in accordance to guidelines by the Institutional Animal Care and Use Committee (IACUC) for Case Western Reserve University. The 4T1 breast cancer models was prepared by injecting 3 million cells into the mammary fat pad of Balb/c mice, 4–6 weeks of age. The MDA-MB-231 model was developed by injecting 3 million cells subcutaneously into the flank of mice. The 4T1 metastatic tumor model was developed by injecting 1 million cells into the left ventricle of the heart. Bioluminescence imaging was used to monitor the growth of metastatic tumors in whole body after i.p. injection of D-Luciferin (Gold Biotechnology, St Louis, MO, USA). At about 3 weeks after injection, mice were used for MRI. MDA-MB-231 metastatic tumor was constructed as described previously (23). Briefly, MDA-MB-231 cells, which were stimulated with TGFβ and engineered to express luciferase, were engrafted in the mammary fat pad of female nude mice. Primary tumors were resected at week 9 and growth of metastatic tumors were monitored with BLI after injection of D-Luciferin. Mice bearing metastases were used for MRI and fluorescence imaging at week 16.
Ex vivo fluorescence imaging
MDA-MB-231 and 4T1 tumor-bearing mice were subjected to injection of 10 nmol ZD2-Cy5.5 or CREKA-Cy5.5. At 4 h post-injection, the mice were sacrificed using cervical dislocation, with tumor and normal tissues dissected. Images of the organs were examined using the Maestro Imaging System (Caliper life Sciences, Waltham, MA, USA) and a yellow filter set with exposure time of 1000 ms. GFP signal from the tumor was recorded using a blue filter set and exposure time of 500 ms.
Histological analysis
Following the ex vivo fluorescence imaging, tumors and normal tissues were embedded in optimal cutting temperature compound (OCT) and kept frozen at −80 °C until further use. Tissue slides were prepared after cryo-sectioning the tissues at 5 μm thickness. Tissue slices were fixed with 5% PFA, permeabilized with Triton-X and blocked with 1% BSA. BC-1 antibodies were then used to incubate the tissue for 1 h. The ProLong Gold mounting medium was then used to mount the coverslip.
MRI
The Bruker Biospec 7T MRI scanner (Bruker Corp., Billerica, MA, USA) equipped with a volume radio frequency coil was used for in vivo MRI. Mice bearing 4T1 or MDA-MB-231 tumors were anaesthetized with 2% isoflurane. A 30-gauge needle connected with a 1.6-m tubing was fixed into the tail vein. Mice were then placed in the magnet and kept under anesthesia with 1.5% isoflurane. Body temperature was maintained at 36 °C by blowing warm air into the magnet. For primary tumor, an axial T1-weighted multi-slice multi-echo sequence was used with the following parameters: field of view (FOV): 3 cm; slice thickness: 1.2 mm; interslice distance: 1.2 mm; TR: 500 ms, TE: 8.1 ms; flip angle: 90°; average: 2; matrix size: 128 × 128. A dose of 0.1 mmol/kg was injected through the tail vein. For imaging of metastases imaging, a high-resolution fat suppression 3D T1-weighted FLASH sequence with respiratory gating was used. The parameters were as follows: TR: 25 ms, TE: 2.8 ms, average 3, flip angle: 15 °, in-plane FOV: 6 cm, slab thickness: 18-mm, resolution: 0.1172 × 0.0976 × 0.562 mm, scan duration with respiratory gating: 20 min. A dose of 0.2 mmol/kg was used for imaging metastatic tumors in accordance to the previous study (14). For CNR analysis, the ROIs of tumor were selected as the whole tumor regions. The CNR of tumors in the MR images was calculated using the following equation: CNR = (Stumor-Snormal)/(σ), where Stumor and Snormal denote the signal in tumor and its surrounding normal tissue, respectively, and σ is the standard deviation of noise estimated from the background air. For MRI of primary tumors and 4T1 metastatic tumors, each mouse was scanned once with either ZD2-Gd(HP-DO3A) or Gd(HP-DO3A). For MRI of MDA-MB-231 metastatic tumors, one mouse was imaged with ZD2-Gd(HP-DO3A), followed by imaging with Gd(HP-DO3A) on the next day.
Statistical analysis
Data are expressed as mean ± s.e.m. Statistical significance between two groups was evaluated by two-tailed unpaired t-test. Differences with P value of less than 0.05 were considered statistically significant.
Results
Upregulated EDB-FN is a marker of breast cancer of high metastatic potential
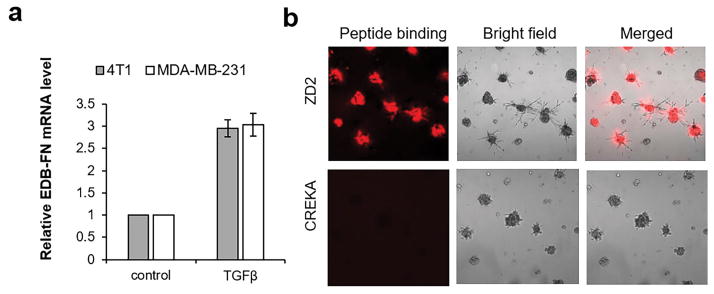
To validate EDB-FN as a molecular target for imaging malignant breast cancer, EDB-FN upregulation was measured in post-EMT 4T1 and MDA-MB-231 breast cancer cells stimulated by transforming growth factor β (TGFβ). TGFβ is known to induce EMT and transform epithelial breast cancer cells into invasive mesenchymal cells. As shown in Figure 1a, EDB-FN mRNA levels increased in TGFβ treated 4T1 and MDA-MB-231 cells, consistent with the role of EDB-FN as a marker of EMT (17). EDB-FN was also highly expressed in 4T1 tumor tissues (Figure S1). Metastatic 4T1 tumors demonstrated even higher EDB-FN expression than that of primary tumors. Negligible EDB-FN expression was seen in normal tissues and organs, including the brain, lung, and liver. The expression of EDB-FN in 3D culture of 4T1 cells was also assessed with a ZD2 peptide fluorescence probe, ZD2-Cy5.5, which specifically bound to EDB-FN. The 3D culture of 4T1 cells was a close mimic of the micrometastases in secondary organs. As shown in Figure 1b, ZD2-Cy5.5 bound to the entire 4T1 3D spheres and their periphery due to EDB secretion into the ECM and periphery of spheres. Binding specificity of ZD2 peptide to EDB-FN was also verified with a control peptide probe CREKA-Cy5.5. CREKA is a pentapeptide binding to the fibronectin-fibrin clots in the microenvironment of aggressive tumors (9). As shown in Figure 1b, there was no binding of CREKA-Cy5.5 to 3D culture of 4T1 breast cancer cells, indicating no formation of fibronectin-fibrin complexes in 3D spheres, which also validated the specific binding of ZD2 peptide. Taken together, the results suggest EDB-FN is highly secreted by post-EMT breast cancer cells and in aggressive breast tumors, and is a promising molecular target abundant in tumor ECM for effective MR molecular imaging of metastatic breast cancer.
Figure 1. Upregulated EDB-FN is a marker of breast cancer of high metastatic potential.
a. Analysis of EDB-FN mRNA levels in 4T1 and MDA-MB-231 cells with or without TGFβ treatment. The level of GAPDH was used as an internal control. Data are normalized to the EDB-FN mRNA level of non-treated 4T1 or MDA-MB-231 cells. **: P<0.01, ***: P<0.001 (n=3, two-tailed t-test) compared to non-treated cells. b. Binding of Cy5.5 labeled ZD2 and CREKA peptides on 3D matrigel culture of 4T1 cells. Peptide binding is shown in red.
MR molecular imaging of TNBC primary tumors
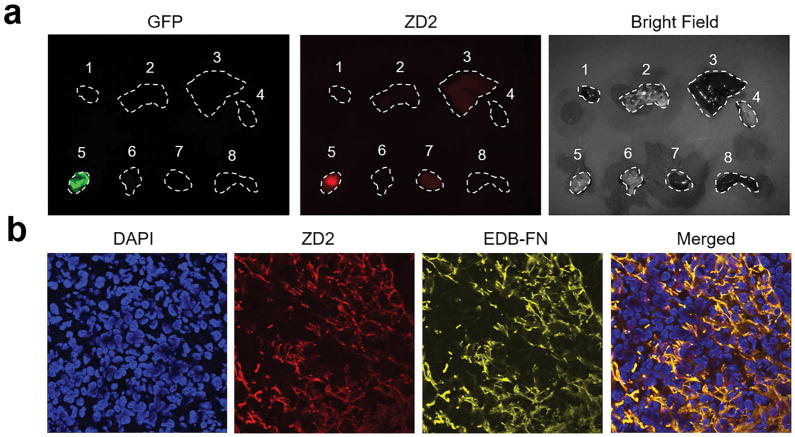
The effectiveness of the EDB-FN specific contrast agent, ZD2-Gd(HP-DO3A), for MR molecular imaging was first tested in mice bearing primary 4T1 breast tumor xenografts. The binding of ZD2-Cy5.5 in green fluorescence protein (GFP) labeled 4T1 primary tumor model was assessed by ex vivo fluorescence imaging (Figure 2a). At 4 h post-injection, substantially high signal was seen in tumors, while signal in the normal tissues and organs was low, suggesting that the EDB-FN targeting probe preferentially accumulated in 4T1 tumors. Immunohistological analysis of the tumor sections showed the co-localization of ZD2-Cy5.5 signal with EDB-FN expression, as probed by an anti-EDB-FN antibody, which distributed in the ECM of tumor in a fibrillary pattern (Figure 2b).
Figure 2. Ex vivo fluorescence imaging and histological analysis of 4T1 tumor.
a. Representative ex vivo fluorescence imaging of tumor and organs harvested from mice bearing 4T1 primary tumor xenografts at 4 h after injection of ZD2-Cy5.5. Numbers denote: 1, heart; 2, lung; 3, liver; 4, muscle; 5, 4T1 tumor; 6, brain; 7, kidney; 8, spleen. b. Histological analysis of the binding of ZD2 peptide and EDB-FN distribution. Merged channel of ZD2 and EDB-FN showed the colocalization of ZD2-Cy5.5 and EDB-FN.
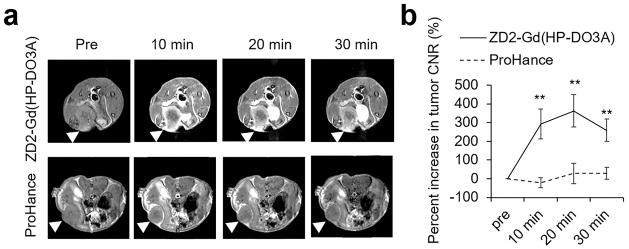
Figure 3a shows the representative axial MR images of 4T1 tumors acquired with a T1-weighted spin-echo sequence before and after intravenous injection of ZD2-Gd(HP-DO3A) at a dose of 0.1 mmol/kg. No apparent contrast could be observed between 4T1 tumors and normal tissues in pre-injection images. At 10 min post-injection, prominent contrast enhancement was visible in the periphery of 4T1 tumors. Contrast enhancement then gradually increases in the inner tumor tissue due to the diffusion of the contrast agent into the tumor (Figure 3a). Non-specific signal from surrounding normal tissues decreased over time due to the clearance of the unbound agent. High tumor contrast-to-noise ratio (CNR), approximately 200% increase from pre-injection, was maintained for at least 30 min post-injection (Figure 3b). In comparison, the administration of a nonspecific clinical contrast agent Gd(HP-DO3A) only resulted in some contrast enhancement mainly in the tumor rim, with less than 50% increase in whole tumor CNR (Figure 3b).
Figure 3. MRI detection of 4T1 primary tumor.
a. Representative axial T1-weighted spin-echo MRI images of 4T1 breast cancer xenografts in mice before and after intravenous injection of ZD2-Gd(HP-DO3A) or Gd(HP-DO3A) at a dose of 0.1 mmol/kg. Tumor locations are denoted with arrowheads. b. CNR analysis of 4T1 tumors (**: P<0.01).
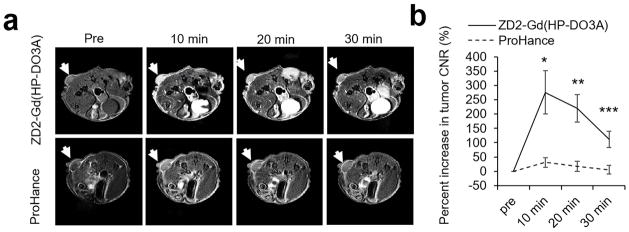
High expression of EDB-FN was also confirmed in MDA-MB-231 primary tumors by the strong binding of ZD2-Cy5.5 in the tumors as shown by fluorescence imaging (Figure S2). Consistently, strong contrast enhancement was also observed in the 2D spin-echo MR images of MDA-MB-231 primary tumors for at least 30 minutes after intravenous injection of ZD2-Gd(HP-DO3A), while much less signal enhancement was observed in the tumors injected with Gd(HP-DO3A) (Figure 4a). The signal enhancement was confined tumor rim and did not produce significant increase in whole tumor CNR after normalizing signal increase in normal tissues. Because the targeted agent bound to the oncoprotein highly expressed in the extracellular matrix of aggressive tumors, it resulted in robust signal enhancement across the whole tumor and 300% increase of CNR in MDA-MB-231 TNBC tumors at 10 minutes post-injection, which gradually reduced to 100% at 30 minutes (Figure 4b). Much less CNR increase was observed in MDA-MB-231 tumors contrast enhanced with Gd(HP-DO3A). CNRs of both 4T1 and MDA-MB-231 tumors remained high (about 100–200% increase) at 30 min after injection. Collectively, these data have demonstrated superior efficacy of ZD2-Gd(HP-DO3A) for MR molecular imaging of primary TNBCs as compared to the clinical agent Gd(HP-DO3A).
Figure 4. MRI detection of MDA-MB-231 primary tumor.
a. Representative axial T1-weighted spin-echo MRI images of MDA-MB-231 breast cancer xenografts before and after injection of ZD2-Gd(HP-DO3A) or Gd(HP-DO3A) at a dose of 0.1 mmol/kg. b. CNR analysis of MDA-MB-231 tumors (*: P<0.05 and **: P<0.01).
MR molecular imaging of TNBC metastases
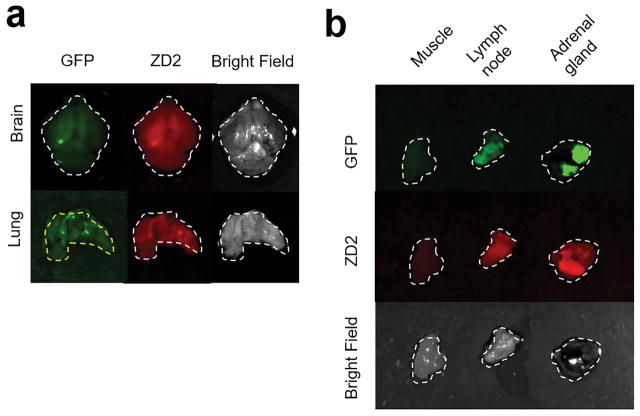
Intracardial injection of 4T1-GFP-Luc cells in the left ventricle induced tumor metastases in various organs as revealed by bioluminescence imaging (Figure 6). The metastatic tumor formation and binding of ZD2-C5.5 were verified by ex vivo fluorescence imaging of after injection of the targeted fluorescence probe. Strong binding of ZD2-Cy5.5 co-localized with the GFP labeled 4T1 metastases, including micrometastases, in different organs and tissues. Brain and lung micrometastases were prominently highlighted by ZD2-Cy5.5 (Figure 5a). Larger metastases in the lymph node and adrenal gland also showed robust binding of the peptide probe, while little binding was seen in the normal muscle (Figure 5b). The results indicate the specific binding of ZD2 peptide to the metastatic tumors.
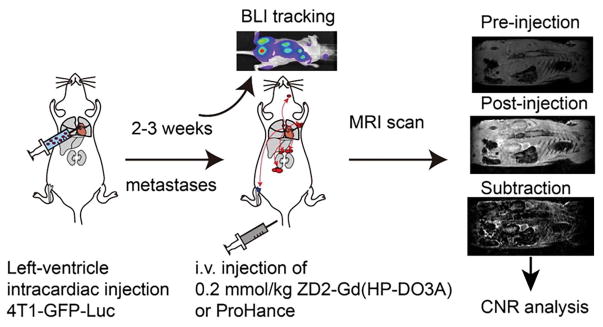
Figure 6. Illustration of 4T1 metastatic tumor model imaging procedures.
Mice were injected with 4T1-GFP-luc cells via the left ventricle of the heart. During 2–3 weeks, BLI was used to track the growth of metastatic tumors throughout the body. MRI was performed before and 20 min after i.v. injection of 0.2 mmol/kg ZD2-Gd(HP-DO3A) or Gd(HP-DO3A). b. Images of muscle, lymph node, and adrenal gland with 4T-GFP-Luc metastatic tumors.
Figure 5.
Representative ex vivo imaging of organs containing 4T1-GFP-Luc metastatic tumors at 5 h after injection of ZD2-Cy5.5. a. Images of brain and lung with GFP denoting the tumor location, ZD2-Cy5.5 signaling demonstrating the specific targeting of the peptide.
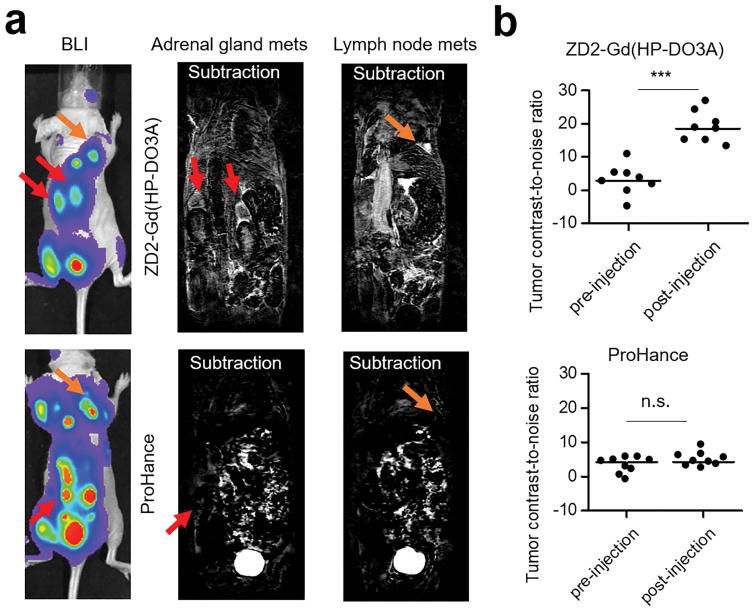
Intravenous injection of ZD2-Gd(HP-DO3A) (0.2 mmol/kg) resulted in strong signal enhancement in 4T1 metastatic tumors. Figure 7a shows the representative whole-body high-resolution MR images, where the head was not covered, acquired after the contrast injection. Significant contrast enhancement was observed in the metastatic tumors in the lymph nodes and adrenal glands, which correlated well with the tumor locations revealed by bioluminescence imaging (Figure 7a). Subtraction of pre-contrast images from the post-contrast images clearly delineated the location and size of these tumors (Figure 7a). Consistently, a substantial CNR increase was observed in these metastatic tumors (Figure 7b and Figure S3). In comparison, the clinical agent Gd(HP-DO3A) was unable to detect any metastatic tumors in MRI (Figure 7a). There was no statistical difference between the CNRs in the pre-injection tumors and post-injection tumors with Gd(HP-DO3A) (Figure 7b).
Figure 7. MRI of 4T1 metastatic tumors.
a. Representative bioluminiscence and subtraction MRI images of mice. Images that covered tumors in the adrenal glands and lymph nodes were presented to compare the efficiency of ZD2-Gd(HP-DO3A) and Gd(HP-DOTA) in highlighting 4T1 metastatic tumors. b. CNR analysis of metastatic tumors before and after contrast agent injection (***: P<0.001 ). Full figure with pre- and post-injection images is shown in Figure S3.
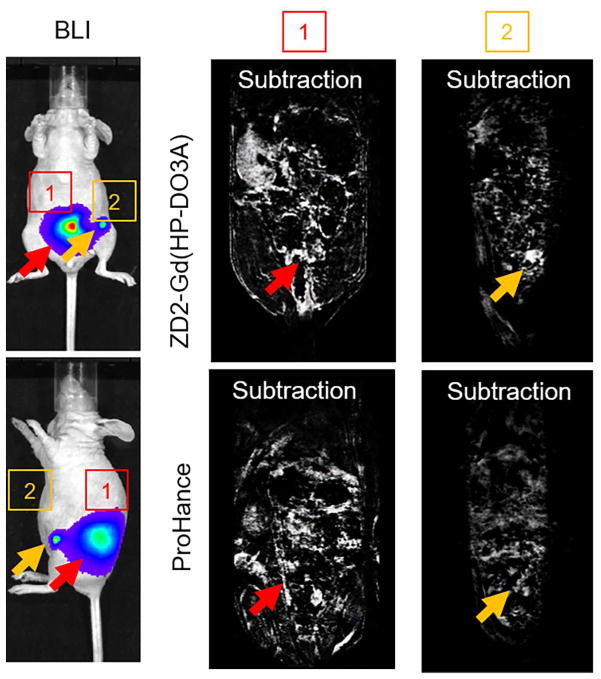
The effectiveness of ZD2-Gd(HP-DO3A) for molecular MRI of metastases was further investigated in mice bearing MDA-MB-231 metastases labeled with a luciferase reporter gene. Strong binding of ZD2 peptide to MDA-MB-231 metastases was verified with ex vivo fluorescence imaging and ZD2-Cy5.5 (Figure S4). Intravenous injection of ZD2-Gd(HP-DO3A) resulted in substantial signal enhancement in metastatic tumors, which correlated well with the tumors localized by bioluminescence imaging (Figure 8). The clinical agent Gd(HP-DO3A) did not produce visible contrast enhancement in the metastatic tumors of the same mouse (Figure 8 and Figure S5). Taken together, these results have demonstrated that MR molecular imaging of EDB-FN with ZD2-Gd(HP-DO3A) is effective for non-invasive detection of both the malignant primary breast cancers and metastases, including micrometastases, in the tested tumor models.
Figure 8. MRI of MDA-MB-231 metastatic tumors.
Representative bioluminescence and subtraction MR images of MDA-MB-231 metastatic tumors in mice before and after the injection of the contrast agents at a dose of 0.2 mmol/kg. Full figure with pre- and post-injection images is shown in Figure S5.
Discussions
Early accurate detection and characterization of breast cancer is critical for physicians to tailor precise treatment of aggressive tumors at the treatable stage to improve therapeutic outcome. Currently, metastatic status of breast cancer is predominantly characterized by biopsy-based histology for staging, treatment planning, and assessing treatment response. Contrast enhanced MRI is also routinely used in the clinical management of breast cancer. High-resolution MRI of the breast is advantageous over other imaging modalities, including mammography, ultrasound, and PET, for detecting and characterizing breast cancer at the earliest possible stage. However, because of the lack of safe and effective tumor-specific contrast agents, clinical application of MRI is limited as a screening tool only in women at high risk based on family history and a genetic predisposition, such as BRCA mutation (24). Development of a safe and effective targeted contrast agent with tumor specificity and the ability to identify breast cancer with metastatic potential could significantly improve accuracy of early detection of malignant breast cancer with MRI.
Here we have demonstrated the effectiveness of ZD2-Gd(HP-DO3A) for MR molecular imaging of both primary and metastatic breast tumors in animal models. ZD2-Gd(HP-DO3A) is a small peptide targeted macrocyclic contrast agent targeting an abundant oncoprotein in the ECM of aggressive tumors. The small size of ZD2-Gd(HP-DO3A) facilitates extravasation, tumor penetration, rapid clearance of unbound agent from the circulation via renal filtration. The highly stable macrocyclic Gd chelate ensures a better safety for clinical use (15). ZD2-Gd(HP-DO3A) has a relaxivity of 3.7 mM−1s−1 at 7T, which is about 35% higher than that of Gd(HP-DO3A) (2.75 mM−1s−1)(15,25). However, the improvement on contrast enhancement brought by ZD2-Gd(HP-DO3A) is much higher than the increase of relaxivity of this targeted agent. The abundant EDB-FN expressed in the tumor ECM is more accessible to the intravenously injected contrast agent than the cell-surface or intracellular biomarkers for effective MR molecular imaging. Consequently, specific binding of the targeted agent to the ECM protein produces robust signal enhancement across whole tumor, while the clinical agent could only result in some non-specific signal enhancement in tumor rim. The specific expression of EDB-FN in malignant tumors also minimizes non-specific background enhancement in normal tissues. As a result, a sufficient amount of the targeted agent binds to malignant tumors to generate detectable signal enhancement to overcome the low-sensitivity of MR molecular imaging. The specific binding of ZD2-Gd(HP-DO3A) also allows prolonged imaging window (as least 30 min post-injection), while non-specific signal enhancement from non-targeted agents only lasts for a few minutes in the tumor rim or other non-targeted tissues. Specific prolonged enhancement window will allow the clearance of unbound agents from untargeted tissues to provide specific tumor imaging ad detection. This is a significant advantage of the molecular MRI with targeted contrast agents for specific cancer molecular imaging. Our technique has great potential to address the limitation of imaging of current imaging modalities for high-resolution, non-invasive and specific detection of metastases in whole body. In our study, accurate tumor detection, particular in whole body, may be affected by the experience of the reader due to some background contrast in blood vessels, gastrointestinal regions, etc. Additionally, whole-body metastasis imaging normally takes longer duration because of the requirement of larger field of view (FOV) and spatial resolution, which may compromise the accuracy of metastasis identification because of motion artifacts. However, this could be addressed by proper design of imaging protocols, e.g. focused MRI scans on the tissues and organs of high metastatic potential, and section-by section scans of the whole body. Further comprehensive work is necessary to optimize the imaging protocols and image analysis criteria to facilitate accurate identification of small tumors. Effective MR molecular imaging of the oncoprotein has the potential to extend the current MRI techniques based on morphological features (2,26,27) for broad applications in clinical management of breast cancer.
Because the extradomain B fragment is conserved in all mammalian species, MRI contrast enhanced with the targeted agent was able to delineate both human and murine TNBC, including both primary and metastatic tumors. This corroborated the broad applicability of the contrast agent. Numerous clinical studies have demonstrated that the high expression of EDB-FN in breast cancer is associated with poor survival of the patients (28). TNBC represents a subclass of highly aggressive breast cancer with high metastatic potential and poor survival (29). EDB-FN had a higher expression in post-EMT cancer cells and metastatic tumors, consistent with the notion that it is a marker of EMT, a biological process associated with metastasis and drug resistance. Consequently, MR imaging of EDB-FN with ZD2-Gd(HP-DO3A) could effectively detect and delineate post-EMT aggressive breast cancers and distant metastases. Because EMT is involved in many types of cancer and EDB-FN overexpression is also observed in these cancers (30–32), MRI with ZD2-Gd(HP-DO3A) is promising for imaging a large array of cancers.
Molecular MRI with our targeted MRI contrast agent has a potential for risk stratification of breast cancer. Our previous study has shown that MR molecular imaging of EDB-FN with ZD2-Gd(HP-DO3A) has the potential to non-invasive characterize the aggressiveness of prostate cancer (15). The ability of our targeted MRI contrast agent for cancer risk-stratification could expand the role of molecular MRI in clinical management of breast cancer (21,33,34). Accurate and non-invasive localization and risk-stratification of malignant breast cancer has the potential to spare many patients from painful and invasive biopsies that exacerbate quality of life. Early and accurate localization of metastases, including micrometastases, could also enable non-invasive assessment of treatment response and tumor relapse, allowing for timely tailoring beast possible treatment strategies.
In summary, we have shown that the EDB-FN specific contrast agent, ZD2-Gd(HP-DO3A), improves sensitivity of molecular MRI in detecting TNBC primary and metastatic tumors. Molecular MRI with ZD2-Gd(HP-DO3A) has great potential to overcome limitations of contrast enhanced MRI in detecting aggressive breast tumors and micrometastases. This could facilitate accurate staging, prognosis, image-guided therapy and therapeutic efficacy assessment in breast cancer management. The high expression of EDB-FN in other types of cancer also opens up avenues for the use of this imaging technology for diagnosis of a broad-spectrum of cancers (32,35). Clinical translation of the targeted MRI contrast agent has the potential to provide more accurate detection and characterization of breast cancer.
Supplementary Material
Figure S1.Western blot images showing the relatively higher EDB-FN expression in 4T1 metastatic tumors in comparison to that in primary tumor and normal tissues. β-actin was used as a loading control.
Figure S2. Representative ex vivo fluorescence imaging of tumor and organs from mice bearing MDA-MB-231 primary breast cancer xenografts. Numbers denote: 1, tumor; 2, muscle; 3, brain; 4, heart; 5, lung; 6, liver; 7, spleen; 8, kidney.
Figure S3. Full figure of Figure 7 with pre- and post-injection images.
Figure S4. Representative bioluminescence imaging and ex vivo fluorescence imaging of MDA-MB-231 metastatic tumors at 5 h after injection. Numbers denote: 1, lung; 2, brain; 3, liver; 4, lymph node with tumor; 5, heart; 6, muscle.
Figure S5. Full figure of Figure 8 with pre- and post-injection images.
Acknowledgments
This work was supported, in part, by a grant from the National Institutes of Health to Z-R.L (R01CA194518).
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. The New England journal of medicine. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer-current status and future directions. Ann Oncol. 2009;20(12):1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 3.Hosseini H, Obradovic MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, Patwary N, Haunschild G, Guzvic M, Reimelt C, Grauvogl M, Eichner N, Weber F, Hartkopf AD, Taran FA, Rucker SYB, Fehm T, Rack B, Buchholz S, Spang R, Eister GM, Aguirre-Ghiso JA, Klein CA. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540(7634):552. doi: 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. Journal Of Clinical Oncology. 2004;22(14):2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 5.Lee CH, Dershaw D, Kopans D, Evans P, Monsees B, Monticciolo D, Brenner RJ, Bassett L, Berg W, Feig S, Hendrick E, Mendelson E, D’Orsi C, Sickles E, Burhenne LW. Breast Cancer Screening With Imaging: Recommendations From the Society of Breast Imaging and the ACR on the Use of Mammography, Breast MRI, Breast Ultrasound, and Other Technologies for the Detection of Clinically Occult Breast Cancer. Journal Of the American College Of Radiology. 2010;7(1):18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Rakha EA, El-Sayed ME, Green AR, Lee AHS, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109(1):25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 7.Baum RP, Prasad V, Müller D, Schuchardt C, Orlova A, Wennborg A, Tolmachev V, Feldwisch J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In-or 68Ga-labeled affibody molecules. J Nucl Med. 2010;51(6):892–897. doi: 10.2967/jnumed.109.073239. [DOI] [PubMed] [Google Scholar]
- 8.Metzger O, Tutt A, de Azambuja E, Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA, Ellis P, Di Leo A, Baselga J, Sotiriou C, Piccart-Gebhart M. Dissecting the Heterogeneity of Triple-Negative Breast Cancer. Journal Of Clinical Oncology. 2012;30(15):1879–1887. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, Wu X, Kresak A, Griswold M, Lu ZR. Peptide targeted tripod macrocyclic Gd(III) chelates for cancer molecular MRI. Biomaterials. 2013;34(31):7683–7693. doi: 10.1016/j.biomaterials.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan M, Burden-Gulley SM, Li W, Wu X, Lindner D, Brady-Kalnay SM, Gulani V, Lu Z-R. MR molecular imaging of prostate cancer with a peptide-targeted contrast agent in a mouse orthotopic prostate cancer model. Pharm Res-Dord. 2012;29(4):953–960. doi: 10.1007/s11095-011-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu XM, Burden-Gulley SM, Yu GP, Tan MQ, Lindner D, Brady-Kalnay SM, Lu ZR. Synthesis and Evaluation of a Peptide Targeted Small Molecular Gd-DOTA Monoamide Conjugate for MR Molecular Imaging of Prostate Cancer. Bioconjugate Chem. 2012;23(8):1548–1556. doi: 10.1021/bc300009t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye FR, Wu XM, Jeong EK, Jia ZJ, Yang TX, Parker D, Lu ZR. A Peptide Targeted Contrast Agent Specific to Fibrin-Fibronectin Complexes for Cancer Molecular Imaging with MRI (vol 19, pg 2300, 2008) Bioconjugate Chem. 2009;20(2):402–402. doi: 10.1021/bc800211r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Z, Lu Z-R. Targeting fibronectin for cancer imaging and therapy. J Mater Chem B. 2017 doi: 10.1039/C6TB02008A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z, Qutaish M, Han Z, Schur RM, Liu Y, Wilson DL, Lu Z-R. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nature communications. 2015;6:7984. doi: 10.1038/ncomms8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Z, Li Y, Roelle S, Zhou Z, Liu Y, Sabatelle R, DeSanto A, Yu X, Zhu H, Magi-Galluzzi C, Lu ZR. Targeted Contrast Agent Specific to an Oncoprotein in Tumor Microenvironment with the Potential for Detection and Risk Stratification of Prostate Cancer with MRI. Bioconjug Chem. 2017 doi: 10.1021/acs.bioconjchem.6b00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiefner A, Gebauer M, Skerra A. Extra-domain B in oncofetal fibronectin structurally promotes fibrillar head-to-tail dimerization of extracellular matrix protein. J Biol Chem. 2012;287(21):17578–17588. doi: 10.1074/jbc.M111.303131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrini I, Barachini S, Carnicelli V, Galimberti S, Modeo L, Boni R, Sollini M, Erba PA. ED-B fibronectin expression is a marker of epithelial-mesenchymal transition in translational oncology. Oncotarget. 2017;8(3):4914–4921. doi: 10.18632/oncotarget.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybak JN, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain a of fibronectin is a vascular marker of solid tumors and Metastases. Cancer Res. 2007;67(22):10948–10957. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 19.Han Z, Zhou Z, Shi X, Wang J, Wu X, Sun D, Chen Y, Zhu H, Magi-Galluzzi C, Lu Z-R. EDB Fibronectin Specific Peptide for Prostate Cancer Targeting. Bioconjugate Chem. 2015;26(5):830–838. doi: 10.1021/acs.bioconjchem.5b00178. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Lv D, Kim HJ, Kurt RA, Bu W, Li Y, Ma X. A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells. Cell Res. 2013;23(3):394–408. doi: 10.1038/cr.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton AM, Foster PJ. In vivo magnetic resonance imaging investigating the development of experimental brain metastases due to triple negative breast cancer. Clinical & experimental metastasis. 2017 doi: 10.1007/s10585-016-9835-5. [DOI] [PubMed] [Google Scholar]
- 22.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nature methods. 2007;4(4):359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parvani JG, Gujrati MD, Mack MA, Schiemann WP, Lu ZR. Silencing beta3 Integrin by Targeted ECO/siRNA Nanoparticles Inhibits EMT and Metastasis of Triple-Negative Breast Cancer. Cancer Res. 2015;75(11):2316–2325. doi: 10.1158/0008-5472.CAN-14-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans DG, Kesavan N, Lim Y, Gadde S, Hurley E, Massat NJ, Maxwell AJ, Ingham S, Eeles R, Leach MO, Howell A, Duffy SW, Grp M. MRI breast screening in high-risk women: cancer detection and survival analysis (vol 145, pg 663, 2014) Breast Cancer Res Tr. 2014;147(3):691–691. doi: 10.1007/s10549-014-2931-9. [DOI] [PubMed] [Google Scholar]
- 25.Vander Elst L, Raynaud J-S, Vives V, Santus R, Louin G, Robert P, Port M, Corot C, Muller R. Comparative relaxivities and efficacies of gadolinium-based commercial contrast agents. 2013 [Google Scholar]
- 26.Uematsu T, Kasami M, Yuen S. Triple-Negative Breast Cancer: Correlation between MR Imaging and Pathologic Findings 1. Radiology. 2009;250(3):638–647. doi: 10.1148/radiol.2503081054. [DOI] [PubMed] [Google Scholar]
- 27.Chen JH, Agrawal G, Feig B, Baek HM, Carpenter PM, Mehta RS, Nalcioglu O, Su MY. Triple-negative breast cancer: MRI features in 29 patients. Ann Oncol. 2007;18(12):2042–2043. doi: 10.1093/annonc/mdm504. [DOI] [PubMed] [Google Scholar]
- 28.Kaczmarek J, Castellani P, Nicolo G, Spina B, Allemanni G, Zardi L. Distribution Of Oncofetal Fibronectin Isoforms In Normal, Hyperplastic And Neoplastic Human Breast Tissues. Int J Cancer. 1994;59(1):11–16. doi: 10.1002/ijc.2910590104. [DOI] [PubMed] [Google Scholar]
- 29.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/”triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Tr. 2007;105(3):319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 30.Berndorff D, Borkowski S, Moosmayer D, Viti F, Müller-Tiemann B, Sieger S, Friebe M, Hilger CS, Zardi L, Neri D. Imaging of tumor angiogenesis using 99mTc-labeled human recombinant anti-ED-B fibronectin antibody fragments. J Nucl Med. 2006;47(10):1707–1716. [PubMed] [Google Scholar]
- 31.Santimaria M, Moscatelli G, Viale GL, Giovannoni L, Neri G, Viti F, Leprini A, Borsi L, Castellani P, Zardi L, Neri D, Riva P. Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cancer. Clin Cancer Res. 2003;9(2):571–579. [PubMed] [Google Scholar]
- 32.Locher R, Erba PA, Hirsch B, Bombardieri E, Giovannoni L, Neri D, Durkop H, Menssen HD. Abundant in vitro expression of the oncofetal ED-B-containing fibronectin translates into selective pharmacodelivery of (131)I-L19SIP in a prostate cancer patient. Journal of cancer research and clinical oncology. 2014;140(1):35–43. doi: 10.1007/s00432-013-1538-6. [DOI] [PubMed] [Google Scholar]
- 33.Kim EJ, Kim SH, Kang BJ, Choi BG, Song BJ, Choi JJ. Diagnostic value of breast MRI for predicting metastatic axillary lymph nodes in breast cancer patients: diffusion-weighted MRI and conventional MRI. Magnetic resonance imaging. 2014;32(10):1230–1236. doi: 10.1016/j.mri.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Serres S, Soto MS, Hamilton A, McAteer MA, Carbonell WS, Robson MD, Ansorge O, Khrapitchev A, Bristow C, Balathasan L, Weissensteiner T, Anthony DC, Choudhury RP, Muschel RJ, Sibson NR. Molecular MRI enables early and sensitive detection of brain metastases. P Natl Acad Sci USA. 2012;109(17):6674–6679. doi: 10.1073/pnas.1117412109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scarpino S, Stoppacciaro A, Pellegrini C, Marzullo A, Zardi L, Tartaglia F, Viale G, Ruco LP. Expression of EDA EDB isoforms of fibronectin in papillary carcinoma of the thyroid. J Pathol. 1999;188(2):163–167. doi: 10.1002/(SICI)1096-9896(199906)188:2<163::AID-PATH335>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.Western blot images showing the relatively higher EDB-FN expression in 4T1 metastatic tumors in comparison to that in primary tumor and normal tissues. β-actin was used as a loading control.
Figure S2. Representative ex vivo fluorescence imaging of tumor and organs from mice bearing MDA-MB-231 primary breast cancer xenografts. Numbers denote: 1, tumor; 2, muscle; 3, brain; 4, heart; 5, lung; 6, liver; 7, spleen; 8, kidney.
Figure S3. Full figure of Figure 7 with pre- and post-injection images.
Figure S4. Representative bioluminescence imaging and ex vivo fluorescence imaging of MDA-MB-231 metastatic tumors at 5 h after injection. Numbers denote: 1, lung; 2, brain; 3, liver; 4, lymph node with tumor; 5, heart; 6, muscle.
Figure S5. Full figure of Figure 8 with pre- and post-injection images.