Abstract
Purpose of review
Here we review the current understanding of the functional neuroanatomy of neurons expressing Agouti-related peptide (AgRP) and the angiotensin 1A receptor (AT1A) within the arcuate nucleus (ARC) in the control of energy balance.
Recent findings
The development and maintenance of obesity involves suppression of resting metabolic rate (RMR). RMR control is integrated via AgRP and proopiomelanocortin neurons within the ARC. Their projections to other hypothalamic and extrahypothalamic nuclei contribute to RMR control, though relatively little is known about the contributions of individual projections and the neurotransmitters involved. Recent studies highlight a role for AT1A, localized to AgRP neurons, but the specific function of AT1A within these cells remains unclear.
Summary
AT1A functions within AgRP neurons to control RMR, but additional work is required to clarify its role within subpopulations of AgRP neurons projecting to distinct second-order nuclei, and the molecular mediators of its signaling within these cells.
Keywords: Obesity, Bioenergetics, Metabolism, Renin-Angiotensin System, Leptin, Agouti-Related Peptide
Introduction
Obesity rates have reached pandemic levels both in the United States and worldwide [1, 2]. Current FDA-approved anti-obesity drugs all work primarily through the suppression of caloric intake or absorption, despite increasing evidence demonstrating that the development of obesity involves (and obesity is maintained by) the suppression of resting metabolic rate (RMR) [3].
Several hypothalamic nuclei have been implicated in the regulation of energy balance [4]. The arcuate nucleus (ARC), located adjacent to the bottom of third ventricle, is a key site for integration of peripheral metabolic signals on energy status [5]. Two distinct types of neurons within the ARC regulate energy homeostasis in rodents: Agouti-related peptide (AgRP) neurons, which co-express neuropeptide Y (NPY), and proopiomelanocortin (POMC) neurons. Acute activation of AgRP/NPY neurons stimulates feeding, while chronic, but not acute, POMC neuron activation suppresses feeding. AgRP is a unique endogenous inverse agonist of the melanocortin 4 receptor (MC4R) [6, 7], a well-established regulator of energy homeostasis. Recent evidence suggests that AgRP may also function as a biased agonist of MC4R [8]. Leptin is an adipokine that promotes satiety through its actions on distinct populations of hypothalamic neurons including arcuate AgRP- and POMC-expressing neurons [9]; furthermore, it is an important modulator of sympathetic nervous activity (SNA) [10] and drives brown adipose tissue (BAT)-mediated non-shivering thermogenesis that contributes to energy balance [5]. In addition to neuropeptides AgRP and NPY, AgRP neurons also release fast-acting neurotransmitter γ-aminobutyric acid (GABA). Mice with specific deletion of the vesicular GABA transporter (Vgat) in AgRP neurons display resistance to weight gain and increased oxygen consumption when fed a high fat diet (HFD) [11], suggesting a role for AgRP neurons in the modulation of energy expenditure.
Recent evidence points to a role of the brain renin-angiotensin system (RAS) in the modulation of RMR [12–14]. Our group has shown that angiotensin II 1A (AT1A) receptors on AgRP neurons are necessary for leptin’s actions on RMR [15]. Indeed, mice lacking AT1A receptors in cells expressing leptin receptor (AT1ALepR-KO mice) that were fed a HFD gained significantly more weight than control littermates without any difference in food intake or digestive efficiency, and, in contrast to littermate controls, did not exhibit increased RMR in response to HFD. Similar results were obtained with DOCA-salt treatment, which is known to stimulate RMR in wildtype animals [16]. Furthermore, AT1A is co-localized more specifically to the subset of leptin receptor-expressing cells within the ARC that also express AgRP (Figure 1A). Mice engineered to lack AT1A receptors specifically in AgRP-expressing cells (AT1AAgRP-KO mice) exhibited a normal RMR on standard chow diet, but suppressed BAT SNA in response to icv leptin, as well as suppressed RMR responses to α-melanocyte stimulating hormone (α-MSH), the stimulatory peptide neurotransmitter derived from the POMC precursor. Interestingly, these mice had upregulated ARC expression of the glutamate decarboxylase enzymes Gad1 and Gad2 as well as the vesicular GABA transporter Vgat, which are involved in synthesis and intracellular transport of GABA [15]. These mice also exhibit increased expression of both AgRP and NPY within the ARC (Figure 1B). These results underscore a major role for the RAS within the ARC in the integrative control of RMR, and support the overall working hypothesis that AT1A within AgRP neurons of the ARC functions to suppress the production of neurotransmitters including AgRP, NPY and GABA.
Figure 1. Angiotensin AT1A localization to AgRP neurons: molecular consequences, and projections that may mediate RMR control.
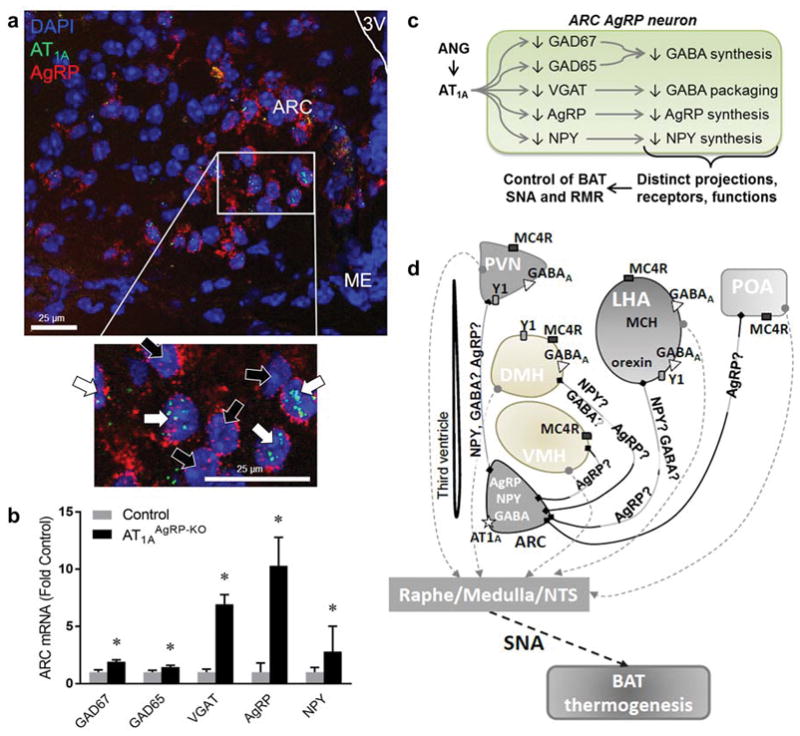
(A) Fluorescent in situ hybridization, demonstrating localization of AT1A to a subset of neurons expressing AgRP within the ARC ([15], with permission from J Clin Invest). White arrows identify cells which express AgRP and AT1A, whereas black arrows identify cells which express AgRP but not AT1A. (B) Expression of GAD65, GAD67, VGAT, AgRP and NPY in mice with genetic disruption of AT1A specifically within cells that express AgRP (AT1AAgRP-KO) ([15], with permission, and unpublished). (C) Schematic representation of the effects of angiotensin II signaling via AT1A in AgRP neurons in the arcuate nucleus. GAD glutamic acid decarboxylase; VGAT vesicular GABA transporter; ARC arcuate nucleus; ANG angiotensin II. (D) Control of resting energy expenditure by ARC AgRP neurons: neurocircuitry and neurotransmitters involved. Question marks indicate putative neurotransmitters. ARC arcuate nucleus; PVN paraventricular nucleus; DMH dorsomedial hypothalamus; VMH ventromedial hypothalamus; LHA lateral hypothalamus; MCH melanin-concentrating hormone; POA preoptic area; NTS nucleus tractus solitarius; Y1 NPY receptor 1; MC4R melanocortin 4 receptor; AT1A angiotensin receptor 1A; SNA sympathetic nervous activity; BAT brown adipose tissue.
While the general neurocircuitry of AgRP neurons is essentially known [17, 18], relatively few studies have focused on elucidating the efferent projections of AgRP neurons involved in the control of RMR. Most lacking is a clear outline of the links between AgRP neurons and other pre-sympathetic hypothalamic and extrahypothalamic nuclei that project to sympathetic centers in the medulla and spinal cord and ultimately innervate the BAT.
As our recent work has highlighted a major role for AT1A function specifically within AgRP neurons in the control of RMR, this review summarizes the current knowledge about the functional neuroanatomy that underlies RMR regulation by AgRP neurons, as well as the neurotransmitters involved (Figure 1C). Our primary focus is to outline the connections between AgRP neurons and their targets in the paraventricular nucleus (PVN) and the dorsomedial hypothalamus (DMH), as well as other hypothalamic and extrahypothalamic nuclei that are known to send direct projections to either sympathetic preganglionic neurons in the intermediolateral cell column of spinal cord (IML) or brainstem nuclei containing sympathetic premotor neurons that regulate BAT SNA [19–21] (Figure 1D).
ARC → Paraventricular Nucleus of the Hypothalamus (PVN) connection
Anatomic studies
The PVN is a heterogeneous nucleus composed of several distinct peptidergic cells generating various hormones (oxytocin, vasopressin, corticotropin-releasing hormone, thyrotropin-releasing hormone), as well as non-peptidergic cells. The majority of hormone-producing cells project to the median eminence and modulate pituitary function, while another population (parvocellular section) projects to other areas of the brain.
The PVN is strongly implicated in the control of feeding, as recently reviewed [22], but less is known about the role of the PVN in the control of energy expenditure. Retrograde tracing studies with transsynaptic pseudorabies virus (PRV) injected into the interscapular BAT resulted in labeling of PVN neurons [19, 23, 24], highlighting the existence of PVN-to-BAT pathways. In the last 20 years, several studies have identified direct projections from ARC AgRP neurons to the PVN. Early studies relied on immunohistochemistry and were performed in mice [25], rats [26, 17, 27], monkeys [27] and human brains [26]. All have demonstrated a broad distribution of AgRP immunoreactive terminals, with a high density of projections to the PVN. With the advent of more advanced techniques in recent years, increasing information about both anatomic connections and their function has been obtained. Transgenic mice engineered to express channel-rhodopsin-2 in AgRP neurons via a Cre-dependent viral vector demonstrated light-evoked feeding via activation of projections from AgRP neurons to PVN [28]. More recent studies have also used viral tracing techniques, which allow for more detailed mapping of neuronal connections. In particular, the presence of anatomical connections between ARC, PVN and BAT were observed using retrograde transsynaptic studies with PRV [19]. Strong innervation of AgRP neurons to the PVN was confirmed by adenovirus associated virus (AAV)-mediated anterograde tracing in AgRP-Cre mice [18]. Finally, Shi et al. [29], using both a Cre-dependent AAV-mCherry injected into the ARC and retrograde tracing with cholera toxin B injected into the RVLM in AgRP-IRES-Cre mice, recently demonstrated that 36% of RVLM-projecting neurons in the PVN are closely associated with AgRP fibers originating in the ARC. In this study, modulation of AgRP neuron activity by chemogenetics led to changes in splanchnic sympathetic nervous activity, but BAT SNA was not assessed.
Thus, a large body of evidence supports the presence of anatomic links between AgRP neurons and PVN that likely contribute to RMR control.
Neurotransmitters
Most of the studies examining the role of specific neurotransmitters produced by AgRP neurons in their communication to second-order neurons in the PVN have tested their roles in the control of feeding behavior (reviewed in [22]). The few studies focused on modulation of energy expenditure by the AgRP→PVN pathway have identified NPY as a contributor, while studies testing roles for GABA and AgRP in this circuit remain scant.
Intracerebroventricular NPY administration causes obesity and hyperphagia in rats [30]. However similar experiments performed under conditions of pair feeding also caused the NPY-treated animals to gain more weight than controls, suggesting an inhibitory effect of brain NPY on energy expenditure [30]. Furthermore, intrahypothalamic NPY administration was reported to decrease BAT thermogenesis in rats [31]. Finally, mice lacking both AgRP and leptin receptors in AgRP neurons, but with intact NPY expression, exhibited decreased oxygen consumption in the second half of the light phase, independent of changes in physical activity [32]. Additional information about the neuronal networks mediating the effects of NPY on energy expenditure was obtained by selective reintroduction of NPY expression in ARC of NPY−/− mice (ARC NPY mice) [33]. This manipulation led to decreased oxygen consumption and BAT temperature compared to NPY−/− mice. Moreover, the PVN was identified as a crucial site for ARC NPY output, as the ARC NPY mice had decreased expression of thyrosine hydroxylase in the PVN, a result also observed as a consequence of HFD-induced obesity. These effects were mediated by NPY Y1 receptors in the PVN, as demonstrated by the fact that ARC NPY injection in Y2−/− mice led to changes in thyrosine hydroxylase expression, while the same experiment in Y1−/− mice did not, and by evidence of Y1 colocalization in a majority of thyrosine hydroxylase-positive PVN neurons [33].
The physiological role of GABAergic signaling in the control of energy expenditure was demonstrated in mice with AgRP neuron-specific deletion of the Vgat. Such mice were resistant to diet-induced obesity due to increased energy expenditure, especially HFD-induced thermogenesis [11]. Furthermore, blockade of GABAA receptors through bicuculline microinjection into the PVN in male Sprague Dawley rats inhibited the cooling-evoked increase in BAT SNA, demonstrating that the PVN → BAT SNA pathway is under tonic GABAergic inhibition [34]. However the origin of this GABAergic input to the PVN still remains to be elucidated, as many GABAergic neurons are present in the hypothalamus [35].
Conflicting results have been reported with regard to the role of the melanocortin system in the PVN-mediated regulation of energy expenditure. Intra-PVN injection of melanotan II (MTII), an MC3R/MC4R agonist, caused increased resting energy expenditure in wild type mice [36]. However, selective restoration of MC4R expression in the PVN of MC4R-null mice failed to normalize resting oxygen consumption [37], and chemogenetic activation of MC4R neurons in the PVN had no effect on oxygen consumption [38]. On the other hand, MC3R/MC4R blockade in the PVN was recently reported to blunt the increase in BAT SNA and BAT temperature in adult male Wistar rats microinjected with N-methyl-D-aspartate (NMDA) in the ARC [39]. In this report, energy expenditure was not measured. Also, given that AgRP neurons were not specifically targeted, these effects may be mediated by other types of neurons within the ARC.
ARC → Dorsomedial Nucleus of Hypothalamus (DMH) connection
Anatomic studies
Strong evidence supports a role for the DMH in the central control of BAT thermogenesis, achieved via modulation of SNA (reviewed in [40, 41]). As BAT thermogenesis contributes a significant portion of energy expenditure in rodents, the DMH is likely to play an important role in the modulation of energy homeostasis.
As with the PVN, ARC→DMH projections were initially reported in immunohistochemical studies [17, 27]. Injection of PRV, a retrograde transsynaptic tracer, into BAT resulted in progressive infection in several hypothalamic nuclei including the DMH [19, 23, 24]. A more recent report of whole brain mapping of axonal projections of AgRP neurons using AAV-mediated anterograde tracing also identified connections between ARC and DMH [18]. Finally, Shi et al. [29] reported that 31% of DMH neurons were closely associated with AgRP fibers originating in the ARC, using a Cre-dependent AAV-mCherry injected into the ARC plus retrograde tracer cholera toxin B injected into the RVLM of AgRP-IRES-Cre mice.
Neurotransmitters
Chitravanshi et al. [39] injected FluoroGold into the DMH and observed retrograde labeling of ARC neurons. Neuronal labeling demonstrated that 30 to 40% of all POMC-positive neurons, α-MSH-positive neurons and Vglut3-positive neurons contained FluoroGold. Unfortunately the authors did not assess retrograde AgRP neuronal labeling. However, supporting the concept that inhibitory (GABA/AgRP/NPY-expressing) AgRP neurons may directly innervate the DMH, DMH neurons express GABAA receptors [23], MC4R [42] and Y1 receptors for NPY [43]. Yet, to the best of our knowledge, the specific contribution of AgRP, GABA and NPY signaling to the regulation of energy expenditure by the ARC→ DMH pathway has not been examined.
It is interesting to note that Chitravanshi et al. have demonstrated that microinjection of NMDA into the ARC resulted in increased BAT SNA, and that this effect is blunted by microinjection of muscimol (GABAA agonist), a glutamate receptor antagonist, or an MC3R/MC4R blocker into the DMH [39]. Liu et al. previously demonstrated that both AgRP and POMC neurons express NMDA receptors, but that genetic disruption only in AgRP (not POMC) neurons had effects on energy homeostasis [44]. Importantly the study by Liu demonstrated that disruption of NMDA receptors in AgRP neurons caused reduced body weight, adiposity and food intake. The authors comment that, although oxygen consumption was measured, no conclusion could be drawn regarding differences in energy expenditure because of these changes in body composition. As these results appear to contradict the conclusions of Chitravanshi, additional work to clarify this issue is required.
Other AgRP projections potentially relevant for the control of RMR
ARC → lateral hypothalamic area (LHA)
The LHA is another hypothalamic nucleus known to be important for the homeostatic control of energy balance. Like in the PVN, there are heterogenous and neurochemically distinct types of neurons residing in the LHA, including those expressing orexin/hypocretin, melanin-concentrating hormone (MCH) or anoretic neuropeptide neurotensin [45]. Patients with narcolepsy, a condition characterized by orexin deficiency, have higher BMI despite lower food intake compared to controls [46, 47]. In mice, orexin deficiency is associated with impaired BAT thermogenesis [48]. Several groups have reported that both orexin- and MCH-expressing neurons in the LHA receive dense projections from ARC AgRP/NPY neurons (reviewed in [45]). Furthermore, orexin fibers were shown to connect to neurons of the rostral raphe pallidus (rRPa) that in turn multisynaptically connected to the BAT [49, 50]. Orexin neurons express NPY receptors type 1 (Y1), and selective Y1 agonists were found to hyperpolarize orexin neurons in ex vivo whole-cell current-clamped recordings of mouse hypothalamus neurons [51]. Orexin neurons express both GABAA and GABAB receptors [52, 53] while MCH neurons express GABAA receptors [54]. These results support a potential role of the ARC → LHA projection in the modulation of energy expenditure.
On the other hand, only low levels of Y5 receptor expression were reported in MCH neurons in the LHA [55], and MC4R expression was found only in neurotensin-positive, but not orexin- and MCH-positive, neurons in the LHA as assessed in MC4R-GFP mice in which GFP expression is under MC4R gene promoter [56].
ARC→ ventromedial hypothalamus (VMH)
The ventromedial hypothalamus (VMH) was shown to influence energy balance by modulating sympathetic outflow as well as peripheral glucose and lipid metabolism [57, 58]. Direct projections exist from VMH neurons to the rRPa [59, 57]. Evidence of projections originating from the ARC to the VMH was obtained from anterograde tracing studies [26, 18]. Recently, microinjections of the melanocortin agonist MTII into the rat VMH were reported to increase skeletal muscle as well as BAT thermogenesis, both at rest and during exercise [58], implicating the melanocortin system, and potentially AgRP, in VMH-mediated modulation of energy expenditure.
ARC → preoptic area (POA)
The preoptic area is the body’s main thermostat and activates BAT thermogenesis in response to cold as well as pyrogens [41]. A high concentration of AgRP-immunoreactive fibers in the medial (MPO) and median (MnPO) nuclei of the POA was reported [17]. These findings were confirmed by a more recent study using viral vectors for both retrograde and anterograde tracing [18]. Furthermore, POA neurons, especially in the MPO, are rich in MC4R [60] and infusion of MTII into the MPO activates BAT thermogenesis in rats [61]. However, to the best of our knowledge, the importance of connections between AgRP neurons and POA in the regulation of resting energy expenditure remains largely unexplored.
Reciprocal input to ARC AgRP neurons from other hypothalamic nuclei
Although beyond the scope of this review, it should also be mentioned that projections from several hypothalamic nuclei to ARC AgRP neurons have been identified [18, 62, 63]. One PVH → ARC AgRP projection [62] and one DMH → ARC AgRP projection [63] were shown to modulate feeding behavior. Projections from the LHA orexin neurons to the ARC have also been reported [45]. However, the impact of these reciprocal connections on energy expenditure, if any, remains to be examined.
Projections to extrahypothalamic nuclei involved in BAT thermogenesis
ARC AgRP neurons have been shown to project to two extrahypothalamic regions that are involved in BAT thermogenesis: locus ceruleus (LC) [17] and periaqueductal gray (PAG) [17], both of which in turn project to sympathetic premotor neurons in the RPa [64, 65]. Indeed, retrograde tracing studies performed with PRV injection into the interscapular BAT identified intense labeling in these two nuclei [19].
As mentioned earlier, selective reintroduction of NPY expression in ARC of NPY−/− mice led to decreased oxygen consumption and BAT temperature compared to NPY−/− mice [33]. In addition thyrosine hydroxylase neurons in the PVN, thyrosine hydroxylase neurons in the LC were also identified as downstream targets of ARC NPY contributing to the modulation of energy expenditure [33]. However, while Y1 receptors in the PVN appear to mediate NPY’s effects on energy expenditure, no direct evidence of Y1 receptors in the TH neurons of the LC is provided in this report. Another study examining brain Y1 receptor immunoreactivity and expression did not find any in the LC [66].
Intra-PAG muscimol microinjection leads to increased BAT SNA, suggesting the presence of GABAA receptors on PAG neurons [67]. However the precise origin of the GABAergic tone on PAG neurons is unknown. On the other hand, the presence of Y1 receptors was reported in the PAG [66]. Similarly, MC4R presence was observed by immunohistochemistry in both the LC and PAG [68].
The parabrachial nucleus (PBN) is another nucleus that could potentially be involved in the control of energy expenditure. The PBN receives afferents from skin temperature receptors via the spinal cord and projects to the POA [40]. It also receives direct projections from ARC AgRP neurons [17, 69, 70, 28] and expresses Y1 receptors [66, 71], MC4R [68] and GABAA receptors [69].
To the best of our knowledge, the importance of these projections from ARC AgRP neurons on the modulation of energy expenditure has not been studied.
Implications for studies of the effects of angiotensin upon AgRP neurons in the control of RMR
As this review illustrates, much remains to be elucidated regarding the regulation of RMR by AgRP neurons, as well as the precise mechanisms through which ANG is implicated in this process. In particular, future studies should examine the following questions:
Which projections from ARC AgRP neurons mediate energy expenditure control in response to ANG (versus other stimuli)?
Which neurotransmitters within AgRP neurons are modulated by ANG, and are there distinct roles for each neurotransmitter in the projections to various second-order nuclei?
Are specific efferent projections involved in the pathogenesis of obesity, the resistance to weight loss in obese patients, and the pathogenesis of obesity-related cardiovascular disorders, such as selective leptin resistance?
We recently performed an in silico reanalysis of a of a recently published, publicly-available single-cell RNA-sequencing dataset (GSE74672) describing the transcriptome of individual populations of cells within the mouse hypothalamus [72]. We described the existence of two distinct subsets of AgRP/NPY/GABA neurons: one population with high expression of somatostatin (“Sst-3”) and another population with low somatostatin expression (“GABA-14”). Interestingly, only the Sst-3 subset expressed AT1A at detectable levels [73]. In addition, we recently published images from fluorescent in situ hybridization studies examining the localization and co-localization of AgRP and AT1A within the ARC, and close examination of these images highlights expression of AT1A in many, but not all AgRP neurons [15] (Figure 1A). This raises the following additional questions:
Does the subset of AgRP neurons that expresses AT1A (i.e. the Sst-3 subset) selectively contribute to RMR control, while feeding is regulated by the other subset (GABA-14 subset)?
Do the Sst-3 and GABA-14 subsets of AgRP-expressing cells exhibit differential projection patterns?
Do chronic pathological states such as obesity result in selective alterations in the distinct subsets of AgRP neurons, with regard to expression of AT1A, use of specific neurotransmitters, or anatomical projections – and can such changes explain the adaptation (suppression) of RMR that is documented during long-term obesity?
Conclusions
In conclusion, evidence accumulated in the last 2 decades supports a role for ARC AgRP neurons in the modulation or resting energy expenditure, and more recent studies have implicated ANG/AT1A as a key regulator of this process. Future studies aimed at further understanding the functional neurocircuitry of AgRP neurons may identify potential pharmacologic targets for the treatment of obesity and obesity-associated pathologies, including selective leptin resistance and obesity-associated hypertension.
Acknowledgments
The authors were supported by grants from the National Institutes of Health (HL134850, HL084207, HL007638, HL127673, MH109920, HL007121, DK117510), the American Heart Association (14PRE20380401, 15SFRN23730000, 18EIA33890055), the American Physiological Society, and the UIHC Center for Hypertension Research.
Footnotes
Authors’ Contributions
LLM drafted the manuscript, and all co-authors edited and approved the final version of the manuscript.
Conflict of Interest
Drs. Morselli, Claflin, Cui and Grobe declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
- 1.Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. The Lancet. 2017 doi: 10.1016/S0140-6736(17)32129-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring, Md) 2016;24(8):1612–9. doi: 10.1002/oby.21538. This study highlights the major role that RMR plays in weight maintenance in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rui L. Brain regulation of energy balance and body weight. Rev Endocr Metab Disord. 2013;14(4):387–407. doi: 10.1007/s11154-013-9261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Pandit R, Beerens S, Adan RAH. Role of leptin in energy expenditure: the hypothalamic perspective. Am J Physiol Regul Integr Comp Physiol. 2017;312(6):R938–R47. doi: 10.1152/ajpregu.00045.2016. A comprehensive review of the role of leptin in the regulation of energy homeostasis. [DOI] [PubMed] [Google Scholar]
- 6.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 7.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11(5):593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Tao YX. Biased signaling initiated by agouti-related peptide through human melanocortin-3 and -4 receptors. Biochim Biophys Acta. 2016;1862(9):1485–94. doi: 10.1016/j.bbadis.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Andermann ML, Lowell BB. Toward a Wiring Diagram Understanding of Appetite Control. Neuron. 2017;95(4):757–78. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui H, Lopez M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nature reviews Endocrinology. 2017;13(6):338–51. doi: 10.1038/nrendo.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience. 2008;11(9):998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, et al. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. American journal of physiology Heart and circulatory physiology. 2012;303(2):H197–206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, et al. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33(11):4825–33. doi: 10.1523/JNEUROSCI.3806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claflin KE, Grobe JL. Control of energy balance by the brain renin-angiotensin system. Current hypertension reports. 2015;17(5):38. doi: 10.1007/s11906-015-0549-x. [DOI] [PubMed] [Google Scholar]
- 15••.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, et al. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest. 2017;127(4):1414–24. doi: 10.1172/JCI88641. This study demonstrates the critical role of AT1A, localized to AgRP-expressing neurons, in RMR control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, et al. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension. 2011;57(3):600–7. doi: 10.1161/hypertensionaha.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19(18):RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460(3):303–26. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 20.Morrison SF, Cao W-H, Madden CJ. Dorsomedial hypothalamic and brainstem pathways controlling thermogenesis in brown adipose tissue. Journal of Thermal Biology. 2004;29(7):333–7. doi: https://doi.org/10.1016/j.jtherbio.2004.08.006. [Google Scholar]
- 21.Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133(4):1039–46. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Sutton AK, Myers MG, Jr, Olson DP. The Role of PVH Circuits in Leptin Action and Energy Balance. Annu Rev Physiol. 2016;78:207–21. doi: 10.1146/annurev-physiol-021115-105347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126(1):229–40. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110(3):515–26. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 25.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95(25):15043–8. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402(4):442–59. [PubMed] [Google Scholar]
- 27.Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, et al. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology. 1999;140(3):1408–15. doi: 10.1210/endo.140.3.6544. [DOI] [PubMed] [Google Scholar]
- 28.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–50. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Z, Madden CJ, Brooks VL. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. The Journal of clinical investigation. 2017;127(7):2868–80. doi: 10.1172/jci92008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jeanrenaud B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 1993;133(4):1753–8. doi: 10.1210/endo.133.4.8404618. [DOI] [PubMed] [Google Scholar]
- 31.Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am J Physiol. 1991;260(2 Pt 2):R328–34. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- 32.Luo N, Marcelin G, Liu SM, Schwartz G, Chua S., Jr Neuropeptide Y and agouti-related peptide mediate complementary functions of hyperphagia and reduced energy expenditure in leptin receptor deficiency. Endocrinology. 2011;152(3):883–9. doi: 10.1210/en.2010-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, et al. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17(2):236–48. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R831–43. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, O’Brien WG, 3rd, Lee CC, Myers MG, Jr, Tong Q. Role of GABA release from leptin receptor-expressing neurons in body weight regulation. Endocrinology. 2012;153(5):2223–33. doi: 10.1210/en.2011-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155–63. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 37.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 38.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nature neuroscience. 2015;18(6):863–71. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chitravanshi VC, Kawabe K, Sapru HN. Stimulation of the hypothalamic arcuate nucleus increases brown adipose tissue nerve activity via hypothalamic paraventricular and dorsomedial nuclei. Am J Physiol Heart Circ Physiol. 2016;311(2):H433–44. doi: 10.1152/ajpheart.00176.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Contreras C, Nogueiras R, Dieguez C, Rahmouni K, Lopez M. Traveling from the hypothalamus to the adipose tissue: The thermogenic pathway. Redox Biol. 2017;12:854–63. doi: 10.1016/j.redox.2017.04.019. A comprehensive review of the neurocircuitry of non-shivering thermogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31(34):12189–97. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, et al. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J Comp Neurol. 2005;482(3):217–43. doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- 44.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73(3):511–22. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104(1):29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lammers GJ, Pijl H, Iestra J, Langius JA, Buunk G, Meinders AE. Spontaneous food choice in narcolepsy. Sleep. 1996;19(1):75–6. doi: 10.1093/sleep/19.1.75. [DOI] [PubMed] [Google Scholar]
- 47.Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251(2):85–9. doi: 10.1007/s004060170057. [DOI] [PubMed] [Google Scholar]
- 48.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14(4):478–90. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123(2):147–56. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- 50.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31(44):15944–55. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24(40):8741–51. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, et al. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23(5):1557–62. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie X, Crowder TL, Yamanaka A, Morairty SR, Lewinter RD, Sakurai T, et al. GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol. 2006;574(Pt 2):399–414. doi: 10.1113/jphysiol.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guyon A, Conductier G, Rovere C, Enfissi A, Nahon JL. Melanin-concentrating hormone producing neurons: Activities and modulations. Peptides. 2009;30(11):2031–9. doi: 10.1016/j.peptides.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 55.Parks GS, Wang L, Wang Z, Civelli O. Identification of neuropeptide receptors expressed by melanin-concentrating hormone neurons. J Comp Neurol. 2014;522(17):3817–33. doi: 10.1002/cne.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, et al. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. The Journal of comparative neurology. 2012;520(18):4168–83. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19(5):741–56. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavini CK, Jones WC, 2nd, Novak CM. Ventromedial hypothalamic melanocortin receptor activation: regulation of activity energy expenditure and skeletal muscle thermogenesis. J Physiol. 2016;594(18):5285–301. doi: 10.1113/JP272352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Contreras C, Gonzalez F, Ferno J, Dieguez C, Rahmouni K, Nogueiras R, et al. The brain and brown fat. Ann Med. 2015;47(2):150–68. doi: 10.3109/07853890.2014.919727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R417–28. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monge-Roffarello B, Labbe SM, Lenglos C, Caron A, Lanfray D, Samson P, et al. The medial preoptic nucleus as a site of the thermogenic and metabolic actions of melanotan II in male rats. Am J Physiol Regul Integr Comp Physiol. 2014;307(2):R158–66. doi: 10.1152/ajpregu.00059.2014. [DOI] [PubMed] [Google Scholar]
- 62.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507(7491):238–42. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 2016;19(12):1628–35. doi: 10.1038/nn.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6(3):254–85. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13(1):1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 66.Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, et al. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111(3):443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 67.Rathner JA, Morrison SF. Rostral ventromedial periaqueductal gray: a source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006;1077(1):99–107. doi: 10.1016/j.brainres.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 68.Gelez H, Poirier S, Facchinetti P, Allers KA, Wayman C, Bernabe J, et al. Neuroanatomical distribution of the melanocortin-4 receptors in male and female rodent brain. J Chem Neuroanat. 2010;40(4):310–24. doi: 10.1016/j.jchemneu.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137(7):1225–34. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–7. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alhadeff AL, Golub D, Hayes MR, Grill HJ. Peptide YY signaling in the lateral parabrachial nucleus increases food intake through the Y1 receptor. Am J Physiol Endocrinol Metab. 2015;309(8):E759–66. doi: 10.1152/ajpendo.00346.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72••.Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nature neuroscience. 2017;20(2):176–88. doi: 10.1038/nn.4462. This study utilized single-cell RNAsequencing to map transcriptomes of individual neurons of the hypothalamus, enabling dissection of traditional classes of neurons (such as ‘AgRP’ neurons) into distinct subsets such as those that do, versus do not, express AT1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sapouckey SA, Deng G, Sigmund CD, Grobe JL. Potential Mechanisms of Hypothalamic Renin-Angiotensin System Activation by Leptin and DOCA-salt for the Control of Resting Metabolism. Physiological genomics. 2017 doi: 10.1152/physiolgenomics.00087.2017. physiolgenomics 00087 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


