Abstract
The Hippo pathway plays prominent and widespread roles in various forms of human carcinogenesis. Specifically, the Yes-associated protein (YAP), a downstream effector of the Hippo pathway, can lead to excessive cell proliferation and the inhibition of apoptosis, resulting in tumorigenesis. It was reported that the YAP is strongly elevated in multiple types of human malignancies such as breast, lung, small intestine, colon, and liver cancers. Recent work indicates that, surprisingly, Hippo signaling components’ (SAV1, MST1/2, Lats1/2) mutations are virtually absent in human cancer, rendering this signaling an unlikely candidate to explain the vigorous activation of the YAP in most, if not all human tumors and an activated YAP promotes the resistance to RAF-, MAPK/ERK Kinase (MEK)-, and Epidermal growth factor receptor (EGFR)-targeted inhibitor therapy. The analysis of YAP expressions can facilitate the identification of patients who respond better to an anti-cancer drug treatment comprising RAF-, MEK-, and EGFR-targeted inhibitors. The prominence of YAP for those aspects of cancer biology denotes that these factors are ideal targets for the development of anti-cancer medications. Therefore, our report strongly indicates that the YAP is of potential prognostic utility and druggability in various human cancers.
Keywords: Cancer, Drug-resistant, Hippo pathway, Tumor, YAP
INTRODUCTION
During the past decade, research on the biology and regulation of the Hippo pathway gained impetus from pioneering Drosophila studies (1–4). Intensive research on Drosophila genetics has been instrumental for our current knowledge about the Hippo pathway (5–8). Many of these genes were in the Hippo pathway, suggesting that the Hippo plays a vital role in the development and growth of Drosophila (4, 9–12). In a recent study, the Hippo pathway functioned as a regulator of tissue growth that would be considered a tumor suppressor pathway of human cancer in neoplastic organs (8, 13–16). In addition, the growing body of evidence connecting YAP to cancer biology encourages the translation of preclinical findings into clinical research (13, 17–20). Therefore, in this review, we examine human carcinogenesis related to the Hippo pathway.
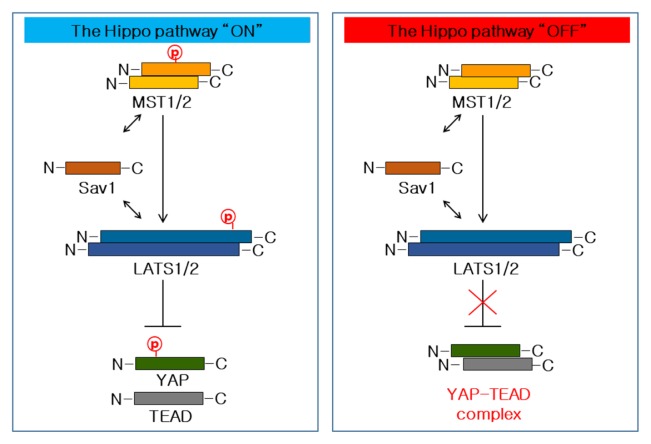
The Hippo pathway is a highly conserved regulator of organ size as well as of stem cell proliferation and maintenance (6, 14, 21). It regulates the Yes-associated protein – a co-transcriptional factor – in a negative manner. The Hippo core component, composed of the kinase MST1/2, LATS1/2, and the adaptor protein SAV1 inhibitively phosphorylates the Hippo pathway terminal downstream YAP (Fig. 1). Mechanically, activated MST1/2 kinase associates with its scaffolding partners SAV1 and phosphorylate LATS1/2, resulting in a LAT1/2 activation (6, 8, 15). The activated LATS1/2 kinase then advances to phosphorylate YAP on the phosphorylation site, leading to the inactivation of YAP by cytoplasmic sequestering and degradation (Fig. 1) (22, 23). In case that the SAV1-MST1/2-LATS1/2 cascade axis is inactive, YAP can accumulate in the nucleus and function as co-transcription factors by interacting with the transcriptional enhancer factor (TEF) family of transcription factors (TEAD) (Fig. 1) (24). The inactivation of the Hippo pathway could lead to excessive cell proliferation and the inhibition of apoptosis, resulting in tumorigenesis. It has been confirmed that the expression of YAP was increased in various organs of human cancer (14, 17, 19). Therefore, abnormal YAP expressions are strongly associated with the occurrence, development, and prognosis of cancers, which is a phenomenon that has become a favorite topic of cancer research.
Fig. 1.
Models of the Hippo pathway in mammals. The Hippo pathway regulation is shown here: When the YAP is relieved from inhibition through phosphorylation-dependent or -independent mechanisms in mammals, its nuclear translocation leads the target gene expression into the regulation of cellular proliferation, apoptosis, and differentiation.
For many years, the development of new effective therapies has progressed and dramatically improved survival rates (25–29). Nevertheless, it occurs even in significantly positive clinical responses to chemotherapeutic and targeted therapies that a complete remission is hardly durable, since almost all cancers acquire an anti-cancer drug resistance (17, 30–34) which can even be considered a key property of malignant growths. The drug resistance mechanisms are not fully understood, but they are particularly important in designing novel targeted therapies aimed at preventing this resistance (30, 35). To address this issue, we identify YAP as a key survival input that mediates drug resistance in parallel to several known independent pathways of tumorigenesis (17, 36–47).
PART 1. YAP IN HUMAN CANCER
The analysis of clinical-pathological and biological results demonstrates that the increased activation and expression of YAP was correlated with the stage and prognoses of tumors (42, 44, 47–55). Specifically, these current views about the Hippo pathway are consistent with numerous previous studies reporting that a YAP activation or expression corresponds to cancer evolution and progression (14, 18–20, 56). Thus, we suggest that the YAP – as a potential oncogene – is a promising independent prognostic biomarker of various cancers.
Lung cancer
Lung cancer includes several subtypes, including lung adenocarcinomas (LACs), lung squamous cell carncinomas (LSCCs), and large-cell lung carcinomas, which are all generally defined as non-small-cell lung cancers (NSCLCs). NSCLCs are responsible for most mortalities among cancers (57–59). Recent clinical research reported that the expressions or nuclear staining of YAP were elevated in NSCLCs (14, 39, 60, 61). A hyper-activation of the YAP level was associated with an overall shorter patient survival rate. NSCLC immunohistochemistry found that a YAP up-regulation is relevant for a high histology score, the lung cancer stage, metastases, and a poor cancer progression. The increased expression of YAP target genes correlated with a poor disease progression as evidenced in a cancer database of NSCLC patients (14, 39, 42, 55). In addition, Chaeuk chung groups reported that an elevated YAP in NSCLC specimens obtained from the development of acquired EGFR inhibitors resistance (60, 61). Among seven cases, six drug-resistant patients exhibited increased nuclear YAP staining as compared with their baseline. Additionally, they and other groups showed that the combination therapy of an EGFR inhibitor and a YAP inhibitor overcame the EGFR inhibitors’ resistance in acquired EGFR inhibitor-resistant lung cancer cells (42, 62, 63). These results implicate that the YAP is closely related to the EGFR inhibitor resistance development and might itself be a critical therapeutic target. Therefore, a pre-clinical analysis with a YAP inhibitor and an EGFR inhibitor is needed to validate the ability of such regimen to overcome the EGFR inhibitor resistance in cancer therapy.
Breast cancer
Breast cancer is the most common cancer type among US-American women with one out of eight developing it. A breast cancer is a malignant tumor arising from the cells of the breast (64). There are many breast cancer types that differ in their capability of spreading (metastasize) to other body tissues. Breast cancer can be classified by the tumor’s site of origin, the stroma surrounding the gland, or the ability to grow in the lumen of the gland (64). Breast cancer cells have receptors on their surface as well as in their cytoplasm and nucleus (64). Chemical messengers such as hormones bind to receptors which causes cell changes. Breast cancer cells may or may not have three important receptors: Estrogen receptor (ERs), Progesterone receptor (PRs), and Human epidermal growth factor receptor 2 (HER2). The most common forms of breast cancer can be classified into hormone hormone-receptor-positive breast cancer (involving estrogen and/or progesterone), HER2-positive breast cancer, and breast cancers that are negative for all three receptors (triple-negative breast cancer [TNBC]) (54, 64). In this review, we demonstrated that the hyper-activation of YAP in breast cancer tissues related directly to the PR status, a luminal subtype, and inversely with HER2 and Ki67 levels. TAZ, as the paralog of the YAP, also plays an important role in the tumorigenesis of breast cancer cells and is overexpressed in about 20% of all human breast cancers (54). In breast cancer, the hyper-activation of a YAP/TAZ-transcriptional program feeds various tumorigeneses (36, 54). An important oncogenic function of TAZ relates to its association with BC stem cells (BCSCs) (65–68). In particular, several studies reported a connection between TAZ and the self-renewal of BCSCs (66, 68, 69). However, several recent controversial results showed that mention relates to the biological significance of YAP in breast cancer. The YAP regulation has shown opposite or different results (70, 71). There are directly opposing ideas about the tumor-promoting function of YAP that are, in turn, countered by some clinical evidence, at least with regard to triple-negative BCs (36, 54). Indeed, another research group has recently reported the relation between a reduced YAP expression/activation and decreased recurrence-free survival in luminal tumors (50, 53, 72). This clinical evidence supports that, at least in TNBC, YAP may possess an oncogenic character. It is plausible that a YAP acts differently in a distinct breast cancer class. The growing interest in YAP related to breast cancer has promoted a wave of research on other breast cancer subtypes. The results from these studies will explain the exact mechanisms underlying the clinical significance of YAP/TAZ in individual breast cancer subtypes.
Colon cancer
Colorectal cancer (CRC) remains a leading cause of cancer death, with one million new cases each year worldwide and as many as half a million cancer deaths annually. The disease begins as a benign adenomatous polyp which develops into an advanced adenoma with high-grade dysplasia and then progresses to an invasive cancer. The development of colorectal cancer (CRC) involves the accumulation of genetic alterations and epigenomic changes that affect cell growth, cell death, and the tumor’s microenvironment (73, 74). YAP play key roles in the development of colorectal cancer as evidenced by biological and clinical colon cancer data (20, 75, 76). In microarray datasets of colorectal cancer patients, an increased expression of gene signatures for YAP activity related to a high histological grade, an enrichment of colon stem cell signatures, metastasis characteristics, and cancer progression. Recently, Keun-Wook Lee and colleagues demonstrated that the up-regulation of YAP is strongly related with resistance in cetuximab therapy of colorectal cancer patients (77). Among the cancer patients with wild-type KRAS, only those without a YAP activation benefited from the cetuximab treatment. This clinical result provides robust evidence for the YAP activation as an important prognostic marker of EGFR inhibitor therapy. Similarly, several studies also identified YAP as potential biomarkers for EGFR inhibitor resistance in head and neck cancers (78). In addition, we demonstrated for the first time that elevated expressions of the YAP and PGE2 are highly correlated with human colitis-associated cancers and colorectal cancer (75). These results demonstrated that inhibitors of YAP may create synergy with non-steroidal anti-inflammatory drugs (COX inhibitors), both in colon cancer prevention and possibly in the inhibition of tumor growth. Therefore, our review suggests that YAP are strongly associated with a poor prognosis and development for colorectal cancer.
Liver cancer
A cancer that originates in the liver is called a primary liver cancer. There is more than one kind of primary liver cancer. Examples are the hepatocellular carcinoma (HCC), cholangiocarcinomas (CCs), and hepatoblastomas (HBs). The most frequent liver cancer, accounting for approximately 75% of all primary liver cancers, is a hepatocellular carcinoma (HCC) (79–81). A number of pre-clinical and clinical investigations demonstrates that the expression or nuclear staining of YAP was elevated in HCCs, CCs, and HBs. A YAP hyper-activation was associated with an overall shorter patient survival rate. HCC immunochemistry led to increased nuclear staining of YAP, relevant for a high histology score, the cancer stage, metastasis, and a poor cancer progression (82–86). Also, hepatic tumors including HCC and CC are a remarkable feature of the Hippo pathway in genetically modified mice (MST1/2, Sav1 and LATs1/2 knock-out mouse, YAP overexpression mouse) (14, 39, 55, 84). Not long ago, the research group of Eek-Hoon Jho demonstrated that high levels of YOD1 – an intrinsic positive regulator of YAP which functions as an oncogene – in liver cancers of both mouse and human liver tumor tissues exhibited a strong correlation with YAP levels (82). AREG, a secreted protein and a member of the epidermal growth factor family, was recently reported to be a target gene of YAP. YAP was correlated with a high serum AFP level and an elevated AFP expression in HCC (87). This information suggests that other signaling networks promote up-regulation, even in the presence of normal Hippo pathway tumor suppressor kinases. It is an important task to develop predictive biomarkers for therapies that are targeted at the Hippo pathway in clinical trials.
Stomach cancer
Stomach cancer, also called gastric cancer, is a cancer that derives from the glandular epithelium of the stomach. These gastric cells can grow into a tumor. It has been reported that increased YAP mRNA and protein levels in gastric cancers are correlated with metastases, the cancer stage, and poor outcomes for the patients (88–90). In addition, the expression of YAP target genes and of YAP mRNA itself was elevated in a preclinical model of gastric cancer, generated by an infection with helicobacter pylori which is a common risk factor for stomach cancer development in humans (18). VGLL4 is a natural antagonist of YAP and its TDU region suffices for a YAP inhibition, allowing the development of a peptide-based YAP inhibitor (18, 47). This peptide drug strongly inhibits stomach cancer growth which presents an opportunity for treating gastric cancer. Such a peptide drug development strategy can be extended to YAP-dependent cancer types.
Anti-cancer drug resistance
Cancer cells with up-regulated YAP exhibit a resistance to anti-cancer drugs. An activated YAP promotes drug resistance to RAF, MEK, and EGFR inhibitors in various cancer cell lines as well as in human cancer patients with activating-BRAF, K-RAS, and mutant EGFR cancer (17, 42, 45, 60–63, 77, 78). In immunostaining of patients’ BRAF mutation melanoma tissues, up-regulated YAP expressions were correlated with the responsiveness to RAF/MEK inhibitors. A knock-down of the YAP-elevated cancer cell sensitivity to RAF and MEK inhibitors with this effect demonstrated a many of cancer cell – lung, melanoma, and colon cancer cell lines. A therapy combining YAP and RAF or MEK inhibitors terminated both BRAF and RAS-mutant cancer cell lines. Therefore, YAP activity predicts the therapy effect of RAF and MEK inhibitors in activated MAPK-signaling cancer patients. An activated YAP is related to anti-cancer drug resistance in three non-small cell lung cancer (NSCLC) lines (HCC827, H1975, and A549), generated to induce resistance to EGFR inhibitors (42, 60, 61). These cell lines elevated the expression or translocation of YAP in the nucleus. Silencing of YAP resulted in re-sensitizing the drug-resistant cells to an EGFR inhibitor (gefitinib and erlotinib) while the combination of an EGFR inhibitor and a YAP inhibitor significantly overcame the EGFR inhibitor resistance. Thus, YAP emerged as critical oncogenes in drug resistance.
PART 2. THE HIPPO PATHWAY – YAP AS A POTENTIAL THERAPEUTIC ANTI-CANCER TARGET
As discussed above, YAP inhibitors present important challenges, including the identification of druggable components (17, 38, 40, 43, 45, 55). The most attractive therapeutic target is the essential oncogene YAP as the terminal protein of the Hippo pathway. The inhibition of YAP in various cancers is of interest as an anticancer therapeutic strategy. The therapeutic effect of a YAP inhibition is mostly based on genetic (knock-out) studies with mice which demonstrate that the heterozygosity of YAP represses cancer development. For instance, colon cancer development in Sav1 and MST1/2-deficient colons was repressed by a YAP knock-out or heterozygosity (91, 92). For successful developments of YAP inhibitor strategies, a human cancer must be depend on the Hippo pathway-YAP in targeted therapy.
The co-crystal structure of the YAP-TEAD binding domain was identified (52, 93, 94). and therefore, disrupting YAP transcriptional mechanisms block the interaction of YAP to TEAD binding. Verteporfin (VP) which is a FDA-approved photosensitizing small molecule compound used in the photodynamic therapy of neo-vascular macular degeneration that blocks YAP-TEAD binding was identified by the John Hopkins Drug Library via high-throughput screening (85). VP inhibited liver overgrowth resulting from the overexpression or hyper-activation of YAP by silencing Hippo kinase components.
Another strategy of YAP-TEAD inhibition involves an inhibitor peptide competing with the YAP for TEAD binding. The inhibitor peptide was identified in the TDU domain of VGLL4, a YAP antagonist, which potently inhibits the YAP-dependent tumorigenic potential of gastric cancer cells, both in vitro and in vivo (18, 47). This VGLL4-mimicking peptide, which was designed with a VGLL4 sequence, interacts with TEAD in a way that excludes YAP. In sum, such results demonstrate that the pharmacological intervention with a YAP-TEAD complex formation is a potential therapeutic approach with few side effects.
DISCUSSION
Many study results provide evidence for the Hippo pathway component’s role in cancer development and in the elevated expression or hyper-activation of YAP in human tumors regarding various aspects of cancer biology at the cell and tissue level. A dysregulation of this mechanism is unlikely to explain the YAP activation in cancers. For example, with the exception of NF2, mutations of upstream Hippo components are rarely found in cancers. In addition, not a single case of a YAP mutation by itself has been reported. The mRNA level of YAP correlates with target gene expressions and cancer progressions. This YAP activity is strongly related to cancer development and anti-cancer drug resistance. During the past few years, our knowledge of the Hippo pathway in both mice and humans has largely increased. Also, important drugs were proven to modulate the YAP and the development of new medications in pre-clinical trials and genetic mouse models is ongoing. The YAP may represent an essential molecular target for cancer therapy, although further research is underway to identify other YAP regulators. Particularly the inhibition with a YAP-TEAD complex is of central interest in the development of new anti-cancer drugs. Since the YAP-TEAD complex is regulated by a protein-protein interaction (PPI) which could serve potentially as a target for inhibition, more selective PPI drugs might also be needed to treat cancer patients. VP as a drug already approved by the FDA could serve as reference to develop new YAP inhibitors. Finally, several promising novel YAP inhibitors are currently pre-clinically tested and may soon be subjected to clinical trials.
ACKNOWLEDGEMENTS
This work was supported by grants from the Ministry of Trade, Industry and Energy (MOTIE, Republic of Korea) in the Industrial Technology Innovation Program (No. 10063408), the Ministry of Health and Welfare, Republic of Korea (No. HI14C1090), and the Asan Institute for Life Sciences (No.2017-559).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 2.Meignin C, Alvarez-Garcia I, Davis I, Palacios IM. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr Biol. 2007;17:1871–1878. doi: 10.1016/j.cub.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Milton CC, Humbert PO, Harvey KF. Transcriptional output of the Salvador/warts/hippo pathway is controlled in distinct fashions in Drosophila melanogaster and mammalian cell lines. Cancer Res. 2009;69:6033–6041. doi: 10.1158/0008-5472.CAN-08-4592. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Kango-Singh M, Singh A. Singh, Regulation of organ size: insights from the Drosophila Hippo signaling pathway. Dev Dyn. 2009;238:1627–1637. doi: 10.1002/dvdy.21996. [DOI] [PubMed] [Google Scholar]
- 6.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Yue T, Jiang J. Hippo signaling pathway and organ size control. Fly (Austin) 2009;3:68–73. doi: 10.4161/fly.3.1.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Degerny C, Xu M, Yang XJ. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol. 2009;87:77–91. doi: 10.1139/O08-114. [DOI] [PubMed] [Google Scholar]
- 9.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 10.Pantalacci S, Tapon N, Léopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 11.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 13.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 14.Harvey KF, Zhang X, Thomas DM. Thomas, The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 15.Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res. 2013;19:4925–4930. doi: 10.1158/1078-0432.CCR-12-3172. [DOI] [PubMed] [Google Scholar]
- 17.Keren-Paz A, Emmanuel R, Samuels Y. YAP and the drug resistance highway. Nat Genet. 2015;47:193–194. doi: 10.1038/ng.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao S, Wang H, Shi Z. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Xie C, Li Q, Xu K, Wang E. Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumour Biol. 2013;34:2169–2174. doi: 10.1007/s13277-013-0751-x. [DOI] [PubMed] [Google Scholar]
- 21.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 23.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 24.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flaherty KT, Hodi FS, Fisher DE. From genes to drugs: targeted strategies for melanoma. Nat Rev Cancer. 2012;12:349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 26.Eggermont AM, Robert C. Melanoma in 2011: a new paradigm tumor for drug development. Nat Rev Clin Oncol. 2012;9:74–76. doi: 10.1038/nrclinonc.2011.201. [DOI] [PubMed] [Google Scholar]
- 27.Jones PS, Jones D. New regulatory framework for cancer drug development. Drug Discov Today. 2012;17:227–231. doi: 10.1016/j.drudis.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Mullard A. The Roadmap Epigenomics Project opens new drug development avenues. Nat Rev Drug Discov. 2015;14:223–225. doi: 10.1038/nrd4582. [DOI] [PubMed] [Google Scholar]
- 29.Vukicevic S. Current Challenges and Hurdles in New Drug Development. Clin Ther. 2016;38:e3. doi: 10.1016/j.clinthera.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 30.De Angelis ML, De Maria R, Baiocchi M. How to Assess Drug Resistance in Cancer Stem Cells. Methods Mol Biol. 2018;1692:107–115. doi: 10.1007/978-1-4939-7401-6_10. [DOI] [PubMed] [Google Scholar]
- 31.Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106:20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guang MHZ, McCann A, Bianchi G, et al. Overcoming multiple myeloma drug resistance in the era of cancer ‘omics’. Leuk Lymphoma. 2018;59:542–561. doi: 10.1080/10428194.2017.1337115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norouzi-Barough L, Sarookhani MR, Sharifi M, Moghbelinejad S, Jangjoo S, Salehi R. Molecular Mechanisms of Drug Resistance in Ovarian Cancer. J Cell Physiol. 2017 doi: 10.1002/jcp.26289. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Siegfried Z, Karni R. The role of alternative splicing in cancer drug resistance. Curr Opin Genet Dev. 2017;48:16–21. doi: 10.1016/j.gde.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv Pharm Bull. 2017;7:339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin CH, Pelissier FA, Zhang H, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin L, Sabnis AJ, Chan E, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo L, Teng L. YAP/TAZ for cancer therapy: opportunities and challenges (review) Int J Oncol. 2015;46:1444–1452. doi: 10.3892/ijo.2015.2877. [DOI] [PubMed] [Google Scholar]
- 39.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Y, Yang Y, Wang F, Wei Q, Qin H. Hippo-YAP signaling pathway: A new paradigm for cancer therapy. Int J Cancer. 2015;137:2275–2286. doi: 10.1002/ijc.29073. [DOI] [PubMed] [Google Scholar]
- 41.Kim MH, Kim J, Hong H, et al. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2016;35:462–478. doi: 10.15252/embj.201592081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu PC, You B, Yang YL, et al. YAP promotes erlotinib resistance in human non-small cell lung cancer cells. Oncotarget. 2016;7:51922–51933. doi: 10.18632/oncotarget.10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanconato F, Battilana G, Cordenonsi M, Piccolo S. YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol. 2016;29:26–33. doi: 10.1016/j.coph.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andl T, Zhou L, Yang K, Kadekaro AL, Zhang Y. YAP and WWTR1: New targets for skin cancer treatment. Cancer Lett. 2017;396:30–41. doi: 10.1016/j.canlet.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Kim MH, Kim J. Role of YAP/TAZ transcriptional regulators in resistance to anti-cancer therapies. Cell Mol Life Sci. 2017;74:1457–1474. doi: 10.1007/s00018-016-2412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun PL, Jin Y, Chung JH. Reply: YAP is a Key Factor to Improve the Management of Cancer Treatments. Ann Surg Oncol. 2017;24:644–645. doi: 10.1245/s10434-017-6205-8. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Shen H, Withers HG, et al. VGLL4 Selectively Represses YAP-Dependent Gene Induction and Tumorigenic Phenotypes in Breast Cancer. Sci Rep. 2017;7:6190. doi: 10.1038/s41598-017-06227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed AA, Mohamed AD, Gener M, Li W, Taboada E. YAP and the Hippo pathway in pediatric cancer. Mol Cell Oncol. 2017;4:e1295127. doi: 10.1080/23723556.2017.1295127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avril T, Chevet E. Proteostasis trumps YAP in colon cancer. Sci Signal. 2015;8:fs18. doi: 10.1126/scisignal.aad3123. [DOI] [PubMed] [Google Scholar]
- 50.Cao L, Sun PL, Yao M, Jia M, Gao H. Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues. Hum Pathol. 2017;68:166–174. doi: 10.1016/j.humpath.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 51.Eibl G, Rozengurt E. YAP, and obesity in pancreatic cancer: A signaling network with multiple loops. Semin Cancer Biol. 2017 doi: 10.1016/j.semcancer.2017.10.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng J, Gou J, Jia J, Yi T, Cui T, Li Z. Verteporfin, a suppressor of YAP-TEAD complex, presents promising antitumor properties on ovarian cancer. Onco Targets Ther. 2016;9:5371–5381. doi: 10.2147/OTT.S109979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HM, Jung WH, Koo JS. Expression of Yes-associated protein (YAP) in metastatic breast cancer. Int J Clin Exp Pathol. 2015;8:11248–11257. [PMC free article] [PubMed] [Google Scholar]
- 54.Maugeri-Saccà M, Barba M, Pizzuti L, et al. The Hippo transducers TAZ and YAP in breast cancer: oncogenic activities and clinical implications. Expert Rev Mol Med. 2015;17:e14. doi: 10.1017/erm.2015.12. [DOI] [PubMed] [Google Scholar]
- 55.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou GX, Li XY, Zhang Q, et al. Effects of the hippo signaling pathway in human gastric cancer. Asian Pac J Cancer Prev. 2013;14:5199–5205. doi: 10.7314/APJCP.2013.14.9.5199. [DOI] [PubMed] [Google Scholar]
- 57.Formisano L, Jansen VM, Marciano R, Bianco R. From biology to therapy: Improvements of therapeutic options in Lung cancer. Anticancer Agents Med Chem. 2017 doi: 10.2174/1871520617666170912123416. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: Biology and treatment options. Biochim Biophys Acta. 20151856:189–210. doi: 10.1016/j.bbcan.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suda K, Mitsudomi T. [Molecular Biology for Surgical Treatment of Lung Cancer]. Kyobu Geka. 2017;70:4–8. [PubMed] [Google Scholar]
- 60.Lee BS, Park DI, Lee DH, et al. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem Biophys Res Commun. 2017;491:493–499. doi: 10.1016/j.bbrc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Lee JE, Park HS, Lee D, et al. Hippo pathway effector YAP inhibition restores the sensitivity of EGFR-TKI in lung adenocarcinoma having primary or acquired EGFR-TKI resistance. Biochem Biophys Res Commun. 2016;474:154–160. doi: 10.1016/j.bbrc.2016.04.089. [DOI] [PubMed] [Google Scholar]
- 62.Karachaliou N, Chaib I, Pilotto S, et al. 76P An innovative co-targeting of signal transducer and activator of transcription 3 (STAT3) and Src-YAP pathways in EGFR mutant non-small cell lung cancer (NSCLC) J Thorac Oncol. 2016;11:S87–S88. doi: 10.1016/S1556-0864(16)30189-7. [DOI] [Google Scholar]
- 63.Wang H, Lu B, Castillo J, et al. Tankyrase Inhibitor Sensitizes Lung Cancer Cells to Endothelial Growth Factor Receptor (EGFR) Inhibition via Stabilizing Angiomotins and Inhibiting YAP Signaling. J Biol Chem. 2016;291:15256–15266. doi: 10.1074/jbc.M116.722967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parsa Y, Mirmalek SA, Kani FE, et al. A Review of the Clinical Implications of Breast Cancer Biology. Electron Physician. 2016;8:2416–2424. doi: 10.19082/2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan SW, Lim CJ, Guo K, et al. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- 66.Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 67.Díaz-Martín J, López-García MÁ, Romero-Pérez L, et al. Nuclear TAZ expression associates with the triple-negative phenotype in breast cancer. Endocr Relat Cancer. 2015;22:443–454. doi: 10.1530/ERC-14-0456. [DOI] [PubMed] [Google Scholar]
- 68.Li YW, Shen H, Frangou C, et al. Characterization of TAZ domains important for the induction of breast cancer stem cell properties and tumorigenesis. Cell Cycle. 2015;14:146–156. doi: 10.4161/15384101.2014.967106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartucci M, Dattilo R, Moriconi C, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 70.Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 71.Kim SK, Jung WH, Koo JS. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int J Clin Exp Pathol. 2014;7:3224–3234. [PMC free article] [PubMed] [Google Scholar]
- 72.Jaramillo-Rodríguez Y, Cerda-Flores RM, Ruiz-Ramos R, López-Márquez FC, Calderón-Garcidueñas AL. YAP expression in normal and neoplastic breast tissue: an immunohistochemical study. Arch Med Res. 2014;45:223–228. doi: 10.1016/j.arcmed.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Kagawa Y, Ishii H, Sekimoto M, Doki Y, Mori M. [Molecular biology of colon cancer]. Nihon Rinsho. 2011;69:67–71. [PubMed] [Google Scholar]
- 74.Zhang L, Yu J. Role of apoptosis in colon cancer biology, therapy, and prevention. Curr Colorectal Cancer Rep. 2013;9 doi: 10.1007/s11888-013-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim HB, Kim M, Park YS, et al. Prostaglandin E2 Activates YAP and a Positive-Signaling Loop to Promote Colon Regeneration After Colitis but Also Carcinogenesis in Mice. Gastroenterology. 2017;152:616–630. doi: 10.1053/j.gastro.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ling HH, Kuo CC, Lin BX, Huang YH, Lin CW. Elevation of YAP promotes the epithelial-mesenchymal transition and tumor aggressiveness in colorectal cancer. Exp Cell Res. 2017;350:218–225. doi: 10.1016/j.yexcr.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 77.Lee KW, Lee SS, Kim SB, et al. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin Cancer Res. 2015;21:357–364. doi: 10.1158/1078-0432.CCR-14-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jerhammar F, Johansson AC, Ceder R, et al. YAP1 is a potential biomarker for cetuximab resistance in head and neck cancer. Oral Oncol. 2014;50:832–839. doi: 10.1016/j.oraloncology.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Hernanda PY, Pedroza-Gonzalez A, Sprengers D, Peppelenbosch MP, Pan Q. Multipotent mesenchymal stromal cells in liver cancer: implications for tumor biology and therapy. Biochim Biophys Acta. 20141846:439–445. doi: 10.1016/j.bbcan.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Kudo M. Targeted therapy for liver cancer: updated review in 2012. Curr Cancer Drug Targets. 2012;12:1062–1072. [PubMed] [Google Scholar]
- 81.Oishi N, Yamashita T, Kaneko S. Molecular biology of liver cancer stem cells. Liver Cancer. 2014;3:71–84. doi: 10.1159/000343863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim Y, Jho EH. Deubiquitinase YOD1: the potent activator of YAP in hepatomegaly and liver cancer. BMB Rep. 2017;50:281–282. doi: 10.5483/BMBRep.2017.50.6.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Targeting YAP and Hippo signaling pathway in liver cancer. Expert Opin Ther Targets. 2010;14:855–868. doi: 10.1517/14728222.2010.499361. [DOI] [PubMed] [Google Scholar]
- 84.Liu AM, Xu Z, Luk JM. An update on targeting Hippo-YAP signaling in liver cancer. Expert Opin Ther Targets. 2012;16:243–247. doi: 10.1517/14728222.2012.662958. [DOI] [PubMed] [Google Scholar]
- 85.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yimlamai D, Fowl BH, Camargo FD. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol. 2015;63:1491–1501. doi: 10.1016/j.jhep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han SX, Bai E, Jin GH, et al. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res. 20142014:261365. doi: 10.1155/2014/261365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cervantes A, Rodríguez Braun E, Pérez Fidalgo A, Chirivella González I. Molecular biology of gastric cancer. Clin Transl Oncol. 2007;9:208–215. doi: 10.1007/s12094-007-0041-4. [DOI] [PubMed] [Google Scholar]
- 89.Dreznik A, Purim O, Idelevich E, et al. Gastric cancer: biology and clinical manifestations in Israel. J Surg Oncol. 2012;105:316–322. doi: 10.1002/jso.22078. [DOI] [PubMed] [Google Scholar]
- 90.El-Rifai W, Powell SM. Molecular biology of gastric cancer. Semin Radiat Oncol. 2002;12:128–140. doi: 10.1053/srao.2002.30815. [DOI] [PubMed] [Google Scholar]
- 91.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou D, Zhang Y, Wu H, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–E1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaan HYK, Chan SW, Tan SKJ, et al. Crystal structure of TAZ-TEAD complex reveals a distinct interaction mode from that of YAP-TEAD complex. Sci Rep. 2017;7:2035. doi: 10.1038/s41598-017-02219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Z, Lin Z, Zhou Z, et al. Structure-Based Design and Synthesis of Potent Cyclic Peptides Inhibiting the YAP-TEAD Protein-Protein Interaction. ACS Med Chem Lett. 2014;5:993–998. doi: 10.1021/ml500160m. [DOI] [PMC free article] [PubMed] [Google Scholar]