Abstract
Hippo signaling plays critical roles in regulation of tissue homeostasis, organ size, and tumorigenesis by inhibiting YES-associated protein (YAP) and PDZ-binding protein TAZ through MST1/2 and LATS1/2 pathway. It is also engaged in cross-talk with various other signaling pathways, including WNT, BMPs, Notch, GPCRs, and Hedgehog to further modulate activities of YAP/TAZ. Because YAP and TAZ are transcriptional coactivators that lack DNA-binding activity, both proteins must interact with DNA-binding transcription factors to regulate target gene’s expression. To activate target genes involved in cell proliferation, TEAD family members are major DNA-binding partners of YAP/TAZ. Accordingly, YAP/TAZ were originally classified as oncogenes. However, YAP might also play tumor-suppressing role. For example, YAP can bind to DNA-binding tumor suppressors including RUNXs and p73. Thus, YAP might act either as an oncogene or tumor suppressor depending on its binding partners. Here, we summarize roles of YAP depending on its DNA-binding partners and discuss context-dependent functions of YAP/TAZ.
Keywords: LATS1/2, MST1/2, RUNX, TAZ, TEAD, YAP
INTRODUCTION
The Hippo pathway was first characterized in Drosophila mosaic genetic screens (1–3). It regulates organ size by controlling cell proliferation, differentiation, and survival (4, 5). Subsequent genetic studies have revealed that Yki, the fly homolog of mammalian YAP, is a major target of the Hippo pathway. Overexpression of Yki induces cell growth and inhibits apoptosis by promoting transcription of diap1 and cycE (6). On the other hand, Yki is inactivated by Wts-mediated phosphorylation (6). Specifically, Hippo signaling results in phosphorylation of Yki at multiple sites, inactivating its oncogenic activities. Accordingly, YkiS168A which harbors a mutation in the key phosphorylation site is constitutively active (3, 6). Eye-specific overexpression of YkiS168A had led to enormous overgrowth of the eye, analogous to the phenotype resulting from knockdown of Hippo kinases (5). Together, these results demonstrate that Yki is an oncoprotein.
The mammalian homolog of Yki was initially identified as Yes-associated protein (YAP) (7). YAP contains WW domains capable of interacting with a PPXY motif and a PDZ-binding motif (Post-synaptic density, Discs large, Zonula occludens-1-binding motif) at the C-terminus (8). It is expressed as two alternatively spliced isoforms: YAP-1 and YAP-2 (9) (Fig. 1). TAZ (transcriptional coactivator with PDZ motif) was initially identified through its ability to interact with 14-3-3 proteins (10). TAZ, a YAP homolog, also contains a conserved WW domain that interacts with the PPXY motif as well as the PDZ domain. Consequently, YAP and TAZ have similar structures and functions (8) (Fig. 1). Yap-knockout mice have exhibited early embryonic lethality (11). ApoE/rtTA-driven liver-specific overexpression of Yap has resulted in hepatomegaly at early stage that ultimately progresses to hepatocellular carcinoma (HCC) at later time points (5). The oncogenic activity of YAP has been further confirmed in various cultured cell lines. For example, high YAP activity promotes the proliferation and survival of cultured ovarian cancer cells (12). It increases the invasiveness of non-small-cell lung cancer cell lines (13). Conversely, knockdown of YAP suppresses invasion and metastasis in gastric cancer cell lines (14). Similarly, TAZ contributes to tumorigenesis of breast cancer cells by promoting cell migration, invasion, and anchorage-independent growth (15).
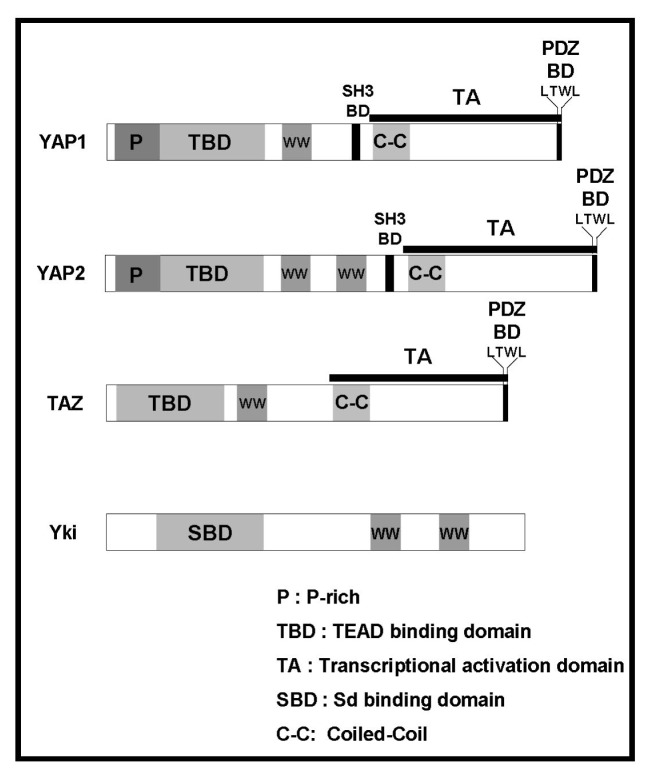
Fig. 1.
Schematic diagram of YAP, TAZ, and Yki. P: Proline-rich region, TBD: TEAD-binding domain, SBD: Sd (Drosophila homolog of mammalian TEADs)-binding domain, WW: WW domain, C-C: coiled-coil region, TA: transactivation domain, PDZ BD: PDZ-binding domain.
YAP/TAZ shuttles between the nucleus and cytoplasm depending on extracellular signaling and growth conditions. For example, YAP is phosphorylated and localized to the cytoplasm at high cell density. However, it is de-phosphorylated and localized to the nucleus at low cell density (3). Such cell density–dependent regulation of YAP phosphorylation is controlled by LATS kinase which is inhibited by GPCR/G-protein signaling (16) or activated by MST1/2 (Fig. 2). Stimulation of protease-activated receptors (PARs) also activates YAP/TAZ by decreasing level of phosphorylation. For example, PAR1 inhibits LATS1/2 kinase via G12/13 and Rho GTPase (17).
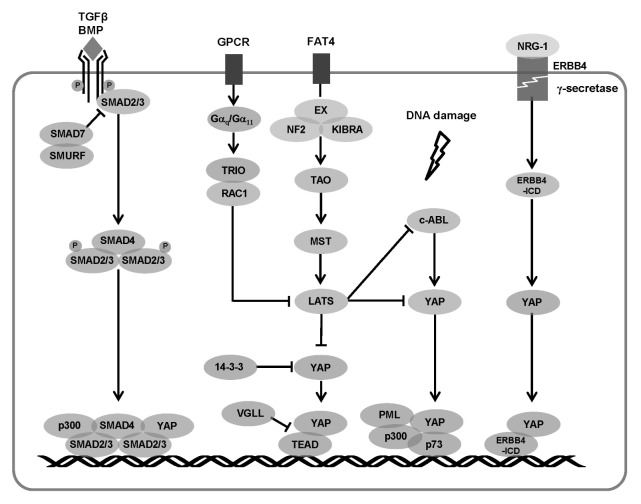
Fig. 2.
Summary of signaling pathways that regulate interactions between YAP and its partners. SMADs activated by TGF-β translocate into the nucleus and bind to YAP, thus promoting the expression of target gene. TEAD is a representative transcription factor that binds to YAP and promotes cell proliferation. YAP is inactivated by LATS kinase which is activated by MST or inactivated by TRIO-RAC1 signaling. In DNA damage, YAP phosphorylated by c-ABL binds to p73 in the nucleus and promotes apoptosis. YAP-ERBB4 activated by NRG1 regulates cell growth by promoting the expression of target genes, including CTGF, CYR61, and ANKRD1.
Because YAP/TAZ are transcriptional coactivators that lack DNA-binding activity, these proteins require DNA-binding transcription factors to regulate target gene’s expression. Initial studies have shown that the oncogenic activity of YAP/TAZ is primarily mediated by interactions with TEAD family transcription factors (18, 19). For example, the YAP-TEAD complex plays a central role in promoting cell proliferation and transformation (20). Although YAP/TAZ mostly interact with TEAD family members in response to various stimuli, they also interact with other DNA-binding transcription factors, including p73 (21), ERBB4 (22), EGR-1 (23), RUNXs (24, 25), and SMADs (26, 27). Binding of YAP to one of these DNA-binding transcription factors results in cellular context–dependent activities that can be either oncogenic or tumor-suppressive. For example, in response to DNA damage, YAP interacts with p73 and induces apoptosis, thereby suppressing tumorigenesis (21). In this review, diverse roles of YAP/TAZ depending on identities and functions of their DNA-binding partners are summarized.
TEADs
TEAD transcription factors are the best-characterized binding partners of YAP/TAZ (28). TEADs were originally identified as transcription enhancer factors (TEFs) (29). Mammals have four TEAD genes (TEAD1–4) that encode four homologs with the same domain structure (30). Despite their structural similarities, TEAD family members are expressed in distinct patterns, suggesting that each member has a unique function (30, 31).
Physical interactions between YAP and TEADs are mediated through the N-terminal region of YAP and the C-terminal region of TEAD protein (28, 32). In human YAP, residue S94 (S79 in mouse Yap) forms a hydrogen bond with Y429 of TEAD4 (Y422 in mouse Tead4) that is critical for YAP-TEAD4 interaction (33). YAP-S94A mutation abolishes YAP-TEAD binding (34). Physiologically, AMP-activated protein kinase (AMPK), a master regulator of cellular energy homeostasis, phosphorylates YAP-S94 and interferes with the YAP-TEAD interaction (35).
TEAD family members play key roles in normal cell growth. Dysregulation of these genes is associated with tumorigenesis (36). Expression of TEADs is frequently elevated in several types of cancer (37), including breast cancer (38, 39), lung cancer (40), prostate cancer (41), osteosarcoma (42), pancreatic ductal adenocarcinoma (43), ovarian cancer (44), glioblastoma (45), melanoma (46), colon cancer (47), hepatocellular carcinoma (48, 49), medulloblastoma (50), and mesothelioma (51). In ovarian cancer initiated cells (OCICs), high expression of TEAD1/3/4 is associated with elevated expression of YAP/TEAD target genes such as RUNX2, ITGB2, and ERBB4 (52). In HCC, dominant-negative TEAD potently suppresses YAP-mediated hepatomegaly and tumorigenesis, indicating that the YAP-TEAD complex plays critical roles in cellular transformation (49).
The transforming activity of TEADs is disrupted by mutations that abolish the YAP-TEAD interaction. Chen et al. have shown that K297A, W299A, F337A, and Y429A mutations of TEAD4 can disrupt its interaction with YAP and abrogate YAP-TEAD4-mediated transformation of MCF10A cells (53). Disruption of YAP-TEAD4 interaction is genetically linked to Sveinsson’s chorioretinal atrophy (SCRA), an autosomal dominant eye disease characterized by bilateral chorioretinal degeneration (54). All SCRA patients harbor the Y421H point mutation in TEAD1 (corresponding to Y429 of TEAD4) which abolishes the YAP-TEAD1 interaction (55).
Through interactions with TEADs, activated YAP/TAZ will localize to the nucleus and bind to promoters of target genes involved in cell proliferation, growth, and survival (4, 5, 15, 28, 56). Target genes of the YAP/TAZ-TEAD complex include CYR61 (20), CTGF (34), AREG (57), MYC (58), Gli2 (59), Vimentin (60), and AXL (61).
p73
p73 is related to p53 tumor-suppressor protein. Like p53, p73 induces cell cycle arrest or apoptosis. It is therefore classified as a tumor suppressor (62). YAP functions as a transcriptional coactivator of p73 (21). It induces p73-mediated apoptosis (63) by inducing BAX and p53AIP1 (64). Binding domains for YAP-p73 interaction have been mapped to the WW domain of YAP and the PPPY motif of p73 (21). The PDZ-binding motif of YAP is required for stabilization of p73 and for p73-mediated pro-apoptotic activity of YAP (65). The YAP-p73 complex stabilizes p73 and prevents its ITCH-mediated degradation (65). The interaction between p73 and ITCH is mediated through the PPPY motif of p73 and the WW domain of ITCH (66). The PPPY motif of p73 is recognized by both YAP and ITCH, consistent with the idea that YAP competes with Itch for binding to p73, thus inhibiting ITCH-mediated ubiquitination of p73 (67).
The activity of the p73-YAP complex is controlled by multiple mechanisms. Promyelocytic leukemia (PML) promotes the apoptosis-inducing activity of the complex by associating with the p73-YAP complex. PML promotes p300-mediated acetylation of p73 and inhibits YAP degradation via the ubiquitin-proteasome pathway (64, 68, 69). Upon treatment with interferon-β (IFN-β), PML is induced, thus promoting accumulation of YAP-p73 in the nucleus (70). Therefore, YAP functions as a tumor suppressor when it is complexed with p73.
The YAP-p73 complex formation and complex-mediated transcription are enhanced by c-ABL (71). In multiple myeloma, YAP is deleted or consistently downregulated to evade apoptosis despite pervasive DNA damage (71). Re-expression of YAP in multiple myeloma cells induces c-ABL-mediated apoptosis and reduces cell proliferation. These results cause the formation of p73-YAP complex by c-ABL (71). In response to DNA damage, c-ABL is activated. It then phosphorylates YAP on residue Y357 (72) (Fig. 2). The resultant phospho-Y357 YAP accumulates in the nucleus (72). In the nucleus, YAP interacts with p73 and induces pro-apoptotic target genes such as BAX and PIG3 (73). Notably, c-ABL-mediated YAP phosphorylation causes dissociation of other YAP partners to facilitate formation of the YAP-p73 complex. In particular, c-ABL dissociates both RUNX and ITCH from YAP (72). Thus, c-ABL dictates the binding partner of YAP by controlling its phosphorylation status. It acts as a “switch” between different transcriptional programs, i.e., between oncogenic and tumor-suppressor functions of YAP. The Y357 residue of YAP is a potential phosphorylation target for several other tyrosine kinases, including Yes and Src. This indicates that these kinases may also affect the choice of YAP partner (72, 74, 75). YAP-TEAD interaction is also affected by LATS1/2-mediated phosphorylation. Therefore, the identity of YAP-binding partner might be affected by these kinases.
ERBB4
ERBB-4 (EGFR family member v-Erb-b2 avian erythroblastic leukemia viral oncogene homolog 4) receptor protein tyrosine kinase is proteolytically processed by membrane proteases (γ-secretase) in response to ligand or 12-O-tetradecanoylphorbol-13-acetate stimulation. The resultant soluble intracellular domain (ICD) of ERBB-4 is translocated to the nucleus, functioning as a transcription regulator (22) (Fig. 2).
The ICD of ERBB4 is a binding partner of YAP. ERBB4 co-immunoprecipitates with YAP and TEAD that might form a ternary complex. Accordingly, ERBB4 could either aid the assembly of binary YAP-TEAD complex or participate in ERBB4/YAP/TEAD ternary complex. In the latter scenario, ERBB4 could modulate transcription at TEAD target sites by recruiting or displacing transcription factors (22, 76, 77). The interaction between YAP and ERBB4 is mediated through WW domains of YAP and PPXY motifs located within the ICD of ERBB4 (78). YAP-ERBB4 regulates organ and tissue growth by promoting the expression of target genes, including CTGF, CYR61, and ANKRD1 (77). YAP-ERBB4 is activated by NRG1 (a member of the neuregulin family that acts on the EGFR family of receptors). This activation is inhibited by the Hippo pathway (77).
The YAP-ERBB4 interaction is inhibited by WW domain–containing oxidoreductase (WWOX) which contains two WW domains that interact with several binding partners of YAP, such as p73 (79), ERBB4 (80), and RUNX2 (81). WWOX sequesters ERBB-4 in the cytoplasm and antagonizes the function of YAP (82). WWOX is frequently inactivated in osteosarcoma. Restoration of WWOX osteosarcoma cell lines decreases the expression of YAP-RUNX2 target genes involved in cell adhesion and motility (83). Thus, WWOX modulates YAP activity by competing with YAP for binding to p73, ERBB4, and RUNX2. The association between YAP and ERBB4 suggests the existence of cross-talk between EGFR and Hippo-YAP networks because ERBB4 is a key member of the EGFR family of receptor tyrosine kinases (RTKs) (22, 76).
EGR-1
EGR-1 (Early growth response protein 1) is also known as Zif268 (zinc finger protein 225) or NGFI-A (nerve growth factor–induced protein A). EGR-1 is a nuclear protein that functions as a transcriptional regulator. EGR-1 induces BAX expression and apoptosis in cancer cells, thus functioning as a tumor suppressor (23, 84). To induce expression of BAX, EGR-1 interacts with YAP via the PPXY motif of EGR-1 and WW domains of YAP (23). In PC3 cell xenografts treated with adenoviral EGR-1, irradiation can result in induction of BAX and significant regression in tumor volume, indicating that radiation-induced pro-apoptotic activity of EGR-1 (inducing BAX expression) can lead to cell death through interaction of EGR-1 with YAP (23).
TBX5
YAP, β-catenin, and TBX5 form a complex and induce the expression of transcriptional targets such as BCL2L1 and BIRC5. This complex is required for the survival and transformation of β-catenin-active cancer cell lines (74). TAZ directly interacts with TBX5, p300, and PCAF, thereby acting as a central component in TBX5-dependent transcriptional complexes. TAZ-related protein YAP also stimulates TBX5 activity. Its influence on TBX5 is potentiated by TAZ, with which it forms a heterodimer (85). TBX5 recruits TAZ/YAP to downstream target genes, resulting in remarkable augmentation of transcription. Physical association of TAZ with p300 and PCAF stimulates TBX5-dependent transcription presumably by promoting acetylation of histones associated with TBX5 target genes (85).
SMADs
SMADs are intracellular proteins that transduce extracellular signals from TGF-β or BMP to the nucleus where they activate transcription of downstream target genes (86). SMADs phosphorylated by receptor kinases form trimers of two receptor-regulated SMADs (Smad1, 2, 3, 5, 8) and one co-SMAD (SMAD4). SMAD6 and 7 are inhibitory SMADs that attenuate TGF-β and BMP signals. YAP/TAZ also regulate TGF-β-SMAD signaling by dictating the localization of receptor-activated SMADs in response to polarity complexes formed in response to cell density (87–89) (Fig. 2). At low cell density, YAP/TAZ and SMAD2/3 accumulate in the nucleus. By contrast, at high density, the Hippo pathway drives cytoplasmic localization of YAP/TAZ which sequesters SMAD2/3, thereby suppressing TGF-β signaling (3, 88). SMAD7 also interacts with YAP and increases the inhibitory activity of SMAD7 against TGF-β signaling (26). In the nucleus, TAZ forms a complex with SMAD2/3-SMAD4 and couples the complex to the transcriptional machinery (87, 88). This complex in turn binds to promoters of SMAD7 and plasminogen activator inhibitor 1 (PAI-1) genes to activate their transcription (87). Because SMAD7 is inhibitory, as noted above, activation of SMAD7 transcription by the SMAD2/3-SMAD4 complex suppresses TGF-β signaling (86).
RUNXs
RUNX family members are DNA-binding transcription factors that serve as master regulators of development. Among three RUNX family members (RUNX1, RUNX2, and RUNX3), RUNX2 functions as an osteogenic master regulator that governs skeletal development and homeostasis (90, 91). The interaction between YAP and RUNX2 was first identified by Yagi et al. (25). Interacting regions have been mapped into the PPPY motif of RUNX2 and the WW domain of YAP (25). Subsequent work has shown that the YAP-RUNX2 complex plays critical roles in regulating skeletal gene expression (75). Src/Yes tyrosine kinase signaling contributes to regulation of bone homeostasis (92, 93). One of the underlying mechanisms is mediated by inhibition of YAP-RUNX2 interaction (25). YAP interacts with native RUNX2 protein and suppresses RUNX2 transcriptional activity. Inhibition of Src/Yes kinase blocks tyrosine phosphorylation of YAP and dissociates YAP-RUNX2 complexes, thereby inducing expression of osteocalcin gene (92). These observations suggest that Src/Yes signals are integrated via organization of YAP-RUNX2 transcriptional complexes to attenuate skeletal gene expression (75). Hong et al. have also reported that TAZ can direct interact with Runx2 and induce the transcription of osteocalcin gene, a late marker of osteoblast development (94). This complex represses PPARγ-dependent gene transcription (94).
RUNX3 interacts with YAP and TEAD4 to form a YAP-TEAD4-RUNX3 ternary complex. RUNX3 interacts with TEAD4 through the C-terminal region of TEAD4 and the Runt domain of RUNX3. However, RUNX3 interacts with YAP through the WW domain of YAP and the PPPY motif of RUNX3. The pattern of YAP-TEAD4-RUNX3 ternary complex formation is very similar to that of the YAP-TEAD-ERBB4 ternary complex (77). Notably, association of RUNX3 with YAP-TEAD4 markedly decreases the DNA-binding ability of TEAD (24). Consistent with this, ectopic expression of RUNX3 in a gastric cancer cell line also attenuates the oncogenic activity of YAP-TEAD4 (24). Conversely, expression of RUNX3-R122C (mutated RUNX3 at Arginine 122 to Cysteine, previously identified in gastric cancer (95)) impairs the interaction between RUNX3 and TEAD (24). Thus, RUNX3 antagonizes the oncogenic activity of YAP-TEAD4.
Jang et al. have reported that the interaction between YAP and RUNX3 is promoted when cell growth is inhibited (96). When cells are grown at high density or cultured under serum-starved conditions, LATS1/2-mediated YAP phosphorylation is elevated. In addition, RUNX3 interacts with phosphorylated YAP (96). Mutation of LATS1/2-mediated phosphorylation sites in YAP can abolish the YAP-RUNX3 interaction without affecting YAP-TEAD4 interaction. LATS1/2-mediated phosphorylation of YAP causes dissociation of the YAP-TEAD4 complex (Fig. 3). Thus, YAP phosphorylation status controlled by cell cycle governs the switching of YAP binding between TEAD4 and RUNX3 (96). It is worth mentioning that the mechanism underlying phosphorylation-dependent partner choice is very similar to that of regulation by c-ABL. As noted above, c-ABL-mediated YAP phosphorylation dissociates RUNX and ITCH from YAP, thereby facilitating formation of the YAP-p73 complex (72). Therefore, the oncogenic or tumor-suppressive activity of YAP is determined by its DNA-binding partner proteins which in turn are governed by cellular status.
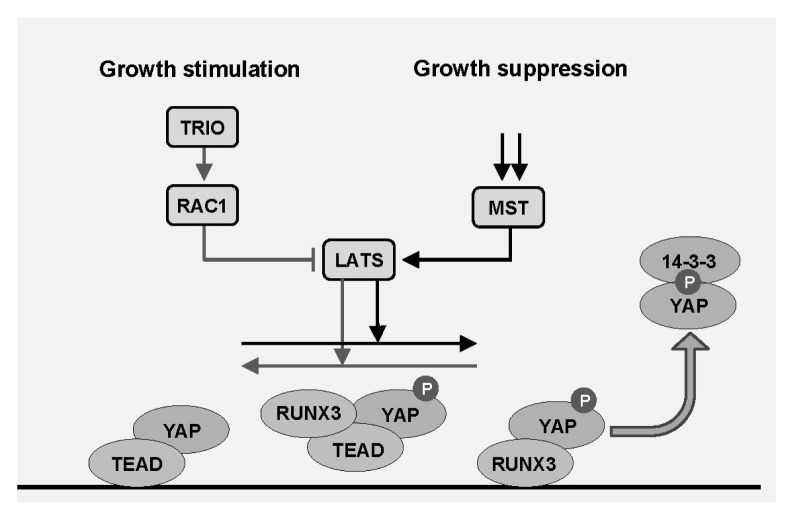
Fig. 3.
Reciprocal regulation of YAP activity by TRIO-RAC1 signaling and the Hippo pathway. RUNX3 interacts with YAP and TEAD4 to form a YAP-TEAD4-RUNX3 ternary complex. When TRIO-RAC signaling is activated, kinase activity of LATS is inhibited while YAP activity is increased. RUNX3 then dissociates from the ternary complex, resulting in the formation YAP-TEAD complex. When cell growth is inhibited, TEAD dissociates from the ternary complex through LATS-mediated YAP phosphorylation, resulting in the formation YAP-RUNX3 complex. Therefore, LATS1/2-mediated YAP phosphorylation not only inhibits YAP-TEAD complex, but also facilitates YAP-RUNX3 complex formation.
PROSPECTS
The role of YAP/TAZ in cancer development remains controversial. Initially, YAP/TAZ were described as oncogenes (6). Consistent with this, overexpression of YAP induces cell proliferation. In addition, YAP expression is elevated in human HCC and many other malignancies (97). On the other hand, recent studies have shown that YAP induces apoptosis in response to DNA damage in collaboration with p73 and PML (64). It also suppresses human colorectal cancer (98). These observations indicate that YAP could be defined as a tumor suppressor as well as an oncogene. The opposing roles of YAP in oncogenesis might be due to its lack of DNA-binding activity. This feature causes YAP target gene selection to be dictated by its DNA-binding partners which in turn are determined by their phosphorylation status. To further understand the roles of YAP in oncogenesis, it is important to study the molecular partners that interact with YAP as well as the kinases that regulate complex formation.
ACKNOWLEDGEMENTS
S-C. Bae was supported by a Creative Research Grant (2014R1A3A2030690) through the National Research Foundation (NRF) of Korea and Research Grant. J-W. Jang was supported by the Basic Science Research Program (NRF-2018R1C1B6009179) through the NRF funded by the Ministry of Science and ICT, Republic of Korea. Min-Kyu Kim was supported by the Basic Science Research Program (2017R1A6A3A11028050) through the NRF funded by the Ministry of Education, Republic of Korea.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 2.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 3.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Fang KS, Barker K, Sudol M, Hanafusa H. A transmembrane protein-tyrosine phosphatase contains spectrin-like repeats in its extracellular domain. J Biol Chem. 1994;269:14056–14063. [PubMed] [Google Scholar]
- 8.Zhu C, Li L, Zhao B. The regulation and function of YAP transcription co-activator. Acta Biochim Biophys Sin (Shanghai) 2015;47:16–28. doi: 10.1093/abbs/gmu110. [DOI] [PubMed] [Google Scholar]
- 9.Sudol M, Bork P, Einbond A, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995;270:14733–14741. doi: 10.1074/jbc.270.24.14733. [DOI] [PubMed] [Google Scholar]
- 10.Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morin-Kensicki EM, Boone BN, Howell M, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall CA, Wang R, Miao J, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z, Zhu JS, Xu ZP. RNA interference mediated YAP gene silencing inhibits invasion and metastasis of human gastric cancer cell line SGC-7901. Hepatogastroenterology. 2011;58:2156–2161. doi: 10.5754/hge11234. [DOI] [PubMed] [Google Scholar]
- 15.Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem. 2009;284:14347–14358. doi: 10.1074/jbc.M901568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69:1089–1098. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strano S, Munarriz E, Rossi M, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 22.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 23.Zagurovskaya M, Shareef MM, Das A, et al. EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene. 2009;28:1121–1131. doi: 10.1038/onc.2008.461. [DOI] [PubMed] [Google Scholar]
- 24.Qiao Y, Lin SJ, Chen Y, et al. RUNX3 is a novel negative regulator of oncogenic TEAD-YAP complex in gastric cancer. Oncogene. 2016;35:2664–2674. doi: 10.1038/onc.2015.338. [DOI] [PubMed] [Google Scholar]
- 25.Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999;18:2551–2562. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrigno O, Lallemand F, Verrecchia F, et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–4884. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- 27.Kurisaki A, Kose S, Yoneda Y, Heldin CH, Moustakas A. Transforming growth factor-beta induces nuclear import of Smad3 in an importin-beta1 and Ran-dependent manner. Mol Biol Cell. 2001;12:1079–1091. doi: 10.1091/mbc.12.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao JH, Davidson I, Matthes H, Garnier JM, Chambon P. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell. 1991;65:551–568. doi: 10.1016/0092-8674(91)90088-G. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko KJ, DePamphilis ML. Regulation of gene expression at the beginning of mammalian development and the TEAD family of transcription factors. Dev Genet. 1998;22:43–55. doi: 10.1002/(SICI)1520-6408(1998)22:1<43::AID-DVG5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Jacquemin P, Sapin V, Alsat E, Evain-Brion D, Dolle P, Davidson I. Differential expression of the TEF family of transcription factors in the murine placenta and during differentiation of primary human trophoblasts in vitro. Dev Dyn. 1998;212:423–436. doi: 10.1002/(SICI)1097-0177(199807)212:3<423::AID-AJA10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Zhao B, Wang P, et al. Structural insights into the YAP and TEAD complex. Genes Dev. 2010;24:235–240. doi: 10.1101/gad.1865810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Loh PG, Song H. Structural and functional insights into the TEAD-YAP complex in the Hippo signaling pathway. Protein Cell. 2010;1:1073–1083. doi: 10.1007/s13238-010-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo JS, Meng Z, Kim YC, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Huang T, Cheng AS, Yu J, Kang W, To KF. The TEAD Family and Its Oncogenic Role in Promoting Tumorigenesis. Int J Mol Sci. 2016;17:1–15. doi: 10.3390/ijms17010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. J Biol Chem. 2014;289:13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashidian J, Le Scolan E, Ji X, et al. Ski regulates Hippo and TAZ signaling to suppress breast cancer progression. Sci Signal. 2015;8:ra14. doi: 10.1126/scisignal.2005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You B, Yang YL, Xu Z, et al. Inhibition of ERK1/2 down-regulates the Hippo/YAP signaling pathway in human NSCLC cells. Oncotarget. 2015;6:4357–4368. doi: 10.18632/oncotarget.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen LT, Tretiakova MS, Silvis MR, et al. ERG Activates the YAP1 Transcriptional Program and Induces the Development of Age-Related Prostate Tumors. Cancer Cell. 2015;27:797–808. doi: 10.1016/j.ccell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene. 2014;33:4857–4866. doi: 10.1038/onc.2013.433. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Nandakumar N, Shi Y, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal. 2013;11:31. doi: 10.1186/1478-811X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artinian N, Cloninger C, Holmes B, Benavides-Serrato A, Bashir T, Gera J. Phosphorylation of the Hippo Pathway Component AMOTL2 by the mTORC2 Kinase Promotes YAP Signaling, Resulting in Enhanced Glioblastoma Growth and Invasiveness. J Biol Chem. 2015;290:19387–19401. doi: 10.1074/jbc.M115.656587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu FX, Luo J, Mo JS, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976. doi: 10.1038/ncomms3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Park JS, Wei Y, et al. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPalpha function. Mol Cell. 2013;51:211–225. doi: 10.1016/j.molcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu AM, Xu Z, Luk JM. An update on targeting Hippo-YAP signaling in liver cancer. Expert Opin Ther Targets. 2012;16:243–247. doi: 10.1517/14728222.2012.662958. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez LA, Squatrito M, Northcott P, et al. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujii M, Nakanishi H, Toyoda T, et al. Convergent signaling in the regulation of connective tissue growth factor in malignant mesothelioma: TGFbeta signaling and defects in the Hippo signaling cascade. Cell Cycle. 2012;11:3373–3379. doi: 10.4161/cc.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia Y, Zhang YL, Yu C, Chang T, Fan HY. YAP/TEAD co-activator regulated pluripotency and chemoresistance in ovarian cancer initiated cells. PLoS One. 2014;9:e109575. doi: 10.1371/journal.pone.0109575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L, Chan SW, Zhang X, et al. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290–300. doi: 10.1101/gad.1865310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fossdal R, Jonasson F, Kristjansdottir GT, et al. A novel TEAD1 mutation is the causative allele in Sveinsson’s chorioretinal atrophy (helicoid peripapillary chorioretinal degeneration) Hum Mol Genet. 2004;13:975–981. doi: 10.1093/hmg/ddh106. [DOI] [PubMed] [Google Scholar]
- 55.Kitagawa M. A Sveinsson’s chorioretinal atrophy-associated missense mutation in mouse Tead1 affects its interaction with the co-factors YAP and TAZ. Biochem Biophys Res Commun. 2007;361:1022–1026. doi: 10.1016/j.bbrc.2007.07.129. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Ji JY, Yu M, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang N, Morrison CD, Liu P, et al. TAZ induces growth factor-independent proliferation through activation of EGFR ligand amphiregulin. Cell Cycle. 2012;11:2922–2930. doi: 10.4161/cc.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neto-Silva RM, de Beco S, Johnston LA. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev Cell. 2010;19:507–520. doi: 10.1016/j.devcel.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C, Srivastava RK, Elmets CA, Afaq F, Athar M. Arsenic-induced cutaneous hyperplastic lesions are associated with the dysregulation of Yap, a Hippo signaling-related protein. Biochem Biophys Res Commun. 2013;438:607–612. doi: 10.1016/j.bbrc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Thongon N, Castiglioni I, Zucal C, et al. The GSK3beta inhibitor BIS I reverts YAP-dependent EMT signature in PDAC cell lines by decreasing SMADs expression level. Oncotarget. 2016;7:26551–26566. doi: 10.18632/oncotarget.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pobbati AV, Hong W. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol Ther. 2013;14:390–398. doi: 10.4161/cbt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zawacka-Pankau J, Kostecka A, Sznarkowska A, Hedstrom E, Kawiak A. p73 tumor suppressor protein: a close relative of p53 not only in structure but also in anti-cancer approach? Cell Cycle. 2010;9:720–728. doi: 10.4161/cc.9.4.10668. [DOI] [PubMed] [Google Scholar]
- 63.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/S1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 64.Strano S, Monti O, Pediconi N, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 65.Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP) J Biol Chem. 2008;283:27534–27546. doi: 10.1074/jbc.M804380200. [DOI] [PubMed] [Google Scholar]
- 66.Rossi M, De Laurenzi V, Munarriz E, et al. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 2005;24:836–848. doi: 10.1038/sj.emboj.7600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levy D, Adamovich Y, Reuven N, Shaul Y. The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death Differ. 2007;14:743–751. doi: 10.1038/sj.cdd.4402063. [DOI] [PubMed] [Google Scholar]
- 68.Lapi E, Di Agostino S, Donzelli S, et al. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell. 2008;32:803–814. doi: 10.1016/j.molcel.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 69.Bernassola F, Salomoni P, Oberst A, et al. Ubiquitin-dependent degradation of p73 is inhibited by PML. J Exp Med. 2004;199:1545–1557. doi: 10.1084/jem.20031943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okazaki T, Kageji T, Kuwayama K, et al. Up-regulation of endogenous PML induced by a combination of interferon-beta and temozolomide enhances p73/YAP-mediated apoptosis in glioblastoma. Cancer Lett. 2012;323:199–207. doi: 10.1016/j.canlet.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 71.Cottini F, Hideshima T, Xu C, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Keshet R, Adler J, Ricardo Lax I, et al. c-Abl antagonizes the YAP oncogenic function. Cell Death Differ. 2015;22:935–945. doi: 10.1038/cdd.2014.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenbluh J, Nijhawan D, Cox AG, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaidi SK, Sullivan AJ, Medina R, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–799. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omerovic J, Puggioni EM, Napoletano S, et al. Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Exp Cell Res. 2004;294:469–479. doi: 10.1016/j.yexcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Haskins JW, Nguyen DX, Stern DF. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci Signal. 2014;7:ra116. doi: 10.1126/scisignal.2005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schuchardt BJ, Bhat V, Mikles DC, McDonald CB, Sudol M, Farooq A. Molecular basis of the binding of YAP transcriptional regulator to the ErbB4 receptor tyrosine kinase. Biochimie. 2014;101:192–202. doi: 10.1016/j.biochi.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aqeilan RI, Kuroki T, Pekarsky Y, et al. Loss of WWOX expression in gastric carcinoma. Clin Cancer Res. 2004;10:3053–3058. doi: 10.1158/1078-0432.CCR-03-0594. [DOI] [PubMed] [Google Scholar]
- 80.Aqeilan RI, Donati V, Gaudio E, et al. Association of Wwox with ErbB4 in breast cancer. Cancer Res. 2007;67:9330–9336. doi: 10.1158/0008-5472.CAN-07-2147. [DOI] [PubMed] [Google Scholar]
- 81.Aqeilan RI, Hassan MQ, de Bruin A, et al. The WWOX tumor suppressor is essential for postnatal survival and normal bone metabolism. J Biol Chem. 2008;283:21629–21639. doi: 10.1074/jbc.M800855200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aqeilan RI, Donati V, Palamarchuk A, et al. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005;65:6764–6772. doi: 10.1158/0008-5472.CAN-05-1150. [DOI] [PubMed] [Google Scholar]
- 83.Del Mare S, Aqeilan RI. Tumor Suppressor WWOX inhibits osteosarcoma metastasis by modulating RUNX2 function. Sci Rep. 2015;5:12959. doi: 10.1038/srep12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Das A, Chendil D, Dey S, et al. Ionizing radiation down-regulates p53 protein in primary Egr-1-/- mouse embryonic fibroblast cells causing enhanced resistance to apoptosis. J Biol Chem. 2001;276:3279–3286. doi: 10.1074/jbc.M008454200. [DOI] [PubMed] [Google Scholar]
- 85.Murakami M, Nakagawa M, Olson EN, Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc Natl Acad Sci U S A. 2005;102:18034–18039. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 87.Varelas X, Sakuma R, Samavarchi-Tehrani P, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 88.Varelas X, Samavarchi-Tehrani P, Narimatsu M, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 89.Grannas K, Arngarden L, Lonn P, et al. Crosstalk between Hippo and TGFbeta: Subcellular Localization of YAP/TAZ/Smad Complexes. J Mol Biol. 2015;427:3407–3415. doi: 10.1016/j.jmb.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 90.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 91.Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 92.Marzia M, Sims NA, Voit S, et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. 2000;151:311–320. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-O. [DOI] [PubMed] [Google Scholar]
- 94.Hong JH, Hwang ES, McManus MT, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 95.Li QL, Ito K, Sakakura C, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/S0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 96.Jang JW, Kim MK, Lee YS, et al. RAC-LATS1/2 signaling regulates YAP activity by switching between the YAP-binding partners TEAD4 and RUNX3. Oncogene. 2017;36:999–1011. doi: 10.1038/onc.2016.266. [DOI] [PubMed] [Google Scholar]
- 97.Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barry ER, Morikawa T, Butler BL, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]