Abstract
The Hippo signaling pathway plays an essential role in adult tissue homeostasis and organ size control. Abnormal regulation of Hippo signaling can be a cause for multiple types of human cancers. Since the awareness of the importance of the Hippo signaling in a wide range of biological fields has been continually grown, it is also understood that a thorough and well-rounded comprehension of the precise dynamics could provide fundamental insights for therapeutic applications. Several components in the Hippo signaling pathway are known to be targeted for proteasomal degradation via ubiquitination by E3 ligases. β-TrCP is a well-known E3 ligase of YAP/TAZ, which leads to the reduction of YAP/TAZ levels. The Hippo signaling pathway can also be inhibited by the E3 ligases (such as ITCH) which target LATS1/2 for degradation. Regulation via ubiquitination involves not only complex network of E3 ligases but also deubiquitinating enzymes (DUBs), which remove ubiquitin from its targets. Interestingly, non-degradative ubiquitin modifications are also known to play important roles in the regulation of Hippo signaling. Although there has been much advanced progress in the investigation of ubiquitin modifications acting as regulators of the Hippo signaling pathway, research done to date still remains inadequate due to the sheer complexity and diversity of the subject. Herein, we review and discuss recent developments that implicate ubiquitin-mediated regulatory mechanisms at multiple steps of the Hippo signaling pathway.
Keywords: Deubiquitinases, E3 ligase, Hippo pathway, Protein degradation, Ubiquitin
INTRODUCTION
Balanced protein, especially onco-proteins and tumor suppressors, synthesis and degradation is critical for the cellular homeostasis and broken balance leads to various types of cancers. Hippo signaling pathway is a key signaling pathway which is responsible for the regulation of organ size by controlling cell growth and proliferation (1, 2). When cells perceive that they exist in the favorable conditions conductive to growth (such as high nutrient or low cell density in culture condition) YAP and TAZ, the key transcriptional activators of Hippo signaling pathway, are stabilized and enter into nuclei. YAP and TAZ in the nuclei interact with transcription factors (mainly TEADs) and enhance the expression of target genes, which are related to proliferation and anti-apoptosis (1). However, when cells exist in suboptimal, or conditions unfavorable to growth (such as serum starvation or high cell density in culture condition), a kinase cascade consisting of MST1/2 (mammalian homologs of Hippo in Drosophila) and LATS1/2 are activated. Activated MST1/2 phosphorylates LATS1/2, which enables LATS1/2 to phosphorylate YAP and TAZ. Phosphorylated YAP and TAZ can be sequestered in the cytoplasm by interacting with 14-3-3 protein, and subsequently degraded by β-TrCP mediated ubiquitin-proteasome system (1). In addition to YAP and TAZ, recent data revealed that the respective levels of several components in the Hippo signaling pathway are controlled by ubiquitin-proteasome system. In this mini-review, we will discuss recent developments on how ubiquitin mediated post-translational modifications regulate the Hippo signaling pathway.
THE UBIQUITIN MODIFICATION SYSTEM
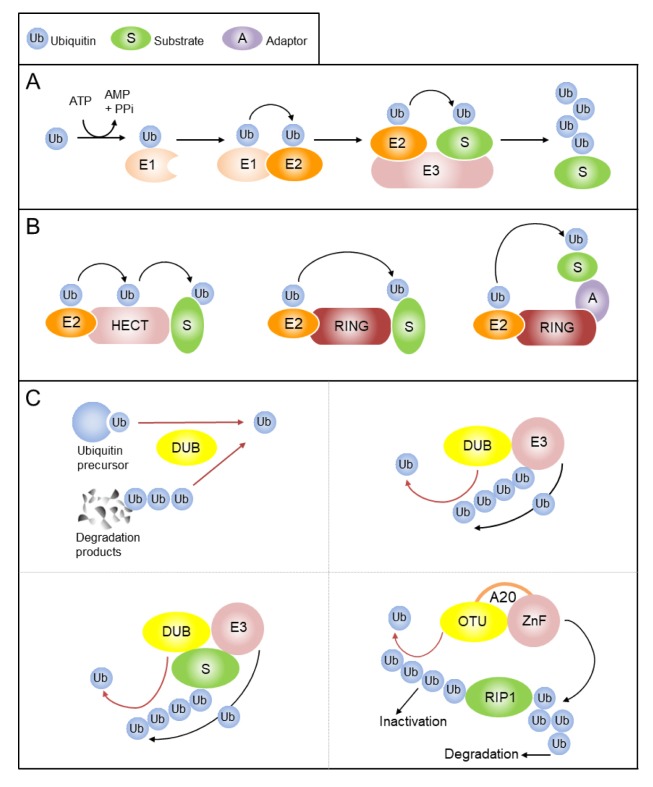
Ubiquitination is a post-translational modification of proteins in which single ubiquitin (Ub), or multiple ubiquitins, are attached to a substrate protein via its C-terminal glycine (Gly76) residue. Ubiquitin is produced in a precursor of linear chains of ubiquitin moieties or a single copy of ubiquitin fused to ribosomal proteins. The activity of deubiquitinating enzymes (DUBs) is required to produce free ubiquitin, either by processing precursor ubiquitin chains or by recycling ubiquitin via removing it from its targets (Fig. 1C, upper left panel). The ubiquitination reaction is catalyzed by the sequential action of three types of enzymes: Ub-activating enzymes (E1s); Ub-conjugating enzymes (E2s); and, Ub ligases (E3s) (Fig. 1A) (3). In certain cases, an additional conjugation factor, named E4 is requited for efficient multi-ubiquitination (4). The nature of ubiquitin conjugation to substrates is diverse, and the various ubiquitin forms determine the role of substrates in distinct cellular pathways and mechanisms. A monomeric ubiquitin can be conjugated at a single lysine residue (mono-ubiquitination), or at several lysine residues of substrate (multi-ubiquitination). Conjugation of extended ubiquitin chains on a substrate can occur through sequential attachment of ubiquitin to lysine of the preceding ubiquitin (poly-ubiquitination). Ubiquitin is conjugated using internal lysines (K), including K6, K11, K27, K29, K33, K48 and K63 (5, 6). Each of the seven lysines in ubiquitin can be used in the formation of polyubiquitin chains, generating diverse molecular signals which determine the fate of proteins. The substrate specificity of ubiquitination is determined by the E3 ligases which mediate substrate recognition and ubiquitin transfer from E2 enzymes to the substrate. The fate of ubiquitinated proteins depends on the type of ubiquitin linkage. The K48-linked ubiquitin chains target proteins for proteasomal degradation, whereas other types of ubiquitin linkages mediate proteolytic as well as non-proteolytic functions including protein trafficking, protein-protein interaction and activation of proteins.
Fig. 1.
The ubiquitin system. (A) Ubiquitin is activated by a ubiquitin-activating enzyme (E1) and transferred to a ubiquitin-conjugating enzyme (E2). E3 ligase binds target substrate and coordinates the covalent attachment of ubiquitin. Target proteins may be mono-ubiquitinated or, as in this example, poly-ubiquitinated. (B) The HECT E3 ligase acts as an acceptor of ubiquitin from E2 enzyme. Ubiquitin is then transferred to a specific Lys residue in the substrate. Conversely, RING E3 ligase acts as scaffold by facilitating interaction between E2 and substrate. These E3s can be a single chain or as large multiprotein complexes. (C) Generation of free ubiquitin from the precursors is a key function of deubiquitinases (DUBs). DUBs have a crucial role in maintaining ubiquitin homeostasis and preventing degradation of ubiquitin together with substrates of the proteasomal or lysosomal pathways (recycling of ubiquitin) (upper left panel). DUB-E3 interactions can rescue E3s or common substrates from degradation, or remove a non-degradative ubiquitin signal (upper right and lower left panels). The A20 combines DUB activity with E3 activity in one single polypeptide chain to modulate the ubiquitination status of key adaptors in NF-κB signaling (lower right panel). E3 ligase and DUB activity is indicated with black arrows and red arrows, respectively.
The human genome encodes two potential E1s, approximately 30 E2s and over 600 E3s. As E3s primarily determine the specificity of the ubiquitin system, a large volume of E3s are necessary, but only a few E1s and E2s. The E3 ligases are categorized in two groups on the basis of their catalytic domain: HECT (homologous to E6-associated protein Carboxy-terminus), and RING (“really interesting new gene”) finger, differ in the manner(s) by which each transfers ubiquitin to the substrate (Fig. 1B). The HECT E3s contain a conserved catalytic Cys, which acts as an acceptor of ubiquitin from E2s. Ubiquitin is then transferred to a specific Lys residue in the substrate. Unlike HECT E3s, RING E3s do not have a direct catalytic role in protein ubiquitination. Instead, RING E3s facilitate interaction between E2s and substrates via acting as scaffolds. These E3s exist as a single protein, or as large multiprotein complexes that determine specificity and regulatory complexity (Fig. 1B). Of the human E3s, most (~95%) belong to the RING family and only ~5% of E3s belong to the HECT family (7).
Ubiquitination is a reversible modification. Disassembly of ubiquitin chains is mediated by DUBs, also known as deubiquitinases. The approximately 100 DUBs encoded in the human genome are categorized in five subclasses on the basis of their Ub-protease domain structures: Four Cys protease classes (ubiquitin-specific protease, USP; ubiquitin C-terminal hydrolase, UCH; Otubain protease, OTU; Machado-Joseph disease protease, MJD); and, one metalloprotease class. All DUBs, which are metalloproteases and have a Ub-protease domain, are called “JAMM” (JAB/MPN/Mov34 metalloenzyme). The diverse functions of DUBs can be classified into three categories. First, DUBs generate free ubiquitin from linear polyubiquitin precursor proteins, as well as a ubiquitin fused to ribosomal proteins. Second, DUBs remove the ubiquitin chain from posttranslational modified proteins, leading to protein stabilization or reversal of ubiquitin signaling. Third, DUBs can edit the ubiquitin chains to alter ubiquitin signaling. As expected from their function, DUBs have been widely implicated in cellular and pathogenic processes (8). However, the precise mechanisms by which (the “how”) DUBs determine specificity are still poorly understood.
Many DUBs interact with E3 ligases, such coupling between opposing catalytic activities may serve various purposes. For example, DUBs are associated with E3 ligases, which have an intrinsic property of self-ubiquitination. These interactions reverse E3-mediated auto-ubiquitination and antagonize self-inflicted degradation (Fig. 1C, upper right panel) (9–11). As ubiquitination has also been related to the activation of signaling pathways, DUB-E3 ligase interactions allow fine-tuning of the ubiquitination status of a common substrate, and thereby switching on and switching off a signaling pathway (Fig. 1C, lower left panel) (12, 13). In rare cases, extreme coupling of DUB and E3 ligase is seen with A20, which has both DUB and E3 ligase activity in the same polypeptide. Mechanistically, A20 can remove K63-linked polyubiquitin chains from receptor interacting protein 1 (RIP1, key adaptors in the NF-κB signaling) in an OTU-dependent manner and catalyzes formation of K48-linked polyubiquitin chains onto RIP1 to promote proteasome-mediated degradation (Fig. 1C, lower right panel) (14).
REGULATION OF HIPPO SIGNALING COMPONENTS BY UBIQUITIN
The regulation of YAP/TAZ by ubiquitin
The transcriptional co-activator YAP and TAZ are major downstream effectors of the Hippo pathway, which shuttle between cytoplasm and nucleus, where they induce expression of genes involved in cell-proliferation and anti-apoptosis via interactions with transcription factor, TEAD. When the Hippo pathway is on, activated LATS1/2 phosphorylate YAP at five HxRxxS consensus motifs, and TAZ has four of these sites. Of these sites, phosphorylation of YAP Ser381 (TAZ Ser311) has been linked to regulation of YAP/TAZ protein stability, as it primes subsequent phosphorylation in a phosphodegron by Casein kinase 1 (CK1δ/ɛ). The phosphorylated phosphodegron then recruits the β-TrCP E3 ligase, leading to ubiquitination and degradation (15, 16). Fig. 2 shows a schematic diagram for the E3 ligases and DUBs which we discuss in this review. Notably, TAZ contains another phosphodegron located in the N-terminal region, and the N-terminal phosphodegron is unique in TAZ but not shared by YAP (15, 17). Huang et al. showed the N-terminal phosphodegron of TAZ is phosphorylated by GSK3 kinase, which is inhibited by the phosphoinositide 3-kinase (PI3K) pathway. The phosphorylation of TAZ Ser58/62 by GSK3 recruits β-TrCP, leading to ubiquitination and degradation of the TAZ. Moreover, the N-terminal phosphorylation of TAZ is regulated by the PTEN/PI3K/AKT pathway but in a way seemingly independent of the Hippo pathway (17). Besides the β-TrCP induced ubiquitination, the E3 ligase F-box and WD repeat domain-containing 7 (Fbxw7) mediates the ubiquitination of YAP, and thus its subsequent proteasomal degradation. Importantly, Fbxw7 levels inversely correlate with YAP abundance in hepatocellular carcinoma (HCC) tissues, suggesting that Fbxw7 could represent a potentially reliable prognostic marker and YAP may represent a potential therapeutic target for HCC (18).
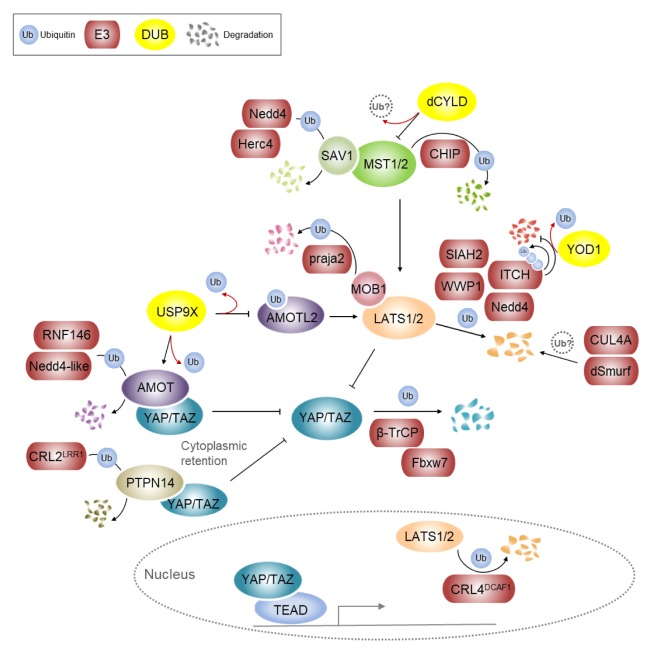
Fig. 2.
Ubiquitin-mediated regulation of the core Hippo pathway components. E3 ligase CHIP targets MST1/2 for ubiquitination. Drosophila CYLD (dCYLD) decreases Hpo (Drosophila ortholog of MST1/2) activity, but it is necessary to investigate whether the suppression is due to deubiquitinase activity of dCYLD. Nedd4 and Herc4 induce SAV1 ubiquitination and degradation in mammals and Drosophila, respectively. Multiple E3 ligases, such as Nedd4, ITCH, SIAH2 and WWP1, target LATS1/2 for degradation, thus negatively regulate Hippo signaling. The activity of LATS1 or level of LATS2 is also regulated in the nucleus by CRL4DCAF1. The activity of LATS1/2 can be indirectly modulated by E3 ligase or DUB. The deubiquitination of AMOTL2 by USP9X depresses LATS1/2 activity and subsequently activates YAP/TAZ. Praja2-mediated ubiquitination targets MOB1 for degradation which leads to inactivation of LATS1/2. β-TrCP and Fbxw7 mediate the ubiquitination of YAP/TAZ, and thus their subsequent proteasomal degradation. Both AMOT and PTPN14 as interacting proteins of YAP/TAZ can undergo ubiquitin-mediated proteolysis by each different E3 ligases, and thus allow YAP to translocate into nuclei. In contrast, deubiquitination of AMOT by USP9X results in stabilization of AMOT and retention of YAP in the cytoplasm, which leads to lower YAP/TAZ activity. E3 ligase and DUB activity is indicated with black arrows and red arrows, respectively.
Despite the presence of a phosphodegron, YAP is a relatively stable and mainly regulated by cytoplasmic-nuclear shuttling. In contrast, TAZ is constantly turned over, indicating that protein degradation may be the main route for TAZ inhibition. Recent studies have shown that YAP activity can be indirectly modulated by several E3 ligases. The nonreceptor tyrosine phosphatase PTPN14 and Angiomotin (AMOT) can antagonize YAP activity by sequestering it in the cytoplasm; this sequestration is achieved through protein-protein interactions mediated by the PY motif of PTPN14/AMOT and WW domains of YAP (19–22). Thus, ubiquitination and degradation of PTPN14 and AMOT through CRL2LRR1 E3 ligase and Nedd4-like E3 ligases (Nedd4, Nedd4-2 and ITCH) positively regulate YAP activity, respectively (19, 23). Moreover, it has been reported that tankyrase inhibitors inhibit YAP activity by stabilizing AMOT family proteins via the tankyrase-RNF146 axis (24). The E3 ligase RNF146 has been shown to recognize the tankyrase-mediated poly-ADP ribosylation (PARsylation) of substrates and promote their ubiquitination and degradation (25). Wang and colleagues showed that tankyrase associates with AMOT family protein, promoting their degradation through RNF146. Thus, tankyrase inhibitors such as XAV939 stabilize AMOT family proteins and suppress YAP activity. These results suggest that the tankyrase-RNF146-AMOT axis acts as an upstream signal, regulating YAP in the Hippo pathway (24). In contrast, Thanh Nguyen et al. identified the DUB USP9X as an indirect negative regulator of YAP/TAZ using a cell-based RNAi screen. USP9X leads to deubiquitinate AMOT at Lys 496, resulting in stabilization of AMOT and reduced YAP/TAZ activity (26).
The regulation of LATS1/2 by ubiquitin
LATS1/2 plays the most critical role in regulation of the Hippo signaling pathway. LATS1/2 phosphorylates YAP/TAZ on Ser127/89 and Ser381/311, respectively, controlling YAP/TAZ on two levels, Ser127/89-mediated cytoplasmic-nuclear shuttling and Ser381/311-mediated phosphodegron mediated degradation (15, 16, 27, 28). Importantly, multiple upstream inputs of the Hippo pathway appear to converge on the LATS1/2. Expanded (Ex) and Merlin (also known as NF2 for neurofibromatosis type 2) behave as a linker for the apical plasma membrane and actin cytoskeleton. Kibra has a C2 domain that interacts with phospholipids and it may target interacting proteins to the cell surface. These three proteins form a complex and then recruit the Hippo pathway kinases to the apical plasma membrane, which leads to activation of LATS1/2 (29–31). In addition, LATS1 can bind or colocalize with F-actin, suggesting that F-actin may directly regulate the LATS1/2 kinase activity (32). GPCR signaling, cell attachment, and cell geometry can also regulate YAP/TAZ activity through LATS1/2 (33–36). Following the establishment of such upstream signals, several protein complexes activate the LATS1/2, which functions as a central player to suppress the YAP/TAZ activity. Therefore, comprehensive understanding of the regulation of LATS1/2 represents a dynamic area of active investigation in the Hippo signaling field.
LATS1/2 contains two PPxY motifs (one motif in LATS2) that are known to interact with WW domains mediating protein-protein interaction by recognizing proline-rich peptide sequences (37). The WW domain-containing E3 ligase, ITCH, catalyzes ubiquitination and subsequent degradation of LATS1/2, thereby promoting cell growth and survival (38, 39). Other WW domain-containing E3 ligases, such as NEDD4, WWP1 and Smurf, also bind to the PPxY motifs and promote degradation of LATS1/2. NEDD4 ubiquitinates and destabilizes LATS1/2 and thus enhances the YAP transcriptional activity (40, 41). WWP1 also inhibit LATS1 by targeting it for ubiquitin-mediated degradation, which in turn leads to promotion of cell proliferation in breast cancer cells (42). In Drosophila, Smurf was also identified as a regulator of Warts (Wts, the Drosophila ortholog of LATS1/2). dSmurf associates with Wts and modulates Wts protein turnover and then Yorkie (Yki, the Drosophila ortholog of YAP) activity. However, the mechanisms as to how dSmurf regulates the turnover of Wts need further investigation. In addition, it remains to be determined whether the dSmurf-mediated ubiquitination is essential for Wts turnover (43). Prior studies have indicated that the closed form, active Merlin enters into the nucleus, binds to E3 ligase CRL4DCAF1 and inhibits its activity (44). The derepressed CRL4DCAF1, which is caused in Merlin/NF2-deficient tumor cells, targets LATS1/2 for ubiquitination and inhibition in the nucleus (45). Whereas LATS1 is poly-ubiquitinated and targeted for proteasomal degradation, LATS2 is oligo-ubiquitinated at multiple sites resulting in loss of kinases activity. Thus active YAP/TAZ accumulates in the nucleus and supports the oncogenic potential of Merlin/NF2-deficient tumor cells (45). It has recently been reported that scaffold protein Cullin4A (CUL4A) interacts with LATS1 and enhances its proteasomal degradation, thereby promoting proliferation and epithelial-mesenchymal transition (EMT) of gastric cancer cells (46). Interestingly, CUL4A is a core component of the Cullin4A-RING E3 ligase (CRL4) complex (47). Although, these observations may explain the means by which CUL4A inhibits LATS1, the precise mechanisms need to be investigated. Ubiquitination of LATS1/2 also plays a role in stress response. Hypoxia-activated E3 ligase, SIAH2, induces LATS1/2 degradation and subsequently de-repressed YAP activity, promoting tumorigenesis. Notably, targeting SIAH2 in tumor cell restores the tumor suppressor function of LATS2 in a xenograft animal model, indicating that the SIAH2-LATS1/2 pathway may have a role in tumorigenesis (48).
Moreover, it has recently been identified that mono-ubiquitination of Angiomotin-like 2 (AMOTL2), which is regulated by the DUB USP9X, can activate LATS2 kinase by supporting the binding of AMOTL2 to the LATS2 UBA domain. Ectopic expression or knockdown of USP9X inhibits or promotes the LATS-mediated phosphorylation of YAP, respectively. These results indicate that deubiquitination of AMOTL2 by USP9X depresses LATS1/2 activity and subsequently activates YAP (49). Recently, deubiquitinase YOD1 has been found to stabilize the E3 ligase ITCH that has an intrinsic tendency of self-ubiquitination. Hence, stabilization of ITCH by YOD1 can promote degradation of LATS1/2. Interestingly, the inducible expression of YOD1 enhances the proliferation of hepatocytes and leads to hepatomegaly in a YAP/TAZ-activity-dependent manner (50).
Other components of the Hippo pathway regulated by ubiquitin
The adaptor protein MOB1 is a key regulator of LATS1/2 kinases in the Hippo pathway. MOB1 is present in an auto-inhibited form and is activated by MST1/2-mediated phosphorylation. The phosphorylated MOB1 assists MST1/2 to recruit and phosphorylate LATS1/2 at hydrophobic motif Thr1079/1041. In addition, the phosphorylation of MOB1 by MST1/2 increases the binding affinity to LATS1/2, which in turn leads to auto-phosphorylation of LATS1/2 on Ser909/872 and increases their kinase activity for full activation (51–53). Lignitto et al. showed that E3 ligase praja2 directly binds to and ubiquitinates MOB1. Degradation of ubiquitinated MOB1 downregulates LATS1/2 activity, attenuating Hippo signaling and sustains glioblastoma growth. Notably, MOB1 levels inversely correlate with glioma malignancy and praja2 abundance, supporting the role of praja2 in control of MOB1 stability in vivo (54).
SAV1, the cofactor of MST1/2, serves as a bridge between MST1/2 and LATS1/2 and enhances LATS1/2 activity upon phosphorylation by MST1/2 (55, 56). SAV1 is also required for MST1 activation and translocation to the nucleus for subsequent LATS1/2 activation and phosphorylation of YAP on S127 upon keratinocyte differentiation (57). Conditional deletion of SAV1 in mouse liver leads to liver size enlargement and tumor formation, but LATS1/2 and YAP phosphorylation are not affected. These results indicate that SAV1 is not absolutely required for MST1/2 activation in hepatocytes and may limit liver growth by other mechanisms (58). Although the precise mechanisms are unknown, SAV1 on its own is less stable than MST1/2-binding form. The association of active MST1/2 with SAV1 leads to phosphorylation and stabilization of SAV1 (56). The E3 ligase NEDD4 destabilizes SAV1 as well as LATS1/2 through ubiquitination and thus activates YAP to promote intestinal stem cell self-renewal (41). Another E3 ligase, Herc4, induces Salvador (Sav, the Drosophila ortholog of SAV1) ubiquitination and degradation in Drosophila, although the function of mammalian Herc4 still requires some elucidation. Interestingly, Hippo (Hpo, the Drosophila ortholog of MST1/2) competes with Herc4 for Sav binding and antagonizes Herc4-mediated ubiquitination of Sav (59).
The Hippo kinase pathway can be initiated by TAO kinases, which phosphorylate the activation loop of MST1/2 at Thr183/180 and thereby lead to full activation of MST1/2 (60, 61). The activation loop phosphorylation is also achieved by MST1/2 auto-phosphorylation (62). Thus, it is possible that upstream kinases are not requisite for MST1/2 activation. A few other kinases, such as mTOR, AKT and ABL, may phosphorylate MST1/2 and regulate the kinase activity by different mechanism. However, the role of MST1/2 phosphorylation by these kinases in the Hippo pathway has not been implicated (63–65). While the activity of MST1/2 in the Hippo pathway has been extensively studied, the stability control of MST1/2 in this pathway is not as well understood. Xiao et al. identified that MST1 is recognized by E3 ligase C terminus of Hsc70-interacting protein (CHIP). Oxidative stress induces the c-Abl-dependent phosphorylation of MST1, which protects it from ubiquitination. Inhibition of c-Abl promotes degradation of MST1 through CHIP-mediated ubiquitination, and thereby attenuates hippocampal neuronal cell death (65). Hpo (the Drosophila ortholog of MST1/2) is also reported to be negatively regulated by Drosophila DUB CYLD (dCYLD). dCYLD decreases Hpo activity through repressing its phosphorylation on Tyr195, thereby increasing activity of Yki (the Drosophila ortholog of YAP). However, whether the deubiquitinase activity of dCYLD is essential for suppressing Hpo activity remains to be seen. In addition, the mechanism as to how dCYLD decreases phosphorylation of Hpo represents an area in need of further investigation (66).
Epithelial cells constitute various tissue architectures and the maintenance of normal tissue architecture is important for size control (67, 68). The Hippo pathway functions as a pivotal regulator of tissue architecture in size control. Inputs from multiple epithelial architectures converge on Hippo signaling, allowing tissues to respond properly to disruptions in cell-cell and cell-matrix interactions (29–32, 35, 36). Based upon several sources of evidence, it has been determined that the apical transmembrane protein Crumb (Crb) antagonizes Yki/YAP activity, both in Drosophila and mammals. In principle, Crb has a large extracellular domain and a short intracellular domain. Expanded (Ex) can interact with the short intracellular domain of Crb, and this interaction modulates Ex localization and stability, which in turn enhances the activity of Hippo pathway core kinases and the phosphorylation of Yki. However, the observation that Crb is required for Ex membrane localization to suppress Yki activity conflicts with the finding that Crb overexpression reduces Ex levels and leads to increased Yki activity (69–72). Ribeiro et al. identified the molecular mechanisms in which Crb recruits Ex to the plasma membrane for phosphorylation and then promotes ubiquitin-dependent degradation via the SCFSlimb/β-TrCP E3 ubiquitin ligase (Slmb). Thus, Crb can perform dual function for the precise tuning of Yki function; recruiting Ex apically to repress Yki activity and to promote phosphorylation dependent Ex turnover. The constant turnover of Ex at the apical membrane may allow that Yki rapidly respond to changing environmental conditions (73).
CONCLUSION REMARKS
The ubiquitin system has emerged as a major tool in the Hippo pathway, regulating the stability of crucial signaling components as well as modulating protein functions through non-proteolytic mechanisms. Notably, the stability of crucial Hippo signaling components, such as YAP/TAZ and LATS1/2, is targeted by diverse E3 ligases and deubiquitinases that are regulated by distinct stimuli, in a tissue specific manner or within specific subcellular compartments. These findings strongly suggest that the abundance and activity of YAP/TAZ and LATS1/2 must be tightly controlled. Aberrant activation or mutation of the ubiquitin regulatory proteins results in human diseases, suggesting that ubiquitin mediated regulation of Hippo signaling pathway can provide potential therapeutic targets.
While previous studies described a framework of ubiquitination network in the regulation of the Hippo pathway, future work is necessary to address the mechanistic details. For example, determination of ubiquitin attachment sites and ubiquitin chain linkages on the components of the Hippo signaling pathway, figuring out the biological significance of these modulations and how the activity of E3 ligases and DUBs are controlled by time- and space-specific manner. In addition, the identification of the ubiquitin-binding proteins that control assembly of complex signaling networks will be critical.
This review focuses on ubiquitin modification, yet other posttranslational modification (PTM), such as phosphorylation, sumoylation, O-GlcNAcylation, and acetylation, onto Hippo signaling components have also been found to be important. The different PTMs can affect each other by recruiting enzymes, orchestrate upstream signal transduction, YAP/TAZ nuclear translocation and transcriptional activation. Thus, in-depth exploration of the impact of PTMs in regulating Hippo signaling will open a new avenue for developing clinical therapeutic applications, as well as the discovery of drugs targeting human diseases.
ACKNOWLEDGEMENTS
This work was supported by the grants from the National Research Foundation of Korea (NRF-2016R1E1A1A01943544 and NRF-2017M3A9B4062421) to E. Jho.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 4.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/S0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. ‘Protein Modifications: Beyond the Usual Suspects’ review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirisako T, Kamei K, Murata S, et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Bengtson MH, Ulbrich A, et al. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS One. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijman SM, Luna-Vargas MP, Velds A, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Canning M, Boutell C, Parkinson J, Everett RD. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J Biol Chem. 2004;279:38160–38168. doi: 10.1074/jbc.M402885200. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Yen L, Irwin L, Sweeney C, Carraway KL., 3rd Stabilization of the E3 ubiquitin ligase Nrdp1 by the deubiquitinating enzyme USP8. Mol Cell Biol. 2004;24:7748–7757. doi: 10.1128/MCB.24.17.7748-7757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hetfeld BK, Helfrich A, Kapelari B, et al. The zinc finger of the CSN-associated deubiquitinating enzyme USP15 is essential to rescue the E3 ligase Rbx1. Curr Biol. 2005;15:1217–1221. doi: 10.1016/j.cub.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 12.Brooks CL, Li M, Hu M, Shi Y, Gu W. The p53--Mdm2--HAUSP complex is involved in p53 stabilization by HAUSP. Oncogene. 2007;26:7262–7266. doi: 10.1038/sj.onc.1210531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 14.Wertz IE, O’Rourke KM, Zhou H, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 15.Liu CY, Zha ZY, Zhou X, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang W, Lv X, Liu C, et al. The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFbeta-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition. J Biol Chem. 2012;287:26245–26253. doi: 10.1074/jbc.M112.382036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu K, Yang W, Li C, et al. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol Cancer. 2014;13:110. doi: 10.1186/1476-4598-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Huang J, Wang X, et al. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26:1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao B, Li L, Lu Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, An J, Zhang P, et al. The Nedd4-like ubiquitin E3 ligases target angiomotin/p130 to ubiquitindependent degradation. Biochem J. 2015;444:279–289. doi: 10.1042/BJ20111983. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Li N, Li X, Tran MK, Han X, Chen J. Tankyrase Inhibitors Target YAP by Stabilizing Angiomotin Family Proteins. Cell Rep. 2015;13:524–532. doi: 10.1016/j.celrep.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Liu S, Mickanin C, et al. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Cell Biol. 2011;13:623–629. doi: 10.1038/ncb2222. [DOI] [PubMed] [Google Scholar]
- 26.Thanh Nguyen H, Andrejeva D, Gupta R, et al. Deubiquitylating enzyme USP9x regulates hippo pathway activity by controlling angiomotin protein turnover. Cell Discov. 2016;2:16001. doi: 10.1038/celldisc.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Lei QY, Zhang H, Zhao B, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–36. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamaratoglu F, Willecke M, Kango-Singh M, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 30.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visser-Grieve S, Zhou Z, She YM, et al. LATS1 tumor suppressor is a novel actin-binding protein and negative regulator of actin polymerization. Cell Res. 2011;21:1513–1516. doi: 10.1038/cr.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 37.Chen HI, Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci U S A. 1995;92:7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho KC, Zhou Z, She YM, Chun A, Cyr TD, Yang X. Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability [corrected] Proc Natl Acad Sci U S A. 2011;108:4870–5. doi: 10.1073/pnas.1101273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salah Z, Melino G, Aqeilan RI. Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res. 2011;71:2010–2020. doi: 10.1158/0008-5472.CAN-10-3516. [DOI] [PubMed] [Google Scholar]
- 40.Salah Z, Cohen S, Itzhaki E, Aqeilan RI. NEDD4 E3 ligase inhibits the activity of the Hippo pathway by targeting LATS1 for degradation. Cell Cycle. 2013;12:3817–3823. doi: 10.4161/cc.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae SJ, Kim M, Kim SH, et al. NEDD4 controls intestinal stem cell homeostasis by regulating the Hippo signalling pathway. Nat Commun. 2015;6:6314. doi: 10.1038/ncomms7314. [DOI] [PubMed] [Google Scholar]
- 42.Yeung B, Ho KC, Yang X. WWP1 E3 ligase targets LATS1 for ubiquitin-mediated degradation in breast cancer cells. PLoS One. 2013;8:e61027. doi: 10.1371/journal.pone.0061027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao L, Wang P, Gao Y, Lin X, Wang F, Wu S. Ubiquitin E3 ligase dSmurf is essential for Wts protein turnover and Hippo signaling. Biochem Biophys Res Commun. 2014;454:167–171. doi: 10.1016/j.bbrc.2014.10.058. [DOI] [PubMed] [Google Scholar]
- 44.Li W, You L, Cooper J, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W, Cooper J, Zhou L, et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014;26:48–60. doi: 10.1016/j.ccr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng J, Lei W, Xiang X, et al. Cullin 4A (CUL4A), a direct target of miR-9 and miR-137, promotes gastric cancer proliferation and invasion by regulating the Hippo signaling pathway. Oncotarget. 2016;7:10037–10050. doi: 10.18632/oncotarget.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sang Y, Yan F, Ren X. The role and mechanism of CRL4 E3 ubiquitin ligase in cancer and its potential therapy implications. Oncotarget. 2015;6:42590–42602. doi: 10.18632/oncotarget.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma B, Chen Y, Chen L, et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17:95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- 49.Kim M, Kim M, Park SJ, Lee C, Lim DS. Role of Angiomotin-like 2 mono-ubiquitination on YAP inhibition. EMBO Rep. 2016;17:64–78. doi: 10.15252/embr.201540809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y, Kim W, Song Y, et al. Deubiquitinase YOD1 potentiates YAP/TAZ activities through enhancing ITCH stability. Proc Natl Acad Sci U S A. 2017;114:4691–4696. doi: 10.1073/pnas.1620306114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26:1772–1781. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 54.Lignitto L, Arcella A, Sepe M, et al. Proteolysis of MOB1 by the ubiquitin ligase praja2 attenuates Hippo signalling and supports glioblastoma growth. Nat Commun. 2013;4:1822. doi: 10.1038/ncomms2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tapon N, Harvey KF, Bell DW, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 56.Callus BA, Verhagen AM, Vaux DL. Vaux, Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee JH, Kim TS, Yang TH, et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27:1231–1242. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aerne BL, Gailite I, Sims D, Tapon N. Hippo Stabilises Its Adaptor Salvador by Antagonising the HECT Ubiquitin Ligase Herc4. PLoS One. 2015;10:e0131113. doi: 10.1371/journal.pone.0131113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell. 2011;21:896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 61.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21:888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381(Pt 2):453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan Z, Kim D, Shu S, et al. Phosphoinositide 3-kinase/Akt inhibits MST1-mediated pro-apoptotic signaling through phosphorylation of threonine 120. J Biol Chem. 2010;285:3815–3824. doi: 10.1074/jbc.M109.059675. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Collak FK, Yagiz K, Luthringer DJ, Erkaya B, Cinar B. Threonine-120 phosphorylation regulated by phosphoinositide-3-kinase/Akt and mammalian target of rapamycin pathway signaling limits the antitumor activity of mammalian sterile 20-like kinase 1. J Biol Chem. 2012;287:23698–23709. doi: 10.1074/jbc.M112.358713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao L, Chen D, Hu P, et al. The c-Abl-MST1 signaling pathway mediates oxidative stress-induced neuronal cell death. J Neurosci. 2011;31:9611–9619. doi: 10.1523/JNEUROSCI.0035-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y, Wang Z, Wang P, Li D, Zhou J, Wu S. CYLD negatively regulates Hippo signaling by limiting Hpo phosphorylation in Drosophila. Biochem Biophys Res Commun. 2014;452:808–812. doi: 10.1016/j.bbrc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 68.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/S0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 69.Chen CL, Gajewski KM, Hamaratoglu F, et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ling C, Zheng Y, Yin F, et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varelas X, Samavarchi-Tehrani P, Narimatsu M, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribeiro P, Holder M, Frith D, Snijders AP, Tapon N. Crumbs promotes expanded recognition and degradation by the SCF(Slimb/beta-TrCP) ubiquitin ligase. Proc Natl Acad Sci U S A. 2014;111:E1980–E19809. doi: 10.1073/pnas.1315508111. [DOI] [PMC free article] [PubMed] [Google Scholar]