Abstract
The Hippo signaling pathway controls nuclear accumulation and stability of the transcriptional coregulator YAP and its paralog TAZ. The activity of Hippo-YAP signaling is influenced not only by biochemical signals, but also by cell shape and mechanical tension transmitted through cell-cell junctions and cell-matrix adhesions. Data accumulated thus far indicates that the actin cytoskeleton is a key mediator of the regulation of Hippo-YAP signaling by means of a variety of biochemical and mechanical cues. In this review, we have outlined the role of actin dynamics and actin-associated proteins in the regulation of Hippo-YAP signaling. In addition, we discuss actin-mediated regulation of YAP/TAZ activity independent of the core Hippo kinases MST and LATS. Although our understanding of the link between Hippo-YAP signaling and the actin cytoskeleton is progressing rapidly, many open questions remain.
Keywords: Actin, Hippo, Mechanotransduction, TAZ, YAP
INTRODUCTION
The Hippo signaling pathway controls organ size in animals through the regulation of cell proliferation and survival (1, 2). YAP and TAZ are central effectors of the Hippo signaling pathway. The Hippo kinase cascade negatively regulates the activity of YAP/TAZ by promoting cytoplasmic retention and proteasomal degradation. Recent in vitro and in vivo studies have made rapid progress in identifying the upstream signals which serve to control the activity of Hippo-YAP signaling (2, 3). Unlike conventional signal transduction pathways which involve dedicated ligand-receptor pairs, Hippo-YAP signaling is influenced by a wide range of architectural and mechanical cues, as well as biochemical signals including extracellular matrix (ECM) stiffness, cell-cell adhesion, cell-matrix adhesion, cell density, cell shape and cell polarity. Although G-protein-coupled receptors (GPCRs) have been shown to control YAP/TAZ activity (4), it is not clear whether the identified GPCRs and their ligands are dedicated to control YAP/TAZ activity in vivo.
Remarkably, the multiple regulatory inputs which determine the activity of YAP/TAZ converge on the actin cytoskeleton (1, 2). Mechanical cues, such as ECM stiffness and cell morphology, strongly influence the architecture and properties of the actin cytoskeleton (5, 6). Cells placed on a stiffer culture substrate respond by assembling contractile actin fibers in order to counteract the rigidity they are experiencing. Mechanical tensions (generated by either the cells themselves or by some force imposed externally at cell-cell and cell-matrix junctions) are closely linked to the re-arrangement of the actomyosin networks (7). Moreover, GPCRs that control YAP/TAZ activity act predominantly through Rho family GTPases, major regulators of cellular actin dynamics (8, 9).
Consistent with the involvement of the actin cytoskeleton in cellular responses to mechanical cues and GPCR signals, several studies have demonstrated that alteration of actin dynamics serves to exert a strong impact on the activity of YAP/TAZ. For example, induction of filamentous actin (F-actin) bundling by knockdown of F-actin capping or severing proteins promotes nuclear enrichment of YAP/TAZ (2, 6, 10). By contrast, treatment of cells with F-actin-disrupting agents causes retention of YAP/TAZ in the cytoplasm in many cellular contexts (11, 12). Because F-actin is known to integrate multiple regulatory signals and participate in diverse cellular activities including migration, polarization and intracellular trafficking, Hippo signaling and YAP/TAZ activity may be fine-tuned by complex interlinked regulatory networks.
ACTIN-RELATED REGULATORY INPUTS WHICH CONTROL HIPPO-YAP SIGNALING
Mechanical stress
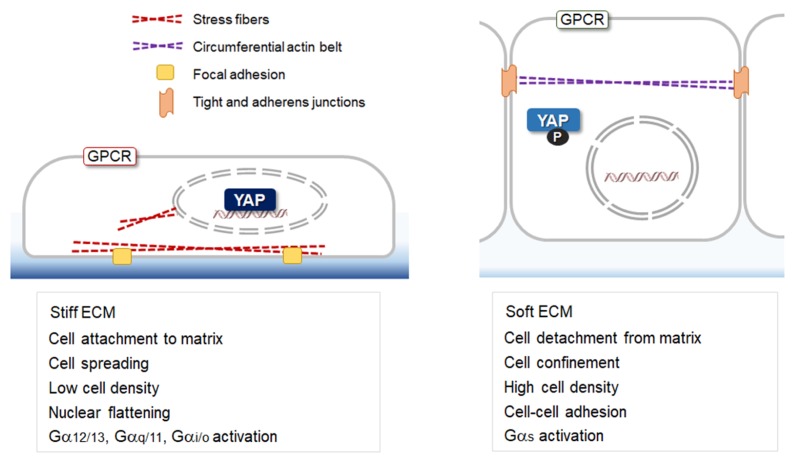
There is increasing awareness that mechanotransduction, the conversion of mechanical stimulations into intracellular biochemical signals, plays crucial developmental and physiologic roles (1, 13). The YAP/TAZ are known as central components of mechanotransduction pathways sensing the local mechanical environment (14, 15). Mechanical inputs, including cell geometry, cell stretching, ECM stiffness, cell adhesion and shear stress, strongly influence the localization and the activity of YAP/TAZ (Fig. 1). Round and compact cell geometry presents with cytoplasmic localization of YAP/TAZ, while cells undergoing spreading exhibit nuclear enrichment of YAP/TAZ (12, 14). YAP, which is normally cytoplasmic in cells grown at high density (16), can re-enter the nucleus upon stretching on an elastic substrate (10). YAP is also activated by fluid share stress in osteoblasts (17) and by disturbed flow in endothelial cells (18). All these mechanical conditions generate intracellular tension and as a consequence, remodeling of the actin cytoskeleton occurs. Modification of cell-cell and cell-matrix junction structures is also involved in the transduction of forces into intracellular signals. Altered junctional structures induce changes in the connection of contractile actin fibers to the junctions. Thus, YAP/TAZ-dependent mechanotransduction occurs in association with actin remodeling.
Fig. 1.
Mechanical and biochemical cues controlling YAP through the actin cytoskeleton. Cell culture conditions known to influence the localization of YAP/TAZ are shown.
Contractile actomyosin
Actomyosin, composed of F-actin and non-muscle myosin II, is visualized as bundles in nonmuscle cells, and Rho GTPases are key regulators of the assembly dynamics and functions of the actomyosin bundles (2, 19). Actomyosin is a main component of cells that respond to, and reflect, mechanical stressors. Moreover, the molecular interactions between F-actin and non-muscle myosin II control the cellular mechanics to modulate cell shape, tension, and contractility (7). Although the molecular mechanisms are not well or fully understood, it has been demonstrated that contractile actomyosin serves a central mediator between mechanical cues and Hippo-YAP signaling in various environments of mechanotransduction. Treatment of cells with cytochalasin D or latrunculin B, which disrupts F-actin, retains YAP/TAZ in the cytoplasm (11, 12). Similarly, blebbistatin and ML-7, which (respectively) inhibit myosin II ATPase and myosin light-chain kinase, reduced nuclear YAP/TAZ localization (12). Complementary results in Drosophila epithelial tissues show that Yorkie (Yki, the Drosophila ortholog of the mammalian YAP) is activated, and causes tissue overgrowth, in response to increased actin polymerization (20).
Extracellular matrix
YAP/TAZ have been shown to respond to the stiffness of the ECM. Cells seeded on stiff substrates display increasing YAP nuclear localization and activity, whereas YAP/TAZ activity is repressed in cells seeded on soft substrates (14). Importantly, actin remodeling is involved in YAP/TAZ response to the mechanical properties of the ECM. Cells in a stiff mechanical environment assemble contractile actin filaments to counteract the forces acting upon them, and cell-matrix junctions are also strengthened (16). Recent studies have noted that the adaptor proteins Talin and Vinculin, which link integrins to F-actin at the focal adhesions, effect the localization of YAP/TAZ. Secondary to the forces generated above a certain “stiffness threshold”, Talin unfolds, binds to Vinculin, and stabilizes the attachment of actin filaments. In this context, YAP/TAZ nuclear translocation is enhanced (21, 22). YAP/TAZ regulation by means of the ECM stiffness has been shown to control cell fate decision. Mesenchymal stem cells tend to differentiate into bone cells by activating YAP/TAZ when cultured on a stiff matrix, which mimicks natural bone environment (14, 23). By contrast, on a soft matrix, they differentiate into fat cells by inactivating YAP/TAZ. It has been noted that the mechanical properties of tumors affect their growth and progression (24). The frequent hyperactivation of YAP/TAZ shown in several types of tumors may be partly due to the altered mechanical properties of the tumor microenvironment (25, 26).
Cell-cell junctions
Attachments to other cells, as well as to extracellular matrix, allow cells to sense and respond to changes in the physical attributes of their environment. “Adherens junctions” and “tight junctions” represent the main structures by which epithelial cells are bound together via protein complexes. Multiple studies have shown that key junctional proteins (such as Crumbs, PATJ, PALS, α-catenin, and E-cadherin) can regulate the activity of YAP/TAZ (27, 28). These junctional proteins are thought to bind and detain YAP/TAZ at cell junctions, thus suppressing their nuclear entry and activity (2, 26). It is also likely that junctional proteins affect the stability of YAP/TAZ by regulating the access of specific phosphatases or kinases (26). Interestingly, mechanical strain applied to quiescent epithelial cells using compliant silicone substrates and stretching devices, induces E-cadherin-dependent YAP activation, thereby promoting cell proliferation (28). By contrast, a recent study showed that contraction of circumferential actin belts underlying adherens junctions suppresses the nuclear translocation of YAP/TAZ in columnar epithelial cells (29). Formation of cell-cell junctions may be associated with the strong influence of the confluency of cultured cells on YAP/TAZ localization (16). The aggregate of these findings indicates that the formation and the integrity of cell junctions, and mechanotransduction through the junctions, are important regulatory inputs of Hippo-YAP signaling.
Nuclear pores
There is growing evidence to substantiate that forces transmitted to the nucleus through the cytoskeleton are important for mechanosensing and YAP/TAZ regulation (30, 31). Transfer of mechanical forces to the nucleus through the actin cytoskeleton is mediated by the linker of nucleoskeleton and cytoskeleton (LINC) complex (30). It has been suggested that forces applied to the LINC complex at the nuclear envelope affects the nuclear pore complex that mediates transport of all macromolecules between the nucleus and the cytoplasm (32). In mesenchymal stem cells, impairment of the cytoskeleton-nucleus connection by knockdown of Nesprin-1-giant (a key component of the LINC complex) decreases YAP response to mechanical stress (30). Moreover, a recent study demonstrated that forces exerted through focal adhesions release mechanical restriction of the nuclear pores, which limits nuclear translocation of YAP and other proteins (33). Force transmission is thought to directly drive YAP nuclear import by flattening the nucleus, stretching nuclear pores and reducing their physical, material resistance (33). This particular mechanism of YAP regulation appears to function independent of the core Hippo kinases.
ACTIN-REMODELING REGULATORS WHICH CONTROL HIPPO-YAP SIGNALING
Rho GTPase
The polymerization and depolymerization of actin filaments is a dynamic process which is controlled by a variety of regulatory proteins including Rho family GTPases (34). Several studies have indicated that Rho GTPases are essential mediators connecting mechanical stimuli and the actin-dependent Hippo-YAP regulation (1, 2). Rho stimulates the assembly of contractile actin stress fibers by the activation of downstream effectors such as Rho-associated kinase (ROCK) and mDia1/2, while Rac and Cdc42 promote the formation of F-actin networks, leading to lamellipodia and fillopodia extensions, respectively (35). It has been shown that activated Rho strongly enhances YAP/TAZ activity (11, 14), and Rac and Cdc42 may modulate the activity of YAP/TAZ (11) somewhat less vigorously. The treatment of human cells with the Rho inhibitor C3 transferase causes retention of YAP/TAZ in the cytoplasm (14). Similarly, treatment of cells with the ROCK inhibitor Y27632 reduces nuclear YAP/TAZ localization (14).
F-actin capping and severing factors
Actin-interacting proteins that restrict actin polymerization or sever actin filaments are also involved in the regulation of actin dynamics, adjusting cell shape and motility in response to environmental factors (36). Studies in Drosophila have showed that inactivation of actin-capping proteins, which results in abnormal accumulation of F-actin, leads to Yki activation and cell proliferation (37). In human mammary epithelial cells, the F-actin-capping protein CapZ and the F-actin-severing proteins Cofilin and Gelosin have been identified as crucial negative regulators of YAP/TAZ activity (10). Soft ECM decreases F-actin levels and suppresses the activity of YAP/TAZ. However, knockdown of Cofilin, Gelsolin, or CapZ increases F-actin levels and rescues the expression of YAP/TAZ target genes, even when cells were grown on soft ECM (10). Interestingly, knockdown of CapZ does not affect the level of YAP phosphorylation, suggesting that the actin cytoskeleton has the capacity to regulate YAP/TAZ activity, independent of the core Hippo kinases (10).
G-protein-coupled receptors (GPCRs)
GPCRs and their wide range of physiological ligands function to modulate a broad spectrum of cellular activities (38). It has been shown that GPCR ligands lysophosphatidic acid and sphingosine-1-phosphate promote YAP/TAZ activity by inhibiting LATS1/2 (4, 39). In contrast, GPCRs activated by glucagon or epinephrine inhibit YAP/TAZ activity (9, 40). Recurrent gain-of-function mutations in the G-protein Gαq/11 have been shown to drive tumorigenesis by activating YAP in uveal melanomas, supporting the importance of GPCR signaling in YAP/TAZ regulation (41). Generally, GPCRs linked to Gα12/13, Gαq/11, and Gαi/o inhibit the activity of LATS and induce YAP/TAZ activity, whereas GPCRs activating Gαs promote LATS activation and repress YAP/TAZ (4, 41). Importantly, GPCR-mediated regulation of Hippo-YAP signaling acts through promotion or inhibition of actin polymerization by Rho GTPases and protein kinase A, respectively (10, 40). Although the involvement of Rho and F-actin in GPCR-mediated YAP/TAZ activation is consistent, experimental evidence suggests that GPCR signaling can activate YAP/TAZ in a Hippo kinase-independent manner (41). The existing understanding of the contribution of the Hippo kinase cascade to the regulation of YAP/TAZ by GPCR signaling is incomplete, and further investigation is necessary.
HIPPO-YAP PATHWAY COMPONENTS REGULATED BY ACTIN REMODELING
Neurofibromin2 (NF2)
NF2, also known as Merlin, was first identified as a tumor suppressor mutated in neurofibromatosis 2, a dominantly inherited disorder characterized by benign tumors of the nervous system (42). NF2 is thought to provide a regulated linkage between membrane associated proteins and the cortical actin cytoskeleton (43). Later, NF2 was shown to function as an upstream regulator of the Hippo pathway in Drosophila (2). NF2 inhibits Yki by promoting plasma membrane association and activation of Warts (the Drosophila ortholog of LATS). Remarkably, disruption of the actin cytoskeleton increases interactions between NF2 and Warts (44). Moreover, inhibitory phosphorylation of Yki induced by treatment with latrunculin B or C3 transferase does not occur in cells depleted of NF2 (44). Therefore, the actin cytoskeleton is an important modulator of the role of NF2 in the Hippo-YAP signaling pathway.
Angiomotin (AMOT)
AMOT binds to F-actin and multiple tight junction components, and plays a role in maintaining cell polarity (2). Moreover, AMOT was identified as a YAP/TAZ binding protein (45). The PPxY motifs of AMOT directly interact with WW domains of YAP/TAZ. AMOT can regulate YAP/TAZ activity by both phosphorylation-independent and -dependent mechanisms (2). AMOT can suppress YAP/TAZ activity by tethering YAP/TAZ to tight junctions independently of their phosphorylation status and also by recruiting the AIP4/Itch ubiquitin ligase to induce YAP/TAZ degradation (46). In addition, it is also known that AMOT promotes YAP/TAZ phosphorylation at LATS target sits (45, 47), and this may be due to a scaffolding function of AMOT for Hippo components including LATS and NF2 (2).
LATS and YAP/TAZ
The activity of LATS1/2 is sensitive to elements of the physical environment such as cell geometry and cell attachment, whereas MST1/2 are not known to be strongly affected by mechanical cues in mammalian cells (2). Experimental evidence also indicates that LATS may be involved in the connection between YAP/TAZ activity and actin remodeling. Treatment of cells with cytochalasin D or latrunculin B induces inhibitory phosphorylation and cytoplasmic retention of YAP (11, 12). However, LATS1/2 inhibition results in YAP nuclear localization, and even after treatment with cytochalasin D (12). Moreover, YAP mutant forms which cannot be phosphorylated by LATS1/2 exhibit nuclear localization in cells treated with latrunculin B (11). It has been shown that in the downstream of the actin cytoskeleton, PKA activates Lats1/2 to phosphorylate Serine 381 of YAP, a residue for controlling the stability of YAP (48).
It is intriguing to note that recent studies have revealed findings thought to suggest that LATS does not play a dominant role in YAP/TAZ regulation in actin-dependent mechanotransduction. Latrunculin A treatment reduces the stability of TAZ, and the deletion of LATS 1/2 does not rescue this phenomenon (10). Consistently, latrunculin A treatment suppresses the activity of the TAZ mutant that is insensitive to LATS, suggesting that the actin cytoskeleton can regulate TAZ independently of LATS (14). Moreover, depletion of LATS1/2 does not rescue YAP/TAZ inhibition by a physically soft environment, demonstrating that mechanical cues can affect YAP/TAZ activity independent of LATS (10). Although LATS does not exert a clearly demonstrable inhibitory effect on YAP/TAZ in a soft environment, its effect can be restored when F-actin networks are re-established by depletion of CapZ (6, 10). Therefore, it has been suggested that the ability of LATS to influence YAP/TAZ activity is overridden by the requirement for a functional cytoskeleton although LATS activity is sensitive to mechanical cues.
CONCLUDING REMARKS
Our understanding of the regulation and function of Hippo pathway components is progressing rapidly. Moreover, recent studies have clearly demonstrated that the architecture and the dynamics of the actin cytoskeleton play key roles in the regulation of YAP/TAZ activity (Fig. 2). However, full and comprehensive understanding of the detailed molecular mechanisms linking the actin cytoskeleton to the regulation of Hippo pathway components still remains elusive. Although both LATS-dependent and -independent YAP/TAZ regulation by actin remodeling have been demonstrated, mechanistic understanding of how F-actin regulates LATS1/2 and YAP/TAZ activity awaits future studies. In addition, it will be important to understand relative contribution and in vivo relevance of the LATS-dependent and -independent mechanisms in various cellular contexts. While AMOT and NF2 are important mediators of the link between actin filaments and Hippo signaling, additional molecules involved in the link are likely to remain unidentified.
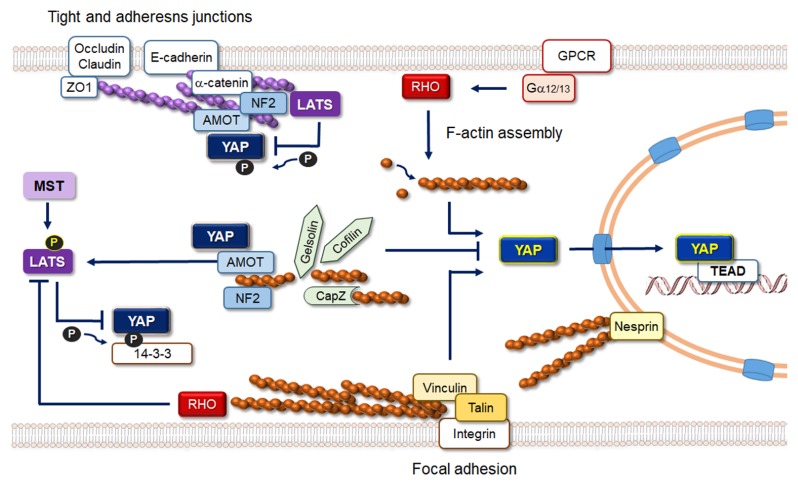
Fig. 2.
Schematic representation of actin-related regulation of Hippo signaling. Actin filaments associated with tight and adherens junctions negatively regulate YAP/TAZ activity, whereas stress fibers associated with focal adhesions promote YAP/TAZ nuclear enrichment. Activation of RHO GTPases either by GPCRs or by mechanical stimuli at focal adhesions promote F-actin assembly and YAP/TAZ nuclear localization. F-actin capping and severing factors inhibit YAP/TAZ activity in a LATS-independent manner. AMOT and NF2 play important roles in cytoplasmic retention of YAP/TAZ and also facilitate YAP/TAZ phosphorylation by LATS.
Cells in developing embryos are subjected to numerous mechanical stresses due to morphogenetic movements, cell migration and active cell proliferation. Normal body movements and gravitational forces also generate mechanical stressors on living tissues. Because YAP/TAZ function as key components of mechanotransduction at the downstream of cell junctions and the actin cytoskeleton, it is tempting to speculate that the YAP/TAZ-dependent transcriptional program might be integrated into developmental and homeostatic programs to determine each organ’s complex morphology, as well as size. Interestingly, YAP/TAZ can regulate the organization of cell junctions and the actin cytoskeleton by controlling the expression of actin/focal adhesion-related genes. This reverse regulation adds additional complexity to the circuitry of Hippo signaling and mechanotransduction (49, 50). Future work, using animal models and unbiased molecule screenings, will continue to provide exciting new insights and discoveries.
ACKNOWLEDGEMENTS
We apologize to colleagues whose work could not be cited due to space constraints. The authors were supported by the Stem Cell Program (2016M3A9B4915821) through the Korean National Research Foundation funded by the Korean Ministry of Science and ICT.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 4.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspar P, Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol. 2014;31:74–83. doi: 10.1016/j.ceb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Matsui Y, Lai ZC. Mutual regulation between Hippo signaling and actin cytoskeleton. Protein Cell. 2013;4:904–910. doi: 10.1007/s13238-013-3084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murrell M, Oakes PW, Lenz M, Gardel ML. Forcing cells into shape: the mechanics of actomyosin contractility. Nat Rev Mol Cell Biol. 2015;16:486–498. doi: 10.1038/nrm4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 11.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 13.Fischer M, Rikeit P, Knaus P, Coirault C. YAP-Mediated Mechanotransduction in Skeletal Muscle. Front Physiol. 2016;7:41. doi: 10.3389/fphys.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 15.Wrighton KH. Mechanotransduction: YAP and TAZ feel the force. Nat Rev Mol Cell Biol. 2011;12:404. doi: 10.1038/nrm3136. [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneko K, Ito M, Naoe Y, Lacy-Hulbert A, Ikeda K. Integrin alphav in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun. 2014;447:352–357. doi: 10.1016/j.bbrc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang KC, Yeh YT, Nguyen P, et al. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A. 2016;113:11525–11530. doi: 10.1073/pnas.1613121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–186. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Sansores-Garcia L, Bossuyt W, Wada K, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elosegui-Artola A, Oria R, Chen Y, et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol. 2016;18:540–548. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda M, Wada H, Kimura Y, Ueda K, Kioka N. Vinculin promotes nuclear localization of TAZ to inhibit ECM stiffness-dependent differentiation into adipocytes. J Cell Sci. 2017;130:989–1002. doi: 10.1242/jcs.194779. [DOI] [PubMed] [Google Scholar]
- 23.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23:803–811. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furukawa KT, Yamashita K, Sakurai N, Ohno S. The Epithelial Circumferential Actin Belt Regulates YAP/TAZ through Nucleocytoplasmic Shuttling of Merlin. Cell Rep. 2017;20:1435–1447. doi: 10.1016/j.celrep.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL. Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophys J. 2015;108:2783–2793. doi: 10.1016/j.bpj.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aureille J, Belaadi N, Guilluy C. Mechanotransduction via the nuclear envelope: a distant reflection of the cell surface. Curr Opin Cell Biol. 2017;44:59–67. doi: 10.1016/j.ceb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Strambio-De-Castillia C, Niepel M, Rout MP. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol. 2010;11:490–501. doi: 10.1038/nrm2928. [DOI] [PubMed] [Google Scholar]
- 33.Elosegui-Artola A, Andreu I, Beedle AEM, et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017;171:1397–1410 e14. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124:679–683. doi: 10.1242/jcs.064964. [DOI] [PubMed] [Google Scholar]
- 35.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinestel K, Wardelmann E, Hartmann W, Grunewald I. Regulators of Actin Dynamics in Gastrointestinal Tract Tumors. Gastroenterol Res Pract. 20152015:930157. doi: 10.1155/2015/930157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 38.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 39.Miller E, Yang J, DeRan M, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Yu FX, Zhang Y, Park HW, et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng X, Degese MS, Iglesias-Bartolome R, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 43.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis--the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 44.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao B, Li L, Lu Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucas EP, Khanal I, Gaspar P, et al. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J Cell Biol. 2013;201:875–885. doi: 10.1083/jcb.201210073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nardone G, Oliver-De La Cruz J, et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun. 2017;8:15321. doi: 10.1038/ncomms15321. [DOI] [PMC free article] [PubMed] [Google Scholar]