Abstract
Angiogenesis is a complex, multistep process involving dynamic changes in endothelial cell (EC) shapes and behaviors, especially in specialized cell types such as tip cells (with active filopodial extensions), stalk cells (with less motility) and phalanx cells (with stable junction connections). The Hippo-Yes-associated protein (YAP)/ transcription activator with PDZ binding motif (TAZ) signaling plays a critical role in development, regeneration and organ size by regulating cell-cell contact and actin cytoskeleton dynamics. Recently, with the finding that YAP is expressed in the front edge of the developing retinal vessels, Hippo-YAP/TAZ signaling has emerged as a new pathway for blood vessel development. Intriguingly, the LATS1/2-mediated angiomotin (AMOT) family and YAP/TAZ activities contribute to EC shapes and behaviors by spatiotemporally modulating actin cytoskeleton dynamics and EC junction stability. Herein, we summarize the recent understanding of the role of Hippo-YAP/TAZ signaling in the processes of EC sprouting and junction maturation in angiogenesis.
Keywords: VEGF, Vascular development, Endothelial cell junction, AmotL1/2, Hecw2
INTRODUCTION
The vascular system is formed by angiogenic expansion that is essential for normal development. Angiogenesis is a complex, multistep process with dynamic changes in endothelial cell (EC) shapes and behaviors. Such changes are thought to represent the appearance of tip cells (with polarized and dynamic filopodial extensions), stalk cells (with less motility and connectivity with parental blood vessels) and phalanx cells (with stable junction connections between neighboring ECs) (1). To elucidate the molecular bases of how ECs dynamically coordinate these cell shapes and behaviors, several mechanistic molecules [e.g., vascular endothelial growth factor (VEGF)/VEGFR, Dll4/Notch, Angiopoietins/TIEs, and platelet-derived growth factor (PDGF)/PDGFR] have be intensely investigated and extensively studied. These molecules activate downstream kinases (e.g., mitogen-activated protein kinases and Akts) and adaptor molecules, and thereafter influence transcription factors (e.g., forkhead box protein 1/3 and Erg), ultimately leading to a variety of gene expression(s) to direct EC shapes and behaviors (2–6). Therefore, understanding the signaling network that controls angiogenesis would play an important role not only in identifying genes whose defects cause angiogenic disorders in the developmental and pathological settings, but also defining therapeutic targets in pathological angiogenesis.
The Hippo signaling comprises evolutionarily conserved kinases and is activated by a variety of contexts, including cell-cell junction formation, the actin cytoskeleton dynamics and mechanical and hormonal regulation (7, 8). The core kinases are MST1/2, SAV1, MOB1, and LATS1/2 (Fig. 1B). MST1/2 interaction with SAV1 activates MST1/2. MST1/2 phosphorylation leads to MOB1 phosphorylation, which in turn phosphorylates LATS1/2. When the Hippo signaling is on, this cascade is sequentially phosphorylated and activated (Fig. 1B). Subsequently, Yes-associated protein (YAP)/ transcription activator with PDZ binding motif (TAZ), effectors of the Hippo signaling, are phosphorylated and retained and/or degraded in the cytoplasm and cell junctions (9). When the Hippo pathway is off, YAP/TAZ is dephosphorylated and shuttled into the nucleus, where it forms a complex with DNA-binding transcription factors including TEAD, Smads, and TBX5 (Fig. 1A) (10). The complex induces the expressions of a wide range of genes (e.g., components of cell-cell junction, cytoskeletal regulators, cellular organelles, kinases and secreted proteins) which are involved in proliferation, survival, migration and cell junction contact.
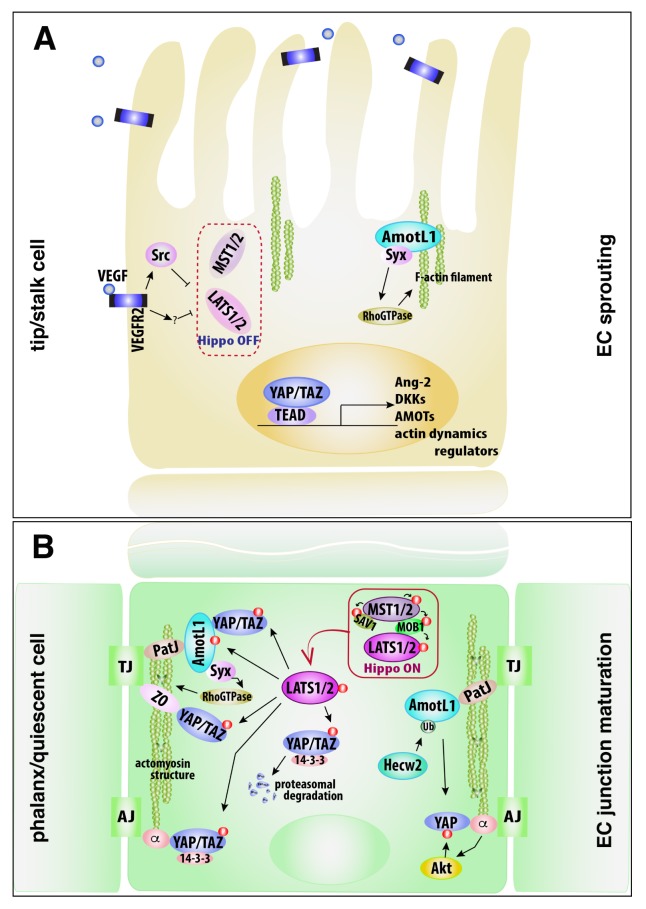
Fig. 1.
Schematic diagram depicting the roles of the Hippo-YAP/TAZ signaling in EC sprouting and junction maturation. (A) VEGF exposure to ECs inactivates LATS1/2, leading to dephosphorylation of AmotL1 and YAP/TAZ in tip and stalk cells. AmotL1 binds with F-actin, regulating the dynamics of F-actin filament via Syx. YAP/TAZ is shuttled into nucleus, increasing expressions of a variety of genes, which are essential for the initiation and expansion of angiogenic sprouting. Syx, Rho GTPase-exchanging factor protein; AmotL1, angiomotin-like 1. (B) Endothelial cell-cell contact induces the Hippo kinase activity. In phalanx cells, LATS1/2 activation phosphorylates AmotL1 and YAP/TAZ. The phosphorylated AmotL1 forms complex with PatJ and Syx to regulate the small Rho GTPase for actomyosin contractility at TJs. The phosphorylated YAP/TAZ leads to the interaction with AmotL1 and ZO proteins at TJs. In addition, the phosphorylated YAP/TAZ is recruited to VE-cadherin-catenin complex at AJs, and degraded in the cytoplasm in the proteasome-dependent proteolysis. Meanwhile, in quiescent ECs, VE-cadherin-mediated Akt activity phosphorylates YAP. AmotL1 is ubiquitinated by Hecw2 and recruited to TJs to promote TJ stability and to seemingly maintain the retention of YAP at EC junction/ cytoplasm. TJ, tight junction; AJ, adherens junction; ZO, zonula occludens; PatJ, polarity-/junction-associated scaffold protein; Hecw2, HECT, C2 and WW domain containing E3 ubiquitin protein ligase 2.
So far, most studies on the Hippo-YAP/TAZ signaling have been focused on the field of epithelial cells. Based upon the remarkable parallels and similarity between the signaling pathway(s) controlling angiogenesis and the development of epithelial tissues, there has been speculation regarding the potential role of Hippo-YAP/TAZ signaling in sprouting angiogenesis. In support of this hypothesis, the physiological relevance of YAP in vascular biology has been underscored by developmental arrest with vascular defects in the yolk sac (11). In particular, new insights into vascular development via the Hippo-YAP/TAZ signaling have been obtained by the finding that YAP is specifically expressed in ECs of the developing mouse retina (12). In addition, studies on differentially expressed genes between proliferating/motile ECs and contacted ECs have shown increased expressions of cyr61, ctgf, Dickkopfs (DKKs) and angiopoietin-2 (Ang-2), which are target genes of YAP/TAZ (12–15). Thus the question of how Hippo-YAP/TAZ signaling in ECs regulates vascular morphogenesis arises. In this review, we outline the current understanding of the role of the Hippo-YAP/TAZ signaling mechanism in the process of angiogenic sprouting and junction maturation in ECs.
Hippo-YAP/TAZ Signaling in Guided Disorganization of EC Junctions
In stable, quiescent blood vessels, ECs typically form a cobblestone-like monolayer and line the luminal surface of the vasculature, acting as a permeability barrier through the coordinated opening and closure of cell-cell junctions. The junctions are effectively provided by adhesive interactions at adherens (AJs) and tight junctions (TJs) (Fig. 1B) (16, 17). These junctions are linked to the contractile actomyosin apparatus of neighboring ECs, and are maintained until the ECs sense angiogenic signals and induce loosening of the junctions.
VEGFA acts as a master regulator in sprouting angiogenesis and regulates actin cytoskeleton dynamics and EC junction integrity (18). When VEGFA binds VEGFR2, the complex activates multiple downstream signalings, leading to the invasive, motile behavior, which turns on the angiogenic switch. VEGF/VEGFR2-mediated Src signaling induces the phosphorylation of vascular endothelial (VE)-cadherin and subsequently triggers the disruption of its assembly. Along with instability of VE-cadherin (an AJ protein), angiogenic stimuli also serves to produce the ablation of TJ proteins. Intriguingly, in vitro studies have shown that VEGF exposure to EC inhibits LATS1/2 activity through stimulation of VEGFR2-Src kinase, although details of the actual mechanisms of VEGF-mediated Hippo kinase inactivation in ECs are poorly understood (Fig. 1A) (19). The inactivation of the Hippo kinase, LATS1/2, is likely to enhance the structural disorganization of AJs and TJs through regulating the activities of AMOTs and YAP/TAZ.
LATS1/2 inactivation regulates cellular localization of the angiomotin family proteins (AMOTs). The AMOTs consist of three members: Amot, angiomotin-like 1 (AmotL1) and AmotL2 (20). AMOTs are characterized by an N-terminal domain with proline-proline-x-tyrosine (PPxY) motif, a WW binding motif, an F-actin binding motif and a C-terminal PDZ-binding motif. Through these motifs, AMOT activity is regulated via a posttranslational mechanism, such as phosphorylation by LATS1/2 and ubiquitination by E3 ligases. Recently, the role of AMOTs in ECs has been determined (21). Of AMOTs, AmotL1 is predominantly located in the TJs and acts as an adaptor to link junction proteins to the actomyosin structure by regulating the local activity of the small Rho GTPases at the junction boundary in stable, quiescent ECs (Fig. 1B) (22). Following VEGF exposure to blood vessels, VEGFR2-mediated LATS1/2 inactivation dephosphorylates AmotL1 and releases it from the TJs (Fig. 1A). This event is likely to potentiate the disruption of the linkage between junction proteins and the actomyosin structure, resulting in ECs losing adherence and attaining motility.
LATS1/2 inactivation also dephosphorylates YAP/TAZ and translocates it from plasma membrane/cytoplasm into the nucleus, facilitating YAP/TAZ’s transcriptional activity to disrupt EC junction stability and induce dynamic changes in EC behaviors (Fig. 1A). Supporting these events, in vivo studies using inducible, EC-specific Yap/Taz and Lats1/2 knockout (Yap/TaziΔEC and Lats1/2iΔEC) mice have recognized that VEGF signaling inhibits the Hippo signaling, inducing YAP/TAZ activity to control the expression of gene subsets (including genes related to junction assembly and EC migration) (19, 23). Another study has shown that YAP is expressed in the front region of the growing vessels and increases the transcription of angiopoietin (Ang)-2, a functional antagonist of ang-1/TIE-2 signaling, to enhance EC sprouting and destabilize EC junction (4, 12). Consequently, LATS1/2 inactivation, AmotL1 dephosphorylation and YAP/TAZ nuclear activity act as determinants of the loosening of EC junction, thereby influencing subsequent initiation and expansion of angiogenic sprouting.
YAP/TAZ in Initiation and Expansion of Angiogenic Sprouting
In sprouting angiogenesis, tip cells are characterized as cells with numerous filopodia to direct the leading front of angiogenic vessels (1). Filopodia are formed by actin filaments cross-linked structures in a Cdc42-dependent manner (24).
Recent findings have revealed that these tip phenotypes are related to the on/off system of Hippo-YAP/TAZ signaling. Deletion of Yap/Taz in mice has shown dramatic defects in the numbers of tip cells, spouts and branching. Hyperactivation of YAP/TAZ induced in Lats1/2iΔEC mice has displayed excessive filopodia and branches and hyperplastic vascular growth (23). Chromatin immunoprecipitation-sequencing analysis and gene set enrichment analysis in Yap/TaziΔEC mice showed increased numbers of subsets of extracellular matrix organization genes, cytoskeleton assembling genes and junction regulators, thus demonstrating the relationship between filopodia/sprouts defects and a decrease in gene subsets by Hippo-YAP/TAZ signaling (19, 23). Furthermore, these reports are likely to consider defects or excessiveness of filopodia and branching as uncontrolled actin cytoskeleton dynamics. Indeed, transcriptional targets detected in Yap/TaziΔEC mice are found to control the Cdc42/Rac1-mediated actin filament activity by increasing the expression of downstream target genes (for example, Mlc2, F-actin filament-related genes, DKK-1/2, and AMOTs) (Fig. 1A).
The DKK-1/2, transcriptional targets of YAP/TAZ, are reported to influence filopodial protrusion (14). The DKK family includes DKK-1, -2, -3 and -4. DKK-1 and DKK-2 are most studied in ECs and act as secreted, potent Wnt antagonists. The DKK-1/2 binds to the Wnt coreceptor LRP5/6. The role of DKK-1 in ECs is known in ocular pathological neovascularization and oxygen-induced retinopathy (25). EC-specific DKK-1 transgenic mice show significant reductions in tip cells and filopodia in the developing vessels. It seems that DKK-1 binds to leucine-responsive regulatory proteins (LRPs) to inhibit the formation of the Wnt-Fz-LRP complex, resulting in the blockade of β-catenin signaling. Contrary to DKK-1, EC-specific expression of DKK-2 increases vessel density, the number of tip cells and filopodia extension (14). This angiogenic activity of DKK-2 occurs through Wnt/β-catenin-independent pathways, seemingly promoting Cdc42 activity through LRP-mediated adenomatous polyposis coli (APC)/APC-stimulated guanine nucleotide exchange factor (Asef) signaling. During angiogenic expansion of vessels, a proper balance of DKK-1/2 expressions by YAP/TAZ might lead to the formation of a fine-tuned vascular patterning.
In vitro studies on AmotL1 in ECs and angiogenesis have suggested that AmotL1 localizes to the leading edge of the cell and binds with F-acitn, affecting cell migration (Fig. 1) (22). To elucidate the mechanistic roles of AMOTs in migrating ECs, AMOTs were applied to identify proteins that specifically interact with the PDZ-binding motif of AMOTs, because AMOTs have several requisite motifs (e.g., PPxY motifs, a WW binding motif, and a PDZ binding motif) necessary for interaction with other proteins. As AMOT-interacting proteins, the polarity-/junction-associated scaffold protein PatJ and the Rho GTPase-exchanging factor protein Syx were identified, suggesting that AMOTs control small Rho GTPase activity (26, 27). Indeed, this function was verified by in vivo studies. AmotL1 deficiency in zebrafish results in the inhibition of migration of sprouting vessels, a decrease in the number of filopodia and the abnormal polarization of tip cells (22). Furthermore, RhoA activities in AmotL1-depleted cells are uniformly dispersed though the plasma membrane, whereas those in wild type cells are confined to the lamelipodia. Thus, it appears that AmotL1-Syx binding seems to facilitate precise localization of small Rho GTPase activity to the leading front of vessels to regulate actin cytoskeleton dynamics in sprouting, migrating blood vessels (Fig. 1A).
Meanwhile, the developing blood vessels generate a lumen for the circulation through intracellular vacuole-mediated cell hollowing (28). Several studies on the involvement of small Rho GTPases and Par proteins in the process have shown that Par3 and Par6 are localized to endothelial cell-cell junctions and associate directly with the VE-cadherin-catenin complex, and that the small Rho GTPase activity is required for Par-VE-cadherin interaction (29, 30). Ras-interacting protein 1 (Rasip1) was also identified as a regulator of Rho GTPase signaling, and its loss resulted in excessive actomyosin contractility and improper Par3 localization (31). Interestingly, AmotL2 was also identified as a transcriptional target of YAP/TAZ and is known to act as a link between VE-cadherin and contractile actin filaments for lumen expansion to extend the lumen diameter (23, 32, 33). In vitro AmotL2 deletion inhibits EC migration and disrupts the vascular tube-like structure (34). Studies on zebrafish and mice have shown that AmotL2 is required for lumen formation via AmotL2-VE-cadherin complex (33). Researches found that in AmotL2iΔEC mice, aortic constrictions were brought close to the ventricular outflow tract because of loss of actomyosin contractile forces. In addition, they discovered that Par3 is needed for localization of AmotL2 to VE-cadherin junctions to organize actin filaments for EC biological processes, such as lumen expansion (32). Hence, AmotL2 seems to be essential for proper lumen expansion; however, more detailed studies for lumen formation during vascular development are still required.
Hippo-YAP/TAZ Signaling in EC Junction Maturation
Upon growing tip/stalk cells change to phalanx cells, ECs inhibit their ability to proliferate and migrate and loosely connected junctions between stalk cells are replaced by EC connections with an assembly of AJ and TJ proteins (Fig. 1B). The VE-cadherin (an AJ protein) and Occludin/Claudins (TJ proteins) initiate the assembly of a primordial endothelial cell-cell junction. These junction proteins form adhesive bonds by trans and/or cis interactions at the plasma membrane and contribute to the protection of the stability and homeostasis of ECs during environmental changes. The ability and strength of these junction proteins are regulated by the extent(s) of their attachment to the actin cytoskeleton. The junction connection requires recruitment of actin-associated proteins and small Rho GTPases, Cdc42/Rac, which facilitate the formation of the actomyosin apparatus by maintaining phosphorylation of non-muscle myosin-II via MRCK-dependent phosphorylation of MLCP (17, 35, 36). As a result, the actomyosin apparatus interacts with VE-Cadherin and TJ proteins, finally leading to intercellular contraction to form a cortical ring structure and stabilize the EC junctions.
Endothelial cell-cell contact and the assembly of AJs and TJs can activate the Hippo signaling in phalanx cells, although the precise mechanism(s) emanating from ECs are poorly understood (37). At the level of TJs, LATS1/2 activation binds and phosphorylates YAP/TAZ and AmotL1 (Fig. 1B). On LATS1/2 activation along with EC junction formation, an F-actin binding motif within AmotL1 protein is phosphorylated by LATS1/2, resulting in AmotL1 dissociation from F-actin and reduced focal adhesion assembly. Phosphorylated AmotL1 forms a complex with PatJ and Syx at the TJs. The AmotL1-PatJ-Syx at TJs controls Cdc42 activity to increase the strength of EC junction connectivity. The localization of AmotL1 at TJs is confirmed by the report that morpholino-mediated knockdown of AmotL1 in developing vessels of zebrafish causes destabilization of the connection between the stalk cells (22). Another report also confirms that posttranscriptional modification of AmotL1 effects on its spatial localization and cellular function. In vitro evidence suggests that AmotL1 is ubiquitinated by Hecw2, a Nedd4-like E3 ligase, and thereby localized to EC junctions, which contributes to the stability of EC junction integrity (38). Furthermore, phosphorylation of YAP/TAZ by LATS1/2 results in the shuttle of YAP/TAZ from the nucleus to the cytoplasm and leads to the interaction with AmotL1-mediated TJs. Phosphorylated YAP can bind with ZO-2, and modulate AmotL1 stability by protecting it from preteasomal degradation by c-Abl at TJs (39). Thus, LATS1/2 activation recruits AmotL1 and YAP/TAZ at the junction structure and is likely to participate in junction maturation.
At the cytoplasmic level, phosphorylated YAP binds to the 14-3-3 protein and is sequestered by binding to VE-cadherin-binding α-catenin at AJs (9). Additional phosphorylation of YAP by casein kinase drives into ubiquitin-dependent proteasomal degradation. This event ultimately serves to produce changes in the expression of a variety of genes governing actin cytoskeleton dynamics, and reduces the motility and proliferative ability of the cells. A recent report has shown the importance of cytoplasmic YAP activity in angiogenic vessels, suggesting crosstalk between Hippo signaling and Cdc42 (40). The idea was initiated by filopodial loss in Cdc42 conditional knockout mice and Cdc42 activation along with YAP phosphorylation in confluent ECs. The authors suggested that a constitutively, nuclear-active form of Yap transgenic mice did not alter neovascularization, although YAP target genes (ctgf and cyr61) were upregulated. Furthermore, overexpression of YAPS127D, the active form of YAP, greatly increased the level of active Cdc42. On the basis of these results, they have demonstrated that phosphorylated YAP indirectly activates Cdc42 by likely regulating Cdc42-GEF or Cdc42-GAP activity, suggesting a distinct cytoplasmic role of YAP. However, the precise mechanism as to how cytoplasmic YAP affects small Rho GTPase activity is still unclear.
In quiescent and contacted ECs, VE-cadherin clustering can induce relatively low and sustained Akt activation (41, 42). As suggested by several studies, this low but sustained activity of Akt causes phosphorylated YAP to maintain vessel quiescence (42–44). In growing blood vessels, YAP is a downstream transcriptional co-activator of the Hippo pathway that is triggered by growth signal mediation. However, in stable and quiescent ECs, the AJ complex can be an upstream regulator of YAP somehow, irrespective of the Hippo pathway (12, 45). Studies have shown that knockdown of VE-cadherin and catenins results in YAP dephosphorylation, EC motility, EC sprouting and Ang-2 increase, although these phenotypes are not affected by LATS1/2, but by Akt. Considering that quiescent ECs are constantly exposed to haemodynamic flow changes, it is thought that Akt-mediated YAP phosphorylation in quiescent ECs may represent a product of a mechanosensing transducer, by which ECs change their shapes and behaviors (46).
CONCLUSION
With recent findings, our understanding of Hippo-YAP/TAZ signaling as a new determinant of blood vessel formation is growing. Hippo-YAP/TAZ signaling modulates EC shapes and behaviors through actin cytoskeleton dynamics (Fig. 1). These actin cytoskeleton dynamics can be governed by spatiotemporal differential activities of LATS1/2, AMOTs, and YAP/TAZ. In the initiation phase of EC sprouting in response to VEGF, Hippo kinases are off and nuclear activity of YAP/TAZ is observed, followed by F-actin polymerization, filopodia extension, and stress fibre formation (Fig. 1A). Thereafter, Hippo kinases turn on and YAP/TAZ activities turn off, followed by enhanced intercellular junction connectivity with the contractile actomyosin network (Fig. 1B). Overall, Hippo-YAP/TAZ reads angiogenic signals and mediates biological effects via a collection of signaling molecules to promote vascular sprouting and junction maturation.
YAP and TAZ are co-transcriptional factors which play multifaceted roles which control a variety of cellular phenomena (10). Thus, in addition to the regulation of actin cytoskeleton dynamics, their transcriptional activities can likely control EC metabolism, energy homeostasis, survival, mitochondrial activity, extracellular matrix organization, inflammation and tissue-specific effects due to the YAP/TAZ tissue-specific transcriptional program. Furthermore, investigation of crosstalk between the Hippo signaling and other signaling pathways (Notch, Wnt, TGF, BMP, and GPCR signals) may shed light on the diverse mechanisms underlying vascular development, as well (43, 47–49). For instance, BMP signaling crosstalks with Hippo signaling by inhibiting YAP target genes. Therefore, a better understanding of upstream regulators and downstream effectors of YAP/TAZ could provide new means of interpreting physiological angiogenesis, and could prove essential for the design of a novel therapeutic target in pathological angiogenesis.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI16C1501) to J.A.P. This work was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) (Grant Number: NRF-2015R1A2A1A05001859) to Y.-G.K. and by the Bio and Medical Technology Development Program of the NRF funded by the MEST (Grant Number: NRF-2015M3A9B6066967) to Y.-G.K.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potente M, Urbich C, Sasaki K, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birdsey GM, Dryden NH, Amsellem V, et al. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–3506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felcht M, Luck R, Schering A, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama M, Nakayama A, van Lessen M, et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol. 2013;15:249–260. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kume T. Foxc2 transcription factor: a newly described regulator of angiogenesis. Trends Cardiovasc Med. 2008;18:224–228. doi: 10.1016/j.tcm.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S, Irvine KD. Cellular Organization and Cytoskeletal Regulation of the Hippo Signaling Network. Trends Cell Biol. 2016;26:694–704. doi: 10.1016/j.tcb.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25:499–513. doi: 10.1016/j.tcb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morin-Kensicki EM, Boone BN, Howell M, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi HJ, Zhang H, Park H, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- 13.Glienke J, Schmitt AO, Pilarsky C, et al. Differential gene expression by endothelial cells in distinct angiogenic states. Eur J Biochem. 2000;267:2820–2830. doi: 10.1046/j.1432-1327.2000.01325.x. [DOI] [PubMed] [Google Scholar]
- 14.Min JK, Park H, Choi HJ, et al. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest. 2011;121:1882–1893. doi: 10.1172/JCI42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park HW, Kim YC, Yu B, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentley K, Franco CA, Philippides A, et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol. 2014;16:309–321. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]
- 17.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 18.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Freire Valls A, Schermann G, et al. YAP/TAZ Orchestrate VEGF Signaling during Developmental Angiogenesis. Dev Cell. 2017;42:462–478 e467. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Moleirinho S, Guerrant W, Kissil JL. The Angiomotins--from discovery to function. FEBS Lett. 2014;588:2693–2703. doi: 10.1016/j.febslet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aase K, Ernkvist M, Ebarasi L, et al. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 2007;21:2055–2068. doi: 10.1101/gad.432007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, Vertuani S, Nystrom S, et al. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ Res. 2009;105:260–270. doi: 10.1161/CIRCRESAHA.109.195156. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Kim YH, Kim J, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. 2017;127:3441–3461. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barry DM, Xu K, Meadows SM, et al. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development. 2015;142:3058–3070. doi: 10.1242/dev.125260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park H, Jung HY, Choi HJ, et al. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2014;17:221–234. doi: 10.1007/s10456-013-9390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garnaas MK, Moodie KL, Liu ML, et al. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in vivo. Circ Res. 2008;103:710–716. doi: 10.1161/CIRCRESAHA.108.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernkvist M, Luna Persson N, Audebert S, et al. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009;113:244–253. doi: 10.1182/blood-2008-04-153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iden S, Rehder D, August B, et al. A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep. 2006;7:1239–1246. doi: 10.1038/sj.embor.7400819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 31.Xu K, Sacharidou A, Fu S, et al. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell. 2011;20:526–539. doi: 10.1016/j.devcel.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hultin S, Subramani A, Hildebrand S, Zheng Y, Majumdar A, Holmgren L. AmotL2 integrates polarity and junctional cues to modulate cell shape. Sci Rep. 2017;7:7548. doi: 10.1038/s41598-017-07968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hultin S, Zheng Y, Mojallal M, et al. AmotL2 links VE-cadherin to contractile actin fibres necessary for aortic lumen expansion. Nat Commun. 2014;5:3743. doi: 10.1038/ncomms4743. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Li Z, Xu P, et al. Angiomotin-like2 gene (amotl2) is required for migration and proliferation of endothelial cells during angiogenesis. J Biol Chem. 2011;286:41095–41104. doi: 10.1074/jbc.M111.296806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kouklis P, Konstantoulaki M, Vogel S, Broman M, Malik AB. Cdc42 regulates the restoration of endothelial barrier function. Circ Res. 2004;94:159–166. doi: 10.1161/01.RES.0000110418.38500.31. [DOI] [PubMed] [Google Scholar]
- 36.Komarova YA, Kruse K, Mehta D, Malik AB. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ Res. 2017;120:179–206. doi: 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 38.Choi KS, Choi HJ, Lee JK, et al. The endothelial E3 ligase HECW2 promotes endothelial cell junctions by increasing AMOTL1 protein stability via K63-linked ubiquitination. Cell Signal. 2016;28:1642–1651. doi: 10.1016/j.cellsig.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Skouloudaki K, Walz G. YAP1 recruits c-Abl to protect angiomotin-like 1 from Nedd4-mediated degradation. PLoS One. 2012;7:e35735. doi: 10.1371/journal.pone.0035735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakabe M, Fan J, Odaka Y, et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc Natl Acad Sci U S A. 2017;114:10918–10923. doi: 10.1073/pnas.1704030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Napione L, Pavan S, Veglio A, et al. Unraveling the influence of endothelial cell density on VEGF-A signaling. Blood. 2012;119:5599–5607. doi: 10.1182/blood-2011-11-390666. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernascone I, Martin-Belmonte F. Crossroads of Wnt and Hippo in epithelial tissues. Trends Cell Biol. 2013;23:380–389. doi: 10.1016/j.tcb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/S1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 45.Giampietro C, Disanza A, Bravi L, et al. The actin-binding protein EPS8 binds VE-cadherin and modulates YAP localization and signaling. J Cell Biol. 2015;211:1177–1192. doi: 10.1083/jcb.201501089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakajima H, Yamamoto K, Agarwala S, et al. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Dev Cell. 2017;40:523–536 e526. doi: 10.1016/j.devcel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 47.Young K, Tweedie E, Conley B, et al. BMP9 Crosstalk with the Hippo Pathway Regulates Endothelial Cell Matricellular and Chemokine Responses. PLoS One. 2015;10:e0122892. doi: 10.1371/journal.pone.0122892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim W, Khan SK, Gvozdenovic-Jeremic J, et al. Hippo signaling interactions with Wnt/beta-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127:137–152. doi: 10.1172/JCI88486. [DOI] [PMC free article] [PubMed] [Google Scholar]