Abstract
Undernutrition and diarrhoeal disease are major causes of infant mortality. We investigated the combined roles of breastfeeding and diarrhoea on infant size in 2940 infants from the Cebu Longitudinal Health and Nutrition Survey. The study aimed to assess whether breastfeeding status modified the deficits associated with diarrhoeal disease. The primary exposures were combinations of current breastfeeding status (yes/no), the presence of diarrhoeal disease in previous week (yes/no) and a categorical survey variable (six surveys taken at bimonthly intervals when infants were 2–12 months of age). Relative weight (weight‐for‐length z‐scores), calculated using the WHO growth standards, was estimated using sex‐stratified, fixed‐effects longitudinal models that also adjusted for energy from complementary foods. Post‐estimation Wald tests were conducted to identify subgroup differences in relative weight. Diarrhoea was associated with reduced relative weight in both breastfed and non‐breastfed infants of 6–12 months. Diarrhoea‐related deficits in relative weight were significantly exacerbated in non‐breastfed girls of 6 and 8 months. Importantly, in infants <6 months, being breastfed and having diarrhoea was still associated with greater relative weight compared with being non‐breastfed and diarrhoea‐free. Breastfeeding emerged as a strong contributor to relative weight in younger infants (<6 months) while diarrhoeal disease strongly contributed to deficits in relative weight in older infants (6–12 months). These findings underscore the importance of breastfeeding for promoting infant nutritional status in infants with or without diarrhoea from birth to 12 months.
Keywords: diarrhoea, infant growth, weight, nutritional status, breastfeeding, complementary feeding
Introduction
Undernutrition and diarrhoeal disease are prominent causes of infant mortality worldwide (UNICEF & WHO 2009, UNICEF et al. 2015). Poor nutrition is related to at least 45% of infant mortality while diarrhoeal disease is the second‐leading cause of child deaths, accounting for 8% of deaths in children under five each year (UNICEF & WHO 2009, UNICEF et al. 2015). These two public health problems are closely related: diarrhoea greatly increases the risk of malnutrition and undernourished infants often suffer more severe cases of diarrhoea (Scrimshaw et al. 1968). In addition to the detrimental impacts that these two pathologies have on infant size, their effects may also extend to reductions in educational attainment and decreased work productivity, among other long‐term consequences (Guerrant et al. 2008).
The infant diet is especially important because both breastfeeding and quality complementary feeding can improve nutritional status. Numerous studies indicate that breastfeeding can reduce diarrhoea risk. Indeed, specific oligosaccharides found in human breastmilk have been linked to reduction in severity and incidence of diarrhoea (Morrow et al. 2004). Conversely, the premature introduction of complementary foods and the concomitant exposure to pathogens via complementary foods can increase the probability of diarrhoea (Scrimshaw et al. 1968; Popkin et al. 1990). As the infant matures, the weaning diet serves as an important complement to breast milk that ideally meets the needs of the growing infant. The weanling's dilemma is characterized by the critical need for nutrients provided by complementary foods—even though said foods may potentially expose the infant to harm (Scrimshaw et al. 1968; Rowland et al. 1978).
Here we sought to characterize the role of breastfeeding in promoting infant nutritional status from 2 to 12 months by exploring whether breastfeeding modified the effects of diarrhoeal disease on relative weight. We hypothesized that (1) breastfeeding would mitigate the effects of diarrhoea on relative weight; (2) breastfed infants with diarrhoea would still fare better than their non‐breastfed peers without diarrhoea.
Key messages.
Mothers reported altered feeding patterns and reduced appetite in infants with diarrhoea.
In infants <6 months, being breastfed while having diarrhoea was associated with better relative weight compared with being non‐breastfed and diarrhoea‐free.
Diarrhoea resulted in deficits for infants between 6 and 12 months, but deficits were more pronounced if infants were not breastfed.
Results support the WHO infant feeding recommendations regarding exclusive breastfeeding in infants until 6 months.
Sophisticated models and detailed data are needed to assess whether breastfeeding and other aspects of the infant diet may modify the impact of diarrhoea on infant relative weight.
Methods
Participants
The Cebu Longitudinal Health and Nutrition Survey (CLHNS) began in 1983 as a study of infant feeding patterns in 3080 pregnant women who gave birth to singletons in the Cebu, Philippines (Adair et al. 2011). Demographic, dietary and anthropometric data were collected at birth and at 12 bimonthly time points until the infants were ~24 months old. Analyses here are restricted to the 6 surveys spanning the 2 to 12‐month period to specifically quantify the effects of breastfeeding and diarrhoea when breastfeeding rates were highest (pre‐6 months) and later after the introduction of complementary foods (post‐6 months). All analyses were stratified by sex because sex‐differences in feeding patterns, morbidity and anthropometry have been reported in this cohort (Popkin et al. 1990). After exclusions because of missing data and implausible reports, 2940 infants were analysed in this study (1386 girls and 1554 boys) each with a mean of 5.5 complete surveys.
Anthropometry
Weight and length were measured at bimonthly intervals using standard techniques (Briscoe 1991). Weight‐for length z‐scores (WLZ) based on the WHO growth standards were used to account for small age differences and to relate WLZ in this population to the global standard (WHO Multicentre Growth Reference Study Group & de Onis 2006). WLZ was chosen as the measure of infant size because relative weight is a strong predictor of mortality (Olofin et al. 2013) and incorporates infant length. Standardized weight (WAZ) and length (LAZ) were secondary outcomes also calculated using the WHO growth standards.
Diet
Dietary data were derived from 24‐h dietary recalls in which mothers reported all liquids and solids fed to the infant in the previous 24 h. Energy intake from complementary foods was calculated using nutrient values derived from the Philippines Food and Nutrition Research Institute (Food and Nutrition Research Institute of the Philippines (FNRI) 1980) and coded continuously in 1000 kilocalorie units. Current breastfeeding status was a binary (yes/no) variable based on whether the infant was breastfed on the previous day, and thus corresponded with the timing of the 24‐h dietary recall.
Diarrhoeal disease
Mothers reported whether the infant had shown symptoms of diarrhoea (yes/no) in the previous 7 days.
Primary exposure
Combinations of current breastfeeding status and diarrhoea status were coded using binary indicators, resulting in a four‐level primary exposure of (1) no breastfeeding and with no diarrhoea, (2) breastfeeding and with no diarrhoea (referent), (3) no breastfeeding and with diarrhoea and (4) breastfeeding and with diarrhoea. Each exposure pattern was mutually exclusive for any given survey, but infants could change exposure patterns over time. These four exposures were then allowed to statistically interact with a six‐level categorical survey variable that corresponded to months 2 to 12 at bimonthly intervals. This survey interaction was included to capture age‐related changes in the impact of breastfeeding and morbidity on nutritional status.
Additional covariates
Two feeding variables were described to provide better context for the relationships of interest, but they were not included in the regression analyses because they presumably lie on the pathway between breastfeeding‐morbidity pattern and infant size. For each survey, mothers were asked whether their infant's appetite in the last week was typical or reduced. This appetite score was on a scale of 1 to 5 (5 represented normal appetite and 1 represented significantly reduced appetite). We also examined changes in feeding patterns during bouts of diarrhoea: mothers were asked whether they fed the infant special foods, or withheld certain foods, or whether they (the breastfeeding mothers) consumed special foods to aid infant's health.
Statistical analyses
A fixed‐effects longitudinal regression model with a robust variance estimator was used to quantify the association between breastfeeding and morbidity exposure patterns with infant nutritional status throughout the 2–12‐month period. A fixed‐effects model was chosen instead of a random‐effects model because a Hausman test showed that the assumption of independence in the individual errors in our sample was violated (StataCorp 2013). The effect of time‐invariant variables such as maternal age, height, education, SES and other potential confounders on infant size is assumed to be constant in a fixed‐effects model so beta coefficients for those variables were not estimated; thus this regression model estimated the population mean of the within‐child effect of time‐varying covariates.
Post‐estimation tests
Coefficients from the longitudinal regression models were used to estimate WLZ, WAZ and LAZ for infants with different breastfeeding‐diarrhoea patterns. The mean energy intake for a given sex, survey and breastfeeding status was used in these calculations. Graphs were created using Microsoft Excel.
Post‐estimation Wald tests were conducted to calculate whether relative weights varied by breastfeeding and diarrhoea patterns (StataCorp 2013). The null hypothesis for these tests was that the relative weights were equivalent. Pursuant to our main hypotheses, we estimated the average diarrhoea‐related deficits in non‐breastfed (WLZ non‐breastfed + no diarrhoea − WLZ non‐breastfed + diarrhoea) and breastfed (WLZ breastfed + no diarrhoea − WLZ breastfed + diarrhoea) infants, with the null hypothesis that these deficits were non‐significant or equivalent to 0. We expected that diarrhoea would reduce relative weight regardless of breastfeeding status, and so expected positive values from these calculations. We then tested whether diarrhoea‐related deficits in WLZ were modified by breastfeeding status by constructing an interaction contrast of the estimated and expected relative weights of non‐breastfed infants with diarrhoea. This interaction contrast tested the null hypothesis that (WLZ deficits estimated) − [WLZ deficits expected] = 0; or (WLZ non‐breastfed + diarrhoea − WLZ breastfed + no diarrhoea) − [(WLZ breastfed + diarrhoea − WLZ breastfed + no diarrhoea) + (WLZ non‐breastfed + no diarrhoea − WLZ breastfed + no diarrhoea)] = 0. We hypothesized that the interaction contrast would be positive, i.e. the average diarrhoea deficits in WLZ would be greater if infants were not breastfed. Finally, a third set of Wald tests were conducted to see whether the average WLZ was different when infants were breastfed and had diarrhoea, compared with when they were non‐breastfed and diarrhoea‐free i.e. whether WLZ breastfed + diarrhoea − WLZ non‐breastfed + no diarrhoea = 0. We expected that despite diarrhoea, breastfeeding would result in superior WLZ and so these calculated values would be positive. All statistical analyses were executed using Stata 14. A P value of less than 0.05 was considered statistically significant.
Results
Study population
Nearly one‐quarter of the study participants dwelled in a rural neighbourhood (Table 1). Mothers were an average of 27 years old when they gave birth, with an average of 8 years of formal education. The sample was 22% first‐born and 53% male. Infants were small at birth, with mean standardized birth length of −0.70 and mean standardized birth weight of −0.25. Infants were relatively small in the first 12 months (Table 2). Mean WAZ was −0.70 at birth, and this decreased to −1.47 by 12 months. Similarly, mean LAZ decreased from −0.25 at birth to −1.74 by 12 months. Mean WLZ ranged from −0.12 to −0.79. Measured anthropometry was available for 3048 infants at baseline, but because of attrition, only 2581 infants were measured at 12 months.
Table 1.
Descriptive statistics of 3080 offspring from the Cebu Longitudinal Health and Nutrition Survey (1983–1985)
| Sample characteristics | Mean | SD |
|---|---|---|
| Urban (%) | 24.67 | 43.12 |
| WAZ at birth | −0.70 | 0.99 |
| Birth weight (kg) | 2.99 | 0.44 |
| LAZ at birth | −0.25 | 1.10 |
| Birth length (cm) | 49.07 | 2.07 |
| Low birth weight (%) | 11.49 | 31.89 |
| Maternal age (years) | 26.59 | 5.99 |
| Maternal height (cm) | 150.56 | 5.00 |
| Maternal education (years) | 7.56 | 3.72 |
| First born (%) | 22.27 | 41.61 |
| Parity | 2.27 | 2.22 |
| Male (%) | 52.99 | 49.92 |
Table 2.
Mean ± standard deviations for relative weight, weight and length z‐scores of Cebu infants* throughout the weaning period
| Age (months) | WAZ | LAZ | WLZ | n |
|---|---|---|---|---|
| 0 | −0.70 ± 0.99 | −0.25 ± 1.10 | −0.74 ± 1.27 | 3048 |
| 2 | −0.85 ± 1.02 | −0.81 ± 1.13 | −0.12 ± 1.29 | 2870 |
| 4 | −0.88 ± 1.06 | −0.99 ± 1.11 | −0.19 ± 1.18 | 2793 |
| 6 | −1.02 ± 1.09 | −1.16 ± 1.11 | −0.30 ± 1.10 | 2708 |
| 8 | −1.20 ± 1.10 | −1.32 ± 1.13 | −0.51 ± 1.07 | 2659 |
| 10 | −1.36 ± 1.12 | −1.52 ± 1.14 | −0.69 ± 1.06 | 2612 |
| 12 | −1.47 ± 1.11 | −1.74 ± 1.15 | −0.79 ± 1.04 | 2581 |
Diarrhoea prevalence
Table 3 shows bimonthly estimates of 7‐day diarrhoea prevalence in infants by breastfeeding status. Diarrhoea prevalence was markedly lower in breastfed infants, relative to their non‐breastfed peers in the first 6 months. For example, at 2‐months, diarrhoea prevalence in non‐breastfed infants (15%) was 10% higher than the prevalence observed in breastfed infants (5%). However, after age 6+ months, there was only a 1–2% difference in diarrhoea prevalence of these two breastfeeding strata. The prevalence of exclusive breastfeeding at months 2, 4 and 6 were 50%, 39% and 8%, respectively (data not shown).
Table 3.
Bimonthly estimates of 7‐day diarrhoea prevalence by breastfeeding status
| Survey | Average age (months) | % of breastfed with diarrhoea | % of non‐breastfed with diarrhoea | % of all infants with diarrhoea | Infants with diarrhoea (n) | Infants without diarrhoea (n) |
|---|---|---|---|---|---|---|
| 1 | 2 | 5.19 | 15.23 | 6.76 | 195 | 2688 |
| 2 | 4 | 11.23 | 15.76 | 12.15 | 341 | 2466 |
| 3 | 6 | 18.78 | 23.74 | 19.97 | 543 | 2176 |
| 4 | 8 | 24.47 | 23.98 | 24.33 | 649 | 2018 |
| 5 | 10 | 23.62 | 25.30 | 24.15 | 635 | 1994 |
| 6 | 12 | 21.35 | 24.97 | 22.73 | 591 | 2009 |
Changes in maternal feeding and infant appetite in response to diarrhoeal disease
Mothers reported feeding special foods (12–20%) or withholding certain foods (7–38%) when their infants had diarrhoea (Table 4). ‘Special’ foods included milk, soups, porridges and fruits. Withheld foods included milks and other complementary foods. Some breastfeeding mothers also reported modifying their diets when infants had diarrhoea. The t‐tests showed that at any given survey, infants with diarrhoea had a lower mean appetite score than those without diarrhoea.
Table 4.
Descriptive statistics of key diarrhoea‐related variables from surveys 1 to 6 of the CLHNS
| All infants | Infants with diarrhoea | Infants without diarrhoea | Mean Difference* in appetite score | |||||
|---|---|---|---|---|---|---|---|---|
| Survey | Average age (months) | % crawling† | % breastfed | % Withheld foods | % special foods‡ | Appetite score in infants with Diarrhoea | Appetite score¶ in Healthy infants | |
| 1 | 2 | 0.42 | 84.29 | 18.37 | 18.37 | 4.835 | 4.973 | 0.139 |
| 2 | 4 | 4.20 | 79.65 | 17.82 | 12.07 | 4.858 | 4.950 | 0.093 |
| 3 | 6 | 40.40 | 75.99 | 20.61 | 16.79 | 4.797 | 4.926 | 0.129 |
| 4 | 8 | 84.82 | 72.50 | 29.26 | 17.78 | 4.729 | 4.881 | 0.152 |
| 5 | 10 | 94.41 | 68.29 | 38.34 | 16.60 | 4.695 | 4.860 | 0.165 |
| 6 | 12 | 97.81 | 61.96 | 29.34 | 19.42 | 4.660 | 4.847 | 0.187 |
Difference in mean appetite scores between those with and without diarrhoea; t‐tests showed that all these differences were statistically significant (P < 0.05).
% of infants allowed to crawl on floor.
% of infants with diarrhoea who received special foods or whose breastfeeding moms consumed special foods with the aim of improving infants' health.
Appetite score ranges from 1 to 5, with 1 indicating severely reduced appetites.
Results of the longitudinal regression
The longitudinal regression analyses revealed many associations of age, breastfeeding and diarrhoea with infant size. Estimated beta coefficients for relative weight as well as infant LAZ and WAZ are presented in Table 5. The coefficients from these regression analyses were plotted to estimate WLZ, WAZ and LAZ by breastfeeding & diarrhoea status from 2 to 12 months (Fig. 1). The curves show that regardless of diarrhoea prevalence, breastfed infants had greater relative weight, weight‐for‐age and length‐for‐age relative to their non‐breastfed counterparts, particularly in the first 6 months of life. Although relative weight was the primary outcome under study, we included weight‐for‐age (Fig. 1, Panel B) and length‐for‐age analyses (Fig. 1, Panel C) to further explain whether any differences in relative weight were likely due differences in infant length or weight. As shown in Fig. 1B, the breastfeeding‐ and diarrhoea‐related differences in growth curves for relative weight strongly resemble weight‐for‐age curves and are only modestly similar to those for infant length (Fig. 1C).
Table 5.
Results of longitudinal regression of WLZ on dietary and diarrhoea patterns during infancy
| Covariate | Girls' WLZ | 95% CI | Boys' WLZ | 95% CI | Girls' WAZ | 95% CI | Boys' WAZ | 95% CI | Girls' LAZ | 95% CI | Boys' LAZ | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 2, BF | Referent | Referent | Referent | Referent | Referent | Referent | ||||||
| Age 2, NBF | −0.63* | (−0.85 −0.41) | −0.92* | (−1.15 −0.68) | −0.72* | (−0.87 −0.57) | −0.88* | (−1.03 −0.72) | −0.42* | (−0.57 −0.26) | −0.40* | (−0.56 −0.24) |
| Age 2, NBF, with diarrhoea | −0.53† | (−1.10 0.04) | −0.70* | (−1.16 −0.25) | −0.78* | (−1.21 −0.35) | −0.64* | (−1.03 −0.26) | −0.37† | (−0.78 0.04) | −0.07 | (−0.48 0.34) |
| Age 2, BF, with diarrhoea | −0.14 | (−0.44 0.15) | −0.15 | (−0.48 0.19) | −0.14 | (−0.31 0.03) | −0.02 | (−0.18 0.15) | 0.04 | (−0.16 0.24) | 0.07 | (−0.16 0.30) |
| Age 4, NBF | −0.46* | (−0.64 −0.29) | −0.84* | (−1.02 −0.67) | −0.59* | (−0.71 −0.48) | −0.70* | (−0.82 −0.58) | −0.49* | (−0.62 −0.36) | −0.45* | (−0.57 −0.32) |
| Age 4, BF | −0.04 | (−0.11 0.03) | −0.14* | (−0.22 −0.07) | −0.05* | (−0.09 −0.02) | −0.07* | (−0.11 −0.03) | −0.16* | (−0.21 −0.11) | −0.22* | (−0.27 −0.17) |
| Age 4, NBF, with diarrhoea | −0.45* | (−0.71 −0.19) | −1.04* | (−1.36 −0.73) | −0.56* | (−0.76 −0.36) | −0.77* | (−0.97 −0.58) | −0.46* | (−0.69 −0.24) | −0.34* | (−0.57 −0.11) |
| Age 4, BF, with diarrhoea | −0.06 | (−0.22 0.09) | −0.15† | (−0.30 0.01) | −0.05 | (−0.14 0.03) | 0.01 | (−0.07 0.10) | −0.12* | (−0.24 −0.01) | −0.11† | (−0.22 0.01) |
| Age 6, NBF | −0.55* | (−0.71 −0.39) | −0.76* | (−0.90 −0.61) | −0.58* | (−0.69 −0.47) | −0.70* | (−0.80 −0.60) | −0.49* | (−0.60 −0.38) | −0.62* | (−0.72 −0.51) |
| Age 6, BF | −0.18* | (−0.26 −0.11) | −0.28* | (−0.37 −0.20) | −0.21* | (−0.25 −0.16) | −0.25* | (−0.30 −0.20) | −0.33* | (−0.39 −0.28) | −0.44* | (−0.50 −0.38) |
| Age 6, NBF, with diarrhoea | −0.48* | (−0.69 −0.27) | −0.91* | (−1.13 −0.69) | −0.56* | (−0.70 −0.41) | −0.73* | (−0.87 −0.59) | −0.54* | (−0.70 −0.37) | −0.54* | (−0.68 −0.39) |
| Age 6, BF, with diarrhoea | −0.34* | (−0.44 −0.24) | −0.41* | (−0.53 −0.29) | −0.29* | (−0.35 −0.23) | −0.33* | (−0.41 −0.25) | −0.27* | (−0.35 −0.18) | −0.39* | (−0.49 −0.30) |
| Age 8, NBF | −0.58* | (−0.73 −0.42) | −0.80* | (−0.95 −0.65) | −0.66* | (−0.76 −0.55) | −0.71* | (−0.81 −0.60) | −0.71* | (−0.81 −0.61) | −0.73* | (−0.84 −0.63) |
| Age 8, BF | −0.45* | (−0.52 −0.37) | −0.60* | (−0.69 −0.51) | −0.44* | (−0.49 −0.39) | −0.48* | (−0.54 −0.43) | −0.52* | (−0.58 −0.46) | −0.55* | (−0.62 −0.49) |
| Age 8, NBF, with diarrhoea | −0.50* | (−0.71 −0.30) | −0.97* | (−1.14 −0.79) | −0.65* | (−0.79 −0.51) | −0.82* | (−0.94 −0.70) | −0.77* | (−0.91 −0.63) | −0.68* | (−0.81 −0.55) |
| Age 8, BF, with diarrhoea | −0.57* | (−0.68 −0.47) | −0.72* | (−0.83 −0.61) | −0.50* | (−0.56 −0.44) | −0.59* | (−0.65 −0.53) | −0.47* | (−0.55 −0.39) | −0.57* | (−0.65 −0.50) |
| Age 10, NBF | −0.69* | (−0.84 −0.54) | −0.92* | (−1.08 −0.77) | −0.71* | (−0.82 −0.60) | −0.82* | (−0.92 −0.72) | −0.82* | (−0.93 −0.71) | −0.93* | (−1.04 −0.83) |
| Age 10, BF | −0.66* | (−0.74 −0.58) | −0.85* | (−0.94 −0.76) | −0.61* | (−0.67 −0.56) | −0.72* | (−0.78 −0.67) | −0.71* | (−0.78 −0.65) | −0.83* | (−0.89 −0.76) |
| Age 10, NBF, with diarrhoea | −0.89* | (−1.06 −0.71) | −1.03* | (−1.21 −0.84) | −0.89* | (−1.02 −0.76) | −0.88* | (−1.01 −0.74) | −0.90* | (−1.02 −0.78) | −0.91* | (−1.05 −0.76) |
| Age 10, BF, with diarrhoea | −0.81* | (−0.92 −0.70) | −1.06* | (−1.18 −0.94) | −0.70* | (−0.77 −0.63) | −0.83* | (−0.90 −0.76) | −0.70* | (−0.79 −0.60) | −0.75* | (−0.84 −0.66) |
| Age 12, NBF | −0.70* | (−0.84 −0.57) | −1.01* | (−1.16 −0.86) | −0.80* | (−0.90 −0.70) | −0.90* | (−1.00 −0.80) | −1.09* | (−1.20 −0.98) | −1.09* | (−1.19 −0.99) |
| Age 12, BF | −0.86* | (−0.95 −0.77) | −0.99* | (−1.08 −0.89) | −0.78* | (−0.84 −0.72) | −0.85* | (−0.91 −0.79) | −0.89* | (−0.96 −0.82) | −1.02* | (−1.09 −0.95) |
| Age 12, NBF, with diarrhoea | −0.93* | (−1.16 −0.69) | −1.22* | (−1.41 −1.02) | −0.96* | (−1.13 −0.78) | −1.02* | (−1.17 −0.88) | −1.13* | (−1.26 −1.00) | −1.15* | (−1.29 −1.01) |
| Age 12, BF, with diarrhoea | −0.93* | (−1.05 −0.80) | −1.14* | (−1.27 −1.01) | −0.87* | (−0.96 −0.78) | −0.99* | (−1.07 −0.91) | −0.97* | (−1.07 −0.88) | −1.08* | (−1.17 −0.98) |
| Complem. energy | 0.27* | (0.16 0.37) | 0.38* | (0.27 0.48) | 0.19* | (0.11 0.26) | 0.30* | (0.23 0.37) | 0.06 | (−0.03 0.15) | 0.12* | (0.04 0.20) |
| Intercept | −0.08* | (−0.14 −0.02) | 0.04 | (−0.02 0.11) | −0.69* | (−0.72 −0.65) | −0.78* | (−0.82 −0.74) | −0.67* | (−0.72 −0.63) | −0.85* | (−0.89 −0.80) |
| Sample size | 1386 | 1553 | 1386 | 1554 | 1554 | |||||||
| Rho | 0.603 | 0.599 | 0.828 | 0.815 | 0.759 | 0.761 | ||||||
P < 0.05.
P < 0.10; BF = breastfed; LAZ= standardized length‐for‐age; NBF = non‐breastfed; WAZ= standardized weight‐for‐age; WLZ= standardized weight‐for‐length.
Figure 1.
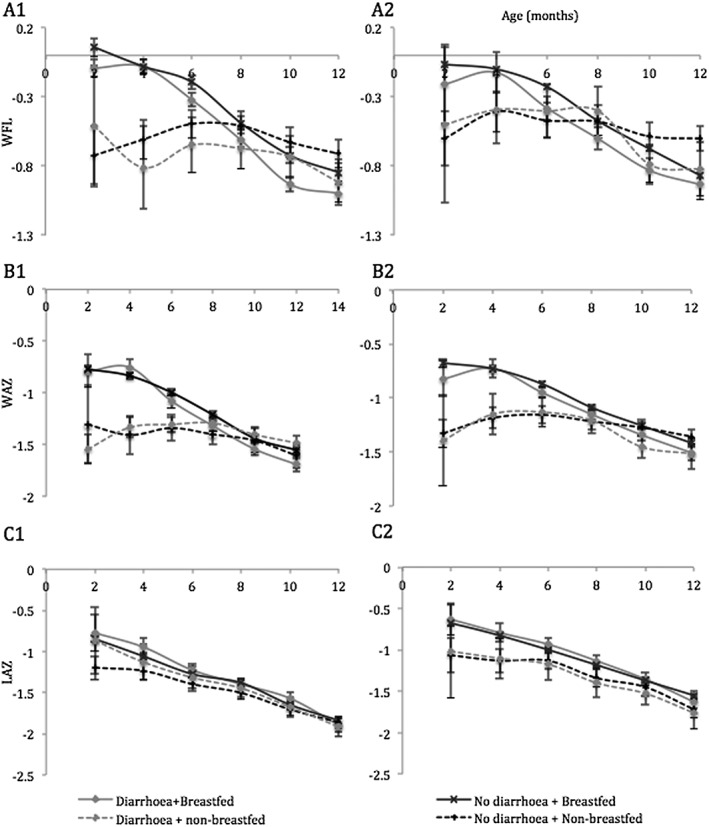
Relative weight, length‐for‐age and weight‐for‐age and 95% CI in male and female infants 2 to 12 months old. Graphs show predicted standardized weight for length (WLZ, A), weight (WAZ, B) and height (LAZ, C) in boys (1) and girls (2) with 95% confidence intervals.
Results of post‐estimation tests
The Wald tests supported our hypothesis that diarrhoea would reduce the relative weights of infants, regardless of breastfeeding status (Fig. 2). Intriguingly, diarrhoea‐related differences in WLZ were not statistically significant before 6 months but differences were marked in infants 6–12 months. For example, we found that when breastfed, boys tended to be heavier when they did not have diarrhoea: Betas which represent standard deviations (SD) of weight‐for‐length z scores (95% CI) were 0.13SD (0.02, 0.23), 0.12SD (0.03, 0.22), 0.21SD (0.10, 0.31) and 0.15SD (0.03, 0.27) at months 6, 8, 10 and 12, respectively. Similarly, when they were not breastfed, girls tended to be heavier if they did not have diarrhoea (Beta = 0.20SD (0.05, 0.35) and 0.22SD (0.02, 0.42) at 10 and 12 months).
Figure 2.
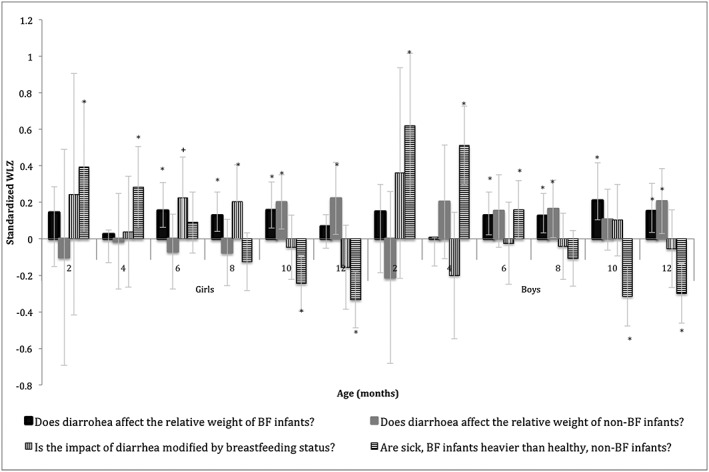
Differences in relative weight between key subgroups of male and female infants. Graph shows the diarrhoea‐related differences in relative weight for breastfed and non‐breastfed infants, as well as the differences in predicted relative weight for sick, breastfed and healthy, non‐breastfed infants with 95% confidence intervals. *P < 0.05, + P < 0.10.
Interaction contrasts were conducted to see whether breastfeeding status modified the effect of diarrhoea on WLZ. As hypothesized, contrasts for girls were positive from months 2–8, indicating that lack of breastfeeding exacerbated the effect of diarrhoea on relative weight. However, only one interaction contrast was statistically significant: at 8 months, girls' interaction contrast was 0.20SD (0.00, 0.41) P < 0.05. The 6‐month contrast also approached statistical significance: 0.22SD (0.00, 0.45), P < 0.10.
We also compared the relative weights of infants when they were breastfed and had diarrhoea to when they were not breastfed and diarrhoea‐free; the advantages of breastfeeding were quite apparent in the younger months. For example, breastfed girls with diarrhoea were 0.39SD (0.04, 0.75) and 0.28SD (0.06, 0.51) heavier at 2 and 4 months, respectively. This advantage was reversed over time. For example, beta estimates for boys showed that being breastfed with diarrhoea was associated with lower relative weight than being non‐breastfed and diarrhoea‐free: −0.31SD (−0.48, −0.15) and −0.30SD (−0.46, −0.14) at 10 and 12 months, respectively.
Discussion
We estimated the impact of diarrhoea on nutritional status and growth in breastfed and non‐breastfed infants. In this large cohort of Filipino infants, we found that diarrhoea was associated with reduced appetites in infants, altered maternal feeding patterns and there were diarrhoea‐related deficits in relative weight. These deficits were sometimes significantly exacerbated in non‐breastfed infants. We also found that prior to 6 months, the joint exposure of being breastfed and having diarrhoea was associated with higher relative weight compared to being non‐breastfed and diarrhoea free. Although previous studies have estimated the individual impact of breastfeeding or diarrhoea on infant nutritional status, ours is the first to explore the combined roles of these two antagonistic exposures on infant growth in such a large cohort of infants.
Consistent with the literature, this study showed breastfeeding‐related benefits, especially in infants <6, and diarrhoea‐related deficits, especially in infants 6–12 months. In younger infants, the high prevalence of exclusive breastfeeding, lower consumption of potentially harmful complementary foods and less physical interaction with the household environment (because of lower prevalence of crawling/walking) all likely contributed to reduced prevalence and severity of diarrhoea. Breastfeeding may improve infant size via two pathways: directly, via its provision of key nutrients and energy required to sustain infant growth, and indirectly, by mitigating risk and impact of diarrhoea. A large ecological study estimated that just 3 months of exclusive breastfeeding may reduce diarrhoea‐related mortality by half (Betrán et al. 2001). Thus it was not surprising that our findings confirmed that breastfed infants with diarrhoea still had superior weights when compared with non‐breastfed infants.
Interestingly, we found that diarrhoea‐related deficits in relative weight were statistically significant only after 6 months. This was surprising because we expected larger deficits in presumably more vulnerable first 6 months. A cohort study of 400 Brazilian infants from Pelotas showed higher daily weight loss in younger infants with diarrhoea: at 2 months, breastfed infants lost 24 g/day but at 6 months they lost 9 g/day (Martines et al. 1994). Our findings are still reasonable given the developmental and dietary changes that occur in infants of ~6 months. First, upwards of 40% of these infants were crawling at 6 months and this might have increased environmental exposure to pathogens that can cause diarrhoea. Second, these older infants consumed higher quantities of complementary foods and this increased opportunities for pathogen exposure. One review found that diarrhoea‐related mortality was more than doubled in the absence of breastfeeding throughout the weaning period (Lamberti et al. 2011). A previous study in this Cebu cohort showed how risk of diarrhoea is doubled or tripled once complementary foods are included in the infant diet (Popkin et al. 1990). At the same time, these infants also reduced breastmilk intake, thus losing the immunological benefits it provides. Taken together, these changes may have increased both risk and severity of diarrhoea and thus explain the significant deficits in relative weight.
The diarrhoea‐related deficits presented here also fit well within the larger body of evidence that the duration (Assis et al. 2005; Weisz et al. 2011), prevalence and cumulative history of diarrhoea (Checkley et al. 2003; Checkley et al. 2008) are inversely related to nutritional status. There are physiological and behavioural reasons for the reduced WLZ seen in infants with diarrhoea. Besides causing accelerated transit of digested material through the gut and increased metabolism of absorbed nutrients, diarrhoea also increases the permeability of the gut mucosa (Lunn et al. 1991), causing injury that is associated with poor nutritional status (Campbell et al. 2002). In addition to these biological impacts, studies show that diarrhoea can also induce anorexia and reduce energy intake of infants thus further reducing growth (Martorell et al. 1980; Bentley 1988; Bentley et al. 1991). Evidence of this behavioural impact was revealed in this present study, as mothers reported altered feeding patterns and significant reduction in appetites of their infants who had diarrhoea.
Concordant with the evidence of the benefits of breastfeeding and the detriments of diarrhoea, we found that diarrhoea‐related deficits were exacerbated in non‐breastfed infants. Indeed, the afore‐mentioned Pelotas cohort study highlighted these important modifications by breastfeeding status. Martines et al. found that at 2 months, non‐breastfed infants lost more weight (58 g/day) during diarrhoea episodes than their partially‐breastfed peers (39 g/day), who also lost more than their predominantly breastfed counterparts (24 g/day) (Martines et al. 1994). Martines et al. also demonstrated the importance of household sanitation: they found that the presence of indoor tap water was associated with reduced length of diarrhoea in the non‐breastfed infant. Although the antagonistic effect of breastfeeding was only statistically significant in some of the contrasts conducted, the weight of the evidence supports prolonged breastfeeding for health promotion throughout the weaning period.
Specifying a model that accurately teases apart the cyclic, interactive roles that nutrition and diarrhoea play is a complex task. Lutter et al. beautifully illustrated how energy intake may modify the impact of diarrhoea on nutritional status: in their proposed model, energy intake promotes nutritional status while the severity of diarrhoea worsens nutritional status; however, at the highest energy intakes, the impact of diarrhoea on nutritional status is nil, even in the most severe cases (Lutter et al. 1992). With this in mind, there are limitations to our models that might explain why the evidence for the mitigating effects of breastfeeding on diarrhoea‐related deficits were not significant in more contrasts.
First, it may be that any‐vs.‐none breastfeeding does not adequately quantify the amount of breast milk being received in these later stages, and that any modification by breastfeeding only becomes more evident for those receiving the highest quantities of energy from breast milk. Second, modification by breast milk may be strongest in infants with the most severe cases of diarrhoea. Neither breast milk energy nor diarrhoea severity was available to be analysed in this study. Third, our calculations used mean energy intake from complementary foods that were based on survey, breastfeeding status and sex. It is possible that energy intake assigned to babies with diarrhoea was much larger than what was actually consumed. However, we were more interested in the non‐energy mediated effect of diarrhoea on growth, and so this approach was appropriate. Thus, the null findings from these analyses may be conservative underestimates of the true impact of diarrhoea on WFL and any modification by breastfeeding.
Fourthly, a single 24‐h recall from the previous day was used to represent dietary intake in the bimonthly interval. However, it is unclear to what extent the dietary recalls captured diarrhoea‐induced anorexia. Given the importance of energy intake in mitigating diarrhoea consequences and the occurrence of anorexia in infants with diarrhoea, dietary intake clearly mediates the association of diarrhoea with WLZ. Alternatively, dietary intake may also be viewed as a confounder, because weaning foods may expose infants to pathogens that promote diarrhoea (exposure) but is also necessary for proper nutritional status (outcome). In light of these two important but conflicting arguments, we opted for a compromise that saw the sole inclusion of a main‐effects term for energy intake. Where detail is available, a more comprehensive exploration of this topic requires (1) clearer estimation of quantity of breast milk consumed, (2) the quantity and types of foods consumed before, during and after diarrhoea episodes, and (3) the severity of diarrhoea. Such information would further elucidate how the weaning diet, as well as breastfeeding, may modify the diarrhoea – nutritional status relationship.
Finally, these data were collected three decades ago and diarrhoea prevalence has changed since then. Although we found no recent studies on infant diarrhoea prevalence in the Cebu population, prevalence estimates from the Philippines Demographic Health Surveys show that between 1993 (Philippines. National Statistics Office 1994) and 2013 (Bersales 2014), the prevalence of diarrhoea has declined from 9.3 to 2.6% for infants <6 months, with more modest declines for 6‐ to 12‐month‐old infants (17.0% to 14.6%). Although these figures indicate significant improvement in diarrhoea prevalence in recent decades, the WHO Global Health Observatory reported that in 2012, diarrhoea was still responsible for 7% of deaths in Filipino children under 5 (Mitchell 2014). Our findings are therefore still relevant in the Filipino context, where diarrhoea is still a major public health burden, and more globally, because diarrhoea still accounts for 8% of deaths in children under five each year worldwide (UNICEF & WHO 2009, UNICEF et al. 2015). Nevertheless, it is clearly policy‐relevant for future studies to test for significant interaction between breastfeeding status and diarrhoea on infant nutritional status in more recent cohorts.
Despite the described limitations of the study, the use of fixed‐effects longitudinal regression models to exploit the longitudinal richness of 12 months of data makes this manuscript a significant contribution to the literature on diarrhoea and infant health. Furthermore, although others have examined the effect of breastfeeding or morbidity on infant size, ours is truly unique in its exploration of the interactive effects of these two important contributors to infant growth in the first 12 months of life. In addition to the statistical approach and longitudinal design of this study, we presented the effects of these breastfeeding‐morbidity patterns on three different measures of nutritional status.
Sources of funding
This project was supported by an educational grant from Wyeth Nutrition Innovation Collaboration (7D6‐N‐3146291). We are grateful to the Carolina Population Centre (R24 HD050924) for general support.
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributions
LA and MW conceived and designed the study; LA provided the databases necessary for the research; MW performed statistical analysis and wrote the paper; MW, MB, MM and LA contributed to the interpretation of results and critically reviewed manuscript; LA had primary responsibility for the final content. All authors read and approved the final manuscript.
Acknowledgements
Financial support: Wyeth Nutrition Initiative Collaboration and the Carolina Population Centre. Wyeth Nutrition had no role in the design, analysis or writing of this article.
Wright, M. J. , Mendez, M. A. , Bentley, M. E. , and Adair, L. S. (2017) Breastfeeding modifies the impact of diarrhoeal disease on relative weight: a longitudinal analysis of 2–12 month‐old Filipino infants. Maternal & Child Nutrition, 13: e12312. doi: 10.1111/mcn.12312.
References
- Adair L.S., Hindin M.J., Popkin B.M., Akin J.S., Guilkey D.K., Gultiano S.A. et al. (2011) Cohort profile: the Cebu longitudinal health and nutrition survey. International Journal of Epidemiology 40 (3), 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis A.M., Barreto M.L., Santos L.M.P., Fiaccone R. & da Silva Gomes G.S. (2005) Growth faltering in childhood related to diarrhea: a longitudinal community based study. European Journal of Clinical Nutrition 59 (11), 1317–1323. [DOI] [PubMed] [Google Scholar]
- Bentley M.E. (1988) The household management of childhood diarrhea in rural North India. Social Science & Medicine 27 (1), 75–85. [DOI] [PubMed] [Google Scholar]
- Bentley M.E., Stallings R.Y., Fukumoto M. & Elder J.A. (1991) Maternal feeding behavior and child acceptance of food during diarrhea, convalescence, and health in the central Sierra of Peru. American Journal of Public Health 81 (1), 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersales L. (2014) Philippines National Demographic and Health Survey 2013. Phillipines National Demographic and Health Survey 2013 129–136. [Google Scholar]
- Betrán A.P., Onís M.d., Lauer J.A. & Villar J. (2001) Ecological study of effect of breast feeding on infant mortality in Latin America. BMJ [British Medical Journal] 323 (7308), 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J. (1991) Underlying and proximate determinants of child health: the Cebu Longitudinal Health and Nutrition Study. American Journal of Epidemiology 133 (2), 185–201. [PubMed] [Google Scholar]
- Campbell D.I., Lunn P.G. & Elia M. (2002) Age‐related association of small intestinal mucosal enteropathy with nutritional status in rural Gambian children. British Journal of Nutrition 88 (5), 499–505. [DOI] [PubMed] [Google Scholar]
- Checkley W., Black R.E., Buckley G., Gilman R.H., Assis A.M., Guerrant R.L. et al. (2008) Multi‐country analysis of the effects of diarrhoea on childhood stunting. International Journal of Epidemiology 37 (4), 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W., Epstein L.D., Gilman R.H., Cabrera L. & Black R.E. (2003) Effects of acute diarrhea on linear growth in Peruvian children. American Journal of Epidemiology 157 (2), 166–175. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Research Institute of the Philippines (FNRI) (1980) Food Composition Tables. Department of Science and Technology (DOST): Manila, Philippines. [Google Scholar]
- Guerrant R.L., Oriá R.B., Moore S.R., Oriá M.O.B. & Lima A.A.M. (2008) Malnutrition as an enteric infectious disease with long‐term effects on child development. Nutrition Reviews 66 (9), 487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti L.M., Fischer Walker C.L., Noiman A., Victora C. & Black R.E. (2011) Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health 11 (Suppl 3, no. 3,), S15–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn P.G., Northrop‐Clewes C.A. & Downes R.M. (1991) Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338 (8772), 907–910. [DOI] [PubMed] [Google Scholar]
- Lutter C.K., Habicht J., Rivera J.A. & Martorell R. (1992) The relationship between energy intake and diarrhoeal disease in their effects on child growth: biological model, evidence, and implications for public health policy. Food & Nutrition Bulletin 14 (1), 36–42. [Google Scholar]
- Martines J., Habicht J., Ashworth A. & Kirkwood B. (1994) Weaning in Southern Brazil—is there a weanling's dilemma? Journal of Nutrition 124 (8), 1189–1198. [DOI] [PubMed] [Google Scholar]
- Martorell R., Yarbrough C., Yarbrough S. & Klein R.E. (1980) The impact of ordinary illnesses on the dietary intakes of malnourished children. The American Journal of Clinical Nutrition 33 (2), 345–350. [DOI] [PubMed] [Google Scholar]
- Mitchell K.K. (2014) Global Health Observatory. American Library Association CHOICE: Middletown. [Google Scholar]
- Morrow A.L., Ruiz‐Palacios G.M., Altaye M., Jiang X., Lourdes Guerrero M., Meinzen‐Derr J.K. et al. (2004) Human milk oligosaccharides are associated with protection against diarrhea in breast‐fed infants. The Journal of Pediatrics 145 (3), 297–303. [DOI] [PubMed] [Google Scholar]
- Olofin I., McDonald C.M., Ezzati M., Flaxman S., Black R.E., Fawzi W.W. et al. (2013) Associations of suboptimal growth with all‐cause and cause‐specific mortality in children under five years: a pooled analysis of ten prospective studies. PloS One 8 (5), e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippines. National Statistics Office (1994) National Demographic Survey 1993/National Statistics Office, Manila, Philippines. Macro International Inc.: Calverton, Maryland USA. [Google Scholar]
- Popkin B.M., Adair L.S., Akin J.S., Black R., Briscoe J. & Flieger W. (1990) Breast‐feeding and diarrheal morbidity. Pediatrics 86 (6), 874–882. [PubMed] [Google Scholar]
- Rowland M.G., Barrell R.A. & Whitehead R.G. (1978) Bacterial contamination in traditional Gambian weaning foods. Lancet 1 (8056), 136–138. [DOI] [PubMed] [Google Scholar]
- Scrimshaw N.S., Taylor C.E. & Gordon J.E. (1968) Interactions of nutrition and infection. Monograph series.World Health Organization 57, 3–329. [PubMed] [Google Scholar]
- StataCorp (2013) Stata 13 Base Reference Manual. Stata Press: College Station, TX. [Google Scholar]
- UNICEF & WHO (2009) Diarrhoea: Why Children Are Still Dying and What Can be Done. United Nations Children's Fund: New York. [Google Scholar]
- UNICEF , WHO , World Bank , UN‐DESA Population Division & United Nations Economic Commission for Latin America and the Caribbean Population Division (2015) Levels and Trends in Child Mortality: Report 2015. Estimates Developed by the UN Inter‐Agency Group for Child Mortality Estimation. United Nations Children's Fund: New York. [Google Scholar]
- Weisz A., Meuli G., Thakwalakwa C., Trehan I., Maleta K. & Manary M. (2011) The duration of diarrhea and fever is associated with growth faltering in rural Malawian children aged 6–18 months. Nutrition Journal 10 (1), 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group & de Onis M. (2006) WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica 95 (Supplement 450), 76–76. [Google Scholar]


