Abstract
Purpose
To determine the binding specificity of 18F-16α-17β-fluoroestradiol (18F-FES) in estrogen receptor-alpha (ERα) positive breast cancer cells and tumor xenografts.
Materials and Methods
Protocols were approved by the Office of Biological Safety and Institutional Animal Care and Use Committee. Using ER-negative MDA-MB-231 breast cancer cells, clonal lines were created that express either wild-type (231 WT ER) or G521R mutant ERα (231 G521R ER), which is defective in estradiol binding. ERα protein levels, subcellular localization, and transcriptional function were confirmed. 18F-FES binding was measured using an in vitro cell uptake assay. In vivo 18F-FES uptake was measured in tumor xenografts using microPET/CT imaging of 24 mice (17 WT ER tumors; 9 mutant G521R ER tumors; 8 MDA-MB-231 tumors; 4 MCF-7 ER+ tumors). Statistical significance was determined using Mann-Whitney/Wilcoxon rank sum test.
Results
ERα transcriptional function was abolished in the mutated 231 G521R ER cells despite appropriate receptor protein expression and nuclear localization. In vitro 18F-FES binding in the 231 G521R ER cells was reduced to that observed in the parental cells. Similarly, there was no significant 18F-FES uptake in the 231 G521R ER xenografts (0.49±0.042 %ID/g), which was similar to the negative control MDA-MB-231 xenografts (0.42±0.051 %ID/g, p=0.20) and nonspecific muscle uptake (0.41±0.0095 %ID/g, p=0.06).
Conclusion
Our studies have shown that 18F-FES retention in ER+ breast cancer is strictly dependent on an intact receptor ligand binding pocket and that 18F-FES binds to ERα with high specificity. These results support the utility of 18F-FES imaging for assessing tumor heterogeneity by localizing immunohistochemically ER+ metastases lacking receptor-binding functionality.
INTRODUCTION
Breast cancers are routinely assayed for biomarkers, including estrogen receptor alpha (ERα), progesterone receptor (PR), and human epidermal growth factor receptor type 2, that provide prognostic and predictive information to guide treatment decisions. Endocrine-based therapies are aimed at directly antagonizing ERα function, depleting endogenous estrogen, or degrading ERα protein to stop estrogen-stimulated tumor growth. Unfortunately, resistance to endocrine therapy is frequent, resulting in a need for accurate methods to determine endocrine sensitivity and ERα functionality.
Molecular imaging of breast cancer is a promising noninvasive approach for guiding treatment decisions and predicting response. Whole-body positron emission tomography (PET) imaging of ERα with radiolabeled estrogen, 18F-16α-17β-fluoroestradiol (18F-FES), can be used to determine the receptor status of all disease sites simultaneously and is particularly helpful when suspected metastatic lesions cannot be biopsied. 18F-FES-PET is also proposed to be a useful predictive imaging biomarker for treatment response. Studies show that patients with metastatic breast lesions with maximum standard uptake value (SUVmax) below 1.5 are unlikely to benefit from endocrine therapy (1, 2). This finding is being prospectively studied through a multi-institutional phase II clinical trial in the Unites States (NCT02398773). Furthermore, 18F-FES PET imaging is increasingly utilized in European clinical practice (3, 4).
Critical to implementation of 18F-FES PET imaging into clinical care is its ability to accurately reflect ERα protein expression. 18F- FES binds the ERα receptor subtype with high binding affinity and selectivity (5, 6). 18F-FES SUVmax correlates well with ERα protein expression (7–10). Overall sensitivity of 18F-FES PET for detection of ER+ breast cancer was 82% (95% CI: 74–88) and overall specificity in histologically benign lesions and ER-negative breast cancer was high at 95% (95% CI: 86–99) (7, 8, 11–14).
High fidelity binding of 18F-FES is important to prevent false-positive interpretation. Binding specificity has been inferred indirectly by demonstrating reduced 18F-FES uptake when co-administered with unlabeled estradiol (5, 15–18). This is also evidenced in animal models and patients receiving ERα antagonists which compete with 18F-FES for the ligand binding domain (19–22). Nonspecific 18F-FES binding may overestimate ERα expression and falsely indicate a high likelihood of response to endocrine therapy or insufficient antagonist drug dosing. Here, we use a direct approach for testing 18F-FES binding specificity by which ERα is genetically altered to determine whether any sites for 18F-FES binding exist beyond the receptor ligand binding pocket. We hypothesized that breast cancer cells and tumors expressing mutant ER protein that is incapable of binding estradiol would show no significant uptake of 18F-FES. The purpose of this study was to determine the binding specificity of 18F-FES in ERα+ breast cancer cells and tumor xenografts.
MATERIALS AND METHODS
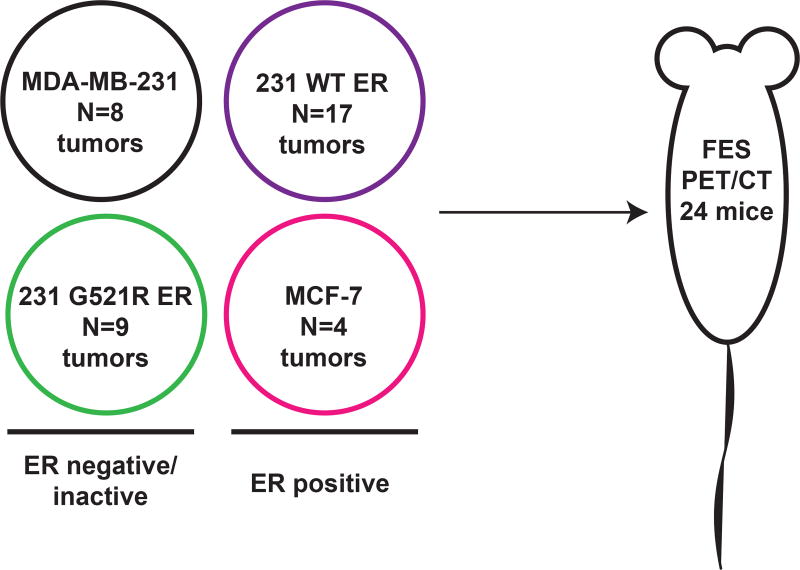
The overall experimental design is illustrated in Figure 1. To test the specificity of 18F-FES for the ligand binding domain of ERα, we first generated stable cell lines constitutively expressing either wild type ERα or G521R mutated ERα. The mutation of glycine521 to arginine in the human ESR1 gene is equivalent to the G525R mutation in the mouse ESR1 gene, which causes loss of estradiol binding (23). To the best of our knowledge, this mutation has not yet been found in human breast cancers but represents a useful tool for modeling a receptor deficient in ligand-binding function despite sufficient protein expression. The generated stable cell lines were then characterized using in vitro assays measuring ERα protein expression, nuclear localization, and transcriptional function. Tumor xenografts were grown in female athymic nude mice and imaged using 18F-FES PET/CT. After imaging, tumors were excised and analyzed for ERα protein expression by immunohistochemistry and Western blot analysis. Due to the inability of the G521R mutant ERα to bind estradiol, we hypothesized that 18F-FES uptake would be completely abolished if the binding of 18F-FES was strictly dependent on the ligand binding pocket. If nonspecific binding of 18F-FES occurred outside of the ligand binding pocket, then residual binding/uptake would be measured in the 231 G521R ER cells.
Figure 1. Overall experimental design.
Stable cell lines were generated and characterized using in vitro assays measuring ERα protein expression, nuclear localization, and transcriptional function. Tumor xenografts were grown in 24 female athymic nude mice (N reflects the number of tumors; 8 for the MDA-MB-231 xenografts, 17 for the 231 WT ER xenografts; 9 for the 231 mutant G521R ER xenografts, and 4 for the MCF-7 ER+ xenografts) and imaged using 18F-FES PET/CT. Nine mice had one tumor xenograft each (right thoracic mammary fat pad) and 15 mice had two tumor xenografts each (one in the right thoracic mammary fat pad, one in the left thoracic mammary fat pad). After imaging, tumors were excised and analyzed for ERα protein expression by immunohistochemistry and Western blot analysis.
Cell culture
Experiments were performed under a protocol approved by the Office of Biological Safety. ERα-positive (MCF-7) and ERα-negative (MDA-MB-231) human breast cancer cell lines were obtained from the Mallinckrodt Institute of Radiology Pre-Clinical PET/CT Imaging Facility (Washington University School of Medicine; St. Louis, MO). Authentication was performed using short tandem repeat analysis. Cells were cultured in Dulbecco’s Modified Eagle medium (DMEM; Corning; Corning, NY), supplemented with 10% fetal bovine serum (FBS; Corning; Corning, NY) and 1% penicillin and streptomycin (P/S; Gibco; Waltham, MA) at 37°C and 10% CO2. For estrogen-depleted conditions, cells were grown in phenol red-free DMEM with 10% steroid-stripped FBS, 2 mM L-glutamine, and 1% P/S at 37°C and 5% CO2.
Stable clonal cell lines expressing either wild-type ERα (231 WT ER) or mutant G521R (231 G521R ER) were created by transfecting MDA-MB-231 cells with an expression plasmid containing wild-type or mutant G521R ERα cDNA (24) using previously described methods (25). Cells were initially selected using 1000 µg/ml and maintained with 200 µg/ml hygromycin B (Life Technologies; Waltham, MA).
In vitro assays
The methods used for immunofluorescence, Western blot analysis, reporter gene assays, and quantitative real-time PCR are included in Appendix A.
Tumor xenografts
Experiments were performed according to the American Association for Laboratory Animal Science guidelines under an approved protocol. Twenty-five female athymic NCr-nu/nu mice, aged 6 weeks were orthotopically injected with 1.5×106 cells at 1:1 ratio with Matrigel (BD Biosciences; San Jose, CA) into the thoracic mammary fat pad. Palpable tumors formed in 24 of the 25 injected mice. Nine mice had one tumor xenograft each (right thoracic mammary fat pad) and 15 mice had two tumor xenografts each (one in the right thoracic mammary fat pad, one in the left thoracic mammary fat pad). The number of tumors was 8 for the MDA-MB-231 xenografts, 17 for the 231 WT ER xenografts; 9 for the 231 mutant G521R ER xenografts, and 4 for the MCF-7 ER+ xenografts. Tumor diameters were measured with calipers and volumes calculated using the formula a × b2/2, where a is the long diameter and b is the short diameter.
MCF-7 cells were used as a positive control to confirm correct 18F-FES radiosynthesis since this ER+ tumor xenograft model has been shown previously to have strong 18F-FES uptake (22). Since estrogen supplementation is required for MCF-7 xenograft formation, mice received 17β-estradiol (10 µg/ml) in the drinking water until 2 days prior to 18F-FES imaging.
18F-FES cell uptake assay and microPET/CT imaging
18F-FES was synthesized by our Radiopharmaceutical Production Facility following a previously reported method with minor modifications (26). Specific activity at the end of synthesis exceeded 55.3 GBq/µmol (1495 mCi/µmol).
For cell uptake assays, cells were plated 1.5 × 105 per well in 24-well plates. After overnight incubation, cells were washed twice with PBS and grown in estrogen-depleted media. The following day, cells were incubated for 1 h at 37°C with 0.037 MBq (1 µCi) of 18F-FES added per well with unlabeled 17β-estradiol (E2; 10−13-10−8M) or ethanol (EtOH) vehicle control. Cells were washed three times with PBS and lysed with 1 N NaOH. Collected radioactivity was measured with a gamma counter (2480 Wizard2, Perkin Elmer; Waltham, MA) and decay-corrected. Data for MCF-7 and 231 WT ER cells is shown as % maximum uptake values (samples containing 18F-FES with no cold E2 added=100%). Since no specific uptake of 18F-FES was observed above background levels (samples without 18F-FES) for MDA-MB-231 and 231 G521R ER cells, these values are expressed relative to MCF-7 cells. Half-maximal inhibitory concentration (IC50) was determined using nonlinear regression:dose response-inhibition. Three independent experiments were performed.
A total of 24 mice were imaged with 18F-FES PET/CT. The number of tumors measured with PET imaging was 8 for the MDA-MB-231 xenografts, 17 for the 231 WT ER xenografts; 9 for the 231 mutant G521R ER xenografts, and 4 for the MCF-7 ER+ xenografts. For imaging, mice with tumor volumes at least 100 mm3 were injected in the tail vein with 9.25 MBq (250 µCi) 18F-FES, anesthetized with 1.5–2.0% isoflurane, and scanned supine in a microPET/CT scanner (Inveon, Siemens Preclinical Solutions; Knoxville, TN) 1 hour following 18F-FES injection. PET/CT images were co-registered and analyzed using Inveon Research Workplace 3.0 (Siemens Medical Solutions; Malvern, PA). One of the authors (M.K.), a research assistant with 3 years of experience in small animal PET/CT imaging, drew the regions of interest with training by the senior author (A.M.F.), who is the principal investigator with 7 years of experience in this area. Regions of interest were drawn around the tumor and within quadriceps and triceps muscles. The average volume for the regions of interest were 146 mm3 (range 16.3 to 630 mm3) for tumors and 12.7 mm3 (range 2.7 to 31 mm3) for muscle. Data is expressed as mean percent injected dose per gram (% ID/g). Tumor-to-muscle (T:M) ratio was calculated as the ratio of % ID/g of tumor to that of muscle. The visual pattern of tumor 18F-FES uptake was also recorded descriptively.
Tissue histology
Excised tumors were fixed in 10% formalin and paraffin-embedded. ERα immunohistochemistry (IHC) was performed using the Discovery XT automated platform (Ventana Medical Systems; Tucson, AZ). Deparaffinization was performed followed by heat-induced epitope retrieval with CC1 buffer (Tris-based, pH 8.5) for 60 minutes at 100°C. ERα antibody (1:100, clone SP1; Thermo Fisher; Waltham, MA) was applied for 28 minutes at 37°C. After rinsing, OmniMap anti-rabbit–HRP antibody was applied for 8 minutes, followed by rinsing. Chromo Map DAB detection was applied, followed by rinsing and application of CAT Hematoxylin (Biocare Medical; Pacheco, CA). Routine staining with hematoxylin and eosin (H&E) was also performed. Slides were scanned at 40× using a whole-slide bright field imaging system (Leica Biosystems; Aperio Image Scope software; Buffalo Grove, IL).
Slides were examined by a pathologist with subspeciality training in breast pathology and 4 years of experience (A.M.M.). Percentage tumor necrosis was determined on the H&E slides. ERα IHC staining intensity (none=0, weak=1, moderate=2, strong=3) and subjective percentage of cells with nuclear staining positive were scored.
Statistical analyses
The nonparametric Mann-Whitney test was used to compare ERα functional activity (reporter gene assay and qPCR data) for the ethanol vehicle control versus estrogen-treated samples. 18F-FES uptake was compared between tumor xenografts using the Wilcoxon rank sum test. P value < 0.05 was considered significant. Statistical analysis were performed in R 3.3.2 and Graphpad Prism 6.05 software (La Jolla, CA).
Sample size calculations indicate that using 6 mice bearing bilateral tumors (12 tumors total) per group, a t-test at a 5% one-sided significance level will have approximately 90% power to detect an effect size of 1.19 (Cohen’s d value defined as the difference between the two means divided by the pooled standard deviation). Xenograft tumors of 231 WT ER cells were initially imaged as part of a control experiment for technical confirmation of 18F-FES uptake and were also included with imaging of the MDA-MB-231 and 231 G521R ER xenografts which accounts for their relatively larger sample size. A relatively smaller number of MCF-7 tumors were imaged with 18F-FES PET/CT simply as a positive control to confirm correct 18F-FES radiosynthesis and were not used for statistical comparison with the WT and mutant ER tumor models using MDA-MB-231 cells.
RESULTS
ERα localization and expression in engineered cell lines
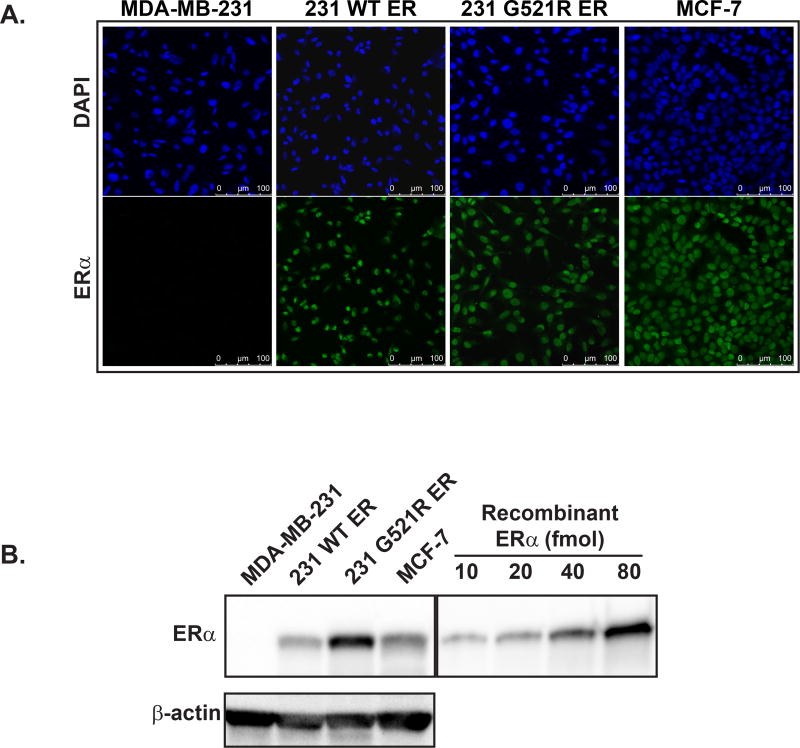
We observed nuclear localization of ERα protein in 231 WT ER and mutated 231 G521R ER cells, as well as in MCF-7 cells which express endogenous wild-type ERα (Figure 2A). No immunofluorescent staining was observed in the parental ER-negative MDA-MB-231 cells.
Figure 2. Stable cell lines created with expression of either wild-type or mutated G521R ERα protein with appropriate nuclear localization for testing 18F-FES binding specificity.
(A) Immunofluorescence of ERα localization. Upper panel: DAPI nuclear staining. Lower panel: Alexa Fluor 488 staining for ERα. Scale bar indicates 100 µm. (B) Western blot analysis of ERα protein expression. Images are representative of three independent experiments.
We then performed Western blot analysis to measure ERα protein levels in each of the cell lines. ERα expression was 0.2±0.2 fmol/mg total protein in MDA-MB-231 cells, 470±140 fmol/mg protein in 231 WT ER cells, 1478±214 fmol/mg protein in 231 G521R ER cells, and 811±180 fmol/mg protein in MCF-7 cells (Figure 2B).
ERα is functionally inactive in 231 G521R ER cells
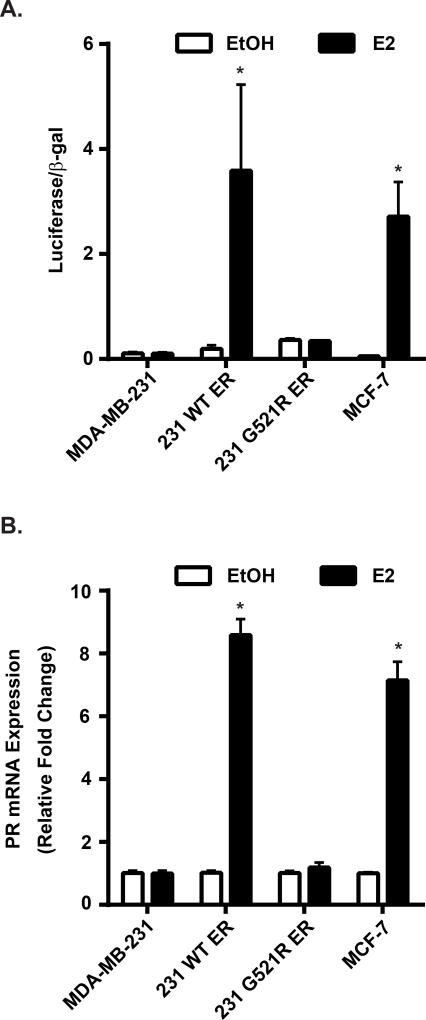
Strong induction of ERα transcriptional activity was measured in the 231 WT ER cells after E2 treatment (luciferase/β-galactoside activity: 0.19±0.069 EtOH vs 3.6±1.6 E2, p=0.03; Figure 3A). However, no significant estrogen-inducible transcriptional activity was measured in 231 G521R ER cells (0.36±0.028 EtOH vs 0.34±0.0046 E2, p=0.70), similar to the parental ER-negative MDA-MB-231 cells (0.11±0.025 EtOH vs 0.10±0.025 E2, p=0.66).
Figure 3. ERα is functionally inactive in 231 G521R ER cells.
(A) Estrogen-deprived cells were transfected with ERE-luciferase and β-galactosidase plasmids and then treated with ethanol (EtOH) vehicle or 10 nM 17β-estradiol (E2) for 24 hours. Luciferase activity was measured and normalized to β-galactosidase activity. (B) PR mRNA expression of estrogen-deprived cells was measured after 24 h treatment with EtOH or 10 nM E2. Values represent the mean±SEM of 3 independent experiments, * p<0.05 compared with the corresponding EtOH control.
There was an 8-fold induction of PR mRNA expression in estrogen-treated 231 WT ER cells (relative fold change: 1.0±0.06 EtOH vs 8.6±0.51 E2, p<0.0001; Figure 3B). This was comparable to PR mRNA expression in MCF-7 cells after estrogen treatment (1.0±0.03 EtOH vs 7.2±0.58 E2, p<0.0001). Similar to our results with the ERE-reporter gene assay, no induction of PR mRNA was observed in 231 G521R ER (1.0±0.06 EtOH vs 1.2±0.16 E2, p=0.50) and parental MDA-MB-231 cells (1.0±0.07 EtOH vs 1.0±0.09 E2, p=0.84) treated with estrogen.
Loss of 18F-FES uptake in 231 G521R ER cells
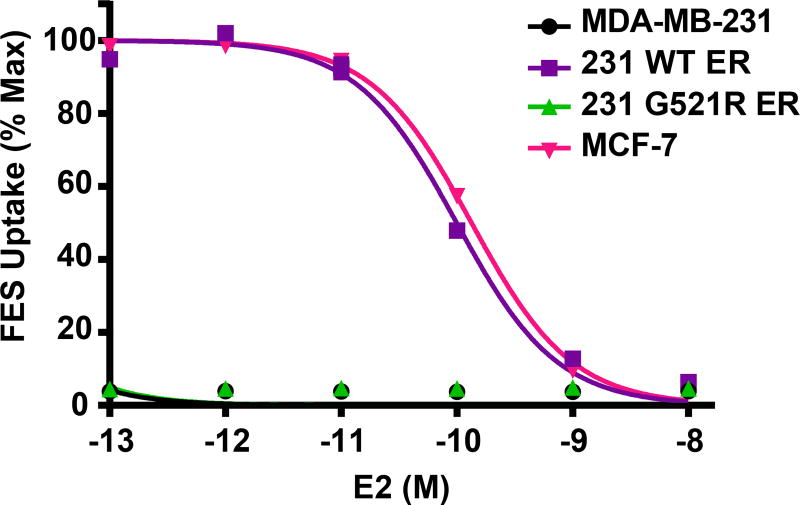
Using a competitive binding assay, we observed similar 18F-FES binding competition curves in the 231 WT ER cells (IC50 0.10 nM; 95%CI 0.09–0.11 nM) as ER+ MCF-7 cells (IC50 0.13 nM; 95%CI 0.12–0.15 nM); Figure 4. However, there was no specific 18F-FES binding in the 231 G521R ER cells, which behaved similar to the parental MDA-MB-231 cells.
Figure 4. Loss of 18F-FES uptake in 231 G521R ER cells.
Estrogen-deprived cells were treated with various concentrations of 17β-estradiol (E2 10−8M-10−13M) prior to addition of 0.037 MBq (1 µCi) 18F-FES for 1 hour. Decay-corrected counts per minute were normalized to wells containing 18F-FES with no cold E2 for % maximum uptake values. MDA-MB-231 and 231 G521R ER cells values were expressed relative to MCF-7. Values represent the mean±SEM of 3 independent experiments.
Loss of 18F-FES uptake in 231 G521R ER tumor xenografts
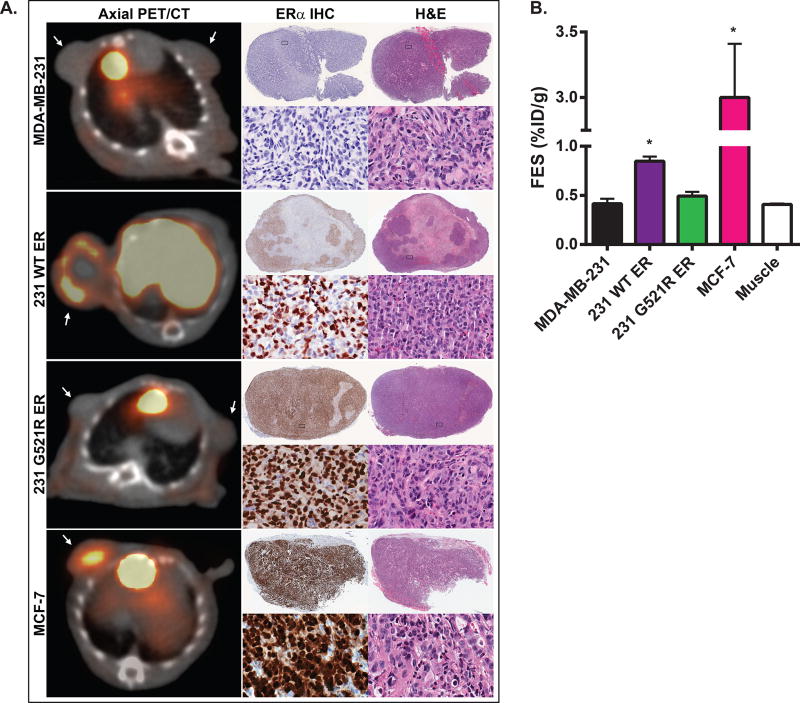
Tumor volumes at the time of imaging (mean±standard deviation) were 174±48, 135±34, and 129±34 mm3 for the MDA-MB-231, 231 WT ER, and 231 G521R ER xenografts, respectively. 18F-FES uptake was observed in the 231 WT ER xenografts and was significantly greater than in the MDA-MB-231 xenografts (0.85±0.045 vs 0.42±0.051 %ID/g, p<0.001) and in muscle (0.85±0.045 vs 0.41±0.0095 %ID/g, p<0.001) (Figure 5A,B). There was no significant 18F-FES uptake in the 231 G521R ER xenografts, which were similar to the negative control MDA-MB-231 xenografts (0.49±0.042 vs 0.42±0.051 %ID/g, p=0.20) and nonspecific muscle uptake (0.49±0.042 vs 0.41±0.0095 %ID/g, p=0.06). Tumor-to-muscle ratios (T:M) were 0.97±0.07 in MDA-MB-231 xenografts, 2.1±0.10 in 231 WT ER xenografts, and 1.1±0.08 in 231 G521R ER xenografts (Figure 5). Strong 18F-FES uptake was observed in the positive control MCF-7 xenografts (T:M ratio 7.2±0.60). Visual assessment of the pattern of tumor 18F-FES uptake demonstrated homogenous central uptake in MCF-7 xenografts and heterogeneous peripheral uptake in the 231 WT ER xenografts (Figure 5A).
Figure 5. Loss of 18F-FES uptake in 231 G521R ER tumor xenografts.
(A) Representative axial 18F-FES-PET/CT images of tumor xenografts grown in athymic nude female mice. Mice were injected with 250 µCi of 18F-FES and images were obtained 1 hour post injection. Arrows point to tumor. 18F-FES uptake is also noted in gall bladder and liver due to physiologic hepatobiliary clearance. Corresponding low and high power magnification images of ERα immunohistochemistry (IHC) and H&E staining of excised tumors. (B) Quantitative 18F-FES uptake assessed by mean %ID/g. Values represent the mean±SEM, * p<0.05 compared with tumor uptake in MDA-MB-231 and 231 G521R xenografts and compared with non-specific muscle uptake. MDA-MB-231 xenografts (N=8); 231 WT ER xenografts (N=17); 231 G521R ER xenografts (N=9).
ERα protein expression in the excised tumors were determined using immunohistochemistry and Western blot analysis (Figure 5A; Table 1). Strong, relatively homogeneous receptor expression was observed in the 231 G521R ER and MCF-7 xenografts. In contrast, we observed central tumor necrosis (40±19%) on H&E stained slides and a heterogeneous ERα immunostaining pattern in the 231 WT ER xenografts which corresponds with the heterogeneous peripheral 18F-FES uptake pattern observed with PET.
Table 1.
ERα Western, immunohistochemistry and H&E staining of tumor xenografts
| Xenograft | ERα Protein Expression (fmol/mg total protein) |
% Cells with ERα Positive Nuclear Staining |
% Tumor Necrosis |
|---|---|---|---|
| MDA-MB-231 | 7.6 ± 9.0 (N=8) | 0.0 ± 0.0a (N=8) | 0.4 ± 0.5 (N=8) |
| 231 WT ER | 240 ± 80.3 (N=8) | 72 ± 10b (N=13) | 40 ± 19 (N=13) |
| 231 G521R ER | 461 ± 48.5 (N=8) | 92 ± 7.6b (N=8) | 12 ± 6.6 (N=8) |
| MCF-7 | 1680 ± 840 (N=4) | 87 ± 15b (N=4) | 28 ± 25 (N=4) |
Values represent the mean±standard deviation.
N = number of xenograft tumors analyzed
Each tumor xenograft was the unit of observation and the level of analysis.
Intensity scores (none=0, weak=1, moderate=2, strong=3) were 0/3 for all samples.
Intensity scores (none=0, weak=1, moderate=2, strong=3) were 3/3 for all samples.
DISCUSSION
We directly tested 18F-FES binding specificity by modification of a single amino acid in the ligand binding domain of ERα that abolishes estradiol binding but preserves the remaining function of the receptor and by expressing this modified receptor in stable cell lines with comparative genetic background and phenotypes. These results support the hypothesis that there is no substantial 18F-FES binding to sites outside the ligand binding pocket of ERα or to nonspecific cellular proteins.
High fidelity binding of 18F-FES to the ligand binding domain of ERα is important to prevent false-positive image interpretation since the presence of nonspecific radiotracer uptake may overestimate receptor expression and falsely indicate a high likelihood of response to endocrine therapy or insufficient antagonist drug dosing. Using genetically modified cell lines with in vitro binding assays and in vivo small animal PET imaging, we demonstrated that 18F-FES binds to ERα with high specificity and is strictly dependent on an intact receptor ligand binding pocket. 18F-FES uptake reflects receptor-ligand binding functionality rather than the amount of receptor protein expression. These results are a direct confirmation of 18F-FES binding specificity and expand the existing literature in the field.
Previously published work provide indirect evidence of 18F-FES binding specificity. These include reports that show positive correlation (r=0.56–0.96) between the amount of ERα protein in breast cancer lesions using 3H-estradiol ligand binding assays and immunohistochemistry and 18F-FES SUVmax (7–10). Likewise, preclinical work using ER+ mouse mammary carcinoma cell lines have demonstrated a 50% decrease in 18F-FES uptake by PET imaging in corresponding stable cells lines when ERα protein levels are decreased by 75% due to RNA interference (17). Similarly, a reduction in 18F-FES uptake paralleled the pharmacologic downregulation of ERα protein in ER+ MCF-7 human breast cancer cells treated for 24 h with fulvestrant (22). 18F-FES uptake was reduced by 86% and ERα protein levels were reduced by 60% at the highest dose of fulvestrant tested.
18F-FES binding specificity has also been shown by preclinical studies demonstrating decreases in uptake measured by tissue biodistribution or PET imaging when unlabeled estradiol is co-injected (5, 15–18). However while estradiol competes with 18F-FES for binding ERα, once bound it induces proteasome-mediated degradation of ERα resulting in approximately 40% decrease in ERα protein within 1 hour (27). This occurs during the same time frame as 18F-FES incubation times which would also result in decreased 18F-FES uptake and is an inherent confounding factor to consider when interpreting these studies.
18F-FES binding specificity can also be inferred from blockade observed with ERα antagonists (fulvestrant and tamoxifen) which also compete similar to estradiol with 18F-FES for the ligand binding domain (19–22). However, in many cases there is incomplete 18F-FES blockade; 44% (7/16 patients) in a retrospective study and 38% (6/16 patients) in a subsequent prospective study (20, 21). Incomplete 18F-FES blockade is likely secondary to inadequate antagonist drug dosing; however, the presence of nonspecific 18F-FES binding cannot be excluded.
Gain-of-function ESR1 mutations have been identified in up to 40% of patients with metastatic, endocrine-resistant ER+ breast cancer and are associated with reduced survival (28, 29). These mutations cluster in the ligand binding domain, but unlike the inactivating G521R mutation, result in constitutively active ERα function. The effect of these activating mutations on 18F-FES uptake is unknown. It is possible that these receptors have reduced 18F-FES binding despite strong protein expression by immunohistochemistry, indicating that an ERα antagonist-based therapy may be less effective.
A potential limitation of our study is that the magnitude of 18F-FES uptake in the 231 WT ER tumor xenografts is relatively small (T:M ratio of 2.1±0.10) compared to other preclinical models (30, 31). This could be due to endogenous circulating estrogen; however, this has not been shown to interfere with 18F-FES uptake in patients (32). Also, the relatively low amount of 18F-FES uptake in vivo may be partially explained by the amount of tumor necrosis which was highest in the 231 WT ER xenografts. Despite this challenge, significant differences in 18F-FES uptake could be observed between the WT and G521R mutant xenografts to confidently demonstrate binding specificity. Lastly, our study does not address factors beyond 18F-FES uptake that may predict clinical benefit from endocrine therapy such as progesterone receptor (33).
In conclusion, our results indicate that 18F-FES uptake reflects the receptor-ligand binding functionality rather than the amount of receptor protein expression. These results support the utility of 18F-FES PET imaging for assessing intra-patient, inter-metastatic tumor heterogeneity by localizing immunohistochemically ER+ lesions lacking receptor binding functionality which could direct further tissue biopsy for genomic analysis and optimize treatment.
Supplementary Material
Advances in Knowledge.
Stable, constitutive expression of wild-type (WT) estrogen receptor alpha (ERα) in MDA-MB-231 cells behaves similar to endogenously expressed ERα; however, the G521R ligand binding domain mutation renders the receptor functionally null despite appropriate protein expression and nuclear localization.
While 18F-Fluoroestradiol (18F-FES) competitive binding curves appeared similar in the 231 WT ER cells as ER+ MCF-7 cells, there was no specific 18F-FES binding in the 231 G521R ER cells, which behaved similar to the parental MDA-MB-231 cells.
Tumor-to-muscle ratios of 18F-FES uptake using PET/CT imaging were 0.97±0.07 in MDA-MB-231 xenografts, 2.1±0.10 in 231 WT ER xenografts, and 1.1±0.08 in 231 G521R ER xenografts demonstrating a lack of 18F-FES retention (p=0.20 compared to parental MDA-MB-231 xenografts) despite robust protein expression of G521R ERα in tumor xenografts.
Implications for Patient Care.
ERα positivity by immunohistochemistry indicates the presence, but not functionality, of ERα protein which may be better assessed with 18F-FES PET imaging.
These results support the utility of 18F-FES PET imaging for assessing intra-patient, inter-metastatic tumor heterogeneity by localizing immunohistochemically ER+ lesions lacking receptor binding functionality which could direct further tissue biopsy for genomic analysis and optimize treatment.
Summary Statement.
18F-FES binds to ERα with high specificity and is strictly dependent on an intact receptor ligand binding pocket.
Acknowledgments
Funding information: This work was supported by the Philips Healthcare/Radiological Society of North America (RSNA) Research Seed Grant #RSD1420, University of Wisconsin (UW) Paul P. Carbone Cancer Center Young Investigator Award, and the UW Institute of Clinical and Translational Research KL2 Scholar Award (5KL2TR000428-09, 4KL2TR000428-10) to A.M.F. Funding was also provided by the School of Medicine and Public Health and the Department of Radiology to A.M.F.
Footnotes
This paper was presented at the RSNA 101st Scientific Assembly and Annual Meeting on December 1, 2015 in Chicago, IL (SSG09-07; ID: 15008367).
References
- 1.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24(18):2793–9. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 2.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19(11):2797–803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 3.Venema CM, Apollonio G, Hospers GA, et al. Recommendations and technical aspects of 16alpha-[18F]fluoro-17beta-estradiol PET to image the estrogen receptor in vivo: the Groningen experience. Clin Nucl Med. 2016;41(11):844–51. doi: 10.1097/RLU.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 4.van Kruchten M, Glaudemans AW, de Vries EF, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53(2):182–90. doi: 10.2967/jnumed.111.092734. [DOI] [PubMed] [Google Scholar]
- 5.Kiesewetter DO, Kilbourn MR, Landvatter SW, Heiman DF, Katzenellenbogen JA, Welch MJ. Preparation of four fluorine- 18-labeled estrogens and their selective uptakes in target tissues of immature rats. J Nucl Med. 1984;25(11):1212–21. [PubMed] [Google Scholar]
- 6.Yoo J, Dence CS, Sharp TL, Katzenellenbogen JA, Welch MJ. Synthesis of an estrogen receptor beta-selective radioligand: 5-[18F]fluoro-(2R,3S)-2,3-bis(4-hydroxyphenyl)pentanenitrile and comparison of in vivo distribution with 16alpha-[18F]fluoro-17beta-estradiol. J Med Chem. 2005;48(20):6366–78. doi: 10.1021/jm050121f. [DOI] [PubMed] [Google Scholar]
- 7.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169(1):45–8. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 8.Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49(3):367–74. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- 9.Gemignani ML, Patil S, Seshan VE, et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med. 2013;54(10):1697–702. doi: 10.2967/jnumed.112.113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chae SY, Kim SB, Ahn SH, et al. A randomized feasibility study of 18F-fluoroestradiol PET to predict pathologic response to neoadjuvant therapy in estrogen receptor-rich postmenopausal breast cancer. J Nucl Med. 2017;58(4):563–8. doi: 10.2967/jnumed.116.178368. [DOI] [PubMed] [Google Scholar]
- 11.van Kruchten M, de Vries EG, Brown M, et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013;14(11):e465–75. doi: 10.1016/S1470-2045(13)70292-4. [DOI] [PubMed] [Google Scholar]
- 12.Dehdashti F, Mortimer JE, Siegel BA, et al. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36(10):1766–74. [PubMed] [Google Scholar]
- 13.Mortimer JE, Dehdashti F, Siegel BA, Katzenellenbogen JA, Fracasso P, Welch MJ. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy. Clin Cancer Res. 1996;2(6):933–9. [PubMed] [Google Scholar]
- 14.Evangelista L, Dieci MV, Guarneri V, Conte PF. 18F-Fluoroestradiol positron emission tomography in breast cancer patients: systematic review of the literature and meta-analysis. Curr Radiopharm. 2016 doi: 10.2174/1874471009666161019144950. [DOI] [PubMed] [Google Scholar]
- 15.Katzenellenbogen JA, Mathias CJ, VanBrocklin HF, Brodack JW, Welch MJ. Titration of the in vivo uptake of 16 alpha-[18F]fluoroestradiol by target tissues in the rat: competition by tamoxifen, and implications for quantitating estrogen receptors in vivo and the use of animal models in receptor-binding radiopharmaceutical development. Nucl Med Biol. 1993;20(6):735–45. doi: 10.1016/0969-8051(93)90160-v. [DOI] [PubMed] [Google Scholar]
- 16.Aliaga A, Rousseau JA, Ouellette R, et al. Breast cancer models to study the expression of estrogen receptors with small animal PET imaging. Nucl Med Biol. 2004;31(6):761–70. doi: 10.1016/j.nucmedbio.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Paquette M, Tremblay S, Benard F, Lecomte R. Quantitative hormone therapy follow-up in an ER+/ERalphaKD mouse tumor model using FDG and [11C]-methionine PET imaging. EJNMMI Res. 2012;2(1):61. doi: 10.1186/2191-219X-2-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benard F, Ahmed N, Beauregard JM, et al. [18F]Fluorinated estradiol derivatives for oestrogen receptor imaging: impact of substituents, formulation and specific activity on the biodistribution in breast tumour-bearing mice. Eur J Nucl Med Mol Imaging. 2008;35(8):1473–9. doi: 10.1007/s00259-008-0745-x. [DOI] [PubMed] [Google Scholar]
- 19.McGuire AH, Dehdashti F, Siegel BA, et al. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. J Nucl Med. 1991;32(8):1526–31. [PubMed] [Google Scholar]
- 20.Linden HM, Kurland BF, Peterson LM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17(14):4799–805. doi: 10.1158/1078-0432.CCR-10-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Kruchten M, de Vries EG, Glaudemans AW, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015;5(1):72–81. doi: 10.1158/2159-8290.CD-14-0697. [DOI] [PubMed] [Google Scholar]
- 22.Heidari P, Deng F, Esfahani SA, et al. Pharmacodynamic imaging guides dosing of a selective estrogen receptor degrader. Clin Cancer Res. 2015;21(6):1340–7. doi: 10.1158/1078-0432.CCR-14-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fawell SE, Lees JA, White R, Parker MG. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990;60(6):953–62. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- 24.Preisler-Mashek MT, Solodin N, Stark BL, Tyriver MK, Alarid ET. Ligand-specific regulation of proteasome-mediated proteolysis of estrogen receptor-alpha. Am J Physiol Endocrinol Metab. 2002;282(4):E891–8. doi: 10.1152/ajpendo.00353.2001. [DOI] [PubMed] [Google Scholar]
- 25.Fowler AM, Solodin NM, Valley CC, Alarid ET. Altered target gene regulation controlled by estrogen receptor-alpha concentration. Mol Endocrinol. 2006;20(2):291–301. doi: 10.1210/me.2005-0288. [DOI] [PubMed] [Google Scholar]
- 26.Zhou D, Lin M, Yasui N, et al. Optimization of the preparation of fluorine-18-labeled steroid receptor ligands 16alpha-[18F]fluoroestradiol (FES), [18F]fluoro furanyl norprogesterone (FFNP), and 16beta-[18F]fluoro-5alpha-dihydrotestosterone (FDHT) as radiopharmaceuticals. J Labelled Comp Radiopharm. 2014;57(5):371–7. doi: 10.1002/jlcr.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alarid ET, Bakopoulos N, Solodin N. Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol Endocrinol. 1999;13(9):1522–34. doi: 10.1210/mend.13.9.0337. [DOI] [PubMed] [Google Scholar]
- 28.Fribbens C, O'Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34(25):2961–8. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 29.Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations-a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12(10):573–83. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler AM, Chan SR, Sharp TL, et al. Small-animal PET of steroid hormone receptors predicts tumor response to endocrine therapy using a preclinical model of breast cancer. J Nucl Med. 2012;53(7):1119–26. doi: 10.2967/jnumed.112.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan SR, Fowler AM, Allen JA, et al. Longitudinal noninvasive imaging of progesterone receptor as a predictive biomarker of tumor responsiveness to estrogen deprivation therapy. Clin Cancer Res. 2015;21(5):1063–70. doi: 10.1158/1078-0432.CCR-14-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson LM, Kurland BF, Link JM, et al. Factors influencing the uptake of 18F-fluoroestradiol in patients with estrogen receptor positive breast cancer. Nucl Med Biol. 2011;38(7):969–78. doi: 10.1016/j.nucmedbio.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammed H, Russell IA, Stark R, et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature. 2015;526(7571):144. doi: 10.1038/nature14959. [DOI] [PubMed] [Google Scholar]
- 34.Danielian PS, White R, Hoare SA, Fawell SE, Parker MG. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol. 1993;7(2):232–40. doi: 10.1210/mend.7.2.8469236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.