Abstract
Pancreatic ductal adenocarcinoma (PDA) has a dismal prognosis and is often discovered at an advanced stage with few therapeutic options. Current conventional regimens for PDA are associated with significant morbidity, decreased quality of life, and a considerable financial burden. As a result, some patients turn to integrative medicine therapies as an alternate option after a diagnosis of PDA. Intravenous pharmacologic ascorbic acid (PAA) is one such treatment. The use of PAA has been passionately debated for many years, but more recent rigorous scientific research has shown that there are significant blood concentration differences when ascorbic acid is given parenterally when compared to oral dosing. This pharmacologic difference appears to be critical for its role in oncology. Here, we report the use of PAA in a patient with poorly differentiated stage IV PDA as an exclusive chemotherapeutic regimen. The patient survived nearly 4 years after diagnosis, with PAA as his sole treatment, and he achieved objective regression of his disease. He died from sepsis and organ failure from a bowel perforation event. This case illustrates the possibility of PAA to effectively control tumor progression and serve as an adjunct to standard of care PDA chemotherapy regimens. Our patient’s experience with PAA should be taken into consideration, along with previous research in cell, animal, and clinical experiments to design future treatment trials.
Keywords: integrative cancer treatment, intravenous ascorbate, intravenous ascorbic acid, intravenous vitamin C, pancreatic cancer treatment, vitamin C
Introduction
Pancreatic ductal adenocarcinoma (PDA) accounts for the vast majority of advanced pancreatic cancer cases and carries an expected 5-year survival rate of less than 6% 1. Standard therapies for unresectable or metastatic pancreatic cancer currently consist of gemcitabine-based regimens, combination therapies such as FOLFIRINOX with or without radiation, and newer agents such as nanoparticle-albumin-bound (nab)-paclitaxel and erlotinib, all of which provide minimal response and a survival advantage measured in a few months 2–6. Furthermore, these regimens are associated with significant side effects, decreased quality of life, and considerable financial costs 2,4,6. The toll exacted by the disease burden and ineffective treatment options for PDA warrant exploration of alternative treatment options.
The use of intravenous ascorbic acid (AA) in oncology has a controversial history and has long been passionately debated 7,8. Notwithstanding the limitations of studies carried out in the late 1970s, the idea of AA as a chemotherapeutic agent seems plausible, stemming from a postulated versatile role in biochemical reactions and its selectivity for abnormal cells 9,10. Our group has elucidated the disproportionate pharmacokinetic properties between oral and intravenous pharmacologic ascorbic acid (PAA) administration in normal human participants 11. PAA achieves a 70-fold higher concentration than that achieved by maximum oral consumption in humans 11–13. This striking concentration difference seems to be dependent on tightly regulated mechanisms that maintain serum AA concentrations when ingested orally, but not when infused intravenously, a characteristic that appears to be critical for the role of PAA in oncology.
We report on a patient with biopsy-proven poorly differentiated metastatic Stage IV PDA who survived for 4 years exclusively on intravenous AA infusions after declining conventional standard of care. Written permission was provided for publication of the case report.
Case report
A 68-year-old man presented in March 2007 with intractable epigastric pain and nausea. A review of symptoms also showed hypersomnia, fatigue, and an ∼20 lb. weight loss. Baseline laboratory testing results included a CA 19–9 tumor marker of 77 (0–37 U/ml), a serum lipase level of 3658 (23–300 U/l), and a serum amylase level of 906 (23–85 U/l). He denied tobacco use and admitted to modest daily alcohol consumption. His family history was significant for PDA, which resulted in the death of his mother. His clinical presentation and laboratory results prompted an abdominal computed tomography (CT) scan, which showed an ill-defined mass in the uncinate process of the pancreas with multiple liver lesions. Several attempts at fine needle biopsy were nondiagnostic.
He was re-evaluated within a month with a repeat CT/PET scan, which showed the previously noted mass to be metabolically active (standardized uptake value or SUV 16) measuring 3.7×3.7 cm with celiac axis and hepatic artery encasement and portal vein abutment (Fig. 1a–d). No significant pancreatic duct or biliary tree dilatation were identified. There were also multiple small mesenteric and peripancreatic lymph nodes. The liver lesions were identified as metabolically active, with the largest measuring 2.3×1.8 cm (SUV 7).
Fig. 1.
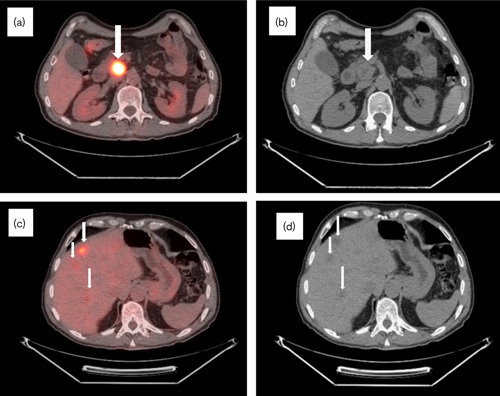
Initial CT/PET at biopsy in April 2007. (a) CT/PET and (b) CT scan: metabolically active (SUV 16) hypodense 4.5×3.7×3.7 cm lesion in the uncinate process of the pancreas (arrows). (c) CT/PET and (d) CT scan: multiple metabolically active lesions throughout liver parenchyma, with the largest measuring 2.8×1.8 cm (SUV 7) (arrows). CT, computed tomography; SUV, standardized uptake value.
He subsequently had an endoscopic ultrasound with biopsy, which identified a 4.5×3.1 cm hyperechoic mass with innumerable liver lesions, consistent with metastatic disease. At the time of the endoscopic ultrasound, the pancreatic duct and biliary tree were not dilated. The biopsy showed poorly differentiated PDA. Cytopathology of the original biopsy was reviewed independently at the University of Alabama and the National Institutes of Health, confirming aggressive poorly differentiated PDA. KRAS/BRAF genotyping was not performed.
At the initial oncology consultation in May 2007, the patient had a recorded weight of 181.7 pounds, persistent epigastric discomfort of 8/10 on the visual analogue scale, and an ECOG status of 1. He was not jaundiced. The patient was given a diagnosis of Stage IV poorly differentiated PDA and was offered chemotherapy consisting of gemcitabine and erlotinib. He declined standard of care with a full understanding of the natural course of metastatic PDA, but not wanting to experience the side effects of chemotherapy and reduced quality of life in the expected short remaining lifespan. After consulting with his family, the patient elected to begin escalating doses of PAA through a port-a-cath, with treatment doses ranging from 75 to 125 g per infusion and administered 2–3 times per week, according to accepted dosing and scheduling protocols 14,15. The patient was prescreened for conditions that might preclude receiving PAA that included G6PD deficiency or abnormal renal function 16.
In November 2007, 8 months after presentation, a follow-up CT/PET was performed (Fig. 2a–d), which noted the pancreatic head mass to be unchanged in size, but with reduced SUV level, indicative of disease regression. The previously identified liver metastases were also noted overall to have decreased SUV levels. Finally, the largest liver mass, initially measuring 1.3 cm with an SUV of 7, had almost entirely resolved, representing improvement.
Fig. 2.
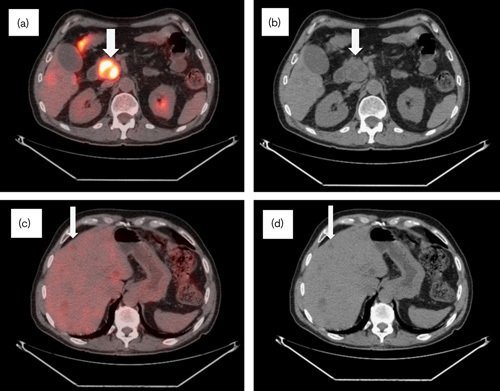
Follow-up CT/PET in November 2007, 8 months after the initial imaging. (a) CT/PET and (b) CT scan: metabolically active lesion in the uncinate process of the pancreas with decrease in activity (SUV 13) and stable measurement 5.3×3×3.3 cm (arrows). (c) CT/PET and (d) CT scan: hepatic parenchymal lesions identified with a decrease in metabolic activity (SUV 3) and resolving a large anterior right hepatic lobe mass (arrows). CT, computed tomography; SUV, standardized uptake value.
During the following year, the patient continued PAA at 75–125 g 2–3 times per week. A follow-up CT/PET scan in June 2008, more than a year from the original diagnosis, showed continued improvement (Fig. 3a–d). There remained persistent, but smaller low-density lesions throughout the liver without hypermetabolic activity, consistent with residual metastatic disease. In addition, the mass in the head of pancreas was noted to be smaller and with less metabolic activity than observed previously. At the time of this scan, the patient had a stable weight, was pain free, and had an ECOG status of 0.
Fig. 3.
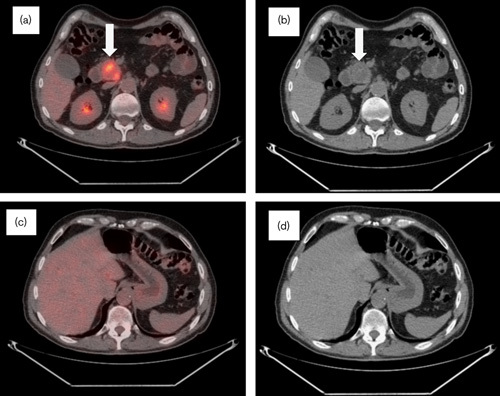
Follow-up CT/PET performed in June 2008 over 1 year after diagnosis. (a) CT/PET and (b) CT scan: metabolically active lesion in the uncinate process of the pancreas with a further decrease in metabolic activity (SUV 9) and stable in size from the previous scan with a necrotic central portion (arrows). (c) CT/PET and (d) CT scan: resolution of metabolic activity throughout hepatic parenchyma, with only small foci of residual disease identified consistent with improvement in metastatic disease. CT, computed tomography; SUV, standardized uptake value.
During the following year, the patient continued to receive PAA at 75–125 g at 2–3 times per week. He had an ECOG status of 0 with stable weight and no pain. A follow-up CT/PET was performed in November 2008, now over 18 months after diagnosis, and imaging was without residual metabolic activity or discrete lesions throughout the liver (Fig. 4a–d). It was reported that there was continued reduction in the size of the pancreatic head mass with increased necrotic components in the central portion of the tumor consistent with continued regression of malignancy.
Fig. 4.
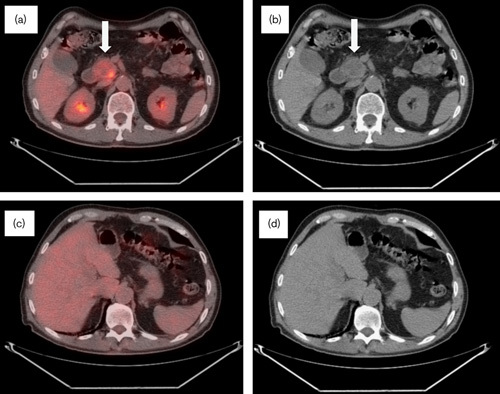
CT/PET follow-up performed in November 2008 at 18 months after diagnosis. (a) CT/PET and (b) CT scan: interval decrease in the size of the pancreatic uncinate process mass measuring 3.9×3.6×3.1 cm with increased necrotic components. The metabolic activity showed decreased activity (SUV 6.4). (c) CT/PET and (d) CT scan: no residual metabolic activity and no discrete lesions identified throughout the hepatic parenchyma. No biliary dilatation noted. CT, computed tomography; SUV, standardized uptake value.
The patient, now 2.5 years after diagnosis, continued infusion therapy receiving 75–125 g at 2–3 times per week, with no change in physical exam, complaints, or ECOG status. Follow-up imaging in November 2009 (Fig. 5a and b) showed an interval decrease in the size of the pancreatic head mass. It was found at this imaging that the SUV increased to 10 and progression of disease was questioned, despite the diminishing size. However, compression of the lesion as a cause of increased SUV was suspected. No other abnormalities were identified on the scan.
Fig. 5.
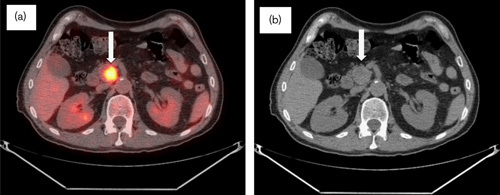
Follow-up CT/PET in November 2009 now 2.5 years after diagnosis. (a) CT/PET and (b) CT scan: interval decrease in the size of the pancreatic uncinate process mass measuring 2.6×2.2×2.3 cm. Hypermetabolic activity showed increase from the previous scan (SUV 10) (arrows). The hepatic parenchyma remained free of hypermetabolic activity or focal lesions (not pictured). No biliary dilatation noted. CT, computed tomography; SUV, standardized uptake value.
In 2010, the patient had routine follow-up blood work after over 450 doses of PAA, which showed normal CA 19–9 levels, pancreatic enzymes, liver enzymes, and renal function. ECOG status remained 0. The patient’s only complaint at this time was arthritic pain in his hands. The last CT/PET was obtained in November 2010 (Fig. 6a and b), which showed stable disease. No new lesions were identified.
Fig. 6.
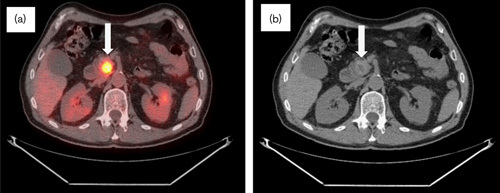
Follow-up CT/PET in November 2010 now 3.5 years after diagnosis. (a) CT/PET and (b) CT scan: no significant change in the pancreatic mass size. Hypermetabolic activity was stable (SUV 9.3) and interpreted as stable disease (arrows). Hepatic parenchyma remained free of metastatic disease (not pictured). No biliary dilatation noted. CT, computed tomography; SUV, standardized uptake value.
A biliary stent was placed in January 2011 because of a suspicion of advancing disease after an increase in liver enzymes, but scanning indicated no evidence for biliary dilatation or increase in the pancreatic mass size. In retrospect, the patient may have had elevated liver enzymes from consuming very high doses of oral vitamin D. In February 2011, 2 weeks after stent placement, the patient presented to the emergency department in septic shock (Fig. 7a and b). At this scan, there was no enlargement of the pancreatic head mass or metastatic disease in the liver parenchyma. He underwent an exploratory laparotomy and was found to have a perforated small bowel segment that required surgical resection. The biliary stent, which had presumably migrated, was found in his cecum. Postoperatively, the patient remained in the ICU in septic shock and in acute respiratory and renal failure. His condition worsened 2 days postoperatively, requiring increasing vasopressors for hemodynamic support, at which point, his wife elected to withdraw support and he died shortly thereafter.
Fig. 7.
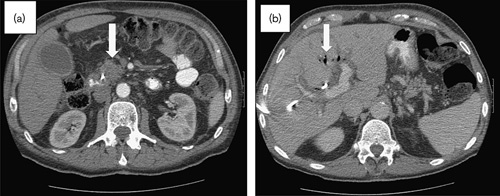
February 2011 presentation to the emergency room, where the patient was found to be in septic shock 2 weeks after biliary stent placement. (a) Contrast-enhanced CT scan: stable size of mass in the uncinate process of pancreas (arrow) and no apparent tumor progression. There was mild diffuse induration of the mesentery consistent with third spacing of fluid. (b) Contrast-enhanced CT scan: hepatic parenchyma with air in the biliary tree (arrow), but no parenchymal lesions identified. CT, computed tomography.
Discussion
Investigation of new treatment agents that are nontoxic, inexpensive, and show clinical promise are in the best interests of our patients, independent of revenue-generating potential. PAA in cancer treatment, with no patentability, is an example. PAA was dismissed as a cancer treatment agent 26 years ago, in retrospect because of a simple pharmacology error 11.
Recent in vitro and animal evidences from many laboratories indicate that PAA, but not oral AA, may have selective cancer-killing properties, by acting as a prodrug to generate hydrogen peroxide selectively in extracellular fluid 10,12,13. For PDA, PAA synergizes with gemcitabine in cell and animal models, and appears to be safe in screened patients 14,16,17. On the basis of these new clinical observations, we hypothesize that PAA treatment may require a much longer time course than conventional treatment agents. Also, we suggest that because of the changing appearance of the tumor mass and the procollagen effects of AA 18, there may be infiltration of the tumor with collagen. These observations could provide insight into the mechanisms of action of PAA in cancer treatment and require further investigation in preclinical and clinical studies.
We report here a case of a 68-year-old man with metastatic PDA whose only oncologic treatment was PAA. He presented in March 2007, with a mean expected survival of 4–6 months. On the basis of emerging science and against his oncologist’s advice, he chose treatment with PAA at doses between 75 and 125 g administered intravenously 2–3 times weekly 10,12. The patient’s weight loss reversed within 4 months; his liver lesions gradually diminished and became undetectable after 1 year; and his primary tumor decreased in size, but did not disappear at 3.5 years after diagnosis. The patient felt well and was active. There are early reports of increased physical and psychological well-being with the use of PAA that could be an additional benefit for patients undergoing chemotherapy 15,19.
In January 2011, the patient had increasing liver enzymes believed to be related to disease progression and had biliary stent placement. In retrospect, the increasing liver enzymes may have been related to ingestion of high doses of vitamin D. He died of a known procedure-related complication that was indirectly related to his underlying diagnosis of PDA, but not from apparent PDA disease progression. Transpapillary or percutaneous placement of biliary stents is a useful adjunct in the management of patients with advanced hepatobiliary malignancies, providing symptom alleviation in the overwhelming majority of patients. However, biliary stents have a known overall complication rate of up to 10% of cases and a mortality of 1%. Stent migration occurs in ∼6% of cases and although benign in most cases, the complications stemming from a migrated stent can be catastrophic. A number of case reports and small series have documented the possibility of stent migration causing bowel perforation, which, under the appropriate circumstances, can be lethal in this patient population 20–22.
Survival of metastatic PDA and regression of metastases are rare 6,23. This single case report clearly does not prove that long-term PAA is valuable in cancer treatment. Rather, this case, raising the possibility of efficacy, is a clue to be coupled with others indicating efficacy of PAA in cell and rodent cancer models, including models of pancreatic cancer 10,13, and synergy in combination with gemcitabine 17. For testing efficacy, prolonged treatment times may be necessary and should be considered in designing treatment trials.
PDA is a human cancer with a poor prognosis. Gemcitabine, a key therapeutic agent for metastatic PDA, and combination treatments have had only a modest impact on extending survival. Adding erlotinib, at an approximate cost of $3000/month, extends survival by mere weeks 2,4,6. We believe that a new treatment agent that shows robust laboratory and animal evidence, coupled with minimal patient toxicity, deserves rigorous clinical investigation without concern that clinical trials might not be supported by industry or have potential to generate profits. Indeed, patients deserve no less. It is our opinion that the current evidence is sufficient to encourage both private and public funding agencies to evaluate support for targeted phase I and II clinical trials of PAA as a complement to standard therapies in the treatment of metastatic PDA.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
Footnotes
Written work prepared by employees of the Federal Government as part of their official duties is, under the US. Copyright Act, a ‘work of the United States Government’ for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government.
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, et al. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer 2016; 57:10–22. [DOI] [PubMed] [Google Scholar]
- 3.Esnaola NF, Chaudhary UB, O’Brien P, Garrett-Mayer E, Camp ER, Thomas MB, et al. Phase 2 trial of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2014; 88:837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hidalgo M, Cascinu S, Kleeff J, Labianca R, Lohr JM, Neoptolemos J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 2015; 15:8–18. [DOI] [PubMed] [Google Scholar]
- 5.Moorcraft SY, Khan K, Peckitt C, Watkins D, Rao S, Cunningham D, Chau I. FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: The Royal Marsden experience. Clin Colorectal Cancer 2014; 13:232–238. [DOI] [PubMed] [Google Scholar]
- 6.Renouf D, Moore M. Evolution of systemic therapy for advanced pancreatic cancer. Expert Rev Anticancer Ther 2010; 10:529–540. [DOI] [PubMed] [Google Scholar]
- 7.Levine M, Espey MG, Chen Q. Losing and finding a way at C: new promise for pharmacologic ascorbate in cancer treatment. Free Radic Biol Med 2009; 47:27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creagan ET, Moertel CG, O’Fallon JR, Schutt AJ, O’Connell MJ, Rubin J, Frytak S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med 1979; 301:687–690. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA 2005; 102:13604–13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 2007; 104:8749–8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt HM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004; 140:533–537. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA 2008; 105:11105–11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr 2011; 2:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monti D, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One 2012; 7:e29794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med 2014; 6:222ra18. [DOI] [PubMed] [Google Scholar]
- 16.Padayatty SJ, Sun YA, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One 2010; 5:e11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, Chen Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med 2011; 50:1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. ‘Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci USA 1981; 78:2879–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr A, McCall C. The role of vitamin C in the treatment of pain: new insights. J Transl Med 2017; 15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abela JE, Anderson JE, Whalen HR, Mitchell KG. Endo-biliary stents for benign disease: Not always benign after all! Clin Pract 2011; 1:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mady RF, Niaz OS, Assal MM. Migrated biliary stent causing perforation of sigmoid colon and pelvic abscess. BMJ Case Rep 2015; 13:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkley M, Wible BC, Hong K, Georgiades C. Colonic perforation by a percutaneously displaced biliary stent: report of a case and a review of current practice. J Vasc Interv Radiol 2009; 20:680–683. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez MJ, Berdiel MJ, Miranda-Massari JR, Lopez D, Duconge J, Rodriquez JL, Androver P. High dose intravenous vitamin c and metastatic pancreatic cancer: two cases. Integr Cancer Sci Therap 2016; 3:1–2. [Google Scholar]


