Abstract
As the influence of diet on health may take place over a period of decades, there is a need for biomarkers that help to identify those aspects of nutrition that have either a positive or a negative influence. The evidence is considered that heart-rate variability (HRV) (the time differences between one beat and the next) can be used to indicate the potential health benefits of food items. Reduced HRV is associated with the development of numerous conditions for example, diabetes, cardiovascular disease, inflammation, obesity and psychiatric disorders. Although more systematic research is required, various aspects of diet have been shown to benefit HRV acutely and in the longer term. Examples include a Mediterranean diet, omega-3 fatty acids, B-vitamins, probiotics, polyphenols and weight loss. Aspects of diet that are viewed as undesirable, for example high intakes of saturated or trans-fat and high glycaemic carbohydrates, have been found to reduce HRV. It is argued that the consistent relationship between HRV, health and morbidity supports the view that HRV has the potential to become a widely used biomarker when considering the influence of diet on mental and physical health.
Keywords: diet, disease, health, heart-rate variability, nutrition
Introduction
Biomarkers are important as proxy measures when studying health or disease states that develop over long periods. As a disease can develop over decades, this is an area where there is a need for biomarkers that identify aspects of life style that are potentially beneficial or problematic. The case will be made for using heart-rate variability (HRV) as an indicator of the physiological response to food by those interested in the association between diet and various health outcomes, and by manufacturers developing functional foods with potential health benefits. HRV is of interest as a wide range of diseases are associated with decreased variability, including diabetes, cardiovascular disease and psychiatric disorders. In addition, there is an increasing literature that reports that HRV responds to various aspects of the diet, raising the possibility that HRV offers a convenient measure of potential benefit.
Traditionally, heart rate (HR) has been considered a product of emotional response or stress, but it is becoming apparent that the interval between beats is a marker of the capacity to regulate internal and external demands. The intervals are not constant, but differ from beat to beat: essentially a higher HRV indicates better general health (Jarczok et al., 2015). In essence, the multitude of ways in which different physiological mechanisms modulate each other ensures that studying one aspect of the body in isolation limits our understanding.
In 1948, the WHO defined health as ‘a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity’. As complete well-being is practically impossible, more recent definitions have emphasized resilience, the capacity to cope and the ability to maintain a sense of well-being. To assess the level of existing health and to evaluate dietary interventions, such definitions need to be operationalized. With this in mind, HRV may serve as a biomarker relating to the above definition, and thus serve as an indicator of the response to diet. In this review, the control of HR and the measurement of HRV are first outlined and then associations between HRV, health and aspects of diet are considered.
Control of heart rate
Figure 1 shows an ECG trace from which the R-to-R intervals are measured, although it is the variability in the differences between these intervals that is of interest. In the brain stem, the medulla oblongata controls HR through the vagus, the tenth cranial nerve: vagal tone reduces HR by inhibiting the sinoatrial node, the heart’s pacemaker. Although there is a tendency to view HR as an involuntary mechanism, the medulla oblongata receives information from the rest of the brain: including the central autonomic network (CAN) (Benarroch, 1993), with the prefrontal cortex playing a leading role (Thayer and Lane, 2009). Indeed, the cortical regulation of the CAN has been well described; there are both direct and indirect pathways (involving the cingulate and insula cortices, amygdala, hypothalamus and medulla oblongata) linking the frontal cortex to autonomic motor circuits responsible for both the excitatory and inhibitory effects on the heart (see Thayer and Lane, 2009 for a review). Therefore, the moment-to-moment control of HR reflects complex interactions between physiology, emotion and cognition (Benarroch, 1993; Thayer and Lane, 2009), encompassing the integration of a wide variety of information. Thus, it has been suggested that HRV reflects the overall capacity of the body to deal with on-going demands (Young and Benton, 2015). ‘HRV may serve as a proxy for the ‘vertical integration’ of the brain mechanisms that guide flexible control over behaviour with peripheral physiology, and as such provides an important window into understanding stress and health’ (Thayer et al., 2012). In this way, HRV may act as a biomarker when considering the influence of diet on health-related mechanisms.
Fig. 1.
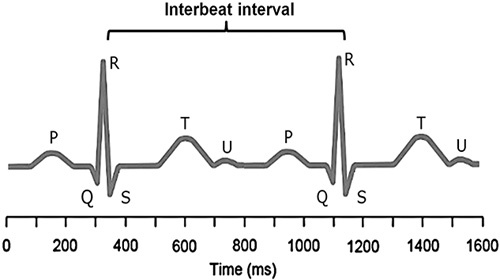
A heart-rate trace. A typical electrocardiogram trace is illustrated. R is the peak of the QRS complex (electrocardiogram trace) and heart-rate variability is measured by considering consecutive R–R intervals. The R–R interval is not constant, but varies within a normal range of 0.6–1.2 s. It is the degree of this R–R variability that is of interest as greater variability is associated with better health.
Indices of heart-rate variability
Three approaches are used to monitor HRV: time-domain and frequency-domain measures are linear in nature, whereas complexity measures offer a nonlinear approach. The simplest approach is to summarize differences in the interbeat interval as a mean and SD, so-called time-domain measures (Fig. 2). Second, a spectral analysis allows frequency domain indices to be calculated and describes the interbeat intervals as a complex sum of waveforms. The variance in HR is distinguished in terms of the underlying rhythms that occur at different frequencies. There is growing evidence that frequency-domain indices measure the functioning of the autonomic nervous system. The high-frequency (HF) measure (power in the range 0.15–0.4 Hz) is more easily understood as it is said to reflect the parasympathetic nervous systems (PNSs) (Katona et al., 1970; Appel et al., 1989; Billman and Hoskins 1989; Camm et al., 1996; Thayer et al., 2010). In contrast, low-frequency (LF) (power in the range: 0.04–0.15 Hz) is less readily interpreted as it is influenced by both the PNS and also baroreceptor activity (Houle and Billman, 1999; Billman, 2013). A frequently considered index is the LF/HF ratio, which is said to reflect the balance between PNS and the sympathetic nervous system activity (Malik, 1998), although this contention has been challenged recently (Billman, 2013).
Fig. 2.

Various measures of heart-rate variability. The variability in consecutive differences in the R–R interval can be expressed using a number of different approaches that are one of three types: time or frequency based or nonlinear. HF, high-frequency; LF, low-frequency.
More recently, nonlinear indices have been developed: as one example, approximate entropy provides an indication of the irregularity or randomness in a data set. The time series 1, 2, 3, 1, 2, 3 has the same variability as the time series 1, 1, 2, 2, 3, 3 and 3, 1, 2, 2, 3, 1, but different underlying patterns. Nonlinear analysis enables the quantification of this extra information. Essentially, the nonlinear approach measures the complexity of the signal; how frequently do similar patterns reoccur? Although frequency measures in particular have been studied widely, it has become apparent that nonlinear indices of complexity reflect different underlying mechanisms (Young and Benton, 2015). For example, a recent study reported that nonlinear measures of HRV were better able to predict the performance of cognitive tests than linear measures (Young and Benton, 2015). In addition, a meta-analysis of the impact of depression and antidepressant treatment on HRV found that the effects were most apparent with nonlinear measures of HRV (Kemp et al., 2010). There is an impression that those capable of generating more complex signals have a greater ability to respond to environmental demands in a more subtle manner (de la Torre-Luque et al., 2016).
Heart-rate variability, inflammation and disease
Inflammation is part of the immune response, with too much and too little inflammation indicating, or leading to, a wide range of diseases. Hotamisligil (2006) noted that metabolic regulation and the immune system are highly integrated: he suggested that this interaction reflects a central homeostatic mechanism that, if dysfunctional, results in chronic metabolic disorders, particularly obesity, type 2 diabetes and cardiovascular disease. An inadequate response creates immunodeficiency that can result in infection or cancer (Mellemkjaer et al., 2002). As the inflammatory response is in part regulated by the autonomic nervous system, HRV is of interest.
The vagus plays a role in the inflammatory reflex that controls innate immune responses when tissue is injured or there is a pathogen invasion (Pavlov and Tracey, 2012). The inflammatory reflex has an afferent component that is activated by cytokines that relay information to the hypothalamus. This input can then initiate an anti-inflammatory response that prevents the release of inflammatory products into the blood stream (Tracey, 2002). It is becoming increasingly clear that the nervous system, through the vagal nerve, both monitors peripheral inflammation and dampens the immune response (Boeckxstaens, 2013). The sympathetic arm of the autonomic nervous system also plays a role: it releases noradrenaline, which appears to act at sites other than the synapse, as many immune cells, including lymphocytes and macrophages, have adrenergic receptors (Kavelaars et al., 1999).
HRV is associated with the levels in the blood of C-reactive protein (CRP), a protein that is produced by the liver as a response to inflammation, with the levels increasing following the secretion of interleukin (IL)-6 from macrophages and T cells. The role of the protein is to bind to lysophosphatidyl choline on the surface of dead cells and to enhance the ability of antibodies and phagocytic cells to remove pathogens. Higher levels of CRP are associated with a greater risk of hypertension, diabetes and cardiovascular disease (Dehghan et al., 2007). Jarczok et al. (2014) reported that the HF component of HRV predicted the level of CRP when it was measured 4 years later; this is important as both inflammation and HRV have been implicated in conditions such as diabetes and cardiovascular disease (Kudat et al., 2006; Thayer et al., 2010).
Madsen et al. (2007) related low-grade inflammation to autonomic dysfunction in those with suspected coronary heart disease. The mean SD of normal to normal R–R (SDNN) was higher in those with lower levels of CRP, with the association being greater in those with a previous history of myocardial infarction. However, CRP remained associated with HRV independent of disease, a finding interpreted as a relationship occurring between low-grade inflammation and autonomic dysfunction. Experimental evidence is beginning to emerge and support these associations. For example, a recent study found that vagus nerve-stimulation in epilepsy patients inhibited peripheral blood production of tumour necrosis factor, IL-1β and IL-6 (Koopman et al., 2016).
Whether by influencing inflammation or by other mechanisms, HRV has been associated with a range of diseases, in some cases predicting subsequent problems and in others being an index of disease progression (RenuMadhavi and Ananth, 2012). As a generalization, a healthy biological system tends to be both variable and complex, characteristics that decline with disease. Irrespective of disease, HRV declines with age, reaching, in some older than 65 years of age, levels that are a risk factor for mortality (Umetani et al., 1998).
The American Heart Association states that diabetes increases the risk of heart disease or a stroke and thus early diagnosis of complications is the key to decreasing mortality. A systematic review of those with diabetes concluded that HRV can help to predict cardiac morbidity and mortality, and that it can be used at an early stage to indicate the future risk of complications (Fakhrzadeh et al., 2012). As one example, Yoshioka and Terasaki (1994) reported that the HF component, which indicates parasympathetic nervous activity, was lower in diabetics rather than controls, and there were inverse correlations between the LF and HF. It was concluded that these measures of HRV are useful when evaluating diabetic autonomic and peripheral neuropathies. The Atherosclerosis Risk in Communities study (Schroeder et al., 2005) also found that diabetics differenced in HF power and that HF in nondiabetics was greater in those with lower levels of fasting insulin. Thus, there was a relationship between insulin resistance, as indicated by higher levels of fasting insulin, and lower HRV. In addition, after a 9-year follow–up, there was a general decline in HRV. Such findings suggest that an impairment of the functioning of the autonomic nervous system, reflected in HRV, occurs during the early stages of diabetes and becomes progressively worse over time.
A more general question is the association between HRV and blood glucose levels. The Framingham Heart Study (Singh et al., 2000) related HRV to fasting levels of blood glucose: LF and HF power was reduced in those with diabetes or impaired levels of fasting glucose. In addition, a recent study found that resting HRV (average R–R interval, HF power, sample entropy) predicted the increase in blood glucose following a high glycaemic load drink (Young and Watkins, 2016). Overall, HRV appears to be associated inversely with plasma glucose levels. It is plausible that the links between insulin resistance, blood glucose and HRV are attributable to deficits in the inflammatory reflex (Pavlov and Tracey, 2012). Vinik (2012) noted that activation of inflammatory cytokines in newly diagnosed type 2 diabetes correlated with changes in sympatho–vagal balance. As, in type 2 diabetes, changes in the autonomic nervous system predict sudden death, such measures offer the chance of an early intervention.
As mentioned above, a reduction in HRV predicts macrovascular disease, for example carotid artery atherosclerosis (Gottsäter et al., 2006). Indeed, a recent study found that insulin resistance mediated the association between HF-HRV and carotid intima–media thickness (Kemp et al., 2016). In fact, it has been known for many years that following myocardial infarction, HRV is related to the risk of consequent morbidity and mortality (La Rovere et al., 1998). In addition, the rate at which congestive heart failure and arrhythmias occur has been related to a reduced HRV (Sandercock and Brodie, 2006) and lower HRV complexity is associated with a worse outcome in cardiac patients (Souza et al., 2015). Although fewer studies have considered those without a history of coronary heart disease, a meta-analysis examined those without any problem at baseline (Hillenbrand et al., 2013). A lower HRV, measured as SDNN, was associated with a subsequent 40% increase in the risk of suffering a first cardiovascular event.
Heart-rate variability, obesity and weight loss
Decreased HRV is associated with a significantly increased risk of death from cardiovascular disease (Bigger et al., 1993; Makikallio et al., 1999). Therefore, Kim et al. (2005) considered whether the high rate of cardiovascular disease in obese individuals might be associated with changes in HRV. They found that the RMSSD (a frequently used index of parasympathetic activity) was correlated negatively with fat mass and the hip-to-waist ratio. Similar effects were noted for LF power, although as noted above, this index is not easily interpretable. Nonetheless, it seems that obesity can alter HRV.
For example, Mouridsen et al. (2013) examined the impact of weight loss on HR and HRV in overweight postmenopausal women. An average weight loss of 3.9 kg was associated with a decrease in HR and increased HRV, as indicated by SDNN and the interbeat interval. Previously, Karason et al. (1999) had considered obese patients, who, after surgery, had lost 28% of their body weight, on average 32 kg. SDNN and HF power were attenuated in those with obesity compared with lean patients; HRV improved with weight loss. Adachi et al. (2011) examined the influence of obesity on autonomic activity during sleep. Volunteers were allocated randomly to a standard diet or one that over 8 weeks increased weight by 4 kg. After weight gain, HF power decreased both when awake and when asleep, changes that resolved with weight loss. A related finding was that caloric restriction may reverse the autonomic changes that occur as we age. A decline in HRV with age is well described: for example, Zulfiqar et al. (2010) compared HRV in patients of ages ranging from 10 to 99 years. Several measures of HRV all decreased rapidly from the second to fifth decades, although the decline then slowed. They concluded that a healthy long life depends on the preservation of autonomic functioning, more specifically, the influence of the PNS on HRV. Stein et al. (2012) found that in patients who had practiced caloric restriction, for on average 7 years, HR was lower and several measures of HRV values were significantly higher. In fact, HRV was comparable to the norms for those 20 years younger. The overall impression is that weight gain adversely influences HRV, although this effect may be reversible with weight loss and/or dietary restriction.
Heart-rate variability and eating behaviour
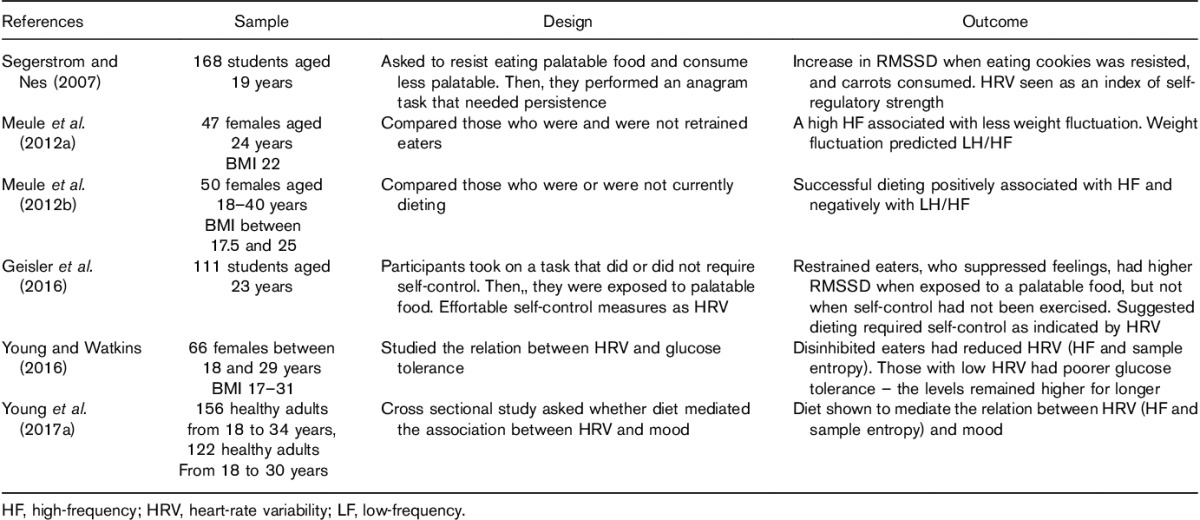
HR variables have also been related to dieting and eating behaviour. A recent review concluded that most, although not all, studies investigating HRV in those with anorexia nervosa find parasympathetic dominance (Mazurak et al., 2011). Similarly, those with bulimia nervosa are characterized by higher vagal activity, particularly HF-HRV (Peschel et al., 2016). In a healthy sample, Meule et al. (2012b) found that restrained eating, which is the intentional restriction of food intake to prevent weight gain or to promote weight loss, was associated with low cardiac vagal control (Table 1). The restraint score and the extent to which weight fluctuated predicted HF-HRV negatively. In a further study, Meule et al. (2012a) qualified this finding by considering successful versus unsuccessful dieters: success was associated positively with HF-HRV. They concluded that vagal–cardiac control reflected the strength of self-regulation such that successful restrained eaters were characterized by higher cardiac vagal control. These findings are in line with two studies that found reduced HR variability and complexity (R–R interval, HF power, sample entropy) in those with a propensity towards disinhibited eating, which is a tendency to overeat in the presence of palatable foods or other disinhibiting stimuli, such as emotional stress (Young and Watkins, 2016; Young et al., 2017a). Importantly, these effects remained after controlling for the healthiness of the diet. Thus, it appears that tonic HRV might index individual differences in the capacity to self-regulate in the face of temptation. Indeed, Geisler et al. (2016) reported that restrained eaters who suppressed their feelings about a distressing film had higher vagally mediated HRV (RMSSD) during subsequent exposure to a palatable food (jelly beans). In an earlier study, Segerstrom and Nes (2007) found an increase in RMSSD when participants were told to resist warm cookies and instead eat carrots. A caveat is that caloric restriction and intermittent fasting, behaviours commonly observed in restrained eaters, have been shown to increase HF oscillatory components in HR (Mager, 2006). Thus, the higher HF-HRV observed in this population may be because of lifestyle factors rather than differences in self-regulatory capacity. Future research should explore this possibility. That said, these associations between eating behaviour and HRV offer the possibility of examining the association between the ability of different types of food to influence HRV and their ability to reduce or prevent pathological eating behaviours.
Table 1.
The relation between heart-rate variability and eating behaviour
Heart-rate variability and psychiatric disorders
A dysfunctional autonomic nervous system, with an associated reduction in HRV, has been found in a wide range of psychiatric disorders, a contention supported by a number of recent meta-analyses. Kemp et al. (2010) examined the association between depression and HRV in those without cardiovascular disease. When depressed patients and healthy controls were compared, the former had a lower HRV; it was particularly lower in those with more severe symptoms. Similar meta-analytic reviews have shown HRV reductions in a range of psychiatric disorders: bipolar disorder (Faurholt-Jepsen et al., 2017), anxiety disorders (Chalmers et al., 2014), post-traumatic stress disorder (Nagpal et al., 2013) and schizophrenia (Clamor et al., 2016). Notably, such effects are most consistently examined using HF-HRV or other vagally mediated HRV indices (e.g. RMSSD), although on occasion, larger effect sizes have been noted with nonlinear indices (Kemp et al., 2010). Importantly, HRV predicted the onset of psychological illness 10 years later (Jandackova et al., 2016). Given the links between HRV, emotion regulation and executive functioning (Williams et al., 2015; Zahn et al., 2016; Holzman and Bridgett, 2017), it has been proposed that HRV is a transdiagnostic biomarker of mental illness (Beauchaine and Thayer, 2015).
This brief overview illustrates that HRV is a risk for, or a marker of, a wide range of disorders. As such, any intervention that impacted positively on HRV has the potential to benefit health. Evidence suggests that lifestyle may be one such factor. Young et al. (2017a) reported that smoking cigarettes and drinking alcohol negatively influenced HRV, measured using linear (HF power) and nonlinear (sample entropy) measures. In addition, these aspects of the HR time series were increased in those who took regular exercise and consumed a healthy diet. Notably, diet quality at least partially explained the association between mood, disinhibition and HRV (Young et al., 2017a); thus, dietary modification may improve HRV in those at risk of psychological disorders.
The nature of the diet and heart-rate variability
In an early study, Lu et al. (1999) examined the post-prandial changes in HRV following a 500 kcal test meal (turkey sandwich: 32.4% fat, 17.5% protein and 50.1% carbohydrate). HF power decreased during the first and second 30 min following consumption, suggesting a reduction in vagal tone. Similarly, after a mixed meal of 501.8 kcal that comprised 31% protein, 18% lipids and 51% carbohydrates, a decrease in HF power was observed from 40 to 120 min after the meal, an effect that correlated negatively with ghrelin concentrations (Chang et al., 2010). In relation to specific macronutrients, Kanaley et al. (2007) studied the influence of an acute glucose load on HRV in obese women with and without diabetes. Total power decreased in response to the glucose challenge compared with what was observed in the fasted state. In addition, a high-carbohydrate meal was reported to augment HRV reactivity (in response to mental stress) compared with a high-protein meal: HR decreased more quickly poststress after the carbohydrate meal (Uijtdehaage et al., 1994). Over a 2-day period, Lima-Silva et al. (2010) examined the impact of a low-carbohydrate diet in those who had been exercising. Compared with a high-carbohydrate diet, the low-carbohydrate diet increased LF and decreased HF power. There were, however, no differences in HR or R–R interval. As this study considered those who had been exercising, there is a need to look at those simply going about their everyday life.
Nagai et al. (2005) reported that a high-fat meal increased the VLF component of HRV. Although the physiological processes underlying VLF-HRV are unclear, Nagai et al. (2005) interpreted this as an indication of thermoregulatory sympathetic nervous system activity. Other components of HRV may also be influenced by the nature of dietary fat, at least in the longer term: Soares-Miranda et al. (2012) reported that in two cohorts, either 19 or 72 years of age, a greater consumption of trans-fats was associated with a less favourable HRV; SDNN, RMSSD and total power were lower. Importantly, trans-fat consumption predicted lower HRV 5 years later.
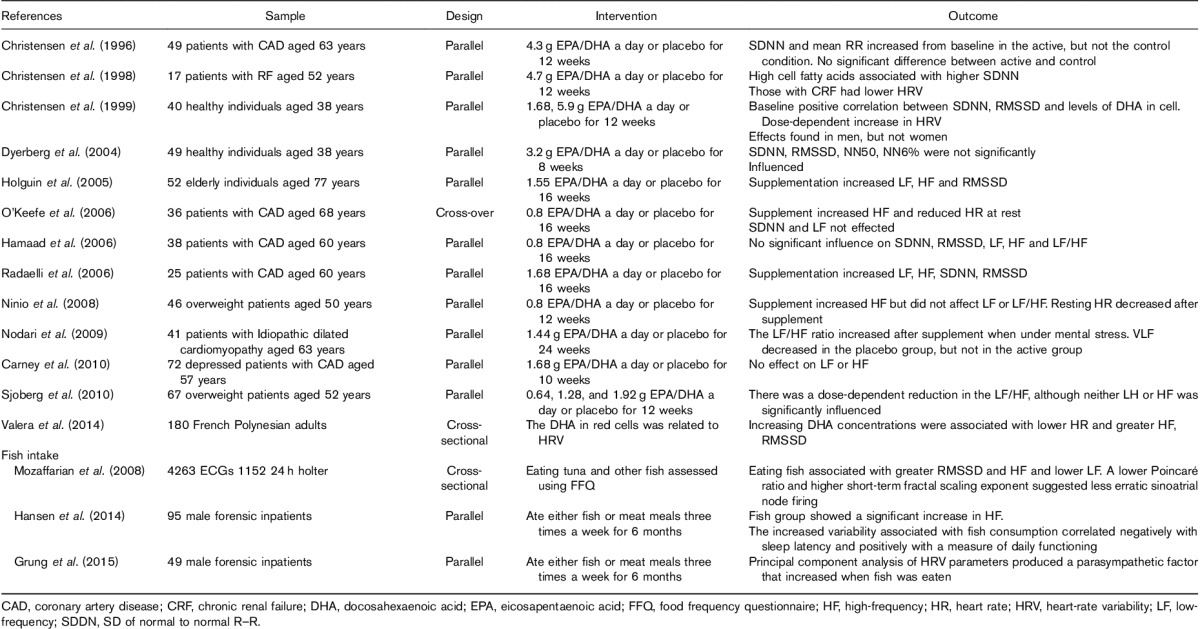
When considering the influence of diet, by far the majority of work has involved supplementation with omega-3 fatty acids. For example, in a secure forensic inpatient facility, Hansen et al. (2014) randomly allocated patients to a diet containing salmon three times a week compared with meat in the control group. The changes in HRV associated with fish consumption correlated negatively with sleep latency and positively with a measure of daily functioning. Table 2 lists a representative sample of studies that show that research has been driven by an interest in disease states, particularly heart disease, and has typically involved the examination of older adults. There is a general impression that omega-3 supplementation resulted in greater parasympathetic activity, although greater confidence in this conclusion comes from a meta-analysis. Xin et al. (2013) integrated fifteen studies. The time-domain measures, SDNN and RMSSD, did not differ after supplementation. However, although there was no effect on the frequency measure LF, fish oil significantly increased HF. The authors concluded that the ‘enhancement of vagal tone may be an important mechanism underlying the antiarrhythmic and other clinical effects of fish oil’. Similarly, the review of Christensen and Schmidt (2007) concluded that: ‘In most (Billman et al., 1994; Christensen et al., 1999; Christensen and Schmidt 2007; Billman and Harris, 2011; Christensen, 2011), although not all, studies, dietary n-3 PUFA levels and n-3 PUFA supplementation are related to improved HR variability’. The findings suggested that an increased parasympathetic regulation of cardiac functioning occurred.
Table 2.
The influence of fish oil supplementation and eating fish on heart-rate variability
More generally, the Mediterranean diet, which has fish as a component, is also associated with HRV. Dai et al. (2010), using a food-frequency questionnaire, established the extent to which middle-aged male twins ate a Mediterranean diet. After adjusting for genetic contributions, those who conformed to the Mediterranean diet had higher HRV across a range of indices: SDNN, RMSSD, ULF, VLF, HF and LF. Although it was unclear which aspect of the diet was beneficial, it is tempting to suggest that one factor is eating fish and in this way increasing the intake of omega-3 fatty acids. Billman (2013) reviewed the evidence that the intake of omega-3 fatty acids influenced cardiac rhythms. He concluded that supplementation with n3-PUFAs affects ion channels and calcium-regulatory proteins, although these depended on the route of administration. Immediately, there is a direct effect of the fatty acids on ion channels, although over the longer term, after the incorporation of the fatty acids into the cell membrane, cardiac electrical activity changes. HR is reduced and HRV increases, reflecting alterations in the intrinsic pacemaker rather than regulation by the activity of the autonomic nervous system.
Other contributions to a Mediterranean diet have also been found to influence HRV. In a randomized trial, the influence of a multivitamin-mineral preparation on HRV was assessed (Pomportes et al., 2015). Faster reactions in a test of the ability to inhibit responses were associated with a stable HF, indicative of PNS activity. In those taking micronutrients, HF remained stable, whereas in the placebo condition, HF decreased over time. Jaatinen et al. (2014) studied the influence of yoghurt enriched with α-lactalbumin, bioactive peptides and B vitamins in individuals with high-trait anxiety. In the active group, vagally mediated HRV (RMSSD) was higher. They concluded that this yoghurt combination may aid coping with stress. As nut consumption is associated with a lower risk of cardiovascular disease, Sauder et al. (2014) studied its influence on HRV. The pistachio diet was associated with a higher HF and greater total variation in beat-to-beat intervals. Similar effects have been observed in relation to the consumption of polyphenol-rich red wine. Intake of wine, but not of spirits or beer, is positively and independently (after controlling for other health behaviours) associated with HRV (SDNN, total power, VLF power, LF power and HF power) in women with CHD (Janszky et al., 2005).
There is also some evidence that particular nutrients influence HRV. Sucharita et al. (2014) considered HRV in a healthy elderly Indian sample with either a high or a low vitamin B12 status. The LF was lower in those with a poorer vitamin B12 status, although no effects on HF power or HR were observed. Supplementation for 3 months increased LF to levels comparable to those with an initially good vitamin B12 status. Sodium intake is also linked to HRV. In a randomized-controlled trial, Allen et al. (2014) asked participants, with normal blood pressure, to consume for 5 days a diet with low (10 mmol/day), normal (150 mmol) or high (400 mmol) levels of sodium. The response to low sodium was consistent with sympathetic activation and reduced vagal activity; LFnu (normalized) increased and SDNN, RMSSD and HFnu (normalized) decreased compared with both normal and high sodium conditions.
In summary, although there has been limited systematic study, there is a series of reports that relate various indices of HRV to the intake of a range of food items. As such, there is good reason to support the further consideration of HRV as a biomarker with the potential to indicate the potential influence of food on health.
Discussion
There is growing evidence that a range of diseases are accompanied by a decrease in HRV, including, amongst others, diabetes (Yoshioka and Terasaki, 1994), cardiovascular disease (Gottsäter et al., 2006) and psychiatric disorders (Kemp et al., 2014). Although indices of HRV do not distinguish between types of disorder, there is a consistent pattern of a reduced variability in HR being associated with disease: a higher HRV is associated with psychological flexibility and allostatic resilience. Indeed, longitudinal studies support the view that reduced HRV predicts psychological and physiological morbidity years later (Carnethon et al., 2003; Jandackova et al., 2016). Thus, HRV may serve as a biomarker for future health.
Various aspects of diet have been found to be associated with HRV. In general, the types of diet and particular foods that have been found to be associated with a healthy life-style are associated with higher HRV. For example, a Mediterranean diet (Mozaffarian et al., 2008; Soares-Miranda et al., 2012), fish consumption (Mozaffarian et al., 2008), multivitamins (Pomportes et al., 2015) and losing weight (Zulfiqar et al., 2010) all increased HRV. However, aspects of diet that are commonly viewed as undesirable, for example a high fat or trans-fat diet, reduced HRV (Soares-Miranda et al., 2012).
Although it is clear that diet influences HRV, the mechanisms and pathways underlying such effects are multifactorial. In relation to physical health, it is clear that vagal tone is central to the regulation of a number of allostatic systems, including the cardiovascular system, glucose regulation, the hypothalamic–pituitary–adrenal axis function, and inflammatory processes (Thayer and Sternberg, 2006; Young and Benton, 2015; Viljoen and Claassen, 2017). Thus, reduced vagal tone may directly contribute towards increased allostatic load (Koopman et al., 2016). However, chronic diseases such as diabetes contribute towards autonomic neuropathy (Vinik et al., 2003), which is associated with reduced HRV (Fakhrzadeh et al., 2012). Such bidirectional effects make causality difficult to determine.
Similarly, it has been argued that vagal afferent signals might mediate the influence that diet has on psychological health (Kemp et al., 2017). Indeed, emerging evidence suggests that cardiac interoceptive sensations contribute towards one’s mental health (Craig, 2003; Critchley et al., 2004; Seth, 2013; Young et al., 2017b); however, it is difficult to isolate diet-related effects on afferent vagal signals. In addition, influential models such as the neurovisceral integration model (Thayer and Lane, 2009) consider HRV to be the product of the CAN, which guides goal-directed behaviour; the prefrontal cortex plays a central inhibitory role (Thayer and Lane, 2000; Smith et al., 2017). Thus, HRV may capture primarily efferent vagal activity. With this in mind, it is plausible that the effects of diet on HRV operate indirectly through changes in mental health. That is, diet influences brain functioning, cognition and mood, which is then reflected in changes in HRV. Indeed, a study supports the view that diet influences psychological health not only acutely (Young and Benton, 2014, 2015) but also chronically (Jacka et al., 2010). However, whether these effects mediate or are meditated by HRV remains to be determined.
Furthermore, the link between HRV and pathological eating behaviours (Young and Watkins, 2016) points towards the possibility of mutual causation. That is, reduced HRV, by virtue of its connections with the prefrontal cortex (Dietrich et al.,, 2006; Chang et al., 2013; Jennings et al., 2016), may indicate a predisposition to make poor dietary choices. In turn, a poor-quality diet could exacerbate the reduction in HRV. In support of this proposition, Young et al. (2017a) found that disinhibited eating and poor diet quality were associated independently with deficits in HRV. Notably, HRV is related positively to interoceptive sensitivity (Ainley et al., 2012), raising the possibility that diet-related reductions in vagal tone may diminish the ability to detect interoceptive signals, including information about one’s current homeostatic state (Attuquayefio et al., 2017, Smith et al., 2017), and contribute towards mental illnesses (Paulus and Stein, 2010). Irrespective of the mechanisms involved, the association between HRV and so many types of disease state suggests that it is a measure of factors with a wide-spread significance. Thus, HRV may serve as a useful biomarker for identifying potentially beneficial or detrimental aspects of diet.
A distinction should be drawn between phasic and tonic HRV as this will impinge upon interpretation of research findings. In relation to emotion, demanding situations may give rise to an increase or a decrease in phasic HRV. The former might arise when an individual successfully self-regulates to deal with the demands of the situation (Park et al., 2014; Geisler et al., 2016) and the latter may arise when the situation is perceived as threatening and an individual shows an autonomic stress response (Segerstrom and Nes, 2007).
The vast majority of research linking health and HRV has focused on tonic resting-state HRV (Chalmers et al., 2014; Kemp et al., 2014, 2016). Although tonic HRV predicts phasic changes in vagal tone, lower tonic HRV was associated with phasic HRV suppression and higher tonic HRV was associated with phasic HRV enhancement (Park et al., 2014). This is an important methodological consideration when considering the effects of diet.
Phasic changes represent real-time adaptations to the external or the internal environment; as such, a temporary decline in HRV need not necessarily be pathological. An example can be considered in relation to the literature on fluid consumption. Hypohydration necessitates a counter-regulatory increase in HR to maintain blood pressure: for every 1% decrease in body mass during exercise, there is an increase in HR of 3.29 bpm (Adams et al., 2014). Even in the absence of hypohydration, water ingestion is followed by an increase in cardiac vagal control (Helen et al., 2002). Under such circumstances, an inability to flexibly alter the variability of HR may be viewed as detrimental. Thus, whether tonic HRV influences one’s ability to effectively counter regulate during the postprandial period is an important question. Young and Watkins (2016) provided the first evidence that this might be the case: individual differences in tonic vagal tone moderated the postprandial response to drinks that differed in glycaemic load. Whether a higher resting-state HRV confers greater metabolic resilience during the post-prandial period is an important question for future research.
Although we have argued that decreased HRV may serve as a biomarker of future health, there are caveats that need to be considered. For example, it has been observed that black individuals have higher HRV, and yet are at greater risk of future cardiovascular disease (Hill et al., 2017). In addition, although most psychiatric disorders are characterized by reduced HRV, those with anorexia nervosa (Mazurak et al., 2011) or bulimia nervosa (Peschel et al., 2016) tend to have parasympathetic dominance (higher HF-HRV). Although both these conundrums may be explained by dietary differences in these populations – for instance, there are clear racial differences in nutritional behaviour – the literature to date has typically not explored this possibility. This will be an important avenue for future research.
There are three types of approach by which HRV can be measured: the linear time-based and frequency-based approaches and also nonlinear methods. To date, limited research has considered nonlinear methods when studying diet, although there are reasons to believe that they contribute additional information to that supplied by the linear methods (Young and Benton, 2015). In addition, there is some evidence that nonlinear HRV indices capture the influence of endogenous hormonal fluctuations and thermoregulation (Bai et al., 2009; Young and Benton, 2015), which may be pertinent when considering the influence of diet. Thus, future studies should incorporate both linear and nonlinear approaches into their designs.
In summary, the consistent relationship between HRV, health and morbidity supports the view that HRV can be considered an index of psychological and physiological resilience. An increasing number of studies report that particular foods, nutrients and dietary styles influence HRV, supporting its future use when examining the impact of what we eat. The insidious influence of diet will be cumulative and take place over a period of many decades (Benton, 2010). As such HRV may prove to be a means for identifying beneficial or detrimental aspects of diet.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- Adachi T, Sert-Kuniyoshi FH, Calvin AD, Singh P, Romero-Corral A, van der Walt C, et al. (2011). Effect of weight gain on cardiac autonomic control during wakefulness and sleep. Hypertension 57:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams WM, Ferraro EM, Huggins RA, Casa DJ. (2014). Influence of body mass loss on changes in heart rate during exercise in the heat: a systematic review. J Strength Cond Res 28:2380–2389. [DOI] [PubMed] [Google Scholar]
- Ainley V, Tajadura‐Jiménez A, Fotopoulou A, Tsakiris M. (2012). Looking into myself: changes in interoceptive sensitivity during mirror self‐observation. Psychophysiology 49:1672–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AR, Gullixson LR, Wolhart SC, Kost SL, Schroeder DR, Eisenach JH. (2014). Dietary sodium influences the effect of mental stress on heart rate variability: a randomized trial in healthy adults. J Hypertens 32:374–382. [DOI] [PubMed] [Google Scholar]
- Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. (1989). Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol 14:1139–1148. [DOI] [PubMed] [Google Scholar]
- Attuquayefio T, Stevenson RJ, Oaten MJ, Francis HM. (2017). A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS One 12:e0172645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Li J, Zhou L, Li X. (2009). Influence of the menstrual cycle on nonlinear properties of heart rate variability in young women. Am J Physiol Heart Circ Physiol 297:H765–H774. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF. (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol 98:338–350. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. (1993). The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68:988–1001. [DOI] [PubMed] [Google Scholar]
- Benton D. (2010). Neuro-developmental and neuro-degeneration - are there critical times for nutritional intervention? Nutr Rev 68 (Suppll 1):S6–S10. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. (1993). The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation 88:927–934. [DOI] [PubMed] [Google Scholar]
- Billman GE. (2013). The effects of omega-3 polyunsaturated fatty acids on cardiac rhythm: a critical reassessment. Pharmacol Ther 140:53–80. [DOI] [PubMed] [Google Scholar]
- Billman GE, Hoskins RS. (1989). Time-series analysis of heart rate variability during submaximal exercise. Evidence for reduced cardiac vagal tone in animals susceptible to ventricular fibrillation. Circulation 80:146–157. [DOI] [PubMed] [Google Scholar]
- Billman GE, Harris WS. (2011). Effect of dietary omega-3fattyacids on heartrate and the heart rate variability responses to myocardial ischemia or exercise. Am J Physiol Heart Circ Physiol 300:H2288–H2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE, Hallaq H, Leaf A. (1994). Prevention of ischemia-induced ventricular fibrillation by ω3 fatty acids. Proc Natl Acad Sci USA 91:4427–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckxstaens G. (2013). The clinical importance of the anti-inflammatory vagovagal reflex. Hand Clin Neurol 117:119–134. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Malik M, Bigger J, Breithardt G, Cerutti R, Cohen P, et al. (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93:1043–1065. [PubMed] [Google Scholar]
- Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. (2003). Prospective Investigation of Autonomic Nervous System Function and the Development of Type 2 Diabetes The Atherosclerosis Risk In Communities Study, 1987–1998. Circulation 107:2190–2195. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Steinmeyer BC, Harris WS, Rubin EH, et al. (2010). Effect of omega-3 fatty acids on heartrate variability in depressed patients with coronary heart disease. Psychosom Med 72:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyerberg J, Eskesen DC, Andersen PW, Astrup A, Buemann B, Christensen JH, et al. (2004). Effects of trans- and n23 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr 58:1062–1070. [DOI] [PubMed] [Google Scholar]
- Chalmers J, Quintana D, Abbott M, Kemp A. (2014). Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiat 5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Ko C, Lien H, Chou M. (2010). Varying postprandial abdominovagal and cardiovagal activity in normal subjects. Neurogastroenterol Motil 22:546. [DOI] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. (2013). Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JH. (2011). Omega-3 fatty acids and heart rate variability. Front Physiol 2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JH, Schmidt EB. (2007). Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovas Med 8:S19–S22. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Gustenhoff P, Korup E, Aaroe J, Toft E, Moller J, et al. (1996). Effect of fish oil on heart rate variability in survivors of myocardial infarction: a double blind randomised controlled trial. BMJ 312:677–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JH, Aarøe J, Knudsen N, Dideriksen K, Kornerup HJ, Dyerberg J, et al. (1998). Heart rate variability and n23 fatty acids in patients with chronic renal failure –a pilot study. Clin Nephrol 49:102–106. [PubMed] [Google Scholar]
- Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. (1999). Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr 70:331–337. [DOI] [PubMed] [Google Scholar]
- Clamor A, Lincoln TM, Thayer JF, Koenig J. (2016). Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br J Psychiatry 208:9–16. [DOI] [PubMed] [Google Scholar]
- Craig A. (2003). Interoception: the sense of the physiological condition of the body. Curr Opinion Neurobiol 13:500–505. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. (2004). Neural systems supporting interoceptive awareness. Nature Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Dai J, Lampert R, Wilson PW, Goldberg J, Ziegler TR, Vaccarino V. (2010). Mediterranean dietary pattern is associated with improved cardiac autonomic function among middle-aged men: a twin study. Circ Cardiovasc Qual Outcomes 3:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, et al. (2007). Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 56:872–878. [DOI] [PubMed] [Google Scholar]
- De la Torre-Luque A, Bornas X, Balle M, Fiol-Veny A. (2016). Complexity and nonlinear biomarkers in emotional disorders: a meta-analytic study. Neurosci Biobehav Rev 68:410–422. [DOI] [PubMed] [Google Scholar]
- Dietrich DF, Schindler C, Schwartz J, Barthélémy JC, Tschopp JM, Roche F, et al. (2006). Heart rate variability in an ageing population and its association with lifestyle and cardiovascular risk factors: results of the SAPALDIA study. Europace 8:521–529. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh H, Yamini-Sharif A, Sharifi F, Tajalizadekhoob Y, Mirarefin M, Mohammadzadeh M, et al. (2012). Cardiac autonomic neuropathy measured by heart rate variability and markers of subclinical atherosclerosis in early type 2 diabetes. ISRN Endocrinol 2012:168264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurholt-Jepsen M, Kessing LV, Munkholm K. (2017). Heart rate variability in bipolar disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev 73:68–80. [DOI] [PubMed] [Google Scholar]
- Geisler FC, Kleinfeldt A, Kubiak T. (2016). Restrained eating predicts effortful self-control as indicated by heart rate variability during food exposure. Appetite 96:502–508. [DOI] [PubMed] [Google Scholar]
- Gottsäter A, Ahlgren AR, Taimour S, Sundkvist G. (2006). Decreased heart rate variability may predict the progression of carotid atherosclerosis in type 2 diabetes. Clin Auton Res 16:228–234. [DOI] [PubMed] [Google Scholar]
- Grung B, Hansen AL, Berg M, Møen-Knudseth MP, Olson G, Thornton D, et al. (2015). Exploratory multivariate analysis of the effect of fatty fish consumption and medicinal use on heart rate and heart rate variability data. Front Psychol 6:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaad A, Kaeng LW, Lip GY, MacFadyen RJ. (2006). Oral omega n3-PUFA therapy (Omacor) has no impact on indices of heart rate variability in stable post myocardial infarction patients. Cardiovasc Drugs Ther 20:359–364. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Dahl L, Olson G, Thornton D, Graff IE, Frøyland L, et al. (2014). Fish consumption, sleep, daily functioning, and heart rate variability. J Clin Sleep Med 10:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helen CS, Chowdhary JH, Coote JN, Townend JN. (2002). Cardiac vagal response to water ingestion in normal human subjects. Clin Sci 103:157–162. [DOI] [PubMed] [Google Scholar]
- Hill LK, Watkins LL, Hinderliter AL, Blumenthal JA, Sherwood A. (2017). Racial differences in the association between heart rate variability and left ventricular mass. Exp Physiol 102:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenbrand S, Gast KB, de Mutsert R, Swenne CA, Jukema JW, Middeldorp S, et al. (2013). Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose–response meta-regression. Europace 15:742–749. [DOI] [PubMed] [Google Scholar]
- Holguin F, Tellez-Rojo MM, Lazo M, Mannino D, Schwartz J, Hernandez M, et al. (2005). Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest 127:1102–1107. [DOI] [PubMed] [Google Scholar]
- Holzman JB, Bridgett DJ. (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev 74:233–255. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. (2006). Inflammation and metabolic disorders. Nature 444:860–867. [DOI] [PubMed] [Google Scholar]
- Houle MS, Billman GE. (1999). Low-frequency component of the heart rate variability spectrum: a poor marker of sympathetic act ivity. Am J Physiol 276:H215–H223. [DOI] [PubMed] [Google Scholar]
- Jaatinen N, Korpela R, Poussa T, Turpeinen A, Mustonen S, Merilahti J, et al. (2014). Effects of daily intake of yoghurt enriched with bioactive components on chronic stress responses: a double-blinded randomized controlled trial. Int J Food Sci Nutr 65:507–514. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, et al. (2010). Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry 167:1–7. [DOI] [PubMed] [Google Scholar]
- Jandackova V, Britton A, Malik M, Steptoe A. (2016). Heart rate variability and depressive symptoms: a cross-lagged analysis over a 10-year period in the Whitehall II study. Psychol Med 46:2121–2131. [DOI] [PubMed] [Google Scholar]
- Janszky I, Ericson M, Blom M, Georgiades A, Magnusson J, Alinagizadeh H, et al. (2005). Wine drinking is associated with increased heart rate variability in women with coronary heart disease. Heart 91:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczok MN, Koenig J, Mauss D, Fischer JE, Thayer JF. (2014). Lower heart rate variability predicts increased level of C-reactive protein 4 years later in healthy, nonsmoking adults. J Intern Med 276:667–671. [DOI] [PubMed] [Google Scholar]
- Jarczok MN, Kleber ME, Koenig J, Loerbroks A, Herr RM, Hoffmann K, et al. (2015). Investigating the associations of self-rated health: heart rate variability is more strongly associated than inflammatory and other frequently used biomarkers in a cross sectional occupational sample. PloS One 10:e0117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Sheu LK, Kuan DCH, Manuck SB, Gianaros PJ. (2016). Resting state connectivity of the medial prefrontal cortex covaries with individual differences in high‐frequency heart rate variability. Psychophysiology 53:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaley JA, Baynard T, Franklin RM, Weinstock RS, Goulopoulou S, Carhart R, et al. (2007). The effects of a glucose load and sympathetic challenge on autonomic function in obese women with and without type 2 diabetes mellitus. Metabolism 56:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karason K, Mølgaard H, Wikstrand J, Sjöström L. (1999). Heart rate variability in obesity and the effect of weight loss. Am J Cardiol 83:1242–1247. [DOI] [PubMed] [Google Scholar]
- Katona P, Poitras JW, Barnett GO, Terry BS. (1970). Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol 218:1030–1037. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, van der Voort CR, Heijnen C. (1999). Adrenergic receptor subtypes in human peripheral blood lymphocytes. Hypertension 34:e5. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. (2010). Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 67:1067–1074. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Brunoni AR, Santos IS, Nunes MA, Dantas EM, de Figueiredo RC, et al. (2014). Effects of depression, anxiety, comorbidity, and antidepressants on resting-state heart rate and its variability: an ELSA-Brasil cohort baseline study. Am J Psychiatry 171:1328–1334. [DOI] [PubMed] [Google Scholar]
- Kemp AH, López SR, Passos VM, Bittencourt MS, Dantas EM, Mill JG, et al. (2016). Insulin resistance and carotid intima–media thickness mediate the association between resting-state heart rate variability and executive function: a path modelling study. Biol Psychol 117:216–224. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Koenig J, Thayer JF. (2017). From psychological moments to mortality: a multidisciplinary synthesis on heart rate variability spanning the continuum of time. Neurosci Biobehav Rev 83:547–567. [DOI] [PubMed] [Google Scholar]
- Kim JA, Park YG, Cho KH, Hong MH, Han HC, Choi YS, et al. (2005). Heart rate variability and obesity indices: emphasis on the response to noise and standing. J Am Board Fam Pract 18:97–103. [DOI] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA 113:8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudat H, Akkaya V, Sozen A, Salman S, Demirel S, Ozcan M, et al. (2006). Heart rate variability in diabetes patients. J Int Med Res 34:291–296. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. (1998). Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 351:478–484. [DOI] [PubMed] [Google Scholar]
- Lima-Silva AE, Bertuzzi R, Dalquano E, Nogueira M, Casarini D, Kiss MA, et al. (2010). Influence of high- and low-carbohydrate diet following glycogen-depleting exercise on heart rate variability and plasma catecholamines. Appl Physiol Nutr Metab 35:541–547. [DOI] [PubMed] [Google Scholar]
- Lu CL, Zou X, Orr WC, Chen J. (1999). Postprandial changes of sympathovagal balance measured by heart rate variability. Dig Dis Sci 44:857–861. [DOI] [PubMed] [Google Scholar]
- Madsen T, Christensen JH, Toft E, Schmidt EB. (2007). C-reactive protein is associated with heart rate variability. Ann Noninvasive Electrocardiol 12:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DE. (2006). Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J 20:631–637. [DOI] [PubMed] [Google Scholar]
- Makikallio TH, Hoiber S, Kober L, Torp-Pedersen C, Peng CK, Goldberger AL, et al. (1999). Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRACE Investigators. TRAndolapril Cardiac Evaluation. Am J Cardiol 83:836–889. [DOI] [PubMed] [Google Scholar]
- Malik M. (1998). Sympathovagal balance: a critical appraisal. Circulation 98:2643–2644. [PubMed] [Google Scholar]
- Mazurak N, Enck P, Muth E, Teufel M, Zipfel S. (2011). Heart rate variability as a measure of cardiac autonomic function in anorexia nervosa: a review of the literature. Eur Eat Disord Rev 19:87–99. [DOI] [PubMed] [Google Scholar]
- Mellemkjaer L, Hammarstrom L, Andersen V, Yuen J, Heilmann C, Barington T, et al. (2002). Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp 130:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A, Lutz A, Vögele C, Kübler A. (2012a). Self-reported dieting success is associated with cardiac autonomic regulation in current dieters. Appetite 59:494–498. [DOI] [PubMed] [Google Scholar]
- Meule A, Vögele C, Kübler A. (2012b). Restrained eating is related to accelerated reaction to high caloric foods and cardiac autonomic dysregulation. Appetite 58:638–644. [DOI] [PubMed] [Google Scholar]
- Mouridsen MR, Bendsen NT, Astrup A, Haugaard SB, Binici Z, Sajadieh A. (2013). Modest weight loss in moderately overweight postmenopausal women improves heart rate variability. Eur J Prev Cardiol 20:671–677. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. (2008). Dietary fish ω-3 fatty acid consumption and heart rate variability in US adults. Circulation 117:1130–1137. [DOI] [PubMed] [Google Scholar]
- Nagai N, Sakane N, Moritani T. (2005). Metabolic responses to high-fat or low-fat meals and association with sympathetic nervous system activity in healthy young men. J Nutr Sci Vitaminol (Tokyo) 51:355–360. [DOI] [PubMed] [Google Scholar]
- Nagpal M, Gleichauf K, Ginsberg J. (2013). Meta-analysis of heart rate variability as a psychophysiological indicator of posttraumatic stress disorder. J Trauma Treat 3:1–8. [Google Scholar]
- Ninio DM, Hill AM, Howe PR, Buckley JD, Saint DA. (2008). Docosahexaenoic acid-rich fish oil improves heart rate variability and heart rate responses to exercise in overweight adults. Br J Nutr 100:1097–1103. [DOI] [PubMed] [Google Scholar]
- Nodari S, Metra M, Milesi G, Manerba A, Cesana BM, Gheorghiade M, et al. (2009). The role of n23 PUFAs in preventing the arrhythmic risk in patients with idiopathic dilated cardiomyopathy. Cardiovasc Drugs Ther 23:5–15. [DOI] [PubMed] [Google Scholar]
- O’Keefe JH, Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS. (2006). Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol 97:1127–1130. [DOI] [PubMed] [Google Scholar]
- Park G, Vasey MW, van Bavel JJ, Thayer JF. (2014). When tonic cardiac vagal tone predicts changes in phasic vagal tone: the role of fear and perceptual load. Psychophysiol 51:419–426. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. (2010). Interoception in anxiety and depression. Brain Struct Funct 214:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. (2012). The vagus nerve and the inflammatory reflex – linking immunity and metabolism. Nat Rev Endocrinol 8:743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel SK, Feeling NR, Vögele C, Kaess M, Thayer JF, Koenig J. (2016). A meta‐analysis on resting state high‐frequency heart rate variability in bulimia nervosa. Eur Eat Disord Rev 24:355–365. [DOI] [PubMed] [Google Scholar]
- Pomportes L, Davranche K, Brisswalter I, Hays A, Brisswalter J. (2015). Heart rate variability and cognitive function following a multi-vitamin and mineral supplementation with added guarana (Paullinia cupana). Nutrients 7:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaelli A, Cazzaniga M, Viola A, Balestri G, Janetti MB, Signorini MG, et al. (2006). Enhanced baroreceptor control of the cardiovascular system by polyunsaturated fatty acids in heart failure patients. J Am Coll Cardiol 48:1600–1606. [DOI] [PubMed] [Google Scholar]
- RenuMadhavi CH, Ananth AG. (2012). A review of heart rate variability and ’it’s association with diseases. Int J Soft Comput Eng 2:2231–2307. [Google Scholar]
- Sandercock GR, Brodie DA. (2006). The role of heart rate variability in prognosis for different modes of death in chronic heart failure. Pacing Clin Electrophysiol 9:892–904. [DOI] [PubMed] [Google Scholar]
- Sauder KA, McCrea CE, Ulbrecht JS, Kris-Etherton PM, West SG. (2014). Pistachio nut consumption modifies systemic hemodynamics, increases heart rate variability, and reduces ambulatory blood pressure in well-controlled type 2 diabetes: a randomized trial. J Am Heart Assoc 3:pii: e000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, et al. (2005). Diabetes, glucose, insulin, and heart rate variability: the atherosclerosis risk in communities (ARIC) study. Diabetes Care 28:668–674. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. (2007). Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci 18:275–281. [DOI] [PubMed] [Google Scholar]
- Seth AK. (2013). Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 17:565–573. [DOI] [PubMed] [Google Scholar]
- Singh JP, Larson MG, O’Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, et al. (2000). Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol 86:309–312. [DOI] [PubMed] [Google Scholar]
- Sjoberg NJ, Milte CM, Buckley JD, Howe PR, Coates AM, Saint DA. (2010). Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. Br J Nutr 103:243–248. [DOI] [PubMed] [Google Scholar]
- Smith R, Thayer JF, Khalsa SS, Lane RD. (2017). The hierarchical basis of neurovisceral integration. Neurosci Biobehav Rev 75:274–296. [DOI] [PubMed] [Google Scholar]
- Soares-Miranda L, Stein PK, Imamura F, Sattelmair J, Lemaitre RN, Siscovick DS, et al. (2012). Trans-fatty acid consumption and heart rate variability in 2 separate cohorts of older and younger adults. Circ Arrhythm Electrophysiol 5:728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza NM, Vanderlei LCM, Garner DM. (2015). Risk evaluation of diabetes mellitus by relation of chaotic globals to HRV. Complexity 20:84–92. [Google Scholar]
- Stein PK, Soare A, Meyer TE, Cangemi R, Holloszy JO, Fontana L. (2012). Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell 11:644–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucharita S, Dwarkanath P, Thomas T, Srinivasan K, Kurpad AV, Vaz M. (2014). Low maternal vitamin B12 status during pregnancy is associated with reduced heart rate variability indices in young children. Matern Child Nutr 10:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. (2002). The inflammatory reflex. Nature 420:853–859. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Dis 61:201–216. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. (2006). Beyond heart rate variability. Ann N Y Acad Sci 1088:361–372. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. (2009). Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 33:81–88. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141:122–131. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 36:747–756. [DOI] [PubMed] [Google Scholar]
- Uijtdehaage SH, Shapiro D, Jaquet F. (1994). Effects of carbohydrate and protein meals on cardiovascular levels and reactivity. Biol Psychol 38:53–72. [DOI] [PubMed] [Google Scholar]
- Umetani K, Singer DH, McCraty R, Atkinson M. (1998). Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 31:593–601. [DOI] [PubMed] [Google Scholar]
- Valera B, Suhas E, Counil E, Poirier P, Dewailly E. (2014). Influence of polyunsaturated fatty acids on blood pressure, resting heart rate and heart rate variability among French Polynesians. J Am Coll Nutr 33:288–296. [DOI] [PubMed] [Google Scholar]
- Viljoen M, Claassen N. (2017). Allostatic load and heart rate variability as health risk indicator. Afr Health Sci 17:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinik AI. (2012). The conductor of the autonomic orchestra. Front Endocrinol (Lausanne) 3:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinik AI, Maser RE, Mitchell BD, Freeman R. (2003). Diabetic autonomic neuropathy. Diabetes care 26:1553–1579. [DOI] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, Thayer JF. (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Front Psychol 6:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Wei W, Li XY. (2013). Short-term effects of fish-oil supplementation on heart rate variability in humans: a meta-analysis of randomized controlled trials. Am J Clin Nutr 97:926–935. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Terasaki J. (1994). Relationship between diabetic autonomic neuropathy and peripheral neuropathy as assessed by power spectral analysis of heart rate variations and vibratory perception thresholds. Diabetes Res Clin Pract 24:9–14. [DOI] [PubMed] [Google Scholar]
- Young H, Benton D. (2014). The glycemic load of meals, cognition and mood in middle and older aged adults with differences in glucose tolerance: a randomized trial. e-SPEN J 9:e147–e154. [Google Scholar]
- Young H, Benton D. (2015). We should be using nonlinear indices when relating heart-rate dynamics to cognition and mood. Sci Rep 5:16619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HA, Watkins H. (2016). Eating disinhibition and vagal tone moderate the postprandial response to glycemic load: a randomised controlled trial. Sci Rep 6:35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HA, Cousins AL, Watkins HT, Benton D. (2017a). Is the link between depressed mood and heart rate variability explained by disinhibited eating and diet? Biol Psychol 123:94–102. [DOI] [PubMed] [Google Scholar]
- Young HA, Williams C, Pink AE, Freegard G, Owens A, Benton D. (2017b). Getting to the heart of the matter: does aberrant interoceptive processing contribute towards emotional eating? PLoS One 12:e0186312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn D, Adams J, Krohn J, Wenzel M, Mann CG, Gomille LK, et al. (2016). Heart rate variability and self-control – a meta-analysis. Biol Psychol 115:9–26. [DOI] [PubMed] [Google Scholar]
- Zulfiqar U, Jurivich DA, Gao W, Singer DH. (2010). Relation of high heart rate variability to healthy longevity. Am J Cardiol 105:1181–1185. [DOI] [PubMed] [Google Scholar]


