Abstract
Objective: Abdominal aortic aneurysm (AAA) is characterized by inflammation and destruction of normal tissue architecture. The present study aimed to evaluate the inflammatory signaling cascade by analyzing the cytokines of AAA tissue.
Materials and Methods: We analyzed the comprehensive cytokine secretion profiles of 52 cytokines from human AAA in four patients with AAA using fluorescent beads-based multiplex assay. Further, the effect of janus kinase (JAK) inhibition by pyridone 6 on cytokine profiles was also evaluated.
Results: Cytokine secretion profiles were found to be similar among the four patients. A high level of JAK/signal transducers and activator of transcription (STAT) pathway activity in AAA tissue in culture was maintained, which may be attributed to the secretion of endogenous JAK-activating cytokines. Inhibition of JAK by pyridone 6 resulted in the suppression of STAT3 phosphorylation and secretion of a subset of chemokines and JAK-activating cytokines. However, the inhibition of JAK had no effect on the secretion of matrix metalloproteinase (MMP)-2, MMP-9, or TGF-β family that is responsible for the metabolism of extracellular matrix.
Conclusion: The findings of the present study suggested that AAA tissue exhibits a stereotypical profile of cytokine secretion, where JAK/STAT pathway may play a role in regulating a subset of cytokines. Identification of such a cytokine profile may reveal potential diagnostic markers and therapeutic targets for AAA.
Keywords: abdominal aortic aneurysm, cytokines, JAK/STAT, pyridone 6
Introduction
Abdominal aortic aneurysm (AAA) is the most prevalent aortic disease in the elderly, with unidentified etiology.1) AAA is primarily characterized by the weakening of aortic walls because of degradation and extensive remodeling of the load-bearing extracellular matrix (ECM), leading to progressive dilatation of the abdominal aorta. Although no symptoms are manifested in most patients, AAA progress over the time and eventually ruptures, leading to high mortality. Presently, the therapeutic strategy for AAA is limited to surgical intervention with open repair or endovascular stent-graft implantation for AAAs >5.5 cm in diameter. However, smaller AAAs are usually managed by “watchful waiting,” as effective non-surgical therapy is unavailable.
Recently, studies have highlighted the significance of inflammatory response in the degradation and remodeling of ECM in AAA lesions.2,3) During vascular remodeling, inflammatory cells secrete proteolytic enzymes including matrix metalloproteinases (MMPs) that are involved in the degradation of ECM in aortic walls.4) Various cell types have been reported to be important in this inflammatory response, including monocytes/macrophages, mast cells, neutrophils, and lymphocytes.2,3) In addition to the secretion of tissue degrading enzymes, these cells secrete a number of cytokines that mediate complex cell–cell interactions and maintain and amplify the inflammation cascade in aortic tissue. Thus, these cytokines regulate migration, proliferation, differentiation, activation, and survival of inflammatory and interstitial cells through interactions with specific receptors on various cell types and activate janus kinase (JAK)/signal transducers and activator of transcription (STAT), nuclear factor-kappa B (NFκB), and mitogen-activated protein kinase (MAP) kinase signaling pathways, thus building a complex network of inflammatory signaling.
Despite the fact that animal studies have identified several potential therapeutic targets in the pathogenesis of AAA, pharmacotherapy for AAA is yet to be established. This is partially because of the complexity of human AAA compared to animal models of AAA.5,6) To overcome the problem, the characterization of inflammatory system in human AAA tissue is necessary. Indeed, previous studies have demonstrated the increased expression of proinflammatory cytokines at the mRNA and protein levels in human AAA tissue.7) However, a number of cytokines secreted from AAA tissue have not yet been analyzed.
Therefore, for better understanding of the inflammatory signaling cascade in human AAA, we examined cytokine secretions from the human AAA tissue in culture. Further, we also studied the effect of pyridone 6, a pan-JAK inhibitor, because JAK/STAT is one of the leading signaling pathways in inflammatory response, reported to be involved in human AAA,8) and hypothesized to have a significant role in the pathogenesis of AAA.9)
Methods
Human AAA tissue culture
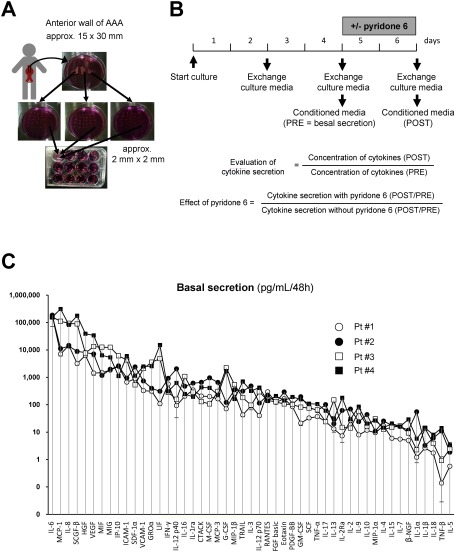
The Institutional Review Board of Kurume University Hospital approved all protocols that involved human specimens, and all the samples were obtained with written informed consent from the patients. Aortic wall specimens were collected from randomly selected four patients (Table 1) during the open aneurysm repair surgery to replace an aneurysm with the artificial graft as described before.10) The anterior aortic wall at the site of transition from the normal diameter to the dilated lesion (approximately 30 mm in length and 15 mm in width) was collected during surgery (Figs. 1A and 1B). The specimens were immediately immersed in ice-cold phosphate buffered saline (PBS, #10010023, Thermo Fisher Scientific, Waltham, MA, USA) containing penicillin/streptomycin (1,000 units/mL each, #15140122, Thermo Fisher Scientific, Waltham, MA, USA) to minimize the proinflammatory effect of microbes, and transported to the laboratory. In the laboratory, the aortic wall specimens were transferred to Dulbecco’s modified Eagle medium (DMEM) supplemented with penicillin and streptomycin (1,000 units/mL each), rinsed and cleaned of the blood clot and loose connective tissue aseptically. Because of heterogeneity in the histopathological features of the aortic aneurysmal walls, the following procedure was performed to minimize variation in tissue content in each well of the 12-well culture plate. The aortic wall was cut into three pieces; proximal, middle, and distal portions of the aortic wall. Each piece was further cut into small pieces (approximately 2 mm×2 mm). Further, the small pieces were transferred to the 12-well tissue culture plate, such that each well received an equal number of pieces from the original proximal, middle and distal portion of the aortic wall. After this preparatory procedure, the specimens were cultured in 2 mL DMEM at 37°C in a humidified incubator with 5% CO2 and 95% room air. After 48 h, the culture media was replaced with fresh DMEM to remove blood clots and tissue debris, and further experiments were conducted. The AAA tissue culture was maintained for 48 h to obtain conditioned media to detect the basal cytokine secretions (PRE group). Furthermore, the culture media was replaced with the fresh DMEM with 0, 1, and 10 µM pyridone 6 (#420099, Millipore, Burlington, MA, USA) or 100 ng/mL IL-6 (#206-IL, R&D Systems, Minneapolis, MN, USA). After 48 h, the conditioned media was collected to measure cytokine secretion (POST group) in the presence or absence of pyridone 6 or IL-6.
Table 1 Patient characteristics.
| Patient | Age | Gender | Maximal diameter (mm) | Smoking | HT | DL | DM | COPD | CVD | Other comorbidities | Medication | Family history of CVD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 76 | M | 53 | + | − | − | − | + | − | Appendicitis, right femur fracture, duodenal ulcer, ocular fundus bleeding | Famotidine, magnesium oxide | − |
| #2 | 66 | M | 46 | + | + | + | − | − | − | Aspirin, doxazosin, nifedipine, ketoprofen, rosuvastatin, probenecid, ifenprodil | − | |
| #3 | 77 | M | 47 | + | + | + | − | − | Coronary artery disease | Chronic kidney disease (Stage 4) | Warfarin, aspirin, methyldigoxin, carvedilol, enalapril, azosemide, spironolactone, pravastatin, ubidecarenone, ethyl icosapentate | Cardiac hypertrophy (father) |
| #4 | 81 | M | 50 | − | + | + | − | − | − | Melanoma, colon cancer, prostate cancer, hepatitis C, cataract, glaucoma | Amlodipine, allopurinol, distigmine, bicalutamide, tamsulosin, ezetimibe, eviprostat | Congestive heart failure (father) |
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease; DL: dyslipidemia; DM: diabetes mellitus; HT: hypertension
Fig. 1 Human AAA tissue culture and cytokine secretion. (A) Depicted is the procedure of human AAA tissue culture. The anterior aortic wall (approximately 30 mm in length and 15 mm in width) was collected during surgery. The aortic wall was cut into three pieces; proximal, middle, and distal portions of the aortic wall. Each piece was further cut into small pieces (approximately 2 mm×2 mm). Further, the small pieces were transferred to a 12-well tissue culture plate, so that each well received an equal number of pieces from the original proximal, middle, and distal portion of the aortic wall. (B) Culture media was replaced with the fresh DMEM to remove blood clots and tissue debris, 48 h after starting the culture, and further experiments were conducted. After another 48 h of incubating the culture with the indicated concentrations of pyridone 6, the culture media was collected to measure the levels of cytokines secreted into the media. (C) Quantitative analysis of the basal secretion in human AAA tissue culture from four patients is demonstrated for a panel of 49 cytokines. Conditioned media was collected after incubating with human AAA tissue in 12-well culture plates for 48 h, and subjected to the beads-based multiplex assay. Data are expressed as means±standard errors from 4–8 wells of a 12-well culture plate with similar culture conditions.
Expression assays
We measured the profiles of 52 cytokines (Table 2) that were secreted into the conditioned media using fluorescent beads-based multiplex assay (Bio-Plex, Bio-Rad, Hercules, CA, USA), using human cytokine 27-plex (#M500KCAF0Y, Bio-Rad), cytokine 21-plex (#MF0005KMII, Bio-Rad), VCAM-1 (#171B6022M, Bio-Rad), ICAM-1 (#171B6009M, Bio-Rad), and human TGF-β 3-plex (#171W4001M, Bio-Rad) assays according to the manufacturer’s protocol. Cytokine secretion was normalized for the wet weight of tissue as measured by the end of culture. The effect of pyridone 6 or IL-6 on cytokine secretion was estimated by calculating the ratio of cytokine secretion in the presence and absence of pyridone 6 or IL-6 (POST) to the basal cytokine secretion (PRE) (Fig. 1). Immunoblotting was performed for phosphorylated STAT3 (Tyr705, #9145, Cell Signaling Technology, Danvers, MA, USA), total STAT3 (#4904, Cell Signaling Technology), and GAPDH (#MAB374, Millipore), which served as the internal reference. Gelatin zymography was performed using the Novex zymogram system (#EC6175BOX, Thermo Fisher Scientific) according to the manufacturer’s instruction.
Table 2 Cytokine panel in this study.
| Abbreviations | Common names |
|---|---|
| CTACK | Cutaneous T cell-attracting chemokine (CCL27) |
| Eotaxin | Eotaxin (CCL11) |
| FGF basic | Fibroblast growth factor basic (FGF2) |
| G-CSF | Granulocyte colony-stimulating factor (CSF3) |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| GROα | Growth-regulated protein alpha (CXCL1) |
| HGF | Hepatocyte growth factor |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IFN-γ | Interferon-γ |
| IL-10 | Interleukin-10 |
| IL-12 p40 | Interleukin-12 p40 subunit |
| IL-12 p70 | Interleukin-12 p70 subunit |
| IL-13 | Interleukin-13 |
| IL-15 | Interleukin-15 |
| IL-16 | Interleukin-16 |
| IL-17 | Interleukin-17 |
| IL-18 | Interleukin-18 |
| IL-1ra | Interleukin-1 receptor antagonist |
| IL-1α | Interleukin-1α |
| IL-1β | Interleukin-1β |
| IL-2 | Interleukin-2 |
| IL-2Ra | Interleukin-2 receptor α chain |
| IL-3 | Interleukin-3 |
| IL-4 | Interleukin-4 |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| IL-7 | Interleukin-7 |
| IL-8 | Interleukin-8 (CXCL8) |
| IL-9 | Interleukin-9 |
| IP-10 | Interferon-γ-induced protein 10 (CXCL10) |
| LIF | Leukemia inhibitory factor |
| M-CSF | Macrophage colony-stimulating factor (CSF1) |
| MCP-1 | Monocyte chemoattractant protein-1 (CCL2) |
| MCP-3 | Monocyte chemoattractant protein-3 (CCL7) |
| MIF | Macrophage migration inhibitory factor |
| MIG | Monokine induced by γ-interferon (CXCL9) |
| MIP-1α | Macrophage inflammatory protein-1α (CCL3) |
| MIP-1β | Macrophage inflammatory protein-1β (CCL4) |
| PDGF-BB | Platelet-derived growth factor-BB |
| RANTES | Regulated on activation, normal T cell expressed and secreted (CCL5) |
| SCF | Stem cell factor |
| SCGF-β | Stem cell growth factor-β (CLEC11A)a |
| SDF-1α | Stromal cell-derived factor 1alpha (CXCL12) |
| TGF-β1 | Transforming growth factor-β1 |
| TGF-β2 | Transforming growth factor-β2 |
| TGF-β3 | Transforming growth factor-β3 |
| TNF-α | Tumor necrosis factor-α (TNF) |
| TNF-β | Tumor necrosis factor-β (lymphotoxin-α) (LTA) |
| TRAIL | TNF-related apoptosis-inducing ligand (TNFSF10) |
| VCAM-1 | Vascular cell adhesion molecule-2 |
| VEGF | Vascular endothelial growth factor |
| β-NGF | β-nerve growth factor |
Statistical analyses
All the statistical analyses were performed using GraphPad PRISM (Ver. 5, GraphPad Software, La Jolla, CA, USA). The data are expressed as mean±standard errors. Mann–Whitney U test was used to compare the difference between the two groups. Kruskal–Wallis test was used to compare three or more groups with Dunn’s correction for multiple comparisons. A p value ≤0.05 was considered statistically significant.
Results
Cytokine profile in AAA tissue culture
We determined the culture media levels for 52 cytokines (soluble mediators) in the cultures of AAA derived from four patients. The cytokine profile revealed minute variation for each cytokine in a set of tissue cultures obtained from a single patient, possibly reflecting the heterogeneity of AAA tissue (Fig. 1C). However, variation among cytokines for a single patient was small as compared to the difference in cytokine levels among the four patients, and the orders of the cytokines were similar among the patients. Functional class of cytokines which had the highest level of secretion were JAK/STAT-activating cytokines: IL-6, MIF, LIF, and IFN-γ, chemokines; MCP-1, IL-8, MIG, IP-10, and GROα, growth factors; SCGF-β, HGF, and VEGF, and cell adhesion molecules; ICAM-1 and VCAM-1. These findings suggested that the intrinsic manifestation of inflammation was similar among the patients.
Effect of JAK inhibition on cytokine secretions
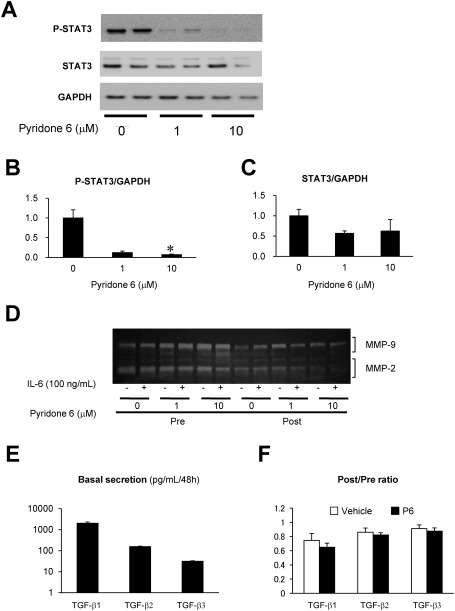
Further, we examined the effect of pyridone 6, a pan-JAK inhibitor, on the activation of STAT3 (phosphorylation at Y705) and cytokine secretions. Pyridone 6 suppressed the phosphorylation of STAT3 during the 48 h of culture, without affecting the expression of STAT3 (Figs. 2A–2C).
Fig. 2 Effect of pyridone 6 on the activity of STAT3, MMPs, and TGF-β in human AAA tissue culture. Effect of pyridone 6 on STAT3 activity was evaluated by immunoblotting for activated (phosphorylated) STAT3 (P-STAT3) and total STAT3 after incubation of the AAA tissue with the indicated concentration of pyridone 6 for 48 h. (A) Representative images of immunoblotting for P-STAT3 and STAT3 are shown, and GAPDH was used as the internal control. (B) Expression levels of P-STAT3 normalized to that of GAPDH are represented. (C) Expression levels of STAT3 normalized to that of GAPDH are represented. (D) Secretions of MMP-9 and -2 were evaluated by gelatin zymography of the conditioned media. After collecting the basal secretion for 48 h, AAA tissue culture was treated with or without 100 ng/mL IL-6 and the indicated concentrations of pyridone 6. (E) Basal secretions of TGF-β family are shown in the conditioned media after 48 h of culture. (F) The effect of 10 µM pyridone 6 on the secretions of TGF-β family of cytokines is represented. Data are expressed as means±standard errors. The sample without pyridone 6 treatment was assigned a value of 1. * p<0.05.
Moreover, to evaluate the role of JAK/STAT pathway in ECM degradation, we examined the effect of pyridone 6 on the secretion of MMP-2 and MMP-9 by gelatin zymography (Fig. 2D). Both MMP-2 and MMP-9 were detected at significant levels in the culture supernatant of human AAA tissue. However, the treatment of AAA culture with pyridone 6 exhibited no apparent effect on the secretion of MMP-9 or MMP-2 at 1 µM and may have caused marginal suppression of MMP-2 at 10 µM. Further, the addition of exogenous IL-6 had no significant effect on the secretion of MMP-9 or MMP-2, probably because of high endogenous IL-6 secretion level during tissue culture. These results suggested that JAK/STAT pathway does not play a significant role in the secretion of these MMPs.
We measured the secretions of TGF-β1, TGF-β2, and TGF-β3 (Fig. 2E), which are important for the regulation of inflammation and ECM metabolism and their sensitivity to pyridone 6 (Fig. 2F) in two sets of human AAA culture from different patients. The secretion of TGF-β isoforms was comparable between the two sets of culture, where TGF-β1 had the highest expression. None of the TGF-β isoforms were sensitive to pyridone 6, indicating that TGF-β secretion was independent of JAK/STAT pathway in this experimental condition.
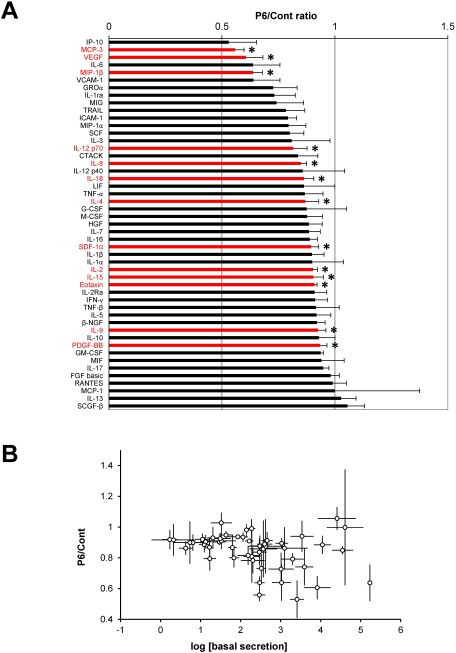
Next, we evaluated the effect of pyridone 6 (10 µM) on a panel of cytokine secretions. The secretion level of a subset of cytokines decreased; however, treatment with pyridone 6 did not increase the secretion levels of any of the cytokines (Fig. 3A). There was no significant correlation between the pyridone 6 sensitivity and cytokine secretion levels (Fig. 3B). The functional classes of pyridone 6-sensitive cytokines were JAK/STAT-activating interleukins (IL-2, IL-4, IL-9, IL-12p70, IL-15, and IL-18), chemokines (MCP-3, MIP-1β, IL-8, SDF-1α, and Eotaxin), and growth factors (VEGF and PDGF-BB). These results indicated that JAK/STAT pathway promotes the secretion of a subset of cytokines.
Fig. 3 Effect of pyridone 6 on cytokine secretions in human AAA tissue culture. Human AAA tissue was cultured for 48 h without any treatment to measure the basal cytokine secretion (basal secretion). AAA tissue was further cultured with or without 10 µM pyridone 6 for another 48 h (secretion after treatment). The effect of pyridone 6 on cytokine secretion was assessed by the P6/Cont ratio as indicated in Fig. 1. Data are expressed as means±standard errors in the order of the P6/Cont ratio. * p<0.05 for the P6/Cont ratio of the corresponding cytokines.
Discussion
In the present study, we examined the cytokine secretion profile of AAA tissue in culture, which revealed reproducible cytokine profiles among the four sets of tissue culture derived from different patients. The results were largely consistent with previous studies that examined either the relative expression levels of a panel of cytokines using immunoassays, quantitative reverse-transcriptase polymerase chain reaction (RT-PCR), and deoxyribonucleic acid (DNA) microarray or the absolute expression levels of specific cytokines by enzyme-linked immunosorbent assays (ELISAs).7) Furthermore, we also evaluated the effect of pyridone 6, a pan-JAK inhibitor, to determine whether or not JAK/STAT pathway regulates the secretion of a subset of cytokines in AAA tissue culture.
Studies indicate that inflammatory responses may contribute to aortic wall destruction in AAA.2,3) Because inflammatory cells and interstitial cells work in concert through various cytokines to maintain and promote inflammation, cytokines and their signaling pathways represent potential diagnostic markers and therapeutic targets.7) It is noteworthy that cytokine therapy has already been applied in clinical practice as neutralizing antibodies and decoy receptors for TNF-α, IL-6, and other cytokines, which resulted in excellent clinical outcomes in inflammatory disorders, including rheumatoid arthritis, inflammatory bowel syndrome, and psoriasis.11) To identify the significance of cytokine therapy for AAA, it is essential to understand how cytokines are involved in the pathogenesis of AAAs.6,7) Previous studies have demonstrated that cytokine inhibition, including MCP-1,12) IP-10,13) TNF-α,14,15) IL-1β,16) IL-4,17) IL-5,18) IL-17,19) IFN-γ,20) SDF-1,21) GM-CSF,22) and VEGF,23) was effective in suppressing the development of AAA in mice. However, other cytokines, including TGF-β,24,25) IL-10,25) IP-10, and IFN-γ,13) have been reported to exhibit protective effects in experimental AAA. Furthermore, studies have also highlighted the complexity of the inflammatory network and cytokine functions as exemplified by the fact that IFN-γ and IP-10 have been reported to have either promotive or suppressive effects in experimental AAA.13,20) Although animal studies have provided critical knowledge in understanding the mechanism of AAA, no single model recapitulates all the characteristics of human AAA. Besides, owing to the heterogeneity in histopathology1) and clinical courses among patients,26) cytokine profiles may be different among patients. Therefore, in addition to animal models of AAA, the role of cytokines should be characterized in the context of human AAA.6) Notably, while the overall profile of cytokine secretions was similar in the four patients in the present study, some of the highly expressed cytokines, including MCP-1, SCGF-β, LIF, and G-CSF, exhibited a difference of >10-fold among the patients. Whether and how differences secretion profiles of specific cytokines reflect AAA activity and status remains to be elucidated in future research.
Our results indicated that STAT3 was found to be highly activated in human AAA tissue for several days in culture. Moreover, the exogenous addition of IL-6 to the tissue culture did not affect P-STAT3 levels (data not shown); it is possible that JAK/STAT pathway was already activated by the endogenous inflammatory cytokines within the AAA tissue as previously reported.8) Indeed, IL-6 accumulated to approximately 100 ng/mL during 48 h of tissue culture, which would be sufficient to fully activate STAT3. In turn, JAK/STAT pathway in AAA tissue may promote the secretion of a subset of cytokines, as previously reported27,28) and as demonstrated in the present study. However, consistent with previous reports,29,30) JAK/STAT pathway did not appear to regulate the secretion of MMP-2, MMP-9, or TGF-β family.
The major limitations of the present study are the small number of patients, which precluded thorough characterization of the inflammatory network in human AAA tissue. Also, the role of cytokines that promote cell infiltration in the pathogenesis of AAA may be underestimated, because tissue in culture does not have a continuous supply of inflammatory cells from the bloodstream and surrounding tissue. Another limitation was the absence of normal tissue or tissue with other aortic diseases for comparison in the experimental group, which precluded the identification of AAA-specific cytokines. Because of these limitations, the present study should be observed as an initial screening for cytokines secreted at high levels among a panel of cytokines to characterize the inflammatory network within human AAA tissue. Besides, it should be noted that isolated tissue in culture differs starkly from aortic tissue in patients. For example, the aortic tissue in culture is free from hemodynamic stress, circulating humoral factors, or neuronal stimuli. Future studies are required to test whether cytokines secreted at high levels and their signaling pathways can serve as diagnostic markers or therapeutic targets for AAA.
Conclusion
In conclusion, we have characterized the profile of the secreted cytokines in AAA tissue, which appeared to be partly maintained by endogenous JAK/STAT pathway. Deciphering such an inflammatory network would lead to the identification of therapeutic targets, including cytokines and signaling molecules, and diagnostic markers for evaluating the activity of chronic inflammation in the pathogenesis of AAA.
Disclosure Statement
The authors have no conflict of interest.
Author Contributions
Study conception: TO, HAo, YF, HT
Sample curation: TO, SH, HAk, HT
Analysis: TO, HA, SO, MN, AF
Writing: TO, HAo
Funding acquisition: HAo, YF, HT
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Curci JA, Baxter BT, Thompson RW. Arterial aneurysms: etiologic considerations. In: Rutherford RB ed. Vascular Surgery. Philadelphia: Saunders, 2005: 475-92. [Google Scholar]
- 2).Aoki H, Yoshimura K, Matsuzaki M. Turning back the clock: regression of abdominal aortic aneurysms via pharmacotherapy. J Mol Med (Berl) 2007; 85: 1077-88. [DOI] [PubMed] [Google Scholar]
- 3).Wang Y, Krishna S, Golledge J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis 2013; 226: 29-39. [DOI] [PubMed] [Google Scholar]
- 4).Aziz F and Kuivaniemi H. Role of matrix metalloproteinase inhibitors in preventing abdominal aortic aneurysm. Ann Vasc Surg 2007; 21: 392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Pearce WH and Shively VP. Abdominal aortic aneurysm as a complex multifactorial disease: interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of MMPs. Ann N Y Acad Sci 2006; 1085: 117-32. [DOI] [PubMed] [Google Scholar]
- 6).Lu H, Rateri DL, Bruemmer D, et al. Novel mechanisms of abdominal aortic aneurysms. Curr Atheroscler Rep 2012; 14: 402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Golledge AL, Walker P, Norman PE, et al. A systematic review of studies examining inflammation associated cytokines in human abdominal aortic aneurysm samples. Dis Markers 2009; 26: 181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Liao M, Xu J, Clair AJ, et al. Local and systemic alterations in Signal Transducers and Activators of Transcription (STAT) associated with human abdominal aortic aneurysms. J Surg Res 2012; 176: 321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Harrison SC, Smith AJ, Jones GT, et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J 2013; 34: 3707-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Yoshimura K, Aoki H, Ikeda Y, et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med 2005; 11: 1330-8. [DOI] [PubMed] [Google Scholar]
- 11).Lee SJ, Chinen J, Kavanaugh A. Immunomodulator therapy: monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J Allergy Clin Immunol 2010; 125 Suppl 2: S314-23. [DOI] [PubMed] [Google Scholar]
- 12).Ishibashi M, Egashira K, Zhao Q, et al. Bone marrow-derived monocyte chemoattractant protein-1 receptor CCR2 is critical in angiotensin II-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol 2004; 24: e174-8. [DOI] [PubMed] [Google Scholar]
- 13).King VL, Lin AY, Kristo F, et al. Interferon-gamma and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation 2009; 119: 426-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Hingorani A, Ascher E, Scheinman M, et al. The effect of tumor necrosis factor binding protein and interleukin-1 receptor antagonist on the development of abdominal aortic aneurysms in a rat model. J Vasc Surg 1998; 28: 522-6. [DOI] [PubMed] [Google Scholar]
- 15).Kaneko H, Anzai T, Horiuchi K, et al. Tumor necrosis factor-alpha converting enzyme is a key mediator of abdominal aortic aneurysm development. Atherosclerosis 2011; 218: 470-8. [DOI] [PubMed] [Google Scholar]
- 16).Johnston WF, Salmon M, Su G, et al. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol 2013; 33: 294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Shimizu K, Shichiri M, Libby P, et al. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest 2004; 114: 300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Xu J, Ehrman B, Graham LM, et al. Interleukin-5 is a potential mediator of angiotensin II-induced aneurysm formation in apolipoprotein E knockout mice. J Surg Res 2012; 178: 512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Sharma AK, Lu G, Jester A, et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation 2012; 126 Suppl 1: S38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Xiong W, Zhao Y, Prall A, et al. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol 2004; 172: 2607-12. [DOI] [PubMed] [Google Scholar]
- 21).Michineau S, Franck G, Wagner-Ballon O, et al. Chemokine (C-X-C motif) receptor 4 blockade by AMD3100 inhibits experimental abdominal aortic aneurysm expansion through anti-inflammatory effects. Arterioscler Thromb Vasc Biol 2014; 34: 1747-55. [DOI] [PubMed] [Google Scholar]
- 22).Son BK, Sawaki D, Tomida S, et al. Granulocyte macrophage colony-stimulating factor is required for aortic dissection/intramural haematoma. Nat Commun 2015; 6: 6994. [DOI] [PubMed] [Google Scholar]
- 23).Kaneko H, Anzai T, Takahashi T, et al. Role of vascular endothelial growth factor-A in development of abdominal aortic aneurysm. Cardiovasc Res 2011; 91: 358-67. [DOI] [PubMed] [Google Scholar]
- 24).Wang Y, Ait-Oufella H, Herbin O, et al. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest 2010; 120: 422-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Meng X, Yang J, Zhang K, et al. Regulatory T cells prevent angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E knockout mice. Hypertension 2014; 64: 875-82. [DOI] [PubMed] [Google Scholar]
- 26).Brady AR, Thompson SG, Fowkes FG, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004; 110: 16-21. [DOI] [PubMed] [Google Scholar]
- 27).Spörri B, Muller KM, Wiesmann U, et al. Soluble IL-6 receptor induces calcium flux and selectively modulates chemokine expression in human dermal fibroblasts. Int Immunol 1999; 11: 1053-8. [DOI] [PubMed] [Google Scholar]
- 28).Qu T, Yang H, Walston JD, et al. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine 2009; 46: 319-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Yoshizaki T, Sato H, Furukawa M, et al. The expression of matrix metalloproteinase 9 is enhanced by Epstein–Barr virus latent membrane protein 1. Proc Natl Acad Sci USA 1998; 95: 3621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Araki Y, Tsuzuki Wada T, Aizaki Y, et al. Histone methylation and STAT-3 differentially regulate interleukin-6-induced matrix metalloproteinase gene activation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol 2016; 68: 1111-23. [DOI] [PubMed] [Google Scholar]