Abstract
Objective: Thoracic aortic aneurysm (TAA) reflects the local expansion of the thoracic aorta; the underlying causal molecular mechanism of TAA is not well understood. Recent studies have shown the importance of transforming growth factor beta (TGFβ) signaling in Marfan and Loeys–Dietz syndromes; however, its role in non-familial, non-syndromic TAA remains unclear.
Materials and Methods: We performed histochemical and immunohistochemical analyses for activated (phosphorylated) SMAD2 (P-SMAD2) as an indicator of TGFβ signaling activities in the ascending TAA tissue as well as in the ascending aortic tissue with a normal diameter obtained from 7 patients without any clinical findings suggesting familial or syndromic TAA.
Results: TAA samples showed a higher P-SMAD2-positive area than samples with a normal diameter. P-SMAD2 signal was higher in the outer zone of the aortic and TAA walls. Within the TAA tissue, P-SMAD2 staining showed the following two distinct patterns: layer-like staining at the border of the medial layer and the thickened intima and a spot-like staining within the medial layer surrounding the microvessels.
Conclusion: These findings suggested that TGFβ signaling is activated in several distinct histopathological contexts in TAA, suggesting a complex role of TGFβ.
Keywords: thoracic aortic aneurysm, TGFβ, SMAD2, inflammation, smooth muscle cells
Introduction
Thoracic aortic aneurysm (TAA) is a serious medical condition caused by local weakening of the thoracic aortic wall, the underlying mechanism for which remains unclear. Although TAA is a relatively rare disorder that reportedly affects 10.4 in 100,000 of the general population in the Western countries and rarely manifests clinical symptoms, it is known to progress gradually over the years, resulting in catastrophic events of rupture and sudden death.1) No medical therapy has been established to stop or slow the growth of TAA; therefore, therapeutic options are limited to surgical interventions that replace the affected aorta with an artificial graft either by open surgery or by thoracic endovascular aortic repair (TEVAR). The location of TAA involves complex branches that connect to the heart and the brain; therefore, surgical replacement of TAA poses a greater risk to patients than abdominal aortic aneurysm.2) An understanding of the molecular pathogenesis of TAA is crucial in order to develop novel, low-risk therapeutic strategies.
Recently, the role of transforming growth factor beta (TGFβ) has gained increasing attention, owing to its causative role in the pathogenesis of aortopathies due to Loeys–Dietz syndrome (LDS)3,4) and Marfan syndrome (MFS).5–7) TGFβ is a member of the multifunctional cytokine family that regulates the differentiation and function of smooth muscle cells (SMCs) as well as the inflammatory response.8) The canonical signaling pathway of TGFβ involves its binding to type 1 and type 2 receptors, resulting in the phosphorylation of SMAD2/SMAD3, leading to the transcriptional regulation of the TGFβ target genes.9) The culprit genes for LDS are TGFBR1 (LDS1), TGFBR2 (LDS2), SMAD3 (LDS3), TGFB2 (LDS4), and TGFB3 (LDS5), all of which are involved in TGFβ signaling.8,10) Although mutations in TGFBR1 and TGFBR2 are predicted to reduce TGFβ signaling, the aortic walls of LDS patients show a paradoxical increase in SMAD2 phosphorylation.3) In addition, causative mutation of FBN1 for MFS results in the abnormal TGFβ signaling activation,11) probably caused by the diminished activity of FBN1 to capture the latent form of TGFβ. Furthermore, neutralizing TGFβ antibodies ameliorated the aortopathies in MFS model mice, demonstrating the disease-promoting action of TGFβ.7) However, the role of TGFβ in aortopathies is controversial, possibly owing to the complex signaling mechanism of TGFβ that involves canonical and non-canonical signaling pathways in aortopathies.5) The conditional deletion of TGFBR2 in the smooth muscle cells resulted in diminished SMAD2 phosphorylation and the development of severe aortopathies, including TAA.12) In MFS model mice, the administration of TGFβ neutralizing antibody exacerbated TAA when imitated prior to TAA formation; however, it mitigated TAA when initiated following the development of TAA.13) In addition, TGFβ neutralization is demonstrated to make mice prone to aneurysm formation by angiotensin II infusion.14) These findings in mice underscore the important and the complex role of TGFβ signaling in the pathogenesis of aortic aneurysm, including TAA. Therefore, it is interesting to assess whether and how TGFβ signaling is activated in human TAA tissue. The above-mentioned studies mainly involved TAA with genetic abnormalities; therefore, the activation status of TGFβ signaling in non-familial TAA warrants investigation. In this regard, human TAA tissue has been reported to show higher SMAD2 phosphorylation,15) and the SMCs derived from TAA walls showed the activation of SMAD2 in culture.16)
Here, we examined the presence and localization of phosphorylated, and hence activated, SMAD2 (P-SMAD2) in the aortae of patients with arch TAA both at the site of the maximal diameter and at the site of normal diameter in the ascending aorta. We assumed that this comparison between the maximal and normal diameter samples would reveal the molecular pathology associated with the destruction of the tissue architecture in TAA. Accordingly, we focused on the histopathological context of P-SMAD2-positive cells in the destructive changes and whether P-SMAD2 is localized in the SMCs.
Methods
Curation of human aortic samples and histological analyses
All the protocols involving human specimens were approved by the Institutional Review Board at the Kurume University Hospital, and all samples were obtained following informed consent from the patients. Human TAA tissue was obtained from 7 randomly chosen patients during open surgery performed for TAA repair (Table 1). TAA patients with an obvious family history or syndromic TAA, as determined using the physical phenotypes, were excluded. Tissues were acquired from the anterior walls of the region with a normal diameter and from the anterior walls of TAA at the maximal diameter.
Table 1 Clinical characteristics of the subjects.
| Age (y.o.) | Sex | Diameter (mm) | Smoking | DM | HT | DL | CKD | IHD | Family history |
|---|---|---|---|---|---|---|---|---|---|
| 82 | M | 60 | Y | N | Y | Y | N | N | N |
| 73 | F | 62 | N | N | Y | N | N | N | N |
| 61 | M | 55 | N | Y | Y | N | Y | N | N |
| 78 | M | 76 | N | N | Y | Y | Y | N | N |
| 89 | M | 89 | Y | N | Y | N | Y | N | N |
| 67 | M | 60 | Y | N | Y | Y | Y | N | N |
| 86 | M | 73 | N | N | Y | Y | N | N | N |
y.o.: years old; DM: diabetes mellitus; HT: hypertension; DL: dyslipidemia; CKD: chronic kidney disease; IHD: ischemic heart disease; M: male; F: female
For histological analyses, TAA tissues were fixed in paraformaldehyde, paraffin-embedded, and sliced into 5-µm thick tissue sections. We stained paraffin-embedded sections of the aortic tissue with elastica van Gieson (EVG) or hematoxylin and eosin (H & E). We performed immunohistochemical staining of the TAA tissue with antibodies specific for SMAD2 phosphorylated at Ser465/467 (P-SMAD2, #3108, Cell Signaling Technology, Danvers, MA, USA) and VECTASTAIN ABC-HRP Kit (#PK-4001, Vector, Burlingame, CA, USA). The P-SMAD2 positive area, as indicated by the brown color of 3,3′-diaminobenzidine (DAB) was quantified using the computerized color area detection using ImagePro Plus Ver. 7.0 (Media Cybernetics, Rockville, MD, USA) with a visually determined threshold that was kept constant throughout the analysis.
We stained the aortic tissue for smooth muscle cells (smooth muscle α-actin; SMA, #A5228, Sigma-Aldrich, St. Louis, MO, USA), endothelial cells (CD34, #NCL-L-END, Leica Biosystems, Wetzlar, Germany), B cells (CD20, Leica Biosystems #NCL-L-CD20-L26), T cells (CD3, Leica Biosystems #NCL-L-CD3-565), neutrophils (neutrophil elastase, #M0752, Dako, Santa Clara, CA, USA), and macrophages (CD68, DAKO #M0814). In order to perform immunofluorescence staining of the aortic tissue, we used antibodies for P-SMAD2 with TSA labeling kit with AlexaFluor 488 tyramide (#T-20922, Thermofisher Scientific, Waltham, MA, USA), (SMA) with Cy3-conjugated goat anti-mouse IgG antibody (#115-165-146, Jackson ImmunoResearch Laboratories, West Grove, PA, USA), and TOPRO-3 (#T3605, Thermofisher Scientific) for nuclear staining.
Statistical analyses
Statistical analyses were performed using the Mann–Whitney test for making comparisons between 2 groups, and Kruskal–Wallis test was used for comparing multiple groups because the data were not normally distributed. P<0.05 was considered statistically significant.
Results
Macroscopic and microscopic characterization of TAA
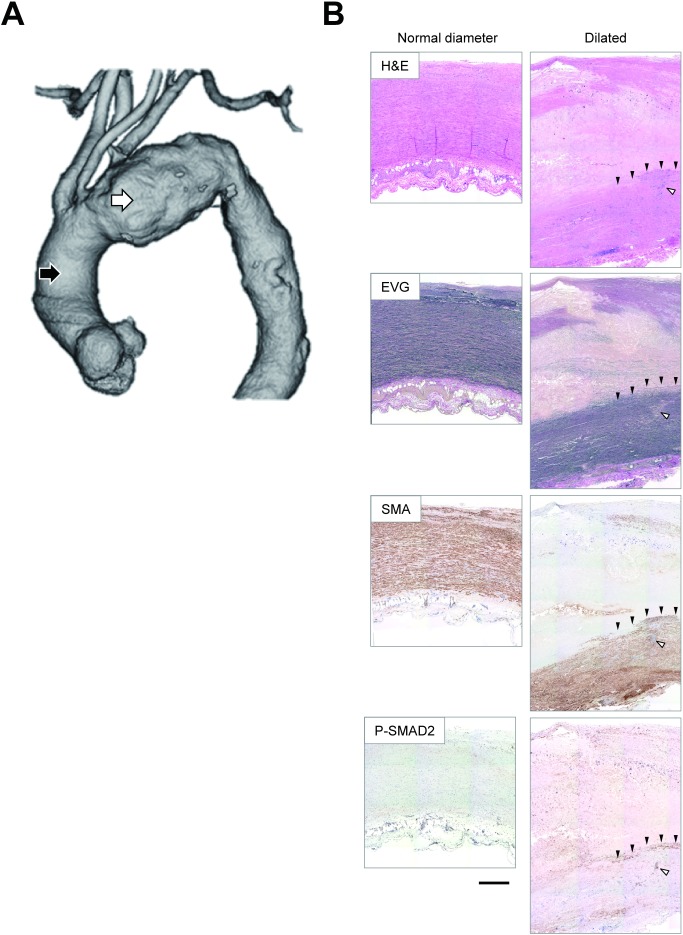
We first examined the histological characteristics of the aortic tissue taken from two locations from TAA patients (Fig. 1); the anterior walls of the ascending aorta with a normal diameter and those of the aneurysmal lesions at the maximal diameters. The aortic wall with a normal diameter showed normal tissue architecture, characterized by thin intima, thick media with ordered elastic lamellae and dense layers of smooth muscle cells, and loose adventitia comprising adipose tissue and small vessels. The aneurysmal wall also showed the medial layer with elastic lamellae and SMC layers. However, the medial layer was thinner than that in the aortic wall with a normal diameter, and focal disruptions of the orderly structure of the elastic lamellae were frequently observed in the TAA wall. By observing the serial sections, the focal disruptions of the elastic lamellae, as observed in EVG staining were associated with increased cellular density, as observed in H & E staining, suggesting focal cellular infiltration.
Fig. 1 Representative macroscopic and microscopic images of TAA. (A) A representative image of the three-dimensional reconstruction of the aorta with TAA, obtained using enhanced computed tomography. The black and white arrowheads indicate the sampling locations of the aortic walls with a normal diameter and those with maximal diameter, respectively. (B) Representative images are shown for the aortic walls with a normal diameter (left panels) and those with maximal diameter of TAA (right panels). Brown staining indicates the positive immunostaining signal of the corresponding antigens for SMA and P-SMAD2. Black arrowheads indicate the area corresponding to the layer-like staining of P-SMAD2. White arrowheads indicate the area corresponding to the spot-like staining of P-SMAD2. Bar 500 µm. H&E: hematoxylin & eosin staining; EVG: elastica van Gieson staining; SMA: smooth muscle α-actin as a marker for smooth muscle cells; P-SMAD2: phosphorylated (activated) SMAD2.
Further, the medial layer was covered by a thick layer of tissue on the luminal side, showing an amorphous structure, deposition of cholesterine crystals, and occasional calcified spots. The thick layer also contained a thin and disorganized fibrous structure stained black in EVG staining. These findings suggested that the thick layer in the TAA tissue comprised a thickened intimal layer with atherosclerotic changes and degenerated medial layer. There were destructive changes in the thickened intima and degenerative media; therefore, we were unable to determine the border between the intimal and medial layers.
When we observed the aortic tissue using immunohistochemical staining for P-SMAD2, the aortic wall with a normal diameter showed only weak staining of the cells scattered throughout the wall thickness. In contrast, P-SMAD2 staining of the aneurysmal wall showed a strong local signal with a layer-like pattern (Fig. 1B, black arrowheads) that corresponded to the border zone between the thick intima-like layer and the media with dense SMCs. In addition, spot-like staining of P-SMAD2 was observed within the medial layer (Fig. 1B, white arrowhead). These findings suggested degenerative changes in the aneurysmal walls with SMAD2 activation in TAA.
Quantitative analyses of SMAD2 activation in TAA
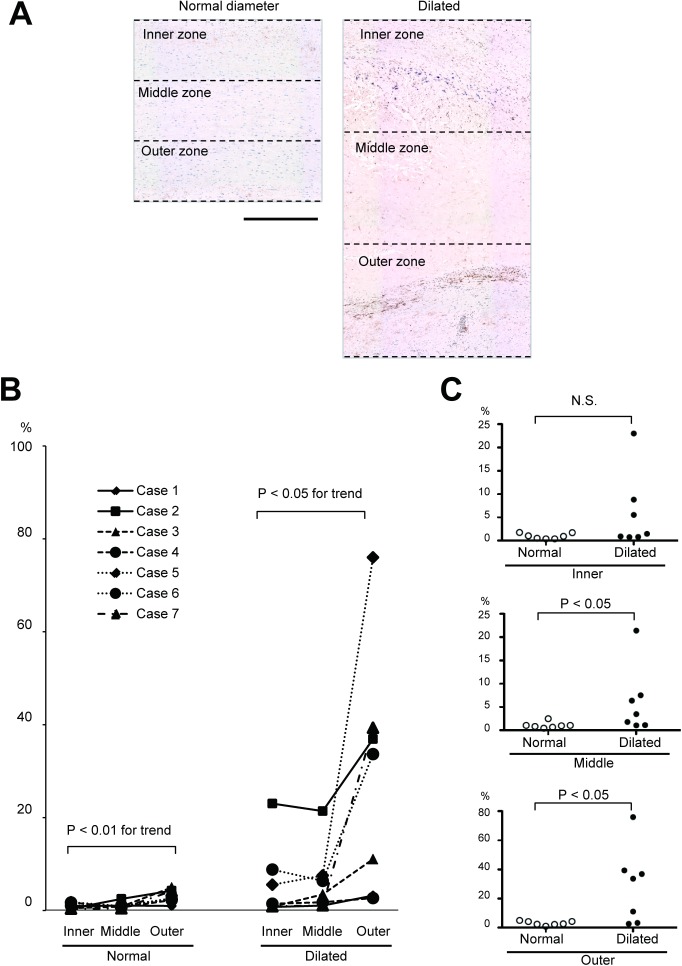
Thereafter, we quantitatively analyzed the local activation of SMAD2 using immunohistochemical staining of the aortic walls either from the region with a normal diameter or from the region with the maximal diameter of TAA. The atherosclerotic thickening of the intimal layer and the degenerative changes in the medial layer that destroyed the border between the intimal and medial layers made it challenging to clearly distinguish between the two layers in several cases. Thus, we decided to divide the total thickness of the aortic walls into three zones with equal thickness and analyze the ratio of the P-SMAD2-positive area to the zonal area (Fig. 2). As shown in Fig. 2B, the aortic wall with a normal diameter region showed very weak P-SMAD2 signal. In contrast, the aneurysmal walls showed a higher ratio of P-SMAD2-positive area, with a trend for stronger signals in the outer zone within the single aneurysmal walls. When the aortic wall with a normal diameter and the aneurysmal wall at the maximal diameter were compared in the three equally thick zones, the inner, middle, and outer zones, the middle and outer zones showed significantly higher ratios of the P-SMAD2-positive area in the aneurysmal wall than the aortic walls with a normal diameter. Moreover, the inner zone did not show any statistical difference. These findings indicate the heterogeneity in SMAD2 activation within the aorta with TAA and within the thickness of the TAA walls.
Fig. 2 Quantitative analysis of SMAD2 activation. (A) Diagrams show the aortic samples with P-SMAD2 staining to indicate the aortic walls with normal diameter (left) and with the maximal diameter of TAA (right) to divide into three zones with equal thickness. Bar 500 µm. (B) A graph shows the ratios of P-SMAD2-positive area to the corresponding zonal area in the samples with a normal diameter (left) and those with maximal diameter of TAA (right). The same symbols indicate the values obtained from the same patient. (C) Graphs show the comparison of P-SMAD2-positive area ratios between the tissues with a normal diameter and those with the maximal diameter of TAA.
Characterization of TAA tissue with activated SMAD2
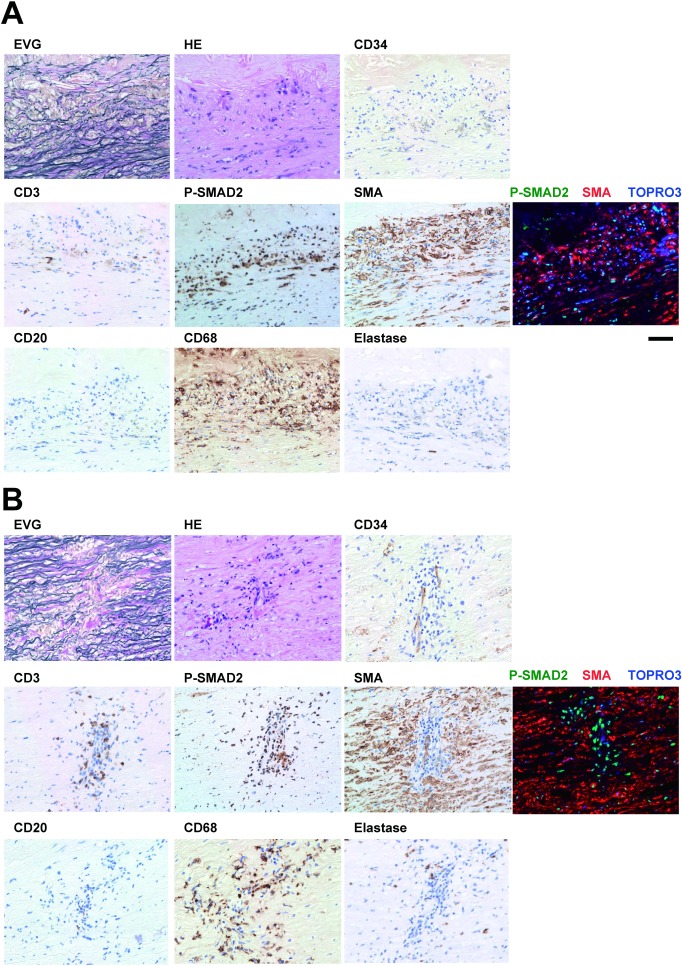
To further characterize SMAD2 activation, we performed a series of immunohistochemical stainings for cell type markers, namely CD34 (endothelial cells), CD3 (T cells), SMA (SMCs), CD20 (B cells), CD68 (macrophages), and neutrophil elastase (neutrophils), along with P-SMAD2. We observed two patterns of the P-SMAD2 area, a layer-like staining at the border of SMA-positive media and the degenerative intimomedial complex (Fig. 1B, black arrowheads) and spot-like staining within the SMA-positive media (Fig. 1B, a white arrowhead), and we analyzed both these patterns.
The layer-like P-SMAD2 staining overlapped with the dense SMA staining (Fig. 3A), suggesting SMAD2 activation in part of the SMCs. Double immunofluorescence staining demonstrated that many of the P-SMAD2-positive nuclei were surrounded by cytosolic staining of SMA, further supporting the SMAD2 activation in SMCs. However, part of the P-SMAD2-positive cells was not stained for SMA. These cells appeared inflammatory cells because this area was infiltrated by many CD68-positive macrophages and occasional CD3-positive T cells. These findings suggested that SMAD2 was activated in the environment where SMCs and macrophages may interact with each other.
Fig. 3 Characterization of the TAA tissue with activated SMAD2. Representative images are shown for the areas corresponding to the layer-like staining of P-SMAD2 (A) and spot-like staining of P-SMAD2 (B). Photomicrograms indicate histochemical staining with EVG and H & E, and immunohistochemical staining for the T cells (CD3), P-SMAD2, smooth muscle cells (SMA), B cells (CD20), macrophages (CD68) and neutrophils (Elastase). Nuclear staining with hematoxylin in immunohistochemical stainings served as counter staining. Photomicrograms of the immunofluorescence staining for P-SMAD2 (green), SMA (red), and nuclei (TOPRO3, blue) are also shown. Bar 50 µm.
In contrast to the layer-like staining of P-SMAD2, the spot-like staining of P-SMAD2 showed a pattern complimentary to SMA staining (Fig. 3B), suggesting that P-SMAD2 was activated in non-SMCs. In fact, double immunofluorescence staining for P-SMAD2 and SMA revealed that P-SMAD2 was almost exclusively stained in the SMA-negative cells. The P-SMAD2-positive cells surrounded the CD34-positive endothelial cells that formed a tube-like structure that was not observed in the layer-like P-SMAD2-positive area. The spot-like P-SMAD2-positive area was also enriched for CD3-positive T cells, CD68-positive macrophages, and elastase-positive neutrophils, with few CD20-positive B cells. These findings suggested the presence of an active inflammatory process, accompanied by the cellular infiltration through the microvessels. The findings also suggested that SMAD2 was activated in the non-SMCs, possibly in the inflammatory cells such as the macrophages and the T cells.
Discussion
In this study, we characterized the activation status of TGFβ signaling using immunostaining for P-SMAD2 at the regions with a normal diameter of the ascending aorta and those with the maximal diameter of the TAA in the arch. In both the regions, P-SMAD2 showed higher signal in the outer zone of the aortic walls. Overall, P-SMAD2 showed higher signal in the TAA regions than in the aortic wall with a normal diameter, especially in the middle and outer zones of the wall. Detailed analyses revealed two distinct patterns of P-SMAD2 signals, including a layer-like staining and a spot-like staining. These findings are consistent with the importance of TGFβ signaling in TAA pathogenesis, as proposed previously.8) The heterogeneity in the staining patterns of P-SMAD2 underscores the complexity of the role that TGFβ signaling may play in TAA pathogenesis.
The layer-like P-SMAD2 was located between the layer with dense elastic lamellae and SMCs and the layer with sparse cellularity and amorphous extracellular matrix. In normal tissue, TGFβ is bound to latent TGFβ-binding proteins (LTBPs) that in turn bind to other extracellular matrix (ECM) proteins.17) When the normal tissue is damaged, TGFβ is released and activated by proteolytic activities. The active TGFβ in turn stimulates SMCs to promote gene expression and ECM protein synthesis, thus making a loop to repair the ECM architecture.18) The released TGFβ also modulates the inflammatory process by regulating the activities of the inflammatory cells. Considering the localization of the layer-like P-SMAD2 staining between the nearly normal medial tissue and the tissue with amorphous ECM, P-SMAD2 may represent active ECM remodeling, including the activation of the ECM repair loop. The finding that P-SMAD2 signal was localized in both SMCs and non-SMCs, possibly in the macrophages, also supports this interpretation.
The spot-like P-SMAD2 staining was in the middle of the medial layer with dense elastic lamellae and SMCs. CD34-positive microvessels were observed at the center of the P-SMAD2-positive area. There was co-localization with the disrupted elastic lamellae and local deficiency in SMCs; therefore, the P-SMAD2-positive area may represent the focus of active inflammation and tissue destruction with neovasculature, where TGFβ plays a pivotal role.18) Consistently, it has been reported that TAA tissue is characterized by the formation of leaky neovessels associated with an imbalance in the angiogenic factors.19) In contrast to the layer-like staining of P-SMAD2 where the majority of P-SMAD2-positive cells were SMCs, the majority of P-SMAD2-positive cells were non-SMCs. At the same time, the spot-like P-SMAD2 positive area contained abundant macrophages and T cells with few B cells and neutrophils. These findings indicate that although the inflammatory process involving TGFβ signaling is ongoing in this area, the surrounding SMCs do not respond to TGFβ signaling. Therefore, TGFβ-dependent repair of ECM may not be active in the SMCs of this area. By contrast, the non-SMCs, possibly including inflammatory cells, showed strong SMAD2 activation. Owing to the context-dependent function of TGFβ signaling, its activity in these cells may be disease-protective/-promoting, similar to the findings in atherogenesis.20)
The major limitation of this study was the relatively smaller number of observations that precluded a comprehensive analysis of the relationship between the activity of TGFβ signaling and clinical conditions, including the TAA size, disease time course, TAA localization (i.e., the ascending, arch, and descending TAA or aneurysm in the thoracoabdominal aorta), and risk factors. Moreover, the manner in which the TAA, including the fusiform and saccular forms, is related to TGFβ signaling remains unclear. It is noteworthy that TGFβ signal is reportedly activated in the ascending aorta with TAA, irrespective of the etiology.21) This finding is consistent with the present findings, although it remains unclear whether the heterogeneous activation of TGFβ signaling as observed in arch TAA in this paper is also applicable to the TAA at other sites. Although we excluded familial or syndromic TAA based on clinical information, we did not perform the analysis for genetic abnormalities or variations in patients. Therefore, in this study, we cannot exclude the influence of genetic factors that may be involved in the pathogenesis of sporadic TAA.22) The aberrant activation of TGFβ signaling is causally involved in TAA pathogenesis8); therefore, the samples with a normal diameter in this study may not be normal with regard to TGFβ signaling. To clarify the issue of TGFβ activation in apparently normal diameter samples, it would be necessary to compare the samples with a normal diameter from TAA patients and those from individuals without TAA. Further studies would be required to clarify these issues and elucidate how different modes of TGFβ signaling in different histopathological environments, as shown in this study, are involved in TAA pathogenesis.
Conclusion
In this study, we compared the TGFβ signal activation in TAA tissue compared to that in the aortic tissue with a normal diameter. The TGFβ signaling showed distinct patterns in terms of histopathology, providing a potential explanation for the complex function of TGFβ signaling in TAA. Deciphering the function of TGFβ signaling in different histopathological contexts in different cell types would enable a deeper understanding of TAA.
Acknowledgments
This work was funded in part by grants JSPS KAKENHI 21390367, 24390334, 24659640, 26670621, and 16H05428 (to HAo); grants from the Daiichi Sankyo Foundation of Life Science, Uehara Memorial Foundation (to HAo), and Vehicle Racing Commemorative Foundation (to HAo). We would like to thank Ms. Kiyohiro, Ms. Nishigata, Ms. Nakao, Ms. Shiramizu, and Dr. Yamamoto for their expertise.
Disclosure Statement
The authors declare no conflict of financial interest concerning this manuscript.
Author Contributions
Study conception: HF, HAo, HT
Sample curation: HF, SY, ST, HO, TS, KT, HAk, HT
Analysis: HF, HAo, SK
Writing: HF, HAo
Funding acquisition: HAo, YF, HT
Critical review and revision: YF, HAk, SK, HT
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Ince H, Nienaber CA. Etiology, pathogenesis and management of thoracic aortic aneurysm. Nat Clin Pract Cardiovasc Med 2007; 4: 418-27. [DOI] [PubMed] [Google Scholar]
- 2).Pitt MP, Bonser RS. The natural history of thoracic aortic aneurysm disease: an overview. J Card Surg 1997; 12 Suppl: 270-8. [PubMed] [Google Scholar]
- 3).Gallo EM, Loch DC, Habashi JP, et al. Angiotensin II-dependent TGF-beta signaling contributes to Loeys–Dietz syndrome vascular pathogenesis. J Clin Invest 2014; 124: 448-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Gillis E, Van Laer L, Loeys BL. Genetics of thoracic aortic aneurysm: at the crossroad of transforming growth factor-beta signaling and vascular smooth muscle cell contractility. Circ Res 2013; 113: 327-40. [DOI] [PubMed] [Google Scholar]
- 5).Holm TM, Habashi JP, Doyle JJ, et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 2011; 332: 358-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358: 2787-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312: 117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).MacFarlane EG, Haupt J, Dietz HC, et al. TGF-beta family signaling in connective tissue and skeletal diseases. Cold Spring Harb Perspect Biol 2017; 9: a022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol 2006; 26: 1712-20. [DOI] [PubMed] [Google Scholar]
- 10).Morisaki T, Morisaki H. Genetics of hereditary large vessel diseases. J Hum Genet 2016; 61: 21-6. [DOI] [PubMed] [Google Scholar]
- 11).Akhurst RJ. The paradoxical TGF-beta vasculopathies. Nat Genet 2012; 44: 838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Li W, Li Q, Jiao Y, et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest 2014; 124: 755-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Cook JR, Clayton NP, Carta L, et al. Dimorphic effects of transforming growth factor-beta signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler Thromb Vasc Biol 2015; 35: 911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Wang Y, Ait-Oufella H, Herbin O, et al. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest 2010; 120: 422-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Nagasawa A, Yoshimura K, Suzuki R, et al. Important role of the angiotensin II pathway in producing matrix metalloproteinase-9 in human thoracic aortic aneurysms. J Surg Res 2013; 183: 472-7. [DOI] [PubMed] [Google Scholar]
- 16).Gomez D, Coyet A, Ollivier V, et al. Epigenetic control of vascular smooth muscle cells in Marfan and non-Marfan thoracic aortic aneurysms. Cardiovasc Res 2011; 89: 446-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Robertson IB, Horiguchi M, Zilberberg L, et al. Latent TGF-beta-binding proteins. Matrix Biol 2015; 47: 44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Roberts AB, Sporn MB. Regulation of endothelial cell growth, architecture, and matrix synthesis by TGF-beta. Am Rev Respir Dis 1989; 140: 1126-8. [DOI] [PubMed] [Google Scholar]
- 19).Kessler K, Borges LF, Ho-Tin-Noe B, et al. Angiogenesis and remodelling in human thoracic aortic aneurysms. Cardiovasc Res 2014; 104: 147-59. [DOI] [PubMed] [Google Scholar]
- 20).Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev 2006; 17: 487-99. [DOI] [PubMed] [Google Scholar]
- 21).Gomez D, Al Haj Zen A, Borges LF, et al. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J Pathol 2009; 218: 131-42. [DOI] [PubMed] [Google Scholar]
- 22).Saratzis A, Bown MJ. The genetic basis for aortic aneurysmal disease. Heart 2014; 100: 916-22. [DOI] [PubMed] [Google Scholar]