Abstract
This study aims to investigate the menstrual recovery outcome of scar pregnancy patients who received uterine artery embolization combined with curettage, and its influencing factors.
The data of 119 patients with scar pregnancy, who received uterine artery embolization combined with curettage between December 2012 and December 2016 in Henan Provincival People's Hospital, were collected. The menstruation recovery of these patients was followed up, and factors that have influence on menstrual blood volume were analyzed using SPSS V.17.0.
Follow-up data were available in 101/119 (84.9%) women. The median follow-up time was 22.7 months (range: 1.6–50.6 months); 58 (57.4%) patients had reduced menstrual blood volume, and 2 patients (2%) had amenorrhea. The proportion of patients with reduced menstrual blood volume, who were embolized with polyvinyl alcohol (PVA), PVA combined with gelatin sponge, and gelatin sponge between < and ≥33 years old was 41.7% versus 66.7%, 40% versus 57.1% and 60.6% versus 68.0%. The average age of patients with reduced menstrual blood volume (34.3 years) was greater than patients with normal menstrual blood volume (31.4 years), but the difference was not statistically significant (P = .07).
Reduced menstrual blood volume can occur in scar pregnancy patients who received uterine artery embolization combined with curettage. The influence of the embolic agent PVA on menstrual blood volume depends on age, but the difference was not statistically significant.
Keywords: cesarean scar pregnancy, curettage, embolic agent, menstrual quantity, uterine arterial embolization
1. Introduction
Cesarean scar pregnancy (CSP) is the gestation where the gestation sac is planted in the scar of the uterus and is a special ectopic pregnancy.[1] Studies have revealed that CSP accounted for 1:1800–1:2216[2,3] of total pregnancies,[2,3] and accounted for 6.1% of ectopic pregnancies.[4] Due to increased connective tissue at the scar, elasticity is poor, and scar pregnancy may cause fatal bleeding, rupture of the uterus, and hysterectomy[5] in the course of the ongoing pregnancy or miscarriage. Present studies have confirmed that uterine artery embolization (UAE) can effectively reduce the risk of massive bleeding and rupture of the uterus during curettage, but recognized its short-term efficacy.[6,7] However, there is very little large-scale, long-term follow-up data on postoperative menstrual recovery in patients, and it remains to be determined whether it affects secondary pregnancy.[8] In the present study, scar pregnancy patients treated with UAE combined with curettage in our hospital in the past 3 years were followed up, and factors that might influence the decrease in menstrual volume were analyzed.
2. Materials and methods
This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Henan Provincival People's Hospital. Written informed consent was obtained from all participants.
2.1. Information
-
(1)
The basic data of 101 patients with scar pregnancy, who underwent UAE combined with curettage in our hospital from December 2012 to December 2016 and were successfully followed up, were collected by cluster sampling, including: age, preoperative human chorionic gonadotropin (hcG) value, the number of cesarean sections and abortions, the interval of the last cesarean section, the types of intraoperatively used embolic agents, the interval between curettage and UAE, curettage tissues, amount of blood loss during curettage, and hospitalization time.
-
(2)
All patients had a history of menopause, a history of caesarean section, and positive hcG, underwent ultrasound or MRI diagnosis in accordance with scar pregnancy, and received UAE after comprehensive evaluation by gynecology and interventional doctors before curettage.
2.2. Therapeutic methods
2.2.1. UAE method
The patient was instructed to lie in the supine position. Routine disinfection was performed and surgical drapes were paved. The puncture site at the right femoral artery was locally anesthetized with 2% lidocaine, the Seldinger technique was used to puncture the right femoral artery, the 5-F artery sheath was placed, and a 4-F Cobra catheter was inserted. The Cobra catheter was successively placed in the bilateral internal iliac arteries for angiography to understand the opening and travel course of the uterine artery, and determine whether there are other branches of blood vessels involved in the uterine blood supply. Utilizing the coaxial catheter technique, a micro catheter (Merit Medical, USA) was superselectively catheterized into the distal segments of the bilateral uterine arteries under the guidance of the micro guidewire (Asahi Intecc, Japan) (ovarian branches that supply ovarian blood were steered) and was contrasted. This revealed that the bilateral uterine arteries were thickened and tortuous, blood flow was accelerated, the number of branches significantly increased, a disorganized vascular mass was visible in the uterus, and the uterus was enlarged at the parenchymal phase. Furthermore, the arteriovenous fistula could be observed in some patients. After confirmation by angiography, 40 mg of methotrexate diluted by physiological saline was slowly perfused through the bilateral uterine arteries. Under fluoroscopy, gelatin sponge particles or PVA particles (Ailcon, Hangzhou, China) suspension mixed with a contrast agent was injected through a micro catheter until the flow of blood in the bilateral uterine arteries was slow or arrested. Then, radiography was performed again. When none of the branches of the distal uterine artery were developed, and no other branch vessels were involved in the uterine blood supply, the embolization was successful. Then, the operation was ended, the catheter and arterial sheath were removed, and the right femoral artery puncture site was treated with compression bandage. Patients were returned to the ward, and laid flat for 24 hours after the operation. The right lower leg was immobilized for 12 hours.
2.2.2. Curettage
At 1–7 days after UAE, ultrasound-guided curettage was performed. Foley catheter and rescue equipments were prepared. The removed tissues and bleeding volume were recorded during the operation. Furthermore, the hcG value was closely monitored, and ultrasonography was performed after curettage. If residue was found, curettage was performed again. The patient was instructed to undergo contraception for 6 months after the operation.
2.3. Follow-up patients were followed up by telephone
The recovery time of hcG, the time of menstruation recovery and changes in volume of menstruation compared with that before the operation were investigated. When patients were found to have amenorrhea, ultrasound, hysteroscopy, and hormonal examinations were done.
2.4. Efficacy evaluation
-
(1)
Short-term efficacy of UAE: The standard was whether UAE could prevent massive bleeding during curettage and whether it could avoid hemorrhagic shock, ruptures of the uterus, and severe hysterectomy.
-
(2)
Long-term efficacy of the combination of UAE and curettage: This was determined based on changes in menstrual volume after menstrual recovery.
2.5. Statistical analysis
All indicators were analyzed using statistical analysis software SPSS V.17.0. The effects of embolic materials used in UAE on menstrual volume were evaluated by χ2 test. Since age, preoperative hcG value, the number of caesarean sections, the number of abortions, the bleeding volume in curettage, and the number of removed tissues in curettage were not normally distributed, the influence of various indexes on menstrual volume was analyzed using a nonparametric rank-sum test. P <.05 was considered statistically significant.
3. Results
3.1. A total of 101 patients were followed up by telephone, and the proportion of patients lost to follow-up was 15.5%
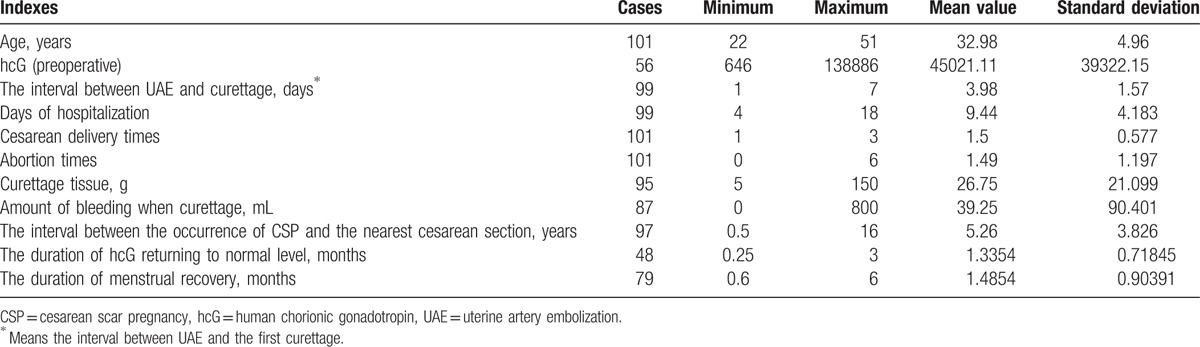
The median follow-up duration was 22.7 months (range: 1.6–50.6 months). Among these 101 patients, preventive embolization was performed in 95 patients, and emergency embolization was performed in 6 patients. Furthermore, among these patients, 34 patients had no vaginal bleeding, and 67 patients had varying degrees of vaginal bleeding. Moreover, among these patients, scar pregnancy was found in 84 patients who were admitted directly to the hospital, and in 17 patients who were admitted to the hospital after developing massive hemorrhage or incomplete abortion after abortion or curettage in local hospitals. The median age of these patients was 33 years old (range: 22–52 years old), the median interval between UAE and curettage was 4 days (range: 1–7 days). The mean preoperative hcG value was 45,021 IU/L (range: 646–138,886 IU/L). The average duration of hospital stay was 9.4 days (range: 4–18 days). Among these patients, 43 patients (42.6%) had a history of ≥2 abortions, and 46 patients (45.5%) had a history of ≥2 cesarean sections. The average interval between the occurrence of caesarean scar pregnancy (CSP) and the nearest cesarean section was 5.26 years (range: 0.5–16 years), the median duration of hcG returning to normal level after curettage was 1.3 months (range: 0.25–3.0 months), and the median duration of menstrual recovery was 1.5 months (range: 0.6–6.0 months) (Table 1).
Table 1.
Statistics in the indexes of 101 patients.
3.2. Short-term curative effect
-
(1)
UAE was successfully performed in all patients, and the success rate of embolization was 100%. All patients underwent curettage under the guidance of ultrasound after UAE, the average interval was 4 days (range: 1–7 days), the median bleeding volume in curettage was 39.3 mL (range: 0–800 mL), and the average weight of tissues removed in curettage was 26.8 g (range: 5–150 g). Among these patients, 2 patients developed massive hemorrhage after UAE, 1 patient developed menopause for 16 days. The 2 patients developed massive hemorrhage at 1 day after UAE, the volume was approximately 1000 mL, Foley catheter for hemostasis was successfully performed by a gynecologist, and symptomatic blood transfusion was given. At the second day, the curettage was successfully performed. The other patient developed menopause for >100 days, and developed massive hemorrhage during curettage in a local hospital. After admission in our hospital, the patient first underwent UAE, and developed massive hemorrhage again during curettage, with a volume of approximately 800 mL. Foley catheter for hemostasis was successfully performed. After blood transfusion for correcting anemia, curettage was performed again, and the process was smooth. The clinical success rate was 98% (99/101). No consequences of massive hemorrhage, ruptures of the uterus, or uterus resection occurred during curettage for the remaining patients.
-
(2)
Four patients (4%, 4/101) underwent curettage again. The longest interval between curettage and UAE was 38 days, and no massive hemorrhage occurred during the curettage in all patients.
3.3. Long-term curative effect
-
(1)
Among these 101 patients, menstrual volume decreased in 58 patients (57.4%), menstrual volume was maintained at normal levels in 41 patients (40.6%), and 2 patients (2%) developed amenorrhea. These 2 patients with amenorrhea had an age of 33 and 36 years, respectively; and PVA and gelatin sponge were used as embolic agents, respectively. The patient who used PVA had ever refused curettage, and underwent 2 times of UAE, hcG level was maintained at >50,000 IU/L, and finally received curettage. The follow-up duration was 14 months, thin endometrium was revealed by ultrasound, no abnormalities were found by hysteroscopy, and hormone levels were normal. The follow-up time for patients who used gelatin sponge as an embolic agent was 12 months, hysteroscopy at 10 months after surgery revealed no endometrium, and hormone levels were normal.
-
(2)
The effects of different indexes on the recovery of menstrual volume: These 101 patients were divided into 2 groups: normal menstrual volume group (n = 60), and decreased menstrual volume group (n = 41). The 2 patients with amenorrhea were assigned into the decreased menstrual volume group.
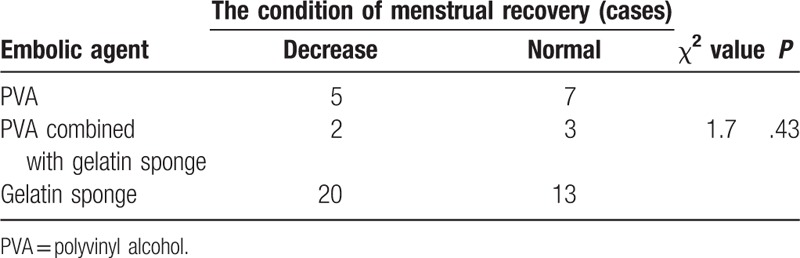
According to the different embolic agent types, these patients were divided into 3 groups: simple PVA group, PVA combined with gelatin sponge group, and simple gelatin sponge group. The difference in reduced menstrual volume among these 3 groups was not statistically significant (P = .58). However, age stratification analysis revealed that the proportion of reduced menstrual volume in patients with an age of <33 years and ≥33 years, and treated with simple PVA, PVA combined with gelatin sponge, and simple gelatin sponge as embolic agents were 41.7% versus 66.7%, 40% versus 57.1% and 60.6% versus 68%, respectively. However, the differences were not statistically significant (Tables 2–4, Figs. 1 and 2).
-
(3)
The average age of patients with reduced menstrual volume (34.3 years) was greater than the average age of patients with normal menstrual volume (31.4 years), but the difference was also not statistically significant (P = .07). The differences in the effects of other indexes (the number of cesarean sections, the number of abortions, preoperative hcG value, the bleeding volume of curettage and the removed tissues in curettage) on menstrual volume were all statistically significant (Table 5).
Table 2.
The influence of different embolic agent types on menstrual recovery.

Table 4.
The influence of different embolic agent types on menstrual recovery of patients (≥33 years old) with caesarean scar pregnancy.

Figure 1.
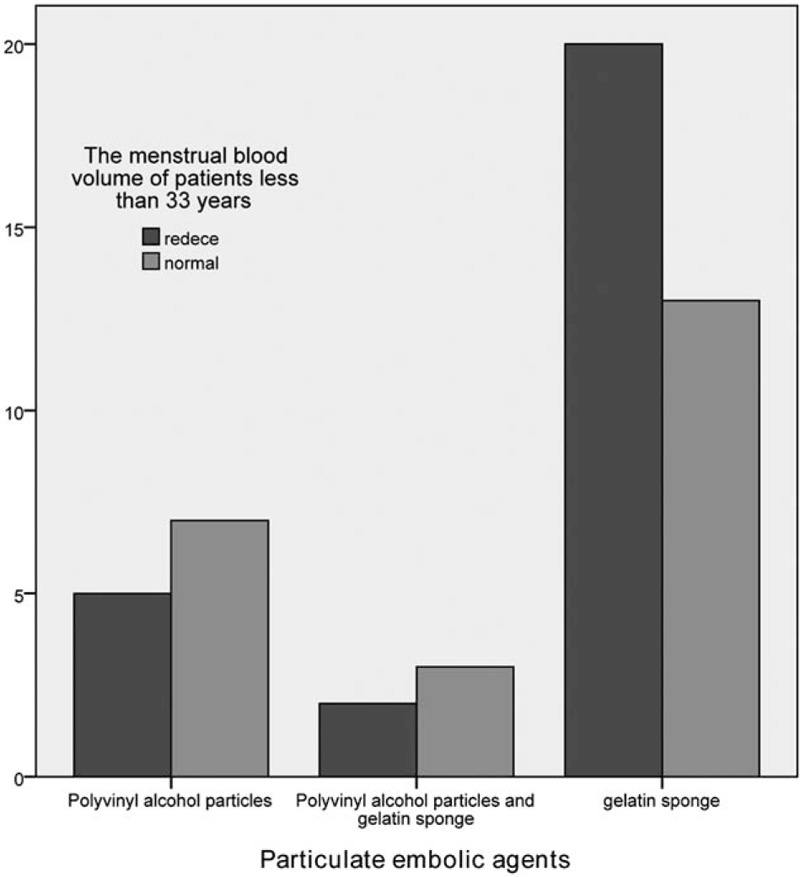
The influence of particulate embolic agents’ embolism on the menstrual amount of patients <33 years old.
Figure 2.
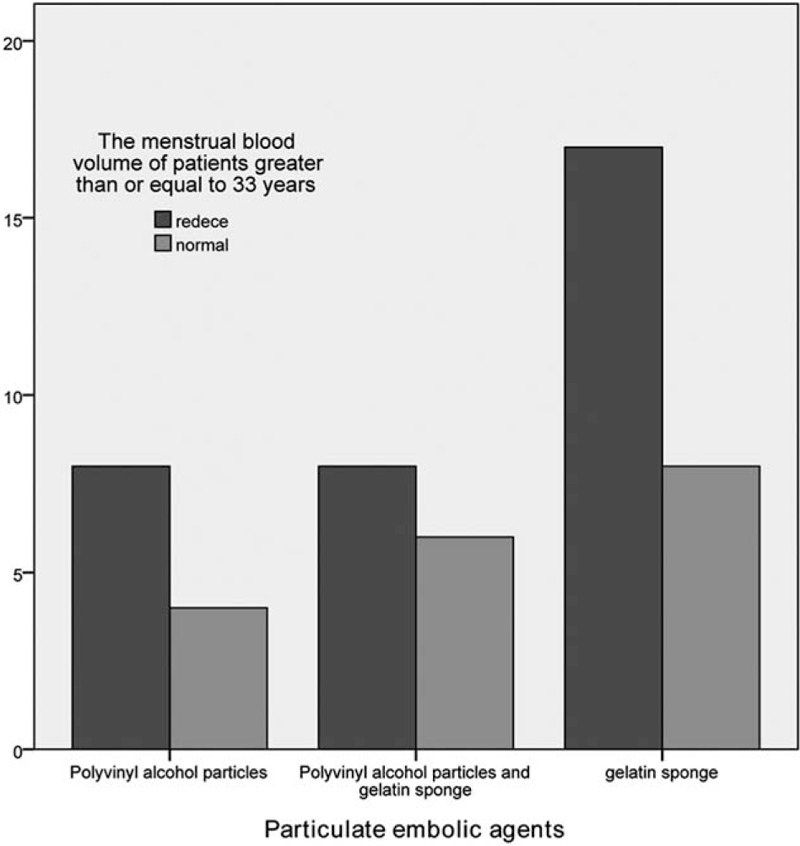
The influence of particulate embolic agents’ embolism on the menstrual amount of patients ≥33 years old.
Table 5.
The influence of different indexes on the menstrual reducing.
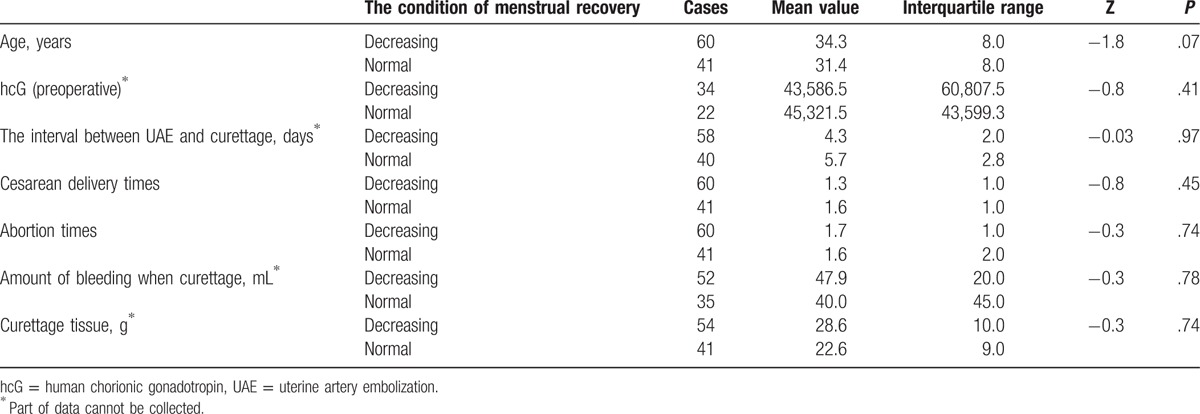
Table 3.
The influence of different embolic agent types on menstrual recovery of patients (<33 years old) with caesarean scar pregnancy.
4. Discussion
CSP is a pregnancy where the gestation sac implants in the scar of the uterus and is a special ectopic pregnancy.[1] This was first reported by Larsen and Solomon[9] in 1978. The increase in CSP cases in recent years was attributed to the increase in cesarean section rates and increase in accuracy of ultrasound diagnosis. Vial et al[10] classified CSP into 2 types: one is when the gestational sac grows in the direction from the scar towards the cervix or uterine cavity, and another is when the gestational sac is implanted deeper and grows in the direction from the scar towards the bladder. The former has the opportunity to continue the pregnancy to a full-term birth, but has a risk of a massive life-threatening hemorrhage; while the latter has a risk of metrorrhexis or massive hemorrhage in early pregnancy. The diagnosis of CSP mainly depends on the history of caesarean section, increase in hcG, and ultrasound. Transvaginal ultrasound is the preferred examination method for the diagnosis of CSP, and the sensitivity is 86.4% to 88.9%.[2,11]
Since the scar site of the scar uterus has more connective tissues, and the intima is poorly developed and even absent, the villi are readily implanted in the muscularis, making the gestational sac easily implanted in scar fissures. Serious consequences of CSP include fatal massive hemorrhage and hysterorrhexis during pregnancy, curettage, or medical abortion; and the patients face a risk of uterine resection or disseminated intravascular coagulation (DIC) or death in severe cases.[5] UAE was first reported in 1999, and was gradually used in the treatment of hemostasis and uterus preservation. UAE causes a reduction in blood flow, thrombosis, vascular occlusion, a decrease or stop of bleeding, and the prevention of embryonic blood supply; causing ischemic necrosis of the embryos, and providing convenience for curettage or abortion. Methotrexate was administered through arterial infusion. Locally higher drug concentrations directly caused degeneration, necrosis, and abscission of trephocytes, achieving the purpose of killing the embryo. At present, there is increasing evidence that applying UAE before curettage can effectively avoid the risk of massive hemorrhage.[5,12] However, there is still no large-scale study on the effect of UAE combined with curettage on the following menstrual volume, menstrual cycle, ovarian function, and subsequent pregnancy. In the present study, 101 CSP patients undergoing UAE combined with curettage were followed-up and statistically analyzed.
The common symptoms of CSP were abdominal pain and irregular bleeding, but at least 1/3 of patients have no symptom.[13] In the present study, 34 patients (33.6%) had no vaginal bleeding, and 67 patients (66.3%) had varying degrees of vaginal bleeding. The mean preoperative hcG value was 45,021 IU/L (range: 646–138,886 IU/L). Previous studies suggest that the occurrence of CSP was not correlated to the number and interval of caesarean sections. Jurkovic et al[3] reported that in CSP patients, patients with a history of ≥2 cesarean sections accounted for 66.7% to 72%. The data in the present study revealed that the average number of abortions and cesarean sections was 1.5 (range: 0–6 and 1–3) in the 101 CSP patients, and patients with a history of ≥2 cesarean sections and abortions accounted for 45.5% (46/101) and 42.6% (43/101), respectively,[2] which were less than those reported in previous literatures. The data in the present study revealed that bleeding occurred in 2 patients after UAE, and the incidence of re-bleeding was 2% (2/101), which was also far less than the 8% to 17% reported in related literatures.[14,15] Du et al[16] reported that the increase in gestational age was a risk factor for re-bleeding after UAE, and a distance of >0.2 cm between the gestational sac and bladder could reduce the risk of bleeding.
Previous studies have reported that UAE may have a certain impact on endometrial and ovarian functions. Tropeano et al[17] and Vashisht et al[18], respectively, reported 1 patient with amenorrhea, in which there was no change in hormone levels in the former, and poor endometrial hyperplasia was found by hysteroscopy; while decreased hormone levels were found in the latter. This suggests that UAE has an impact on endometrial and ovarian functions. In the present study, during the follow-ups of these 101 patients, menstrual volume decreased at varying degrees in 58 patients (57.4%), and amenorrhea occurred in 2 patients. Among these 2 patients, 1 patient refused to undergo curettage at the time of the visit. This patient underwent 2 sessions of UAE, but hcG remained at a high level; and subsequently, these patients agreed to receive curettage. In postoperative follow-ups, hysteroscopy revealed that the endometrium had become thinner, while no endometrium was found in the other patient. Furthermore, hormone levels were normal in both patients. Therefore, the decrease in menstrual volume may be related to the thinning and impairment of the endometrium. Further studies are needed to demonstrate this.
The effects of different embolic agents on menstrual volume during UAE were further analyzed. In the present study, 2 embolic granules, PVA, and gelatin sponge particles were used. The mechanism of these 2 materials was the mechanical embolization of blood vessels. After embolization from the peripheral into the trunk of the artery, the entire arterial lumen was occluded without damaging the capillary network, changing local hemodynamics and gathering platelets, thereby forming thrombi and obstructing the target vessel obstruction. The difference is that PVA is a polymeric material that has good biological safety and is nonbiodegradable in vivo. Therefore, it is considered as a permanent embolic agent, and blood vessels that are embolized with it cannot be recanalized. Gelatin sponge particle is fibrin glue without antigenicity prepared from pigskin gelatin, which is insoluble in water and can be degraded in human blood vessels. It is a medium-term embolic agent, and the complete degradation time is 14 to 90 days. Therefore, the embolic target blood vessel has the possibility of recanalization. In the present study, according to different embolic agent types, patients were divided into 3 groups: simple PVA group, PVA combined with gelatin sponge group, and simple gelatin sponge group. The difference in menstrual volume between these 3 groups was not statistically significant (P = .58). However, age stratification analysis revealed that the proportion of reduction in menstrual volume in patients with an age of <33 years and ≥33 years, who were treated with simple PVA, PVA combined with gelatin sponge, and simple gelatin sponge as embolic agents, were 41.7% versus 66.7%, 40% versus 57.1%, and 60.6% versus 68%, respectively, but the differences were not statistically significant. Theoretically, PVA belongs to permanent embolic agents. It should have a greater impact on menstrual volume than that of the medium-term embolic agent—gelatin sponge. However, the data of facts were just the opposite. Through our analysis, the reasons may be the following: the gelatin sponge was used as an absorbable medium-term embolic agent, its dosage was higher in UAE, the embolism is compact, and it affected the endometrial blood supply and caused damage in some patients; PVA was used as a permanent embolic agent, it was feared to form reflux and inappropriate embolization that would affect the normal branches of the surrounding blood vessels, and its use was usually stopped immediately when the blood flow became slow, thereby the dosage was lower. In an observation on postoperative menstruation in uterine fibroids patients treated with UAE, Liu et al[19] revealed that ovarian damage was easy to avoid and easy to recover, while endometrial damage was relatively difficult to avoid and difficult to recover. Honda et al[20,21] revealed in a study that UAE could cause endometrial damage. However, under the condition of damage, the observed pregnancy rate was 50%. Our median follow-up duration was 22.7 months, the follow-up duration of some patients was up to 4 years, and no obvious recovery in menstrual loss was found. Some patients were conditioned with oral traditional Chinese medicine. However, the condition effect requires further follow-up to understand. Therefore, UAE may cause damage to the endometrium, and this damage is difficult to restore. For young patients or patients who are schedule to have second pregnancy, it remains unknown whether it is a better choice to adopt PVA or to choose gelatin sponge particles. It is recommended that the use of embolic granules should be reduced as much as possible based on effective prevention of bleeding or embolic methods should be improved. For example, Liu et al[19] recommend that complete endometrial damage can be avoided by stratified embolization (using a small-diameter embolic agent first, followed by a large-diameter embolic agent) and a mixed embolism (an admixture of different diameters of embolic agents), in order to protect the endometrium to the maximum extent. Furthermore, other therapies were used to treat the disease, such as direct injection of potassium chloride to the pregnancy sac or using high-intensity focused ultrasound.[22,23]
According to the observation and follow-up data of our hospital, it was revealed that most patients with scar pregnancy treated with UAE combined with curettage have reduced menstrual volume. Therefore, UAE has a certain impact on the endometrium, which is difficult to restore. Since the present study is retrospective in nature, and the sample size was not large, many specific data are incomplete and the preliminary analysis results have only referential meaning. In addition, further statistically analysis is needed to determine whether the decrease in menstrual volume affects further conception. Prospective, randomly controlled large-scale data is needed to guide the next clinical work.
Footnotes
Abbreviations: CSP = cesarean scar pregnancy, DIC = disseminated intravascular coagulation, PVA = polyvinyl alcohol, UAE = uterine artery embolization.
The authors have no conflicts of interest to disclose.
References
- [1].Zahálková L, Kacerovský M. Cesarean scar ectopic pregnancy. Ceska Gynekol Winter 2016;81:414–9. [PubMed] [Google Scholar]
- [2].Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol 2006;107:1373–81. [DOI] [PubMed] [Google Scholar]
- [3].Jurkovic D, Hillaby K, Woelfer B, et al. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment cesarean section scar. Ultrasound Obstet Gynecol 2003;21:220–7. [DOI] [PubMed] [Google Scholar]
- [4].Seow KM, Huang LW, Lin YH, et al. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol 2004;23:247–53. [DOI] [PubMed] [Google Scholar]
- [5].Huang Y, Li Y, Xi R, et al. An application of uterine artery chemoembolization in treating cesarean scar pregnancy. Int J Clin Exp Med 2015;8:2570–7. [PMC free article] [PubMed] [Google Scholar]
- [6].Cao S, Zhu L, Jin L, et al. Uterine artery embolization in cesarean scar pregnancy: safe and effective intervention. Chin Med J (Engl) 2014;127:2322–6. [PubMed] [Google Scholar]
- [7].Peng P, Gui T, Liu X, et al. Comparative efficacy and safety of local and systemic methotrexate injection in cesarean scar pregnancy. Ther Clin Risk Manag 2015;11:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhai JF, Xu M, Zhang B, et al. Treatments of caesarean scar pregnancy and the corresponding results in ten years. Eur Rev Med Pharmacol Sci 2015;19:2523–7. [PubMed] [Google Scholar]
- [9].Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus: an unusual case of postabortal hemorrhage. S Afr Med J 1978;53:142–3. [PubMed] [Google Scholar]
- [10].Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol 2000;16:592–3. [DOI] [PubMed] [Google Scholar]
- [11].Yu HR, Kang J, Wei N, et al. Application of color Doppler ultrasound in the diagnosis and treatment of cesarean scar pregnancy after cesarean section. Shan Dong Yi Yao 2015;55:47. [Google Scholar]
- [12].Feng Y, Chen S, Li C, et al. Curettage after uterine artery embolization combined with methotrexate treatment for caesarean scar pregnancy. Exp Ther Med 2016;12:1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McKenna DA, Poder L, Goldman M, et al. Role of sonography in the recognition, assessment, and treatment of cesarean scar ectopic pregnancies. J Ultrasound Med 2008;27:779–83. [DOI] [PubMed] [Google Scholar]
- [14].Wu X, Xue X, Wu X, et al. Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: a cohort study. Int J Clin Exp Med 2014;7:2793–803. [PMC free article] [PubMed] [Google Scholar]
- [15].Lian F, Wang Y, Chen W, et al. Uterine artery embolization combined with local methotrexate and systemic methotrexate for treatment of cesarean scar pregnancy with different ultrasonographic pattern. Cardiovasc Intervent Radiol 2012;35:286–91. [DOI] [PubMed] [Google Scholar]
- [16].Du YJ, Zhang XH, Wang LQ. Risk factors for haemorrhage during suction curettage after uterine artery embolization for treating caesarean scar pregnancy: a case-control study. Gynecol Obstet Invest 2015;80:259–64. [DOI] [PubMed] [Google Scholar]
- [17].Tropeano G, Litwicka K, Di Stasi C, et al. Permanent amenorrhea associated with endom etrial atrophy after uterine artery embolization for symptomatic uterine fibroids. Fertil Steril 2003;79:132–5. [DOI] [PubMed] [Google Scholar]
- [18].Vashisht A, Studd J, Carey A, et al. Fetal septicemia after myoma embolization. Lancet 1999;354:307. [DOI] [PubMed] [Google Scholar]
- [19].Liu P, Chen CL, Gao LF, et al. Pregnancy and outcomes of patients with uterine leiomyoma after uterine arterial embolization. Chin J Pract Gynecol Obstet 2006;22:446–8. [Google Scholar]
- [20].Honda I, Sato T, Adachi H, et al. Uterine artery embolization for leiomyoma: complications and effects on fertility [J]. Gynecol Obstet Fertil 2003;31:243–5. [PubMed] [Google Scholar]
- [21].Trastour C, Bongain A, Rogopoulos A, et al. Uterine artery embolization for leiomyoma: complications and effects on fertility. Gynecol Obstet Fertil 2003;31:243–5. [DOI] [PubMed] [Google Scholar]
- [22].Pirjani R, Bayani L, Shirazi M. Successful local and systemic medical treatment of cesarean scar pregnancy and a subsequent term pregnancy after treatment: a case series. Iran J Reprod Med 2015;13:445–50. [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu X, Deng X, Wan Y, et al. High-intensity focused ultrasound combined with suction curettage for the treatment of cesarean scar pregnancy. Medicine (Baltimore) 2015;94:e854. [DOI] [PMC free article] [PubMed] [Google Scholar]


