Abstract
Background:
The efficacy of oxaliplatin-based chemotherapy combined with anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) remains controversial in metastatic colorectal cancer (mCRC). This meta-analysis aims to estimate the effect of adding panitumumab or cetuximab to oxaliplatin-based chemotherapy in RAS wild type mCRC patients for the first-line treatment. The primary tumor location is also considered into this meta-analysis.
Methods:
RCT studies were identified by a search of MEDLINE, EMBASE, Cochrane library to October 2017, supplemented by manually retrieving ASCO, ESMO conference abstracts. The pooled hazard ratio (HR) for progression-free survival (PFS) and overall survival (OS), and pooled odds ratios (OR) for the overall response rate (ORR) were calculated by Review Manager 5.3.
Results:
The results indicated that the addition of anti-EGFR mAbs to FOLFOX regimen in RAS wild-type mCRC patients for the first-line treatment resulted in considerable improvements in PFS (HR = 0.70; 95% confidence interval [CI]: 0.59–0.82; P < .0001), OS (HR = 0.79; 95%CI: 0.67–0.92; P = .003), and ORR (OR = 2.56; 95% CI: 1.77–3.70; P < .00001) compared with chemotherapy alone. However, in RAS/BRAF wild patients, no significant differences were observed when anti-EGFR mAb was added to FLOX or XELOX regimen compared with chemotherapy alone with regard to OS and PFS, whereas FOLFOX+anti-EGFR mAb showed a marked superior OS and PFS (OS, HR = 0.77; 95% CI: 0.61–0.98; P = .03; PFS, HR = 0.68; 95% CI: 0.57–0.82; P < .00001). A meta-analysis including TAILOR and PRIME study suggests that primary tumor location (PTL) predicted a survival benefit when adding the EGFR antibody to FOLFOX regimen in RAS-wild mCRC patients (OS, HR for left-sided: 0.71; 95% CI: 0.59–0.85; P = .0002 and HR for right-sided: 0.90; 95% CI: 0.65–1.25; P = .53). However, the HR for PFS and ORR still suggests a benefit from the addition of anti-EGFR mAb in right-sided mCRC patients.
Conclusion:
So these results suggest anti-EGFR mAb and oxaliplatin are good partners in the FOLFOX regimen. The addition of EGFR antibody to FOLFOX markedly improved efficacy in RAS-wild patients with left-sided mCRC. In RAS/BRAF-wild patients, the efficacy is similar. For patients with right-sided tumor, a benefit showing a trendency in favor of anti-EGFR mAb can still seen. The molecular characteristics behind the tumor location need to be more explored urgently.
Keywords: anti-EGFR mAb, metastatic colorectal cancer, oxaliplatin-based chemotherapy, primary tumor location, RAS wild-type
1. Introduction
Colorectal cancer (CRC) is one of the most frequently diagnosed cancer in the world with >1.3 million new diagnoses and 694,000 deaths in 2012.[1] Both as monotherapy or in combination with chemotherapy, biological agents have been widely researched in metastatic colorectal cancer.[2–4] Inconsistent results from clinical trials have been supposed to involve the interaction with chemotherapy partners, including anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (mAb) and anti-angiogenesis inhibitors.[5–7]
Activating mutations in RAS except for the KRAS mutations is also considered to be the negative predictive biomarkers for EGFR antibodies. Based on the existing mutational and biochemical data, it's biologically plausible. More clinical data have also shown mutations in RAS predict a lack of benefit to panitumumab or cetuximab. In a updated analysis of the PRIME trial, in patients with mCRC and mutated RAS, panitumumab plus oxaliplatin-based regimens have no value.[8] BRAF V600E, which is typically exclusive of RAS mutations, is clearly predictive of poor prognosis in mCRC, but not insufficient to justify the exclusion of the EGFR antibodies, in patients with metastatic colorectal cancer.[9–12]
More and more evidences reveal tumors arising from different sides of the colon are molecularly and clinically distinct.[13–17] With the availability of genomic platforms capable of broadly surveying gene expression and methylation, 4 consensus molecular subtypes (CMSs) emerged.[18] CMS1, which is predominantly composed of right-sided CRCs, and enriched for MSI-high, CIMP-high, and BRAF mutation, are associated with worse survival. High tumor expression of AREG and EREG is linked to greater response rates and improved outcomes with anti-EGFR mAb in patients with RAS wild-type mCRCs[19,20] and left-sided CRCs have a significantly higher EREG and AREG expressions.[13,21,22] Differential distribution of these genomic CRC subtypes and other biologic features among right- and left-sided CRCs may contribute to the inferior prognosis of advanced-stage right-sided CRCs and an inferior outcome with anti-EGFR therapy in right-sided CRC.[23]
There are several randomized controlled clinical trials, which have shown confusing findings about whether the efficacy is improved by adding panitumumab or cetuximab to oxaliplatin-based regimens in KRAS wild mCRC.[24–28] Many scholars believe that the efficacy of the EGFR antibody combined with oxaliplatin-based regimen in the treatment of KRAS wild mCRC have been limited, and oxaliplatin may not be the appropriate compatible drug for the combined cetuximab. Some scholars also pointed out that oxaliplatin can strongly and continuously activate Src gene, making cetuximab can not play the desired anti-tumor effect, resulting in drug resistance.[29,30] However, the PRIME trial reveals that in mCRC patients without any RAS mutations, improvements were observed in overall survival by comparing panitumumab plus FOLFOX4 versus FOLFOX4. Considering the same mechanism binding of antibodies on EGFR that prevents the dimerization and the activation of EGFR, it's confused why there is a conflicting result.
The purpose of this study is to evaluate the efficacy of the addition of anti-EGFR mAb to oxaliplatin-based regimens in RAS wild type patients with metastatic colorectal cancer for the first-line treatment. The primary tumor location and BRAF status is considered.
2. Methods
2.1. Search strategy
Search is limited to randomized controlled trials. Medline, EMBASE, Cochrane library were searched using subject headings and key words including: metastatic colorectal cancer, mCRC, cetuximab, panitumumab, oxaliplatin, ras-wild, FOLFOX, XELOX, FLOX. The latest search was done on October, 2017. Further more, major oncological conferences in ASCO, ASCO GI, ESMO were searched manually. Relevant MeSH terms (Medical Subject Headings) were used where possible. The search is limited in English. Duplication and irrelevant studies were excluded.
2.2. Inclusion and exclusion criteria
In this meta-analysis, a study should meet the following criteria: only randomized clinical trials evaluating the oxaliplatin-based regimen with or without EGFR antibodies in the first-line treatment of RAS wild mCRC; a study should include the following information: ORR, PFS, OS. Case reports, reviews, cohort studies, and irrelevant articles were excluded. FOLFOXRI regimen is excluded due to the containing of the irinotecan.
From the results obtained, five randomized controlled trials evaluating the oxaliplatin-based regimens with or without EGFR antibodies in the first-line treatment of mCRC were selected for a meta-analytic evaluation.
2.3. Data extraction and objectives
The following characteristics were collected: first author, year of publication, chemotherapy regimens, number of patients, overall response rate (ORR), progression-free survival (PFS), overall survival (OS), follow-up period, RAS and BRAF status, primary tumor location. The primary objective of this meta-analysis was to analyze the addition of panitumumab or cetuximab to the oxaliplatin-based regimen in the first-line treatment of RAS-wild and RAS/BRAF wild mCRC. ORR, PFS, OS were considered where data are available. The impact of primary tumor location was considered into this meta-analysis. The splenic flexure was used to distinguish tumor sideness. All analyses were done according to previous published studies, so there are no patient consent and ethical approval required. We follow the guidelines by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement.
2.4. Statistical methods
All meta-analyses were carried out with Review Manager 5.3 (Nordic Cochrane Center, Copenhagen, Denmark). Time to event outcomes of PFS and OS were reported using HRs with random-effects model. For ORR, odds radio (OR) was also used with random-effects model. HRs >1 favored the anti-EGFR whereas HRs <1 favored the chemotherapy alone. ORs for ORR >1 reflected a higher overall response in the anti-EGFR mAb arm. Respective 95% CIs and P-values were presented in the forest plot. The heterogeneity among these studies was assessed by the chi-square and I-square test, which was defined as P < .1 or I2 >50%. If the heterogeneity was detected, possible explanation was explored.
Only 5 RCTs were included in this meta-analysis, so we didn’t perform a funnel plot to evaluate the publication bias. However, these are all high quality research, and we believe the result is stable to overcome the publication bias.
3. Results
3.1. Overview of the included trials
Five first-line trials including 7 articles met the inclusion criteria with usable informations through searching the related references and databases: PRIME (NCT00364013),[8,31] NORDIC VII study (NCT00145314),[32] TAILOR (NCT01228734),[33,34] COIN (ISRCTN27286448),[26] OPUS (NCT00125034)[35] (Table 1). They are all of high quality, and the efficacy of cetuximab or panitumumab was analyzed according to the RAS and BRAF status. The OS, PFS, ORR of these patients were extracted from 5 trials where available.
Table 1.
Source of patients for the analyses.
Three trials (TAILOR, PRIME, and OPUS) reported the outcome of differential treatments in RAS-wild patients by comparing FOLFOX plus anti-EGFR mAbs versus FOLFOX. Five trials evaluated the clinical outcomes in EGFR antibodies-treated mCRC for RAS/BRAF wild patients. In this group, a subgroup analysis could be performed according to the fluoropyrimidine regimens.
According to the PTL subgroup, 2 trials (TAILOR and PRIME) reported the outcome in the differential treatment arms (Table 2). A meta-analysis of PRIME and TAILOR study assessed the predictive role of PTL for anti-EGFR mAbs combined with FOLFOX were performed.
Table 2.
Treatment effects within subgroups defined by primary tumor location (PTL) in patients with RAS wild-type metastatic colorectal cancer.
3.2. Meta-analysis results
3.2.1. Anti-EGFR mAb improve the efficacy combined with FOLFOX
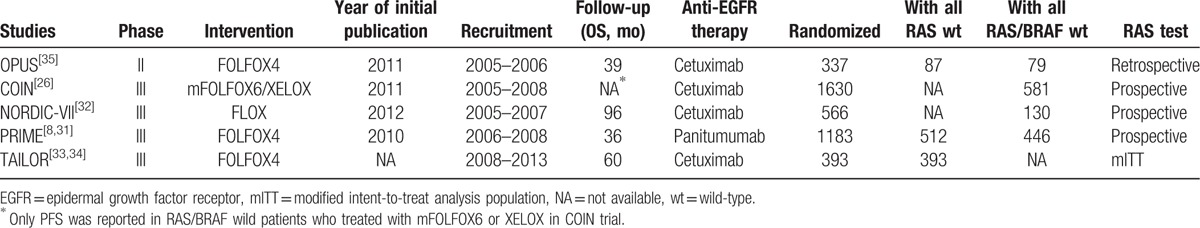
A total of 4109 patients were evaluated in the 5 trials, but the total number of patients included in this meta-analysis was 992 for RAS wild-type and 1236 for RAS/BRAF wild-type. The characteristics of these studies are shown in Table 1. The main result of our meta-analysis is the addition of EGFR antibody to oxaliplatin-based chemotherapy (FOLFOX) in RAS wild-mCRC patients for the first-line treatment lead to significant improvements in PFS (HR = 0.70; 95% CI, 0.59–0.82; P < .0001; Fig. 1A) and OS (HR = 0.79; 95% CI, 0.67–0.92; P = .003; Fig. 1B) compared with chemotherapy alone. The odds ratio for ORR also favored EGFR antibody therapy (OR = 2.56; 95% CI, 1.77–3.70; P < .0001, Fig. 1C).
Figure 1.
Forest plots for predictive analyses in trials comparing chemotherapy plus EGFR antibody therapy with chemotherapy alone in RAS-wild patients. (A) progression-free survival, (B) overall survival, and (C) objective response rate. CI = confidence interval, CT = chemotherapy, EGFR = epidermal growth factor receptor, HR = hazard ratio, OR = odds ratio.
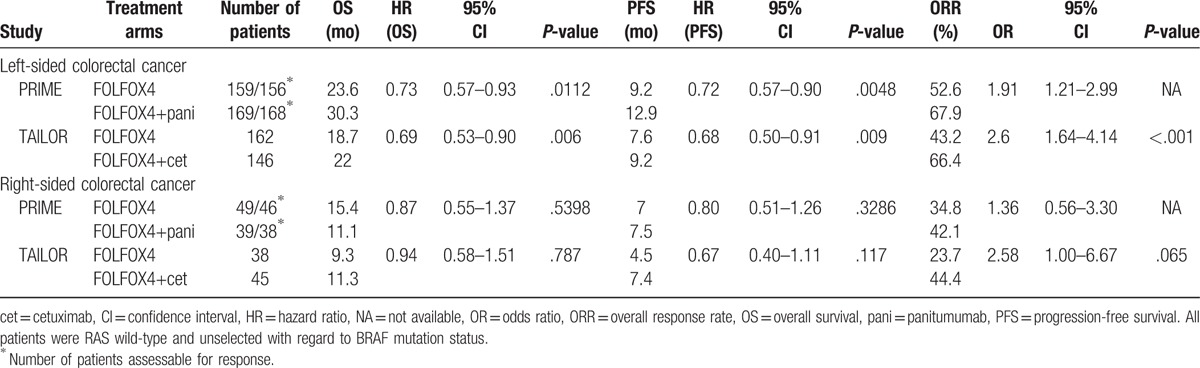
In RAS/BRAF-wild patients, a subgroup analysis of the type of fluoropyrimidine regimen was performed. The HR for PFS and OS were not significant when anti-EGFR mAbs were added to XELOX regimen (PFS, HR = 1.02; 95% CI, 0.82–1.26; P = .88, Fig. 2A) or FLOX (OS, HR = 1.07; 95% CI, 0.74–1.55; P = .72, Fig. 2B; PFS, HR = 1.06; 95% CI, 0.73–1.55; P = .75, Fig. 2A) compared with chemotherapy alone, while PFS and OS obviously improved with an FOLFOX regimen (OS, HR = 0.77; 95% CI, 0.61–0.98; P = .03; PFS, HR = 0.68; 95% CI, 0.57–0.82; P < .0001). There was significantly difference among the 3 subgroups (P = .009) when the PFS of EGFR antibodies plus chemotherapy versus chemotherapy alone was analyzed according to different fluoropyrimidine regimens.
Figure 2.
Forest plots for predictive analyses in trials comparing chemotherapy plus EGFR antibody therapy with chemotherapy alone in RAS/BRAF wild patients. (A) progression-free survival, (B) overall survival. CI = confidence interval, CT = chemotherapy, EGFR = epidermal growth factor receptor, HR = hazard ratio, OR = odds ratio.
3.2.2. Predictive implications of tumor location for anti-EGFR treatment
To evaluate the predictive implications of primary tumor location on differential treatment arms, a meta-analysis based on 2 trials could be performed (TAILOR and PRIME). This meta-analysis analyzed the treatment efficacy on PFS, OS, and ORR by comparing EGFR antibody plus FOLFOX with FOLFOX alone (Fig. 3). With regard to PFS, OS, and ORR, the analysis displayed a significant benefit from anti-EGFR mAb for RAS wild left-sided tumors in the first-line treatment. The HRs for PFS and ORR in right-sided tumor were also favorable of the anti-EGFR mAb+FOLFOX. There was no significant study heterogeneity for the 3 endpoints.
Figure 3.
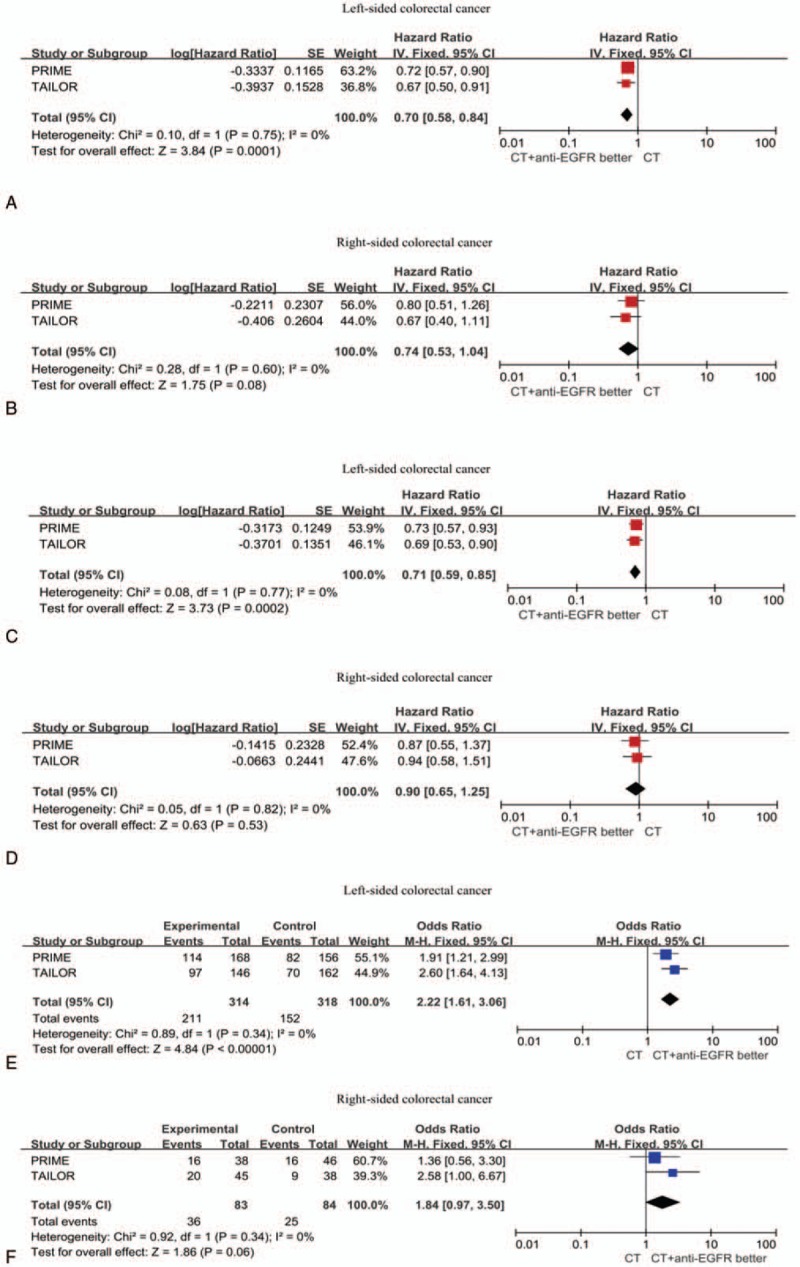
Forest plots showing hazard ratio (HR) for progression-free survival (PFS; A–B), overall survival (OS; C–D), and overall response rate (ORR; E–F) comparing FOLOFX plus anti-EGFR antibody with FOLFOX for the subgroups of left-sided and right-sided colorectal cancer.
4. Discussion
The presented analysis finds that the combination of Anti-EGFR mAb and FOLFOX supports a potential benefit for patients wild RAS-wild mCRC, compared with FOLFOX alone. In RAS/BRAF-wild mCRC, additional subgroup analysis was evaluated according to the type of fluoropyrimidine regimen (FOLFOX, XELOX, and FLOX) in RAS/BRAF wild mCRC. It became evident that the differences in PFS and OS were not significant when EGFR antibody was added to FLOX or XELOX regimen compared with chemotherapy alone, but PFS and OS were improved with FOLFOX treatment. It should, however, be noted that only 1 clinical trial evaluate the XELOX or FLOX plus cetuximab as compared with chemotherapy alone, respectively. Due to the limited clinical data, definitive conclusions cannot be drawn.
There have been 2 other recent meta-analyses evaluating anti-EGFR mAb with oxaliplatin-based chemotherapy regimens in KRAS-wild mCRC.[6,36] The first shows that no survival benefit was observed in KRAS wild mCRC patients in first-line treatment when adding panitumumab or cetuximab to oxaliplatin-based chemotherapy. The second meta-analysis, which included the same 4 trials as the first study, demonstrated that EGFR mAb combined with FOLFOX regimen as first-line treatment was associated with a significant improvement on PFS and OS in KRAS wild mCRC. However, it's still controversial with regard to this issue. The present meta-analysis adds to this by including updated ORR, PFS, OS data restricted to RAS-wild or RAS/BRAF wild mCRC and incorporating an extra data from TAILOR study.
In MRC COIN study, additional predictive factor analysis reveals improved PFS with cetuximab was noticed in fluorouracil-based subgroup while the capecitabine-based therapy shows a negative result. One explanation may be that increased toxicity from capecitabine-based regimen resulted in decreased dose intensity and impaired efficacy. Patients given capecitabine-based therapy (XELOX) in the COIN trial were treated with a shorter treatment duration for median 25 weeks, whereas 29 weeks in fluorouracil-based therapy group (FOLFOX).[26] The higher rate of adverse effects than expected led the reduction dose of capecitabine from 1000 to 850 mg/m2 Bid in a protocol amendment only for patients in XELOX plus cetuximab.[26] It is also particularly noted that in patients treated with XELOX plus cetuximab, 33% of patients reduced the oxaliplatin dose compared with 15% treated with XELOX alone.[37] In fact, some clinical studies demonstrated cetuximab plus XELOX is a effective and tolerable treatment regimen.[38–40]
In view of the result from NORDIC-VII, a FLOX regimen was used. There are no obvious explanations for the discrepant findings. Thus, a positive pharmacodynamic synergism may be existed between the EGFR antibody and fluoropyrimidine administered via the FOLFOX chemotherapy, which was not achieved through a bolus 5-FU regimen as the chemotherapy backbone. Lack of efficacy when cetuximab was added to FLOX strengthens the viewpoint that this combination may be not suitable and strongly implies FLOX regimen has a negative interaction with cetuximab.
Previous researches have suggested that tumor location has a prognostic role and predicts the efficacy of targeted therapy in mCRC patients. This is the first report evaluating the impact of tumor location on clinical outcomes for patients receiving FOLFOX plus EGFR antibody compared with FOLFOX alone. Data from 2 first-line randomized clinical trials (PRIME and TAILOR) were analyzed according to the tumor location. The evidence obviously demonstrates that benefits from EGFR antibody were markedly greater in left-sided tumors compared with those right-sided tumors, however the HR for PFS and the OR for ORR consistently suggested a benefit that presents a trendency in favor of anti-EGFR mAb.
Regarding the predictive role of tumor location on efficacy of EGFR antibodies, most recent data arises from first-line studies through comparing chemotherapy with either cetuximab or bevacizumab in RAS-wild mCRC. In CALGB 80405 clinical trial, clinical outcomes were consistently superior for PFS and OS in left-sided colorectal cancers compared with those right-sided colorectal cancers. Among the cetuximab group, left-sided mCRC was associated with better OS that reaches 25.7 months comparing with right-sided tumors with a statically significant difference (HR, 1.82, P < .001). Furthermore, in left-sided mCRC, FOLFOX plus cetuximab appears to be significantly superior to FOLFOX plus bevacizumab for OS. Conversely, right-sided mCRCs had better outcomes in bevacizumab-treated arm.[41] A meta-analysis conducted by Holch, which assessed the prognostic and predictive role of tumor location in patients with mCRC treated with first-line therapy, demonstrates primary tumor location has a prognostic value in mCRC. Furthermore, it supports the viewpoint that RAS-wild mCRC patients with left-sided tumors should be firstly treated with an EGFR antibody, and in right-sided tumors, bevacizumab-based treatment numerically associates with better survival and the benefits from standard therapy was limited. Interestingly, the contrary was found for ORR which favored anti-EGFR therapy.[42] Another meta-analysis also confirms the observation that anti-EGFR disease control expression signature was associated with left-sided tumor location, and RAS-wild mCRC patients with right-sided tumors might benefit from bevacizumab compared with panitumumab or cetuximab in terms of PFS, OS, but not for ORR.[43] We also notice the addition of cetuximab to FOLFIRI in RAS-wild right-sided patients was linked with non-significant numerical advantage with regard to PFS and ORR in the CRYSTAL trial.[44] This promotes the notion that patients with right-sided tumors might be preferentially treated with anti-EGFR mAb plus chemotherapy if the goal is to reduce the tumor size since the ORR was higher, which is important to increase the resectability of non-resectable liver metastases of colorectal cancer.
We acknowledge several limitations of the present investigation: first, the most studies are retrospective and exploratory. Second only 5 studies were included in this analysis and study-level data were utilized rather than patient-level data. Also, when considering the patients with left-sided and right-sided tumors separately, there are some small imbalances in baseline characteristics. Therefore, we ought to interpret the results with adequate caution.
5. Conclusion
In summary, FOLFOX plus anti-EGFR mAb is an effective first-line treatment for patients with RAS-wild left-sided mCRC. For patients with right-sided disease, it is not possible to draw definitive conclusions on optimal treatment based on the present analyses. Much more prospective clinical trials are required to confirm a potential subgroup of patients with right-sided mCRC who might benefit from anti-EGFR mAb.
Footnotes
Abbreviations: EGFR = epidermal growth factor receptor, mAbs = monoclonal antibodies, mCRC = metastatic colorectal cancer, ORR = overall response rate, OS = overall survival, PFS = progression-free survival, PTL= primary tumor location.
The authors report no conflicts of interest in this work.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–17. [DOI] [PubMed] [Google Scholar]
- [3].Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626–34. [DOI] [PubMed] [Google Scholar]
- [4].Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757–65. [DOI] [PubMed] [Google Scholar]
- [5].Vale CL, Tierney JF, Fisher D, et al. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev 2012;38:618–25. [DOI] [PubMed] [Google Scholar]
- [6].Zhou SW, Huang YY, Wei Y, et al. No survival benefit from adding cetuximab or panitumumab to oxaliplatin-based chemotherapy in the first-line treatment of metastatic colorectal cancer in KRAS wild type patients: a meta-analysis. PLoS One 2012;7:e50925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hurwitz HI, Tebbutt NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist 2013;18:1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34. [DOI] [PubMed] [Google Scholar]
- [9].Phipps AI, Buchanan DD, Makar KW, et al. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidemiol Biomarkers Prev 2012;21:1792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–9. [DOI] [PubMed] [Google Scholar]
- [11].Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol 2010;21:2396–402. [DOI] [PubMed] [Google Scholar]
- [12].Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466–75. [DOI] [PubMed] [Google Scholar]
- [13].Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995–2001. [DOI] [PubMed] [Google Scholar]
- [14].Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut 2012;61:794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012;61:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carethers JM. One colon lumen but two organs. Gastroenterology 2011;141:411–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 1990;113:779–88. [DOI] [PubMed] [Google Scholar]
- [18].Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jacobs B, De Roock W, Piessevaux H, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2009;27:5068–74. [DOI] [PubMed] [Google Scholar]
- [20].Seligmann JF, Elliott F, Richman SD, et al. Combined epiregulin and amphiregulin expression levels as a predictive biomarker for panitumumab therapy benefit or lack of benefit in patients with RAS wild-type advanced colorectal cancer. JAMA Oncol 2016;2:633–42. [DOI] [PubMed] [Google Scholar]
- [21].Lee MS, McGuffey EJ, Morris JS, et al. Association of CpG island methylator phenotype and EREG/AREG methylation and expression in colorectal cancer. Br J Cancer 2016;114:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Adams RA, Fisher D, Farragher S, et al. Use of epiregulin (EREG) and amphiregulin (AREG) gene expression to predict response to cetuximab (cet) in combination with oxaliplatin (Ox) and 5FU in the first-line treatment of advanced colorectal cancer (aCRC) [abstract]. J Clin Oncol 2012;30(Suppl): Abstract 32. [Google Scholar]
- [23].Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw 2017;15:411–9. [DOI] [PubMed] [Google Scholar]
- [24].Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705. [DOI] [PubMed] [Google Scholar]
- [25].Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011;22:1535–46. [DOI] [PubMed] [Google Scholar]
- [26].Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755–62. [DOI] [PubMed] [Google Scholar]
- [28].Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663–71. [DOI] [PubMed] [Google Scholar]
- [29].Kopetz S, Lesslie DP, Dallas NA, et al. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res 2009;69:3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dahan L, Sadok A, Formento JL, et al. Modulation of cellular redox state underlies antagonism between oxaliplatin and cetuximab in human colorectal cancer cell lines. Br J Pharmacol 2009;158:610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Boeckx N, Koukakis R, Op de Beeck K, et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol 2017;28:1862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guren TK, Thomsen M, Kure EH, et al. Cetuximab in treatment of metastatic colorectal cancer: final survival analyses and extended RAS data from the NORDIC-VII study. Br J Cancer 2017;116:1271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Qin S, Xu J, Wang L, et al. Impact of tumor epidermal growth factor receptor (EGFR) status on the outcomes of first-line FOLFOX-4 ± cetuximab in patients (pts) with RAS-wild-type (wt) metastatic colorectal cancer (mCRC) in the randomized phase 3 TAILOR trial. Ann Oncol 2016;27:527. [Google Scholar]
- [34].Qin S, Xu J, Wang L, et al. Impact of primary tumor location (TL) on outcomes of first-line (1L) FOLFOX-4 (F) ± cetuximab (cet) in patients (pts) with RAS wild-type (wt) metastatic colorectal cancer (mCRC) in the phase 3 TAILOR trial. ASCO-GI 2017 abstr 683; 2017. [Google Scholar]
- [35].Bokemeyer C, Köhne CH, Ciardiello F, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer 2015;51:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wen F, Tang R, Sang Y, et al. Which is false: oxaliplatin or fluoropyrimidine? An analysis of patients with KRAS wild-type metastatic colorectal cancer treated with first-line epidermal growth factor receptor monoclonal antibody. Cancer Sci 2013;104:1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Adams RA, Meade AM, Madi A, et al. Toxicity associated with combination oxaliplatin plus fluoropyrimidine with or without cetuximab in the MRC COIN trial experience. Br J Cancer 2009;100:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Soda H, Maeda H, Hasegawa J, et al. Multicenter Phase II study of FOLFOX or biweekly XELOX and Erbitux (cetuximab) as first-line therapy in patients with wild-type KRAS/BRAF metastatic colorectal cancer: the FLEET study. BMC Cancer 2015;15:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Borner M, Koeberle D, Von Moos R, et al. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol 2008;19:1288–92. [DOI] [PubMed] [Google Scholar]
- [40].Hazama S, Maeda H, Iwamoto S, et al. A phase II study of XELOX and Cetuximab as first-line therapy in patients with KRAS wild type metastatic colorectal cancer (FLEET2 Study). Clin Colorectal Cancer 2016;15:329–36. [DOI] [PubMed] [Google Scholar]
- [41].Venook A, Niedzwiecki D, Innocenti F, et al. Impact of primary tumor location on overall suvival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016;34(Suppl): Abstract 3504. [Google Scholar]
- [42].Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 2017;70:87–98. [DOI] [PubMed] [Google Scholar]
- [43].Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 2017;3:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]


