Abstract
The preservation of pituitary stalk during surgery is very important for neurosurgeons. Sometimes, it is hard to identify the pituitary stalk in the operation. The hypothalamo-hypophyseal tract (HHT) projects through the pituitary stalk to the posterior pituitary gland. If the HHT can be identified, the position of pituitary stalk will be visualized. The diffusion tensor imaging (DTI) fiber tracking technique has been widely used for the quantitative assessment of the white matter integrity and thus may be suitable for the evaluation of the HHT.
DTI was used to track the HHT in 11 patients with pituitary adenoma, and the location of the tract was compared with the pituitary stalk of postoperative image in those patients.
The fiber tracking and 3D visualization of the HHT were successfully carried out in all 11 patients. Comparison between the tract and pituitary stalk of postoperative magnetic resonance imaging (MRI) was carried out in 9 patients. The results revealed that the position of tract was consistent with the pituitary stalk of postoperative MRI image in 8 patients (88.9%). The properties of tract showed that the median number of tract was 5.18 ± 7.00, the median fractional anisotropy (FA) was 0.14 ± 0.04, and the median length was 28.81 ± 7.94 mm.
HHT can be tracked and visualized with the DTI-FT technique. It will be helpful to identify the location of pituitary stalk preoperatively.
Keywords: diffusion tensor imaging, fiber tracking, hypothalamo-hypophyseal tract, pituitary adenoma, pituitary stalk
1. Introduction
The pituitary stalk (PS) is a very important structure, which connects the hypothalamus and the pituitary gland. With a crucial role in endocrine function and water–electrolyte equilibrium, the preservation of PS during surgery is a significant issue for neurosurgeons, because the PS resection may induce endocrine dysfunction, water–electrolyte imbalance, diabetes insipidus (DI), and other clinical manifestations. However, under a pathological state, it is difficult to identify the PS due to the distortion of its shape, displacement, or incorporation within the tumor. The PS lesions included neoplastic, inflammatory, congenital anomalies, and unclear etiology.[1] DI and anterior pituitary hormone deficit are the most common symptom. Magnetic resonance imaging (MRI) plays the key role in the diagnosis of PS lesions, however, some remain confirmed by pathology. There were no statistically significant correlations between hypopituitarism and the pattern of enhancement or size of the lesion.[1]
The pituitary locates in the sella turcica. The hypothalamus and the posterior pituitary was connected by the PS. The floor of the third ventricle extends downward into the infundibulum, where the apex of the pituitary is attached. The PS goes through the dura mater of the diaphragma sellae while it brings axons from the hypothalamus down to the posterior pituitary. The HHT produced by paraventricular (PVN) and the supraoptic nuclei (SON) projects through the PS to the posterior pituitary gland. If the HHT is visualized, the position of PS will be identified. Among numerous advanced MR techniques, diffusion tensor imaging (DTI) is an optimal technique for the quantitative assessment of the white matter integrity and thus may be suitable for the evaluation of the HHT. DTI might represent a promising technique to depict the position of the PS. DTI parameters commonly include fractional anisotropy (FA) and mean diffusivity, and FA value reflects the degree of alignment of cellular structures and their structural integrity.[2] So far, DTI has been widely applied to various structures such as the cranial nerve,[3] pyramidal tract,[4] arcuate fasciculus,[5] but also many types of brain diseases including multiple sclerosis,[6] neuromyelitis optica,[7] Parkinson disease,[8] and schizophrenia.[9] However, there is no report about the DTI methods on the assessment of HHT so far.
In this study, DTI was used to assess the HHT in patients with pituitary adenoma, and the location of the tracked HHT was compared with the PS of postoperative image in those patients.
2. Materials and methods
2.1. Patient
From May 1, 2016, to July 30, 2016, totally, 11 patients with pituitary adenoma were enrolled in this study. All patients were diagnosed as pituitary adenoma according to the following criteria. MRI showed the intra and superior sellar lesion, and no obvious PS was visualized. The laboratory examination revealed the abnormal or normal endocrine level. The symptom presented with impairment of visual acuity and headache. All patients accepted the standard procedure of endoscopic endonasal approach surgery. This research was approved by the Ethics Committee of Hospital. The main demographic and clinical characteristics of the patients are listed in Table 1.
Table 1.
Main demographic and clinical characteristics of the 11 patients.
2.2. Data acquisition
Images were acquired preoperatively and postoperatively on a 1.5-T MR system (Espree; Siemens Medical Solution, Erlangen, Germany) in the diagnostic room of iMRI brain suite, which was described in detail previously.[10] The protocol and parameters are listed in Table 2.
Table 2.
MRI parameters.
2.3. Data processing
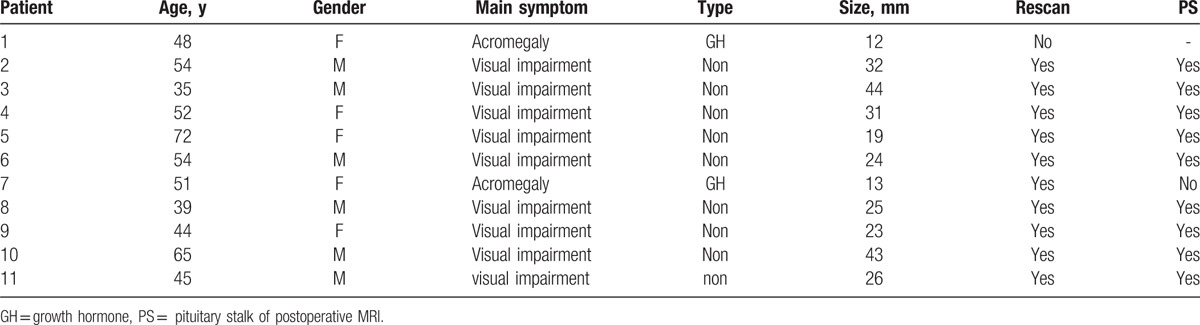
We delineated the tract with the “fibre tracking” module of iPlan3.0 software (BrainLAB, Feldkirchen, Germany), after various image sets were loaded and registered. The tractography was acquired with some regions of interest (ROI): The first ROI was placed between the visual chiasm and base of tumor, and the other was placed between the hypothalamus and upper part of tumor (Fig. 1). The tracking parameters were listed: FA threshold value, 0.02; angle threshold, 30°; shortest fiber bundle length, 5 mm. We used the tracking parameters to track the tracts across the 2 ROIs simultaneously. Tracts were appropriately trimmed on the basis of the anatomical knowledge of the operator, because the tracts often appear as mixed redundant tracts. The reconstruction of 3D image of tumors was based on the T1 images. The contour of tumor and tracts were fused to delineate the course of the tracts in axial, coronal plane. Sometimes, the color of tracts was not clear in the image, so the contour of tract was delineated in the “3D Fiber objects” module to make it visualized apparently. The properties of tracts were acquired through the module “plan content,” including the numbers of tracts, the minimum FA, maximum FA, average FA, minimum length, maximum length, and average length. Tumor contour was delineated according to the anatomical images in the “object creation” module. After selecting the appropriate border, a 3-D object was generated automatically by wrapping each structure with a contour. When the tumor contour and tracts contour was fused, the relation between tumor and tracts was visualized clearly.
Figure 1.
HHT visualization in a patient with pituitary adenoma. (A) The contour of head with 2 ROIs and HHT. (B) The yellow contour of adenoma in axial plane. (C) Two yellow plane show 2 ROIs masks. (D) HHT after proper pruning.
3. Results
The median patient age was 50.8 ± 10.2 years. Among the participants (6 male and 5 female patients), the clinical main symptoms included decreased visual acuity (9 patients), headache (5 patients), acromegaly (2 patients). The disease course ranged from 1 month to 3 years (median 1.4 years). The tumor size: 10–20 mm (3 patients), 20–30 mm (4 patients), 30–40 mm (2 patients), 40–50 mm (2 patients) (Table 2).
All 11 patients underwent total resection under the endoscopic surgery. After operation, 10 patients got rescan of MRI. The PS were visualized in 9 patients (Table 1). For further comparison with the tracts, the axil plane and coronal plane were used to confirm the location of PS.
The preoperative MRI data for the 11 patients were processed using iPlan3.0 software. The fiber tracking and 3D visualization of the tracts were carried out by 1 experienced neurosurgeon. The neural fasciculus courses that simultaneously passed through 2 ROIs were successfully tracked and reconstructed in all patients. Comparison validations between the tracts and PS of postoperative MRI image were carried out in 9 patients. The results revealed that the HHT visualization results were consistent with the PS in 8 patients (88.9%). Three cases are presented with pictures in Figs. 2 to 4. Among the 8 cases, the HHT was located on the left side (n = 5) (Figs. 2), middle position (n = 1) (Fig. 3), and right side (n = 2) (Fig. 4).
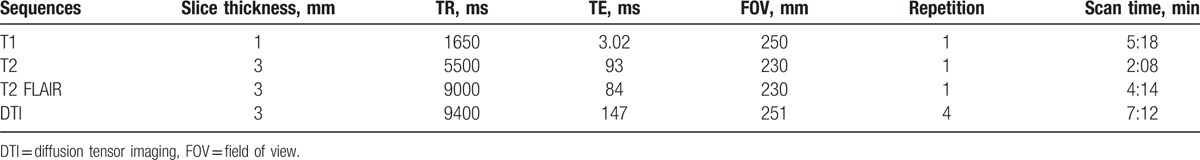
Figure 2.

HHT visualization and the postoperative observations of pituitary stalk. The tracts and pituitary stalk are located in the left. (A) Tracts (blue fibers) visualization with the adenoma. (B) Red contour of the tracts and yellow contour of the adenoma. (C) The green translucent part shows a 3D anatomical model of the tumor, and red part shows the contour of tracts. (D) The image of postoperative pituitary stalk.
Figure 4.
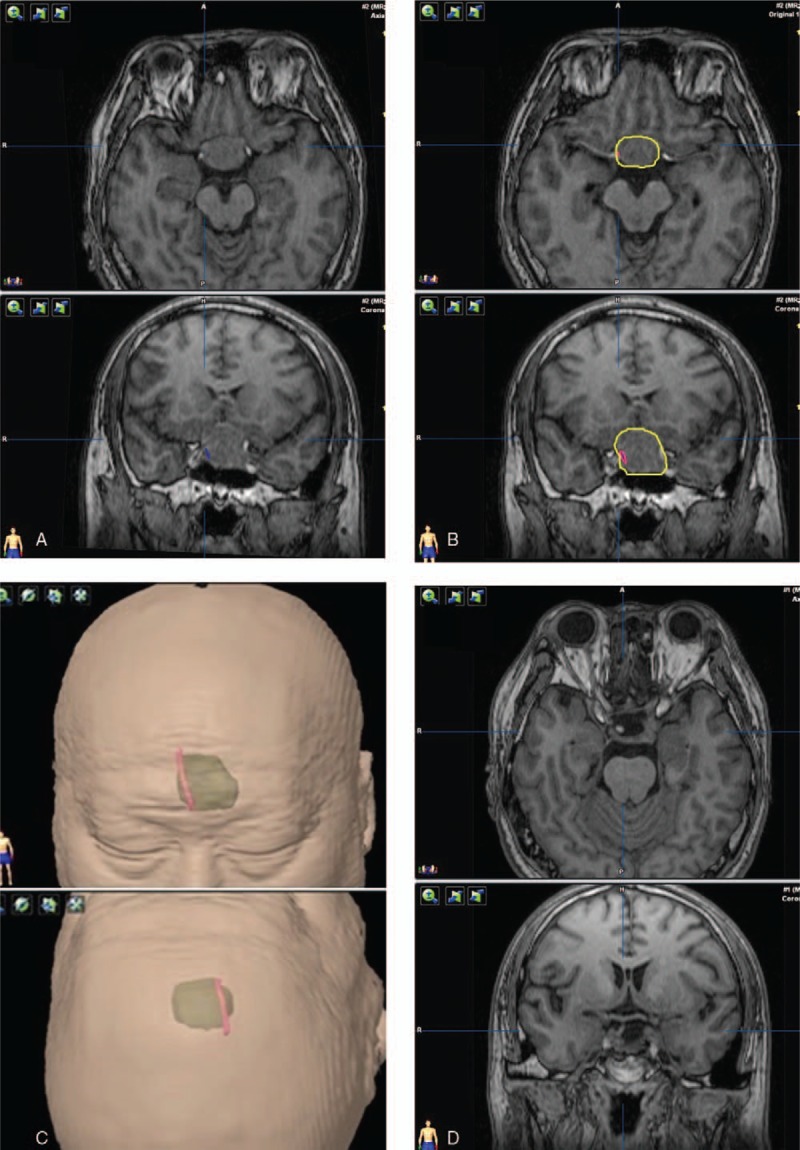
HHT visualization and the postoperative observations of pituitary stalk. The tracts and pituitary stalk are located in the right portion of the tumor. (A) Tracts (blue fibers) visualization with the adenoma. (B) Red contour of the tracts and yellow contour of the adenoma. (C) The green translucent part shows a 3D anatomical model of the tumor, and red part shows the contour of tracts. (D) The image of postoperative pituitary stalk.
Figure 3.
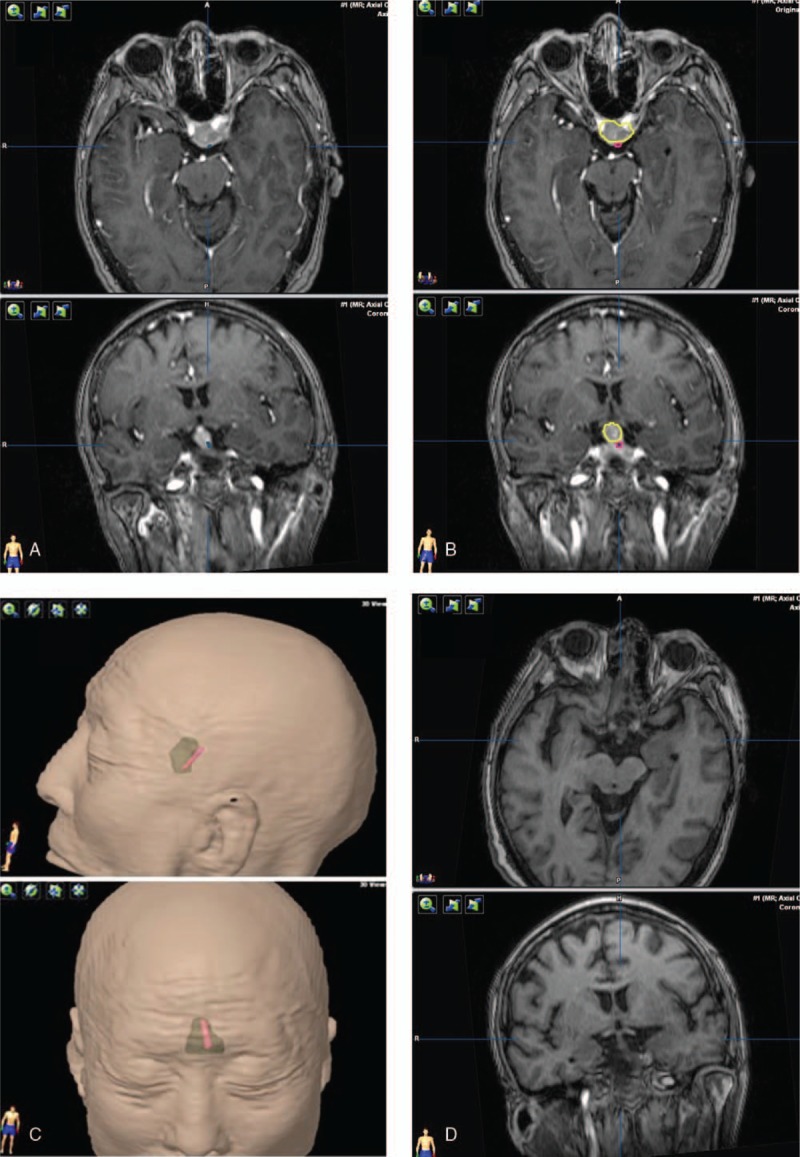
HHT visualization and the postoperative observations of pituitary stalk. The tracts and pituitary stalk are located in the middle portion of the tumor. (A) Tracts (blue fibers) visualization with the adenoma. (B) Red contour of the tracts and yellow contour of the adenoma. (C) The green translucent part shows a 3D anatomical model of the tumor, and red part shows the contour of tracts. (D) The image of postoperative pituitary stalk.
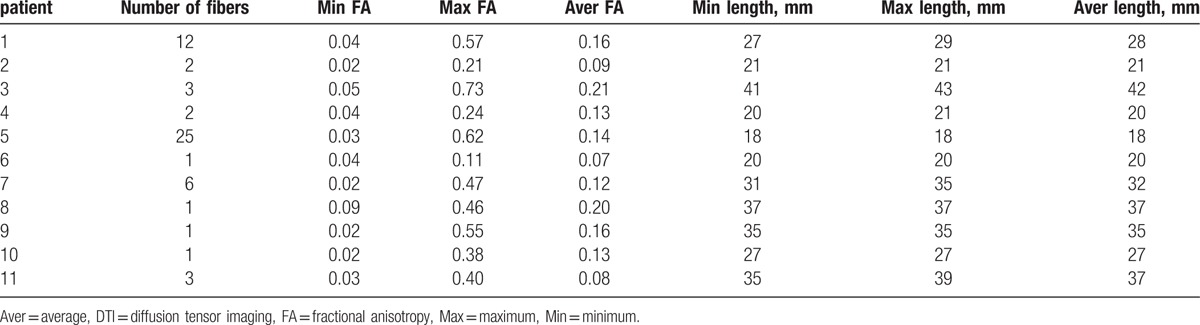
The properties of tracts were listed (Table 3). The median number of tracts was 5.18 ± 7.00 (from 1 to 25), the median FA (average FA) was 0.14 ± 0.04 (from 0.08 to 0.21), and the median length (average length) was 28.81 ± 7.94 mm (from 20 to 42 mm) (Table 3).
Table 3.
Properties of fiber with DTI source in patients with pituitary adenoma (B = 1000 s/mm2).
4. Discussion
The oxytocin (OT) and antidiuretic hormone (ADH) synthesized by the PVN and the SON of the hypothalamus were transported down to the posterior lobe by HHT. Landgraf et al[11] first assumed that the OT and vasopressin was released from nerve terminals in the brain. Morris and Pow[12] showed that OT and ADH could be released from all of the compartments of magnocellular neurons, not just the nerve terminals. Ludwig[13] revealed that release of OT and ADH was from the soma and dendrites, not from nerve terminals. The ADH targets kidneys to enhance water retention, reduce urine, and also functions as a neurotransmitter. The OT functions as labor contractions, lactation, other possible role in sperm transport, emotional bonding.
The HHT is very important for the transport of the OT and ADH. It is hard to detect the HHT with normal technique in vivo. Among advanced MR techniques, DTI has been used to quantitatively assess the white matter integrity and applied to various structures such as the cranial verves, and some important tracts.
For the first time, we make the visualization of human HHT tracts in vivo. The results showed that fiber tracking was successfully performed in all patients. We found that the tracts were consistent with the postoperative view of the PS in 8 patients (88.9%). The possible reasons for this were the FA value of the tumor's substantial part, and low FA threshold value. Consequently, some nonfiber bundle structures were incorrectly tracked, which resulted in the deviation in the visualization course of the tracts. In further study, the problem may be reduced by selecting more accurate ROIs and adjusting the FA threshold value.[14] In the current study, lack of adequate spatial resolution and poor signal-to-noise ratio (SNR) remained the major limitation of the DTI technique.[15] The use of large voxel sizes increased partial volume averaging and contamination from neighboring fiber pathways. Low SNR resulted in overestimation of anisotropy and increased errors in eigenvector estimation, which led to inaccurate white matter connections and false-positive results.[16] Applying higher spatial resolution reduced crossing fibers and susceptibility artifacts encountered in DTI studies,[17] which would help avoiding false-positive or false-negative results and reveal the delicate structure of the tracts. In addition, the tumor characteristics might have an effect on the DTI results. In a big volume tumor, the PS was compressed and stretched and adhered closely to the tumor wall, sometimes may partially fused with the tumor wall. Taoka et al[18] reported that the cystic tumor may affect the tracking of the fasciculus. However, Gerganov et al[19] showed that no important differences were found between DTI results and the tumor characteristics.
The PS plays a vital role in postoperative endocrine function. Patients with preserved PS exhibit less endocrine dysfunction postoperatively. So, it is important to visualize and preserve this structure during microsurgery. Even partial preservation of the PS is important for the production of ADH postoperatively. Accurate identification of the neurohypophysis and the PS as well as adenohypophysis during surgery contributes to pituitary-conserving operations.[20,21] The PS is easily identifiable under normal conditions. However, under a pathological state, it is difficult to identify due to the distortion of its shape, displacement, or incorporation within the tumor. In this study, we found that the tracts were consistent with the postoperative image of PS by 88.9%. So, it will be helpful to preserve the PS if the information of location of HHT by DTI technique was acquired preoperatively. Clinically, our results have the potential to be combined with intraoperative navigation to identify the PS preoperatively and protect the PS during surgery.
The numbers, FA values and length of tracts were acquired in this study. The properties of the tracts in 11 cases were demonstrated. The tracts number was relatively low, only 1 fiber was found in 4 patients, 2 in 2 patients, 3 in 2 patients, 6 in 1 patient, 12 in 1 patient, 25 in 1 patient. The study presented that 3 cases with smaller tumor size (10–20 mm) had more tracts (from 6 to 25) than others, and 2 cases with larger tumors (> 4 mm) have 1 and 3 tracts separately. On the basis of this result, it seemed that the number of the tracts and the size of the tumor inversely correlated. But conflicted results were demonstrated in other cases: 2 cases (30–40 mm) had 2 fibers, and 3 cases (20–30 mm) only had 1 fiber. The reasons may be complicated. In the fiber-tracking algorithm, fiber tracking could be compromised because the infiltrating tumor may damage the HHT. The interference of cerebrospinal fluid (CSF) would affect the resolution of the DTI signals and significantly decreased the tracts number. The results demonstrated that the median FA (average FA) was 0.14 ± 0.04 (from 0.08 to 0.21), which was lower than some cranial nerve[17,22] and other white matter tracts.[23–25] The reasons may be that the HHT was unmyelinated fiber,[26–28] and the compression of tumor may cause low FA value.[22] The median length (average length) of HHT was 28.81 ± 7.94 mm (from 20 to 42 mm), longer than the normal length of PS.[29] The results may be reasonable, because in normal situation, the HHT originated from hypothalamus went through the PS to the posterior lobe, and the length was longer than the PS. Under a pathological state, the tumor may stretch the HHT. The study of properties of HHT may certainly advance our understanding of the pathogenesis of various disease processes affecting the PS in future.
Several limitations should be considered in this study. The number of cases in the current study is too small to arrive at a more comprehensive quantitative assessment of the effects of tumor size, gender, and age. Thus, further studies are required to establish the relationship between DTI tractography and the tumor characteristics. More investigations will be required to determine the statistical significance for clinical applications.
5. Conclusion
This study showed for the first time the feasibility of tracing the HHT in pituitary adenomas. It will be helpful to identify the location of PS preoperatively. The properties of tracked HHT will lead to more understanding of the HHT.
Footnotes
Abbreviations: ADH = antidiuretic hormone, Aver = average, CSF = cerebrospinal fluid, DI = diabetes insipidus, DTI-FT = diffusion tensor imaging–based fiber tracking, FA = fractional anisotropy, GH = growth hormone, HHT = hypothalamo-hypophyseal tract, iMRI = intraoperative magnetic resonance imaging, Max = maximum, Min = minimum, MRI = magnetic resonance imaging, OT = oxytocin, PS = pituitary stalk, PVN = paraventricular, ROI = regions of interest, SNR = signal-to-noise ratio, SON = supraoptic nuclei.
FW, JZ, and PW contributed equally in this study.
The authors have nothing to disclose.
For this type of study, formal consent is not required.
The authors have no conflict of interest.
References
- [1].Turcu AF, Erickson BJ, Lin E, et al. Pituitary stalk lesions: the Mayo Clinic experience. J Clin Endocrinol Metab 2013;98:1812–8. [DOI] [PubMed] [Google Scholar]
- [2].El-Serougy L, Abdel Razek AA, Ezzat A, et al. Assessment of diffusion tensor imaging metrics in differentiating low-grade from high-grade gliomas. Neuroradiol J 2016;29:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Borkar SA, Garg A, Mankotia DS, et al. Prediction of facial nerve position in large vestibular schwannomas using diffusion tensor imaging tractography and its intraoperative correlation. Neurol India 2016;64:965–70. [DOI] [PubMed] [Google Scholar]
- [4].George E, Heier L, Kovanlikaya I, et al. Diffusion tensor imaging of pyramidal tract reorganization after pediatric stroke. Childs Nerv Syst 2014;30:1135–9. [DOI] [PubMed] [Google Scholar]
- [5].Psomiades M, Fonteneau C, Mondino M, et al. Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: a DTI-tractography study. Neuroimage Clin 2016;12:970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ontaneda D, Sakaie K, Lin J, et al. Identifying the start of multiple sclerosis injury: a serial DTI study. J Neuroimaging 2014;24:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen Z, Lou X, Liu M, et al. Assessment of optic nerve impairment in patients with neuromyelitis optica by MR diffusion tensor imaging. PLoS One 2015;10:e0126574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zheng Z1, Shemmassian S, Wijekoon C, et al. DTI correlates of distinct cognitive impairments in Parkinson's disease. Hum Brain Mapp 2014;35:1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wagner G, De la Cruz F, Schachtzabel C, et al. Structural and functional dysconnectivity of the fronto-thalamic system in schizophrenia: a DCM-DTI study. Cortex 2015;66:35–45. [DOI] [PubMed] [Google Scholar]
- [10].Chen X, Xu BN, Meng X, et al. Dual-room 1.5-T intraoperative magnetic resonance imaging suite with a movable magnet: implementation and preliminary experience. Neurosurg Rev 2012;35:95–109. [DOI] [PubMed] [Google Scholar]
- [11].Landgraf R1, Neumann I, Russell JA, et al. Push–pull perfusion and microdialysis studies of central oxytocin and vasopressin release in freely moving rats during pregnancy, parturition, and lactation. Ann N Y Acad Sci 1992;652:326–39. [DOI] [PubMed] [Google Scholar]
- [12].Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Rec 1991;231:437–45. [DOI] [PubMed] [Google Scholar]
- [13].Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol 1998;10:881–95. [DOI] [PubMed] [Google Scholar]
- [14].Song F, Hou Y, Sun G, et al. In vivo visualization of the facial nerve in patients with acoustic neuroma using diffusion tensor imaging–based fiber tracking. J Neurosurg 2016;125:787–94. [DOI] [PubMed] [Google Scholar]
- [15].Salmela MB1, Cauley KA, Nickerson JP, et al. Magnetic resonance diffusion tensor imaging (MRDTI) and tractography in children with septo-optic dysplasia. Pediatr Radiol 2010;40:708–13. [DOI] [PubMed] [Google Scholar]
- [16].Hasan KM1, Kamali A, Kramer LA. Mapping the human brain white matter tracts relative to cortical and deep gray matter using diffusion tensor imaging at high spatial resolution. Magn Reson Imaging 2009;27:631–6. [DOI] [PubMed] [Google Scholar]
- [17].Kamali A, Hasan KM, Adapa P, et al. Distinguishing and quantification of the human visual pathways using high spatial resolution diffusion tensor tractography. Magn Reson Imaging 2014;32:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taoka T1, Hirabayashi H, Nakagawa H, et al. Displacement of the facial nerve course by vestibular schwannoma: preoperative visualization using diffusion tensor tractography. J Magn Reson Imaging 2006;24:1005–10. [DOI] [PubMed] [Google Scholar]
- [19].Gerganov VM, Giordano M, Samii M, et al. Diffusion tensor imaging-based fiber tracking for prediction of the position of the facial nerve in relation to large vestibular schwannomas. J Neurosurg 2011;115:1087–93. [DOI] [PubMed] [Google Scholar]
- [20].Xiao G, Yuan X, Yuan J, et al. Pituitary stalk management during the microsurgery of craniopharyngiomas. Exp Ther Med 2014;7:1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yoneoka Y, Watanabe N, Okada M, et al. Observation of the neurohypophysis, pituitary stalk, and adenohypophysis during endoscopic pituitary surgery: demonstrative findings as clues to pituitary-conserving surgery. Acta Neurochir (Wien) 2013;155:1049–55. [DOI] [PubMed] [Google Scholar]
- [22].Chen F, Chen L, Li W, et al. Pre-operative declining proportion of fractional anisotropy of trigeminal nerve is correlated with the outcome of micro-vascular decompression surgery. BMC Neurol 2016;16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yao Y, Ulrich NH, Guggenberger R, et al. Quantification of corticospinal tracts with diffusion tensor imaging in brainstem surgery: prognostic value in 14 consecutive cases at 3T magnetic resonance imaging. World Neurosurg 2015;83:1006–14. [DOI] [PubMed] [Google Scholar]
- [24].Banaszek A, Bladowska J, Pokryszko-Dragan A, et al. Evaluation of the degradation of the selected projectile, commissural and association white matter tracts within normal appearing white matter in patients with multiple sclerosis using diffusion tensor MR imaging: a preliminary study. Pol J Radiol 2015;80:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ivanova MV, Isaev DY, Dragoy OV, et al. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex 2016;85:165–81. [DOI] [PubMed] [Google Scholar]
- [26].Harris GW, Manabe Y, Ruf KB. A study of the parameters of electrical stimulation of unmyelinated fibres in the pituitary stalk. J Physiol 1969;203:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stone BS, Zhang J, Mack DW, et al. Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann Neurol 2008;64:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Haller S, Xekardaki A, Delaloye C, et al. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. Psychiatry Neurosci 2011;36:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Satogami N, Miki Y, Koyama T, et al. Normal pituitary stalk: high-resolution MR imaging at 3T. AJNR Am J Neuroradiol 2010;31:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


