Abstract
The present study was aimed to investigate the relationship between the expression of collagen type V alpha 2 chain (COL5A2) and clinical outcomes of patients with bladder cancer.
Chi-square test and log-rank-based survival analysis were performed to assess the correlation of COL5A2 expression with clinical characteristics and survivals of patients with bladder cancer using GSE13507. Gene set enrichment analysis was conducted to study the relevant mechanisms.
Bladder cancer patients in COL5A2 low expression group were associated with better invasiveness (P < .0001), tumor grade (P = .001), T staging (P < .0001), N staging (P = .002), cancer specific survival (P < .0001), overall survival (P < .0001), and a trend of better M staging (P = .053) than those in COL5A2 high expression group.COL5A2 might affect the progression of bladder cancer through “Coagulation,” “Hypoxia,” “Apical junction,” “Ultraviolet response,” “Epithelial mesenchymal transition,” “Angiogenesis,” “KRAS (KRAS proto-oncogene, GTPase) signaling,”“Complement,”“IL2-STAT5-signaling,” “Inflammatory response,” “IL6-JAK-STAT3-signaling,” “Myogenesis,” “TNF α signaling,” “Apoptosis,” and “Hedgehog-signaling.”
Our results demonstrated that COL5A2 was correlated with poor clinical outcomes and survivals of patients with bladder cancer, suggesting that it could be regarded as a biomarker of bladder cancer.
Keywords: bladder cancer, clinical significance, COL5A2
1. Introduction
Bladder cancer (BC) is one of the most common malignances, with nearly 74,000 newly diagnosed cases each year in the world.[1,2] In China, BC represents the most frequent cancer of genitourinary system in males, which ranks the 8th in all cancers.[3] Meanwhile, with the development of novel detection and diagnosis options as well as the aging population, the incidence of BC has been increasing year by year.[4] Nevertheless, the management options and the corresponding effectiveness for BC are limited. For patients with superficial BC, which accounts for 70% of BC, transurethral resection of the bladder tumor (TURBT) is regarded as the preferred surgical option, while 45% to 80% of patients with superficial BC may undergo disease relapse and progression.[5,6] For patients with muscle invasive BC, radical cystectomy and TURBT followed by concurrent radiation therapy and systemic chemotherapy (trimodality therapy) are recommended, while the trimodality therapy has the shortcoming of large injure, resulting in poor quality of life and 5 year survival rate of BC patients.[6,7] Thus, it is necessary to develop novel management options for patients with BC.
COL5A2, also known as collagen type V alpha 2 chain, has previously been reported to be involved in the pathological process of colorectal cancer,[8] adenomas,[9] breast cancer,[10] and osteosarcoma,[11] etc. However, these studies did not analyze the relationship the COL5A2 expression and clinical outcomes of patients with corresponding cancers, and no studies investigated the clinical relevance of COL5A2 with BC. In the present study, we therefore analyzed the relationship between the expression of COL5A2 and clinical pathological features of patients with BC.
2. Methods
2.1. Microarray studies
Gene expression omnibus (GEO) BC microarray studies GSE13507[12,13] and GSE31684[14,15] were applied to complete correlation analysis and investigate molecular mechanisms. GSE13507, proposed by Kim et al, was annotated with Illumina human-6 v2.0 expression beadchip, containing 165 primary bladder cancer samples obtained from patients with benign disease, and the raw data of GSE13507 was preprocessed using quantile normalization and log 2 transformation. Besides, the detailed clinical information of BC patients was also attached to GSE13507. GSE31684, contributed by Riester et al, was annotated with Affymetrix Human Genome U133 Plus 2.0 Array including 92 BC samples, and the raw data of GSE31684 was preprocessed using gcRMA method.
2.2. Statistical analysis
We categorized BC samples into COL5A2 low expression group and COL5A2 high expression group based on the median of the COL5A2 expression of these 2 microarray studies. The strength of relationship between the expression of COL5A2 and the clinical features (age, gender, TNM staging, tumor grade, disease recurrence, disease progression, and invasiveness) of BC patients was estimated in GSE13507 using Chi-square analysis. Next, log-rank based survival analysis was conducted to compare cancer specific survival and overall survival of patients in GSE13507. P value < .05 was considered statistically significant for both Chi-square test and survival analysis. Finally, we carried out gene set enrichment analysis (GSEA)[16,17] to identify molecular mechanisms related to the impact of COL5A2 on the growth of BC cells.
3. Results
3.1. The expression of COL5A2 and the clinical features of patients with BC
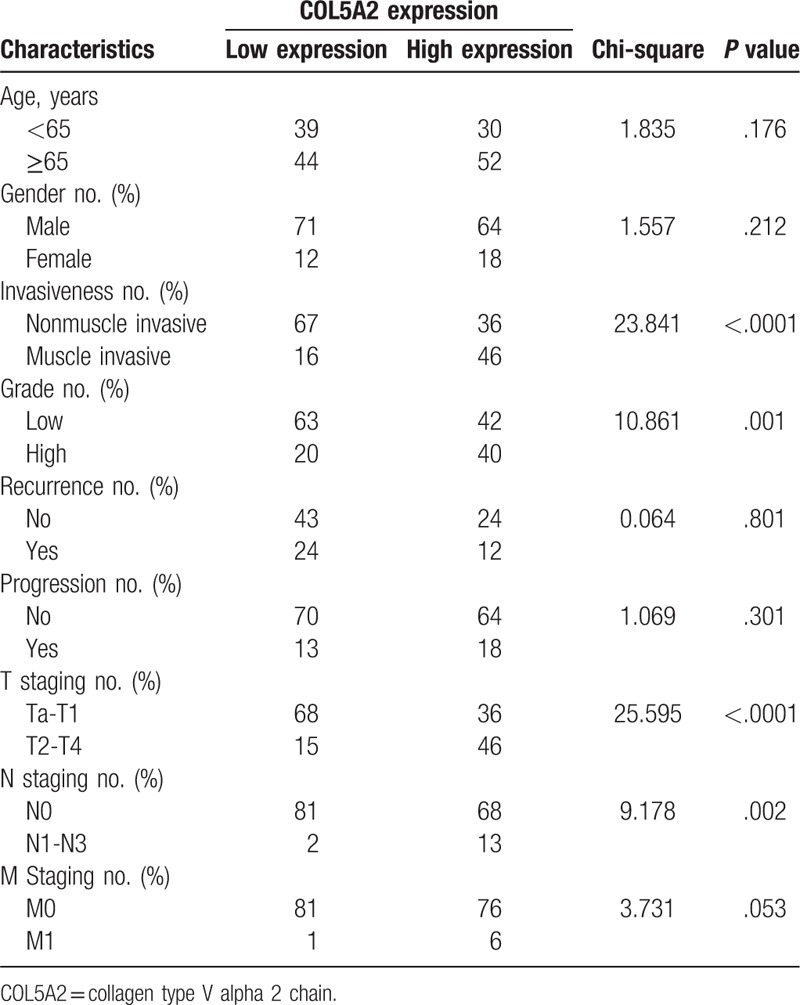
Firstly, we studied the relationship between the expression of COL5A2 and the clinical features of patients with BC. As shown in Table 1, BC patients in COL5A2 low expression group were associated with better invasiveness (P < .0001), tumor grade (P=.001), T staging (P < .0001), N staging (P = .002), and a trend of better M staging (P = .053) than those in COL5A2 high expression group. These results indicated that COL5A2 might promote the progression of BC cells.
Table 1.
The relationship between COL5A2 expression and the clinical characteristics of patients with bladder cancer.
3.2. Patients in COL5A2 low expression group were associated with better survivals
Next, we analyzed the cancer specific survival and overall survival of BC patients in COL5A2 low expression group and COL5A2 high expression group, respectively. As shown in Figure 1, BC patients in COL5A2 low expression group were associated with better cancer specific survival (HR = 0.2084, 95% CI: 0.103–0.4216, P < .0001) and overall survival (HR = 0.3459, 95% CI: 0.2109–0.5671, P < .0001) compared with those in COL5A2 high expression group.
Figure 1.
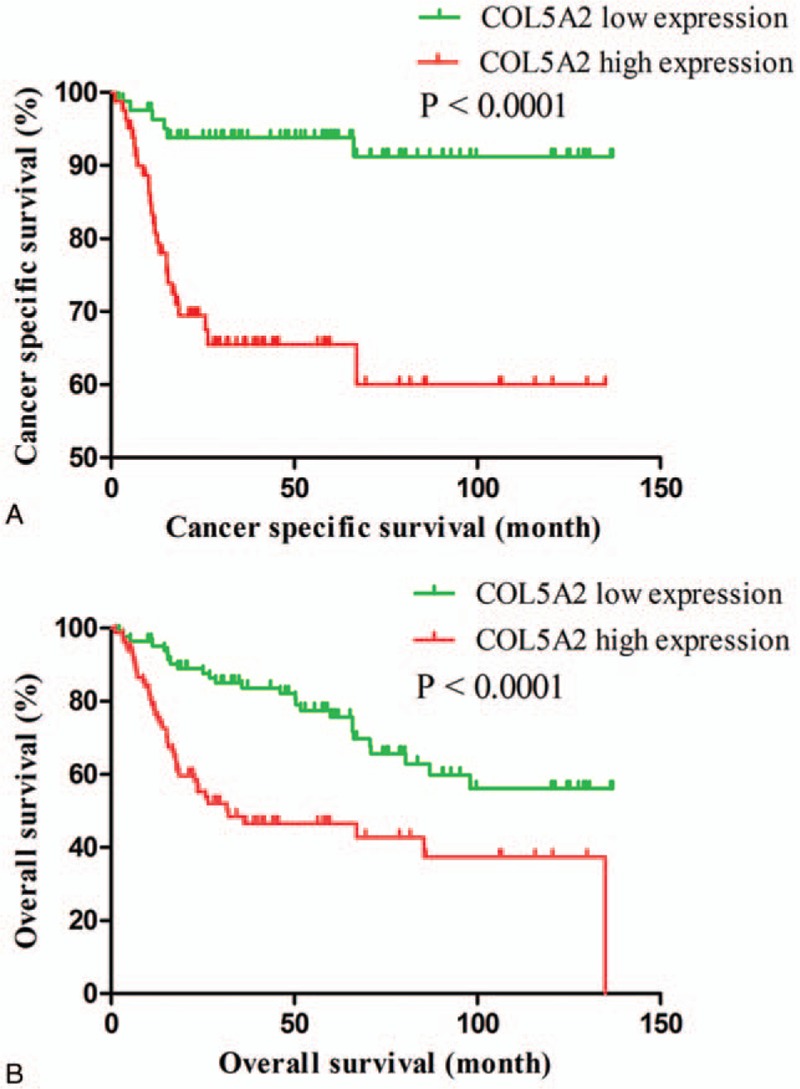
The cancer specific survival (A) and overall survival (B) of bladder cancer patients in COL5A2 low expression group and COL5A2 high expression group. COL5A2 = collagen type V alpha 2 chain.
3.3. Gene set enrichment analysis (GSEA)
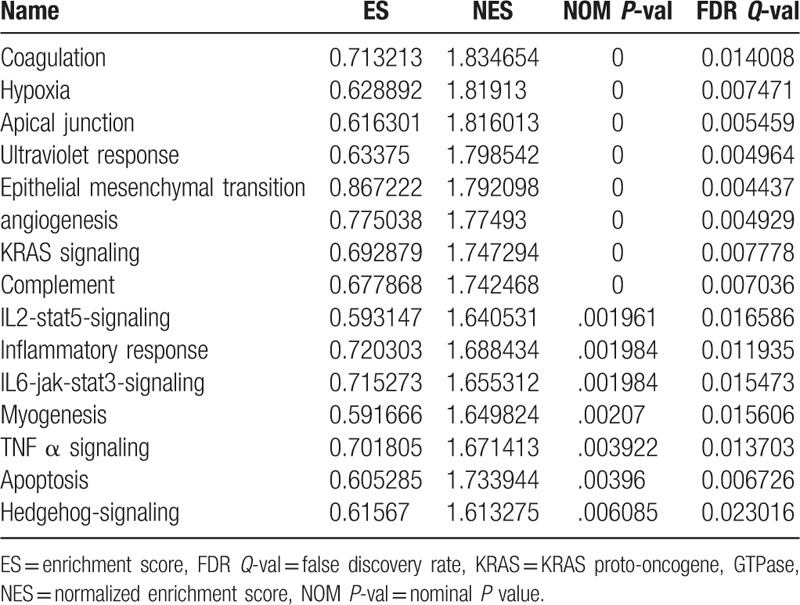
To clarify the molecular mechanisms regarding the expression of COL5A2 on the proliferation of BC cells, another GSE31684[14,15] was used to perform GSEA[16,17] based on expression of COL5A2. The results of GSEA suggested that BC samples in COL5A2 high expression group were enriched in “Coagulation,”“Hypoxia,”“Apical junction,”“Ultraviolet response,”“Epithelial mesenchymal transition,”“Angiogenesis,”“KRAS (KRAS proto-oncogene, GTPase) signaling ,”“Complement,”“IL2-stat5-signaling,”“Inflammatory response,”“IL6-jak-stat3-signaling,”“Myogenesis,”“TNF α signaling,”“Apoptosis,”and“Hedgehog-signaling.” These findings indicated that COL5A2 might impact the growth of BC cells through the above biological processes.
4. Discussion
As stated above, BC is a malignant disease with relatively high incidence, limited management options and poor clinical outcomes. It is of great importance to develop novel biomarkers that can predict the clinical outcomes of BC patients.[6]
The present study demonstrated that patients in COL5A2 low expression group were associated with better clinicopathological phenotypes in terms of tumor grade, invasiveness, T staging, N staging, cancer specific survival, and overall survival. These results uncovered that COL5A2 might predict poor clinical outcomes of BC patients. Actually, previous studies have revealed that COL5A2 might participate in the pathological processes of several human cancers. For instance, Fischer et al[8] found that COL5A2 was expressed in colorectal cancer but not in normal colon, suggesting that COL5A2 was implicated in the carcinogenesis of colorectal cancer. Guillen-Ahlers et al[9] supported that COL5A2 was upregulated in adenoma-derived dysplastic epithelial cells. Furthermore, the results of microarray studies conducted by Vargas et al[10] showed that the expression of COL5A2 was increased in invasive breast cancer compared with that in ductal carcinoma in situ, which was also associated with extracellular matrix (ECM) remodeling, indicating that COL5A2 was related to tumor progression of breast cancer. Wu et al[11] concluded that COL5A2 was upregulated in osteosarcoma cells, and it might considered as a diagnostic marker for osteosarcoma. In sum, the conclusions of literature review confirmed that COL5A2 was correlated with tumorigenesis and tumor progression including BC. Nevertheless, none of the above studies analyzed the correlation between COL5A2 expression and clinical outcomes of patients with corresponding cancers.
Collagens are the main fibrous proteins of connective tissue and are the most abundant proteins of the extracellular matrix (ECM).[18] GSEA, a computational method that determines whether an a priori defined set of genes shows statistically significant, concordant differences between 2 biological states (e.g., phenotypes), has been widely used in various medical and molecular studies to deduce and identify molecular mechanisms that involved in vivo and intro. The results of these have been widely accepted and made a profound influence.[19–21]Our GSEA results indicated that COL5A2 might promote the progression of BC cells through “Coagulation,” “Hypoxia,” “Apical junction,” “Ultraviolet response,” “Epithelial mesenchymal transition,” “Angiogenesis,” “KRAS signaling ,”“Complement,”“IL2-STAT5 signaling,” “Inflammatory response,” “IL6-JAK-STAT3 signaling,” “Myogenesis,” “TNF-α signaling,” “Apoptosis,” and "Hedgehog signaling”. Thereinto, coagulation, hypoxia, apical junction, epithelial mesenchymal transition and angiogenesis were the most enriched gene sets, reflecting that COL5A2 might affect the growth of BC cells through ECM remodelling (Table 2).
Table 2.
Gene sets enriched in BC samples in COL5A2 high expression group.
Moreover, for coagulation pathway, the most significantly enriched biological process, several studies proved that it was concerned with the carcinogenesis of multiple human cancers such as lung cancer,[22] esophageal cancer,[23] ovarian cancer,[24] and colorectal cancer.[25] More enriched biological processes including apical junction, ultraviolet response, epithelial mesenchymal transition, angiogenesis, and other well-known signaling pathway were also reported to be involved in the biological activities of various cancers.
This is a retrospective analysis of gene expression data of bladder cancers, demonstrating that COL5A2 was associated poor clinical outcomes and survivals of patients with BC and could be regarded as a biomarker of bladder cancer, Meanwhile, the evidences and conclusions of our study would be more robust when in vivo and in vitro studies and prospective cohort studies was conducted.
Taken together, our findings demonstrated that COL5A2 was associated with poor clinical outcomes and survivals of patients with BC, implying that it could be regarded as a biomarker of BC.
Footnotes
Abbreviations: BC = bladder cancer, COL5A2 = collagen type V alpha 2 chain, ECM = extracellular matrix, GEO = gene expression omnibus, GSEA = gene set enrichment analysis, TURBT = transurethral resection of the bladder tumor.
Funding: This work was supported by The National Key Research and Development Plan of China (Grant no. 2016YFC0106300).
The authors have no conflicts of interest to disclose.
References
- [1].Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- [2].Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int 2017;119:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pang C, Guan Y, Li H, et al. Urologic cancer in China. Jpn J Clin Oncol 2016;46:497–501. [DOI] [PubMed] [Google Scholar]
- [4].Matsuda T, Okuyama A. Incidence rate for bladder cancer in Japanese in Japan and in the United States from the cancer incidence in five continents. Jpn J Clin Oncol 2017;47:284–5. [DOI] [PubMed] [Google Scholar]
- [5].Kukreja JB, Shah JB. Advances in surgical management of muscle invasive bladder cancer. Indian J Urol 2017;33:106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Clark PE, Spiess PE, Agarwal N, et al. NCCN Guidelines Insights: bladder cancer, version 2.2016. J Natl Compr Canc Netw 2016;14:1213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Browne BM, Vanni AJ. Management of urethral stricture and bladder neck contracture following primary and salvage treatment of prostate cancer. Curr Urol Rep 2017;18:76. [DOI] [PubMed] [Google Scholar]
- [8].Fischer H, Stenling R, Rubio C, et al. Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis 2001;22:875–8. [DOI] [PubMed] [Google Scholar]
- [9].Guillen-Ahlers H, Buechler SA, Suckow MA, et al. Sulindac treatment alters collagen and matrilysin expression in adenomas of ApcMin/+ mice. Carcinogenesis 2008;29:1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vargas AC, McCart Reed AE, Waddell N, et al. Gene expression profiling of tumour epithelial and stromal compartments during breast cancer progression. Breast Cancer Res Treat 2012;135:153–65. [DOI] [PubMed] [Google Scholar]
- [11].Wu D, Chen K, Bai Y, et al. Screening of diagnostic markers for osteosarcoma. Mol Med Rep 2014;10:2415–20. [DOI] [PubMed] [Google Scholar]
- [12].Kim WJ, Kim EJ, Kim SK, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee JS, Leem SH, Lee SY, et al. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J Clin Oncol 2010;28:2660–7. [DOI] [PubMed] [Google Scholar]
- [14].Riester M, Taylor JM, Feifer A, et al. Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clin Cancer Res 2012;18:1323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Riester M, Werner L, Bellmunt J, et al. Integrative analysis of 1q23.3 copy-number gain in metastatic urothelial carcinoma. Clin Cancer Res 2014;20:1873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–73. [DOI] [PubMed] [Google Scholar]
- [18].Symoens S, Syx D, Malfait F, et al. Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum Mutat 2012;33:1485–93. [DOI] [PubMed] [Google Scholar]
- [19].Li L, Wang X, Xiao G, et al. Integrative gene set enrichment analysis utilizing isoform-specific expression. Genet Epidemiol 2017;41:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ng WL, Marinov GK, Chin YM, et al. Transcriptomic analysis of the role of RasGEF1B circular RNA in the TLR4/LPS pathway. Sci Rep 2017;7:12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Del Corvo M, Luini M, Stella A, et al. Identification of additional loci associated with antibody response to Mycobacterium avium ssp. Paratuberculosis in cattle by GSEA-SNP analysis. Mamm Genome 2017;28:520–7. [DOI] [PubMed] [Google Scholar]
- [22].Kim JS, Ryu JS, Jeon SH, et al. Dramatic response of acute disseminated intravascular coagulation to erlotinib in a patient with lung adenocarcinoma with activating EGFR mutation. J Int Med Res 2017;46:533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Matsutani T, Nomura T, Hagiwara N, et al. Salvage endoscopic argon plasma coagulation after chemoradiotherapy for inoperable esophageal cancer. Surg Laparosc Endosc Percutan Tech 2017;27:384–90. [DOI] [PubMed] [Google Scholar]
- [24].Li L, Zheng H, Huang Y, et al. DNA methylation signatures and coagulation factors in the peripheral blood leucocytes of epithelial ovarian cancer. Carcinogenesis 2017;38:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee S, Huh SJ, Oh SY, et al. Clinical significance of coagulation factors in operable colorectal cancer. Oncol Lett 2017;13:4669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


