Abstract
Many studies show that CXC chemokine ligand 14 (CXCL14) is highly expressed in tumor-associated stromal cells, promoting tumor cell growth, and invasion. Because of its unclear receptors, CXCL14-initiated intracellular signal cascades remain largely unknown. However, CXCL14 can regulate nitric oxide synthase 1 (NOS1) as its intracellular molecular target. In this paper, we investigated the expression of CXCL14 and NOS1 in specimens from patients with stage I–IIIA nonsmall cell lung cancer (NSCLC) after curative resection, and evaluated the prognostic significance of this gene expression in stromal fibroblasts and cancer cells.
Immunohistochemistry was used to detect the expression of CXCL14 and NOS1 in 106 formalin fixed, paraffin-embedded specimens from patients with stage I-IIIA NSCLC. The chi-square test was performed to examine the correlation of CXCL14 and NOS1 expression level with clinicopathological features. The effects of the expression of CXCL14 or NOS1 on progression-free survival (PFS) and overall survival (OS) were determined by Kaplan–Meier and Cox hazard proportional model.
The percentages of high CXCL14 expression in stromal fibroblasts and that in cancer cells were 46.2% (49/106) and 23.6% (25/106), respectively. The positive expression rates of NOS1 in cancer cells were 42.5% (45/106). The result indicated that there was a significant positive correlation between CXCL14 expression level in stromal fibroblasts and that in cancer cells (χ2 = 4.158, P = .041). In addition, the expression of CXCL14 in stromal fibroblasts was significantly correlated with NOS1 expression in cancer cells (χ2 = 16.156, P < .001). The 5-year PFS rates with low and high CXCL14 expression in stromal fibroblasts were 66.7% and 14.3% (χ2 = 44.008, P < .001), respectively, and the 5-year OS rates with those were 87.1% and 43.5% (χ2 = 21.531, P < .001), respectively. The 5-year PFS rates with negative and positive expression of NOS1 in cancer cells were 62.3% and 15.6% (χ2 = 33.756, P < .001), respectively, and the 5-year OS rates with those were 86.4% and 40.1% (χ2 = 24.430, P < 0.01), respectively.
Both the high expression of CXCL14 in stromal fibroblasts and the positive expression of NOS1 in cancer cells are independent negative predictors of PFS and OS in patients with stage I–IIIA NSCLC after curative resection.
Keywords: CXCL14, NOS1, NSCLC, prognosis, stromal fibroblasts
1. Introduction
Lung cancer is the leading cause of cancer death worldwide. In 2016, the number of newly diagnosed lung cancer cases was estimated to be 224,390 in the United States, of which lung cancer deaths accounted for 27% of all cancer deaths.[1] About 80% of lung cancer is nonsmall cell lung cancer (NSCLC), mainly including adenocarcinoma and squamous cell carcinoma.[2] In recent years, although multidisciplinary treatment has been widely used in NSCLC patients, the overall prognosis is still poor. Previous studies showed that the prognosis of NSCLC patients after surgical resection depends on following clinical factors: tumor size, histological grade, vascular invasion, and visceral pleural infiltration.[3–5] In addition, several molecular biomarkers can predict the prognosis of patients with NSCLC after surgical resection, such as RAS, MSH2, ERCC1, P27, etc.[6] In recent years, many studies have shown that malignant progression of the tumor depends on both the tumor cells themselves and the surrounding microenvironment.[7,8] The tumor microenvironment is associated with the extracellular matrix, metabolites, blood vasculature, growth factors, cytokines, and mesenchymal cells. Stromal fibroblasts are major cellular components of the tumor microenvironment and promote tumor cell growth, invasion, and metastasis through complex biological interactions between them and tumor cells.[9,10]
Chemokines are 8 to 14 kDa chemoattractant cytokines that mainly regulate cell migration during organogenesis and immune surveillance. Chemokines and their receptors are potentially important factors in oncogenesis, infection, allergy, and autoimmunity by modulating cellular attraction, proliferation, angiogenesis, as well as tumor cell growth and spreading.[11] The chemokine (C-X-C Motif) ligand 14 (CXCL14) gene, also known as breast and kidney-expressed chemokine, is a member of the CXC chemokine family, located on human chromosome 5q31.[12] According to previous studies, enhanced expression of CXCL14 has been found in different types of cancers, including colon cancer,[13] pancreatic cancer,[14] prostate cancer,[15] and breast cancer,[16] where CXCL14 can be expressed by cancer cells or stromal cells or both cell types.[17] Although the role of CXCL14 as a tumor suppressor seems well established,[18,19] recent studies have shown that CXCL14 might actually promote tumor progression.[20,21] Using a systems genetics approach, Williams et al[22] found that the CXCL14, ITGAX, and LPCAT2 genes are identified as novel aggressive prostate cancer susceptibility genes. In another study conducted by Park et al,[23] CXCL14 enhances the proliferation and migration of human lung cancer cell line NCI-H460 by interacting with heparan sulfate proteoglycans and sialic acids. However, previous studies have shown that the effects of CXCL14 on tumor growth depend on the cell type expressing this factor.[24,25] When expressed by stromal fibroblasts, CXCL14 shows the tumor-promoting effect.[24] However, it shows antitumor effect when expressed by malignant epithelial cells.[25] In a study of Augsten et al,[26] CXCL14-expressing fibroblasts promote the growth of prostate tumors by promoting the secretion of nitric oxide synthase 1 (NOS1) in fibroblasts. When the irreversible inhibitor L-NNA (Nw-nitro-L-arginine) of NOS1 was used or the NOS1 gene of fibroblasts was knocked out, the tumor-forming ability of CXCL14-expressing fibroblasts is inhibited, indicating that NOS1 may be an intracellular signaling pathway that CXCL14 promotes the tumor growth. NOS1, also known as neuronal nitric oxide synthase (NOS), is one of the 3 isomers of NOS. NOS1, NOS2 (iNOS), and NOS3 (eNOS), as well as their products, nitric oxide (NO), play an important role in carcinogenesis.[27] Yang et al[28] found that the expression of NOS1 in melanoma cells is higher than that in melanocytes. NOS1 enhances melanoma cell proliferation and invasion by inducing NO overproduction, leading to subsequent tumor metastasis. Similarly, NOS1 is also highly expressed in renal cell carcinoma and is associated with microvascular infiltration and lymph node metastasis.[29]
However, there are few studies examining the correlation of CXCL14 and NOS1 expression level in cancer cells with their clinical significance. In this paper, we explored the immunohistochemical expression of CXCL14 and NOS1 in NSCLC. In addition, we examined the correlation of CXCL14 and NOS1 expression level with clinicopathological features, progression-free survival (PFS), as well as overall survival (OS). Our results show that both the high expression of CXCL14 in stromal fibroblasts and the positive expression of NOS1 in cancer cells are associated with poor PFS and OS for patients with stage I–IIIA NSCLC. Thus, the CXCL14 and NOS1 can be identified as potential prognostic markers for patients with NSCLC.
2. Materials and methods
2.1. Patients and specimens
The postoperative paraffin-embedded tissue specimens were obtained from the archives of the Department of Pathology, Jinling Hospital Affiliated to Nanjing University Medical College, from March 2010 to May 2011. We retrospectively analyzed 106 tumor specimens from patients with stage I–IIIA NSCLC. The study was approved by the Ethics Committee of Jinling Hospital, and written informed consents were obtained from all patients. The main characteristics of all patients are summarized in Table 1. The ages of the patients in this study were from 31 to 80 years (mean age was 60.5 years). According to the American Joint Committee on Cancer and the Union for International Cancer Control stage system (7th edition), there were 45 patients with stage I NSCLC, 27 patients with stage II NSCLC, and 34 patients with stage IIIA NSCLC. All patients underwent radical resection. Thirty-seven patients received postoperative radiotherapy, and 67 patients received postoperative adjuvant chemotherapy. The chemotherapy regimens were cisplatin-based doublet.
Table 1.
Patients’ characteristics.
2.2. Immunohistochemistry
A total of 106 specimens from patients were obtained for immunohistochemistry analyses. The formalin-fixed, paraffin-embedded tissues obtained from patients with stage I–IIIA NSCLC were cut into consecutive 3-μm-thick sections. One slide stained with hematoxylin eosin and the others immunohistochemically stained with a 2-step procedure. After deparaffinization and hydration, the tissue sections were subjected to antigen retrieval by pressurizing for 30 minutes. The sections were then incubated with 3% hydrogen peroxide solution at room temperature for 25 minutes to neutralize endogenous peroxidase activity. Next, the sections were incubated with antihuman CXCL14 rabbit polyclonal antibody (ab46010) (Abcam, Cambridge, UK; diluted 1:100) and antihuman NOS1 rabbit monoclonal antibody (#4231) (Cell Signaling, Boston, MA; diluted 1:100) for overnight at 4 °C. This was followed by incubation with the secondary antibody (ChemMate EnVision/HRP; Gene Tech, Shanghai, China) for 30 minutes at room temperature. Subsequently, the slides were treated with freshly prepared DAB liquid (DAB; Gene Tech, Shanghai, China) for 10 minutes, followed by counterstaining the nuclei with hematoxylin. The negative control used was the IgG2b isotype antibody (Dako), which was consistent with the same immunoglobulins concentration as for the anti-CXCL14 and anti-NOS1.
2.3. Immunohistochemical scoring
The immunohistochemical staining was scored by 2 pathologists. This is a single-blind study, and 2 pathologists read and scored the slides independently. The expression levels of each antibody in cancer cells and stromal fibroblasts were evaluated independently. The expression of CXCL14 and NOS1 was scored by assigning a percentage of positive cells and an intensity score. Immunohistochemical staining intensity was scored as 0, 1, 2, or 3, indicating absent, weak, moderate, or strong expression, respectively. The percentages of positive cells were scored as follows: 0 = no staining, 1 = 1% to 5%, 2 = 5% to 25%, 3 = 25% to 50%, 4 = 50% to 75%, 5 = 75% to 100%. For each marker, the score of percentage and intensity was multiplied for final scores and to classify expression: 0 to 8, CXCL14 low expression; 9 to 15, CXCL14 high expression; 0, NOS1 negative expression; and 1 to 15, NOS1 positive expression.[30]
2.4. Statistical analysis
The correlation of CXCL14 and NOS1 expression level in cancer cells or stromal fibroblasts with clinicopathological variables was examined using chi-square test. In addition, the Kaplan–Meier method and log-rank test were used for OS rate and PFS rate. Multivariate survival analysis was performed by the Cox proportional hazard model to assess the independent factors that may affect the prognosis of patients. Only variables with P < .15 from the univariate analyses or important ones were explored in the Cox regression analysis. PFS was calculated from the beginning date of surgery to the date of any new recurrence or death (without confirmed recurrence). OS was calculated from the date of surgery to the date of death from any cause or last follow-up. All the statistical analysis was analyzed by SPSS 19.0 statistical software, and a 2-sided P < .05 was considered significant.
3. Results
3.1. Expression of CXCL14 and NOS1 in stromal fibroblasts and cancer cells
Immunohistochemical staining showed that CXCL14 and NOS1 were mainly expressed in the cytoplasm. The percentages of high CXCL14 expression in stromal fibroblasts and cancer cells were 46.2% (49/106) and 23.6% (25/106), respectively (Fig. 1). There were 42.5% (45/106) of patients with positive NOS1 expression in cancer cells (Fig. 2). The expression of NOS1 was not observed in stromal fibroblasts (Fig. 2). The analysis revealed a significant positive correlation between CXCL14 expression in stromal fibroblasts and that in cancer cells (χ2 = 4.158, P = .041). In addition, the expression of CXCL14 in stromal fibroblasts was significantly correlated with NOS1 expression in cancer cells (χ2 = 16.156, P < .001).
Figure 1.
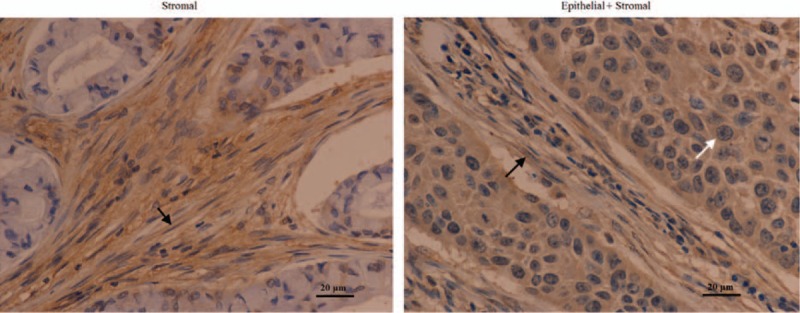
CXCL14 expression by immunohistochemistry in the specimens from patients with stage I–IIIA NSCLC after curative resection (magnification ×400, scale bars = 20 μm). Examples of stromal as well as epithelial and stromal staining of CXCL14 in the specimen from patients with stage I–IIIA NSCLC. Black arrows show stromal CXCL14 expression and white arrows show epithelial CXCL14 expression. CXCL14 = CXC chemokine ligand 14, NSCLC = nonsmall cell lung cancer.
Figure 2.
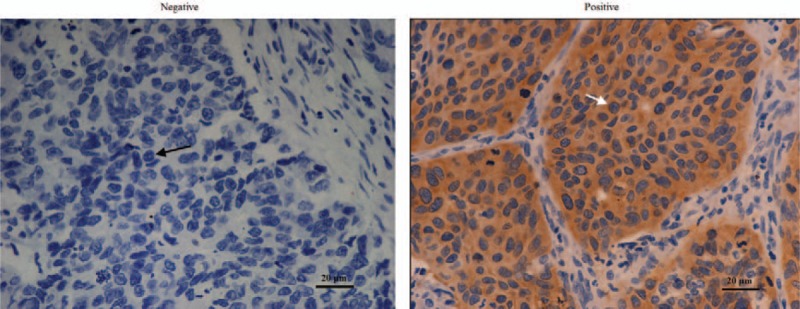
NOS1 expression by immunohistochemistry in the specimens from patients with stage I–IIIA NSCLC after curative resection (magnification ×400, scale bars = 20 μm). Examples of negative and positive staining of NOS1 in cancer cells. Black arrows show negative NOS1 expression and white arrows show positive NOS1 expression. NOS1 = nitric oxide synthase 1, NSCLC = nonsmall cell lung cancer.
3.2. Associations of CXCL14 or NOS1 expression with clinicopathologic characteristics
To explore the clinical relevance of CXCL14 and NOS1, associations between clinicopathological characteristics (age, gender, smoke, pathologic type, pathologic differentiation, tumor category, node category, and pathologic stage) and epithelial or stromal CXCL14 expression, as well as that and NOS1 expression were analyzed in Tables 2 and 3, respectively.
Table 2.
Association between CXCL14 expression in the specimens from patients with stage I–IIIA NSCLC after curative resection and clinicopathologic features of patients.
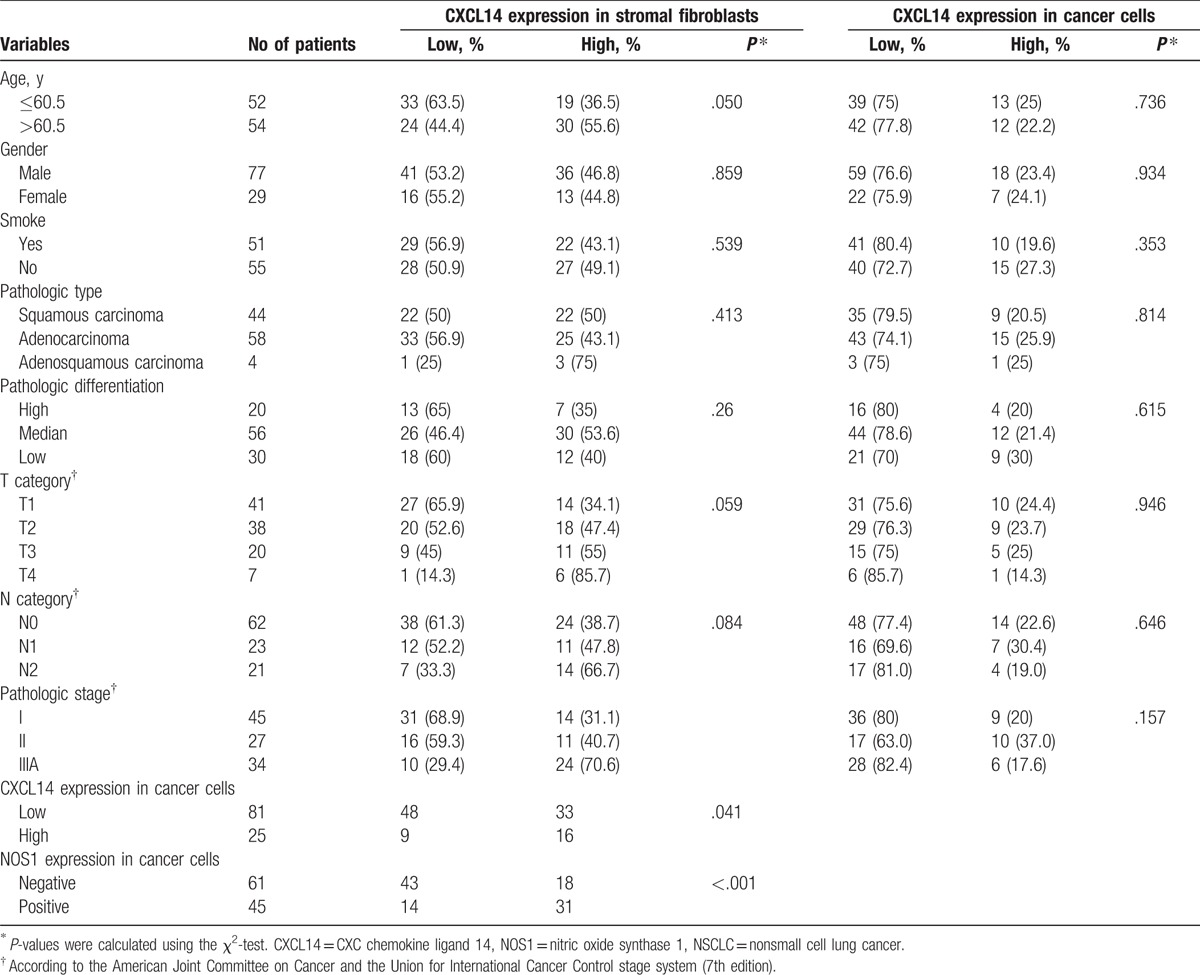
Table 3.
Association between NOS1 expression in the specimens from patients with stage I–IIIA NSCLC after curative resection and clinicopathologic features of patients.
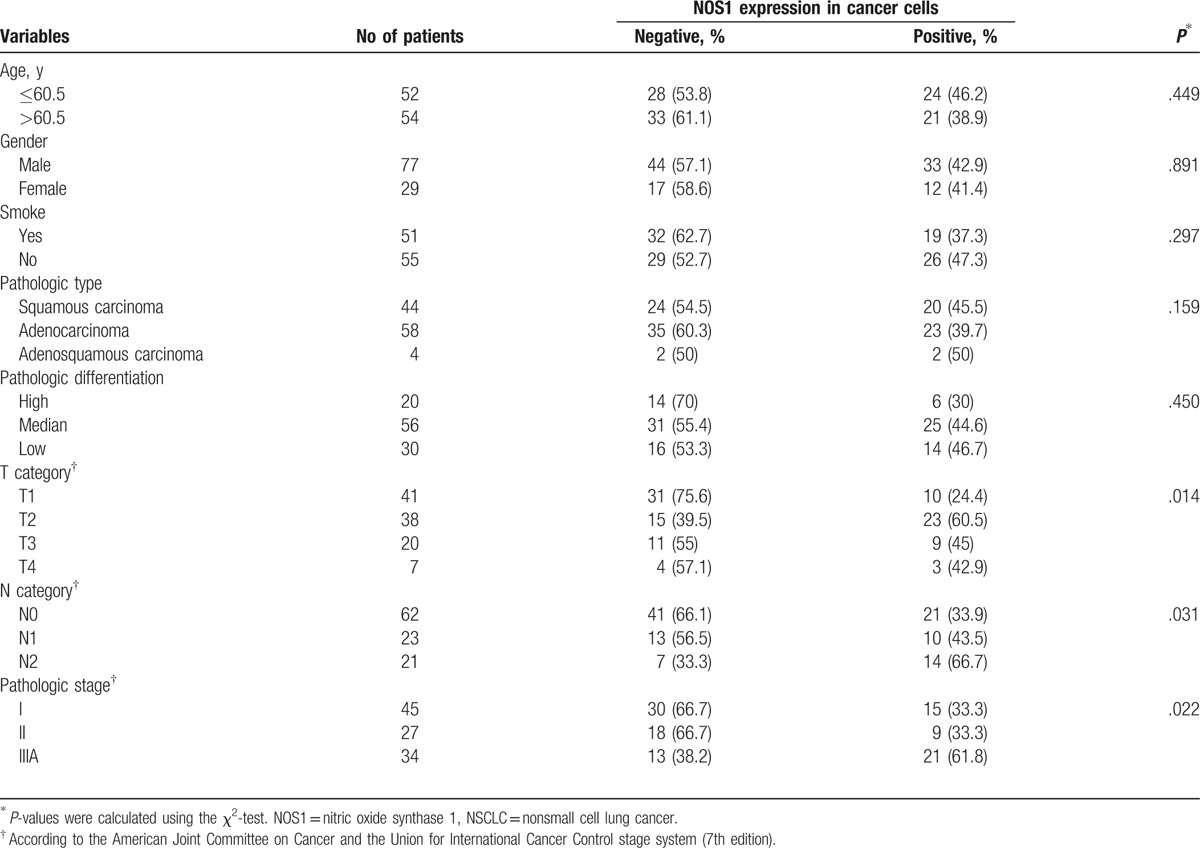
Stromal CXCL14 expression was only significantly associated with advanced pathologic stage (P = .002) (Table 2). However, epithelial CXCL14 expression was not correlated with any clinicopathological characteristics of patients (Table 2). NOS1 expression in cancer cells was correlated with tumor stage in tumor category (P = .014), node category (P = .031), and pathologic stage (P = .022) (Table 3).
3.3. Associations of CXCL14 or NOS1 expression with PFS and OS
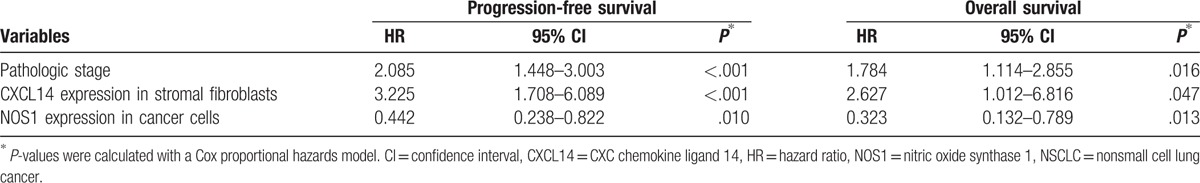
The mean follow-up time for these patients was 60 months (range 8–68 months). The Kaplan–Meier plots showed that the 5-year PFS rates and the 5-year OS rates were 42.5% and 67.7% of all patients, respectively. The 5-year PFS rates of patients with stage I, II, and IIIA were 62.2%, 44.4%, and 17.6%, respectively (χ2 = 33.463, P < .001). The 5-year OS rates of that were 88.5%, 63%, and 41.4%, respectively (χ2 = 19.972, P < .001). The 5-year PFS rates for patients with low and high CXCL14 expression in stromal fibroblasts were 66.7% and 14.3% (χ2 = 44.008, P < .001), respectively, and the 5-year OS rates of that were 87.1% and 43.5% (χ2 = 21.531, P < .001), respectively (Fig. 3). The 5-year PFS rates for patients of negative and positive expression of NOS1 in cancer cells were 62.3% and 15.6% (χ2 = 33.756, P < .001), respectively, and the 5-year OS rates for that were 86.4% and 40.1% (χ2 = 24.430, P < .001), respectively (Fig. 4). However, no significant association was observed between CXCL14 expression in cancer cells with respect to 5-year PFS (χ2 = 1.699, P = .192) and 5-year OS (χ2 = 0.076, P = .783), respectively. Multivariate analysis using the Cox hazard proportional model showed that variables associated with OS and PFS included pathologic stage, CXCL14 expression level in stromal fibroblasts, and NOS1 expression level in cancer cells (Table 4).
Figure 3.
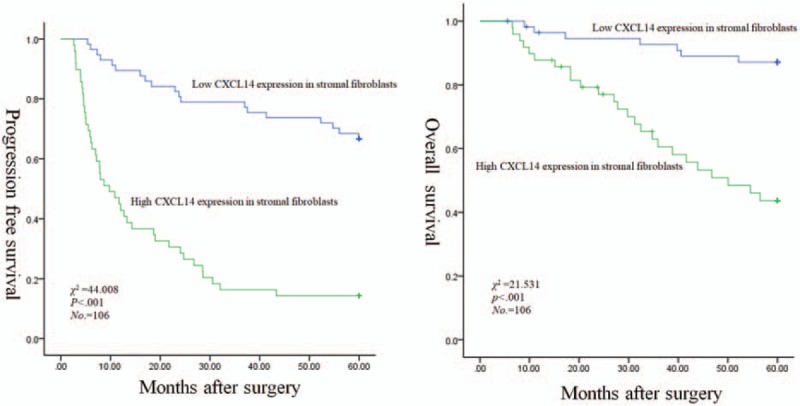
Correlations of stromal CXCL14 expression with worse PFS and OS for patients with stage I–IIIA NSCLC after curative resection: Kaplan–Meier curves for PFS (left panel) and OS (right panel). CXCL14 = CXC chemokine ligand 14, NSCLC = nonsmall cell lung cancer, OS = overall survival, PFS = progression-free survival.
Figure 4.
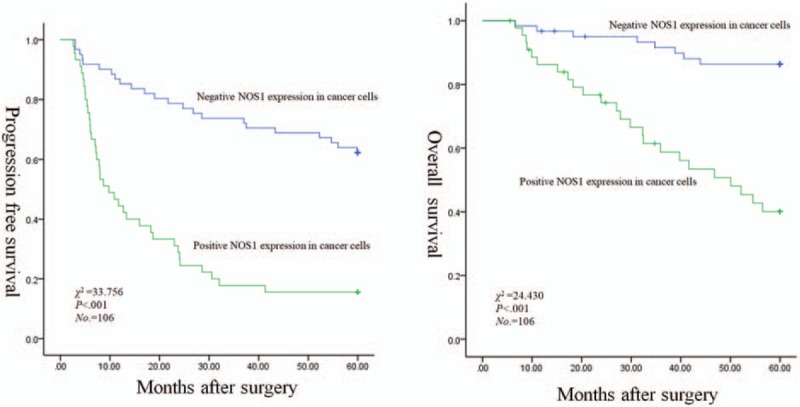
Correlations of NOS1 expression in cancer cells with worse PFS and OS for patients with stage I–IIIA NSCLC after curative resection: Kaplan–Meier curves for PFS (left panel) and OS (right panel). NOS1 = nitric oxide synthase 1, NSCLC = nonsmall cell lung cancer, OS = overall survival, PFS = progression-free survival.
Table 4.
Prognostic value of CXCL14 and NOS1 expression in the specimens from patients with stage I–IIIA NSCLC after curative resection from multivariate analysis using Cox hazard proportional model (n = 106).
4. Discussion
CXCL14 is a novel non-ELR chemokine with nonspecific receptors associated with tumor progression and metastasis. This study found that the high expression of CXCL14 in stromal fibroblasts is identified as an independent novel predictive factor of poor prognosis for patients with stage I–IIIA NSCLC after curative resection. In addition, CXCL14 expression in cancer cells is not associated with clinical outcome. These are consistent with previous studies, indicating that marker proteins expressed by stromal fibroblasts show a prognostic significance. For example, the high expression of podoplanin,[31] FAP,[32] TGF-β, and a-SMA[33] in cancer-associated fibroblasts indicates a short-term survival rate of patients with NSCLC.
This study suggests that the associations between stromal CXCL14 expression and a poor survival are consistent with previous findings.[13,17,34] By analyzing CXCL14 protein expression, Shaykhiev et al[34] found the chemokine to be upregulated in lung adenocarcinoma and lung squamous carcinoma by comparing healthy smokers with smokers having chronic obstructive pulmonary disease. Furthermore, the high expression of CXCL14 indicates worse survival in lung adenocarcinoma. Similarly, Zeng et al[13] demonstrated that CXCL14 is highly expressed in colorectal cancer tissues, promotes colorectal cancer cell proliferation and invasion, and is associated with impaired OS. Other researchers reported correlations between high CXCL14 expression and favorable prognosis in Ewing sarcoma[35] and colorectal cancer.[36] However, none of these studies considered the cell type expressing CXCL14.
It has been identified that activated fibroblasts in the stroma of breast and prostate cancer are the source for CXCL14.[24,37] In a study of Takiguchi et al,[38] CXCL14 can promote bone metastasis from lung cancer by enhancing cancer cell tropism to the bone and/or recruitment of bone marrow cells around metastatic cancer cells in vitro and in vivo. Besides, this study has shown that CXCL14 was not only expressed in tumor cells infiltrating bone marrow but also in the bone marrow stromal cells. In breast cancer, by analyzing CXCL14 mRNA expression, it was found that high stromal CXCL14 expression is an independent marker of worse prognosis.[17] In the present study, we assessed the prognostic significance of stromal CXCL14 expression for patients with stage I–IIIA NSCLC after curative resection. The results showed that the expression level of CXCL14 in stromal fibroblasts was significantly correlated with TNM stage (P = .002). The more advanced pathologic stage had significantly higher CXCL14 expression. In addition, high expression of CXCL14 in stromal fibroblasts was associated with shorter PFS (p < .001) and shorter OS (P < .001).
However, the receptor for CXCL14 has not yet been identified. One study showed that in the prostate cancer and breast cancer animal models, NOS1 is an important determinant for stromal CXCL14 promoting tumorigenesis.[26] Thus, whether CXCL14 affects the prognosis of patients with NSCLC through the NOS1 signaling pathway? This study indicates that NOS1 was only expressed in cancer cells. In addition, the expression of CXCL14 in stromal fibroblasts and NOS1 expression in cancer cells was statistically significant (P < .001). Furthermore, the positive NOS1 expression in cancer cells predicted poor PFS (P < .001) and poor OS (P < .001). Therefore, we need further studies on the molecular mechanisms that CXCL14 and NOS1 promote the tumor malignant progression.
In summary, more experiments should be conducted to identify the potential of CXCL14 and NOS1 as lung cancer biomarkers. In addition, researches on the role of NOS1 in the intracellular signaling of CXCL14 is important. Understanding these mechanisms may lay a solid foundation for developing new therapeutic strategies.
Acknowledgments
The authors thank Hospital Research Foundation of Nanjing General Hospital (no. 2016058) for the support.
Footnotes
Abbreviations: CXCL14 = CXC chemokine ligand 14, NO = nitric oxide, NOS1 = nitric oxide synthase 1, NSCLC = nonsmall cell lung cancer, OS = overall survival, PFS = progression-free survival.
Funding/support: This study was supported by Hospital Research Foundation of Nanjing General Hospital (no. 2016058).
No writing assistance was utilized in the production of this manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011;378:1727–40. [DOI] [PubMed] [Google Scholar]
- [3].Kim MP, Chen Y, Bekele BN, et al. Activating enhancer-binding protein-2 (nucleolar localization predicts poor survival after stage I non-small cell lung cancer resection. Ann Thorac Surg 2011;92:1044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mineo T, Ambrogi V, Baldi A, et al. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB–IIA non-small cell lung cancer. J Clin Pathol 2004;57:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pfannschmidt J, Muley T, Bülzebruck H, et al. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer 2007;55:371–7. [DOI] [PubMed] [Google Scholar]
- [6].Konopa K. Do we have markers to select patients for adjuvant therapies of non-small-cell lung cancer? Ann Oncol 2010;21(suppl 7):vii199–202. [DOI] [PubMed] [Google Scholar]
- [7].Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev 2016;30:1002–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shiga K, Hara M, Nagasaki T, et al. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel) 2015;7:2443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ji X, Zhu X, Lu X. Effect of cancer-associated fibroblasts on radiosensitivity of cancer cells. Future Oncol 2017;13:1537–50. [DOI] [PubMed] [Google Scholar]
- [10].Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Song EY, Shurin MR, Tourkova IL, et al. Epigenetic mechanisms of promigratory chemokine CXCL14 regulation in human prostate cancer cells. Cancer Res 2010;70:4394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lu J, Chatterjee M, Schmid H, et al. CXCL14 as an emerging immune and inflammatory modulator. J Inflamm 2016;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zeng J, Yang X, Cheng L, et al. Chemokine CXCL14 is associated with prognosis in patients with colorectal carcinoma after curative resection. J Transl Med 2013;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wente MN, Mayer C, Gaida MM, et al. CXCL14 expression and potential function in pancreatic cancer. Cancer Lett 2008;259:209–17. [DOI] [PubMed] [Google Scholar]
- [15].Schwarze SR, Luo J, Isaacs WB, et al. Modulation of CXCL14 (BRAK) expression in prostate cancer. Prostate 2005;64:67–74. [DOI] [PubMed] [Google Scholar]
- [16].Kleer CG, Bloushtain-Qimron N, Chen Y-H, et al. Epithelial and stromal cathepsin K and CXCL14 expression in breast tumor progression. Clin Cancer Res 2008;14:5357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sjöberg E, Augsten M, Bergh J, et al. Expression of the chemokine CXCL14 in the tumour stroma is an independent marker of survival in breast cancer. Br J Cancer 2016;114:1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hata R-I, Izukuri K, Kato Y, et al. Suppressed rate of carcinogenesis and decreases in tumour volume and lung metastasis in CXCL14/BRAK transgenic mice. Sci Rep 2015;5:9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tessema M, Klinge DM, Yingling CM, et al. Re-expression of CXCL14, a common target for epigenetic silencing in lung cancer, induces tumor necrosis. Oncogene 2010;29:5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liang W, Yang C, Peng J, et al. The Expression of HSPD1, SCUBE3, CXCL14 and Its Relations with the Prognosis in Osteosarcoma. Cell Biochem Biophys 2015;73:763–8. [DOI] [PubMed] [Google Scholar]
- [21].Pelicano H, Lu W, Zhou Y, et al. Mitochondrial dysfunction and reactive oxygen species imbalance promote breast cancer cell motility through a CXCL14-mediated mechanism. Cancer Res 2009;69:2375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Williams KA, Lee M, Hu Y, et al. A systems genetics approach identifies CXCL14, ITGAX, and LPCAT2 as novel aggressive prostate cancer susceptibility genes. PLoS Genet 2014;10:e1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Park CR, You DJ, Kim DK, et al. CXCL14 enhances proliferation and migration of NCI-H460 human lung cancer cells overexpressing the glycoproteins containing heparan sulfate or sialic acid. J Cell Biochem 2013;114:1084–96. [DOI] [PubMed] [Google Scholar]
- [24].Augsten M, Hägglöf C, Olsson E, et al. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc Nat Acad Sci 2009;106:3414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gu X-L, Ou Z-L, Lin F-J, et al. Expression of CXCL14 and its anticancer role in breast cancer. Breast Cancer Res Treat 2012;135:725–35. [DOI] [PubMed] [Google Scholar]
- [26].Augsten M, Sjöberg E, Frings O, et al. Cancer-associated fibroblasts expressing CXCL14 rely upon NOS1-derived nitric oxide signaling for their tumor-supporting properties. Cancer Res 2014;74:2999–3010. [DOI] [PubMed] [Google Scholar]
- [27].Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer 2006;6:521. [DOI] [PubMed] [Google Scholar]
- [28].Yang Z, Misner B, Ji H, et al. Targeting nitric oxide signaling with nNOS inhibitors as a novel strategy for the therapy and prevention of human melanoma. Antioxid Redox Signal 2013;19:433–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Cássio Zequi S, Fregnani JHG, Favaretto RL, et al. The impact of immunohistochemical expression of nitric oxide synthases on clinical and pathological features of renal cell carcinoma. World J Urol 2013;31:1197–203. [DOI] [PubMed] [Google Scholar]
- [30].Sugihara H, Ishimoto T, Yasuda T, et al. Cancer-associated fibroblast-derived CXCL12 causes tumor progression in adenocarcinoma of the esophagogastric junction. Med Oncol 2015;32:168. [DOI] [PubMed] [Google Scholar]
- [31].Ono S, Ishii G, Nagai K, et al. Podoplanin-positive cancer-associated fibroblasts could have prognostic value independent of cancer cell phenotype in stage I lung squamous cell carcinoma: usefulness of combining analysis of both cancer cell phenotype and cancer-associated fibroblast phenotype. Chest J 2013;143:963–70. [DOI] [PubMed] [Google Scholar]
- [32].Kilvaer TK, Khanehkenari MR, Hellevik T, et al. Cancer associated fibroblasts in stage I-IIIA NSCLC: prognostic impact and their correlations with tumor molecular markers. PLoS One 2015;10:e0134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen Y, Zou L, Zhang Y, et al. Transforming growth factor-β1 and α-smooth muscle actin in stromal fibroblasts are associated with a poor prognosis in patients with clinical stage I–IIIA nonsmall cell lung cancer after curative resection. Tumor Biol 2014;35:6707–13. [DOI] [PubMed] [Google Scholar]
- [34].Shaykhiev R, Sackrowitz R, Fukui T, et al. Smoking-induced CXCL14 expression in the human airway epithelium links chronic obstructive pulmonary disease to lung cancer. Am J Respir Cell Mol Biol 2013;49:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sand L, Scotlandi K, Berghuis D, et al. CXCL14, CXCR7 expression and CXCR4 splice variant ratio associate with survival and metastases in Ewing sarcoma patients. Eur J Cancer 2015;51:2624–33. [DOI] [PubMed] [Google Scholar]
- [36].Lin K, Zou R, Lin F, et al. Expression and effect of CXCL14 in colorectal carcinoma. Mol Med Rep 2014;10:1561–8. [DOI] [PubMed] [Google Scholar]
- [37].Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004;6:17–32. [DOI] [PubMed] [Google Scholar]
- [38].Takiguchi S, Korenaga N, Inoue K, et al. Involvement of CXCL14 in osteolytic bone metastasis from lung cancer. Int J Oncol 2014;44:1316–24. [DOI] [PubMed] [Google Scholar]


