Abstract
Early identification of acute lung injury (ALI) in pediatric patients at risk of mortality is important for improving outcome.
Assessment of soluble form of receptor for advanced glycation end products (sRAGE) as a valid biomarker for diagnosis of ALI among critically ill, pediatric patients in addition to correlating levels of sRAGE and different outcomes of those patients.
A Hospital-based case-control study was conducted in pediatric intensive care units (PICUs) at Cairo University Hospital, along a period of 6 months. Total of 68 pediatric patients following inclusion criteria were classified into: patients with ALI; with both ALI and sepsis; with sepsis and control patients. They were prospectively followed and their laboratory and immunological workup (at days 1 and 9) was done to measure serum sRAGE levels and detect (sRAGE) genotypes.
The age of the included children ranged from 8 to 84 months. Plasma level of sRAGE was significantly higher in plasma from patients with ALI regardless of associated sepsis. Plasma sRAGE levels were positively correlated with lung injury score. When assessing sRAGE genotypes, TA and TT genotypes were significant in most of the ALI with and without sepsis patients.
Monitoring levels of sRAGE and genotypes can significantly affect the survival of ALI children.
Keywords: biomarker, outcome, pediatric intensive care unit (PICU), sRAGE genotypes
1. Introduction
In developing countries, data from population-based epidemiologic studies of critical illness suggest that 0.86% to 7.8% of pediatric intensive care unit (PICU) admissions are due to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Both have a major impact on PICU patients’ morbidity and mortality rates; thus, a substantial impact on human health.[1] ALI cases start as an acute disease then persist for days up to weeks. It is characterized by arterial hypoxemia resistant to oxygen therapy alone, associated with one or more known risk factors that necessitate PICU admissions like sepsis or aspiration.[2]
Recent advances in medicine and biomedical technology have increased the likelihood of diagnosis and treatment of such diseases. Developing protocols for diagnosis has proved to improve outcomes and reduce expenses.[3,4] Such protocols include clinical and laboratory diagnostic tools.[4]
The laboratory diagnostic criteria include exploring biomedical markers, thus enabling early diagnosis and prompt treatment. At this point; arises the importance of the soluble form of receptor for advanced glycation end products (sRAGE) as a biomarker of pulmonary tissue damage.[5] The receptor for advanced glycation end products RAGE is an important factor amplifying the immune and inflammatory responses in several pathophysiological conditions. The sRAGE is the soluble isoform of RAGE which is endogenously secreted in lung and other organs. After release, sRAGE may locally interact with ligands and/or extracellular matrix acting as an autocrine modulator of RAGE pathway. According to; cell type, pathophysiological and genetic factors, sRAGE is expressed in a highly variable regulated pattern. During lung injury, depending on the intensity level of injury, sRAGE is secreted into the broncho-alveolar lavage (BAL) or even to the plasma. Thus, it can be considered a reliable biomarker which could enhance the diagnostic accuracy of pulmonary tissue injury.[6] However, it is important to point out that non-pulmonary sources of sRAGE, for example, cardiovascular diseases, oxidative stress may contribute to plasma levels in cases of ALI.[7]
Identification of genetic variants in select candidate genes has enhanced our understanding of the specific pathways involved in susceptibility, severity, and manifestation of ALI, and the more severe ARDS. RAGE gene is located on chromosome 6p21.3 in the major histocompatibility locus which encodes for multiple factors critically involved in immune and inflammatory responses.[8] The commonest 2 functional polymorphisms that correlated to individual susceptibility to RAGE-mediated pathogenesis were (–429T/C and –374T/A). When comparing studies from an ethnically different population, detection of genetic factors may be important. This reflects inconsistencies in phenotype definition as well as the profound role that environmental factors play as key determinants in ALI development and manifestation.[9] Within specific genetic context, the correlation between circulating sRAGE and inflammatory cytokines reflects inherited tissue RAGE expression rather than endogenous protective response.[8,9]
The present study primarily aims to assess sRAGE as a valid biomarker for diagnosis of ALI among critically ill, pediatric patients in addition to correlating levels of sRAGE and different outcomes of those patients.
2. Materials and methods
2.1. Study design, period, and setting
A prospective, observational case-control study, was conducted at 2 PICUs at Cairo University Pediatric Hospital (CUPH), a university-affiliated teaching hospital in Egypt. The PICUs include 23 and 14 beds, with the same protocol of management. The study took place over a period of 6 months starting from January 2017.
2.2. Working definitions
ARDS is defined by the following criteria: acute onset hypoxemia manifested by a partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio ≤200 in the presence of bilateral infiltrates on chest radiographs and the absence of left atrial hypertension. The concept of acute lung injury (ALI) was introduced when, under the same conditions, the PaO2/FiO2 ratio falls between 200 and 300.[10] In 2012, a new criterion for ARDS was developed, known as the Berlin definition; integrating ALI as a mild subgroup of ARDS based on the degree of observed hypoxemia (mild, moderate, and severe). However, this definition still did not consider the pediatric population.[11] The Pediatric Acute Lung Injury Consensus Conference (PALICC) defined pediatric acute respiratory distress syndrome (PARDS), through the following criteria: age group: (the neonatal period through adolescence), timing: (onset of hypoxemia and radiological changes), myocardial dysfunction: (children with heart disease are not excluded), chest radiographs, and finally hypoxemia.[12]
The severity of lung illness was assessed on the basis of lung injury score which ranges from 0 to 4 with higher scores indicating more severe lung injury.[4] Sepsis was defined as suspected infection in the presence of ≥2 systemic inflammatory response syndrome (SIRS) criteria.[13]
The sequential organ failure assessment (SOFA) score was used for prediction of outcome and indirect assessment of disease severity. Giving a possible score of 0 to 24, higher scores indicate more severe disease. An increase in SOFA score during the first 48 hours in the PICU, predicted a mortality rate of at least 50%, while a decrease was associated with a PICU mortality rate of just 27%.[14]
2.3. Study sample
A total of 68 patients, admitted to the PICUs of CUPH were prospectively enrolled in the study within 24 hours of disease onset, with the following inclusion criteria:
Children aged >1 month up to 14 years were classified into 4 groups as follows: patients who met criteria for ALI only without sepsis n = 18; patients who met criteria of both ALI and sepsis n = 15; patients with sepsis only n = 15; and patients who served as controls n = 20. Controls were recruited from patients admitted to PICU with diseases as Guillain Barre syndrome, respiratory muscle paresis.
Exclusion criteria were: neonates who are <1 month of age, patients after an operative procedure. Only patients with immunodeficiency or concomitant immunosuppressive therapy, cardiac arrest, acute exacerbation of diabetes, chronic renal failure, rheumatoid arthritis, and malignant tumors.
2.4. Data collection
2.4.1. A data collection form was designed to collect and write down the following
Demographic characteristics (age, sex, race, address), the state of patients upon admission (primary diagnosis, associated comorbidity), results of laboratory and immunological workup, and clinical data details. Length of PICU stay (in days) was recorded. The clinical and outcome data of the patients during PICU stay (survived or died) were noted.
2.4.2. Clinical Data
After inclusion in the study, clinical data were obtained for all patients at the selected time points. Clinical outcome was recorded on day 1 and day 9 or discharge from the PICU, whichever occurred first.
The choice of timing of samples collection was at day 1 the day of admission, that is, initial assessment of the cases and controls, while day 9 represents the median maximum length of PICU stay of control patients.
Calculation of SOFA and lung scores is done for routine assessment following patients’ admission to the PICUs and for the first 24 hours following diagnosis. The pulmonary diagnostic criteria included the criteria of acute hypoxemia. Vital signs and ventilator parameters as tidal volume, positive end expiratory pressure (PEEP), respiratory rate, the partial pressure of arterial oxygen to the fraction of inspired oxygen PaO2/FiO2 were monitored.
2.4.3. Laboratory and immunological workup
Laboratory data were obtained for all patients at the selected time points. They included: complete blood count, C reactive protein, and blood culture for suspected sepsis cases. Blood gases for PaO2/FiO2. The sRAGE assessment was used for early diagnosis of ARDS.
Plasma levels of sRAGE were measured using enzyme-linked immunosorbent assay (ELISA) kits. sRAGE polymorphism, single nucleotide polymorphism (SNP) (–429T/C and –374T/A) were measured by polymerase chain reaction (PCR)-RFLP technique.
2.4.4. Specimen collection
Three milliliter of venous blood samples were collected from all participants and divided between 2 plain Vacutainer tube for serum sRAGE level measurement and for CRP measurement. Serum was separated and stored at –70 °C till analysis, also 3 mL of venous blood samples were collected from all participants on EDTA Vacutainer tube for complete blood count and DNA extraction and sRAGE polymorphism SNP (–429T/C and –374T/A) detection using PCR-RFLP technique, extracted DNA was stored at –70 °C until analysis. One milliliter of arterial blood was collected from all participants on the heparinized syringe for blood gases. Blood sample for blood culture for only suspected sepsis cases.
2.4.5. Serum levels of sRAGE
Serum sRAGE was measured using a commercially available BioVendor sRAGE (ELISA Kit, cat # RD191116200R).
2.4.6. Genomic DNA extraction and SNP (–429T/C and –374T/A) polymorphism analysis
Genomic DNA was extracted from peripheral blood leucocytes using the QIA amp DNA Blood mini KIT (Qiagen Inc., Germany). Enzymatic amplification of SNP (–429T/C and –374T/A) was done as proposed by Hudson et al,[15] simultaneously amplification of the –429 T/C, −374 T/A using the primers (F 5′-GGGGGCAGTTCTCTCCTC-3′ R 5′-TCAGAGCCCCCGATCCTATTT-3′). Primers used to amplify from –590 to –246 (total PCR product = 344 bp).
The (PCR) amplification was performed in 25 μL reaction volume containing genomic DNA samples (100 ng), 200 μmol/L dNTPs, 1.5 mM MgCl2, 1× Taq polymerase buffer, 50 pmol of each primer, and 0.5 unit of Taq DNA polymerase. The reaction was performed in a Hybaid thermal cycler (Promega Corporation, 2800 Woods Hollow Road, Madison, WI 153711–5399), programmed as follows: initial denaturation at 94 °C for 5 minutes, 30 cycles of denaturation at 94 °C for 1 minute, annealing at 56 °C for 1 minute, and extension at 72 °C for 1 minute each followed by a 5 minute final extension at 72 °C the PCR product was digested with 3U Alu I for 6 hours at 37 °C followed by overnight digestion with 3U Tsp509 I at 65 °C. Product were resolved on 3% agarose gel at 100 V for 2 hours.
2.5. Ethical disclosures
2.5.1. Protection of human subjects:
The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee at the Faculty of Medicine, Cairo University and with those of the World Medical Association and the Helsinki Declaration of biomedical ethics.
Informed consent was obtained directly from the legal guardian of each patient before sample withdrawal and data collection, after explanation of the study objectives.
2.6. Statistical analysis
Collected data were entered and analyzed using the Statistical Package for Social Science Software (SPSS) program, version 21.0 IBM. Data were summarized using the median and interquartile range for quantitative variables. Box and whisker plots were used to show the evolution of sRAGE levels over time. Comparison between groups was performed using Kruskal–Wallis and Chi-square tests. Receiver operating characteristics (ROC) curve analysis was performed to explore the discriminate ability of sRAGE in predicting ALI. Kaplan–Meier survival function was generated to explore the survival pattern among different groups. P values below .05 were considered statistically significant. Scatter plot diagram was constructed to detect the correlation between the lung and PaO2/FiO2 scores and sRAGE.
3. Results
The study participants were all of the same race (Egyptian). Respiratory diseases were the main diagnosis among the 3 case groups while among the control group were central nervous system diseases (Table 1).
Table 1.
Baseline patient's clinical characteristics, comorbidities, and main outcome of the study groups.
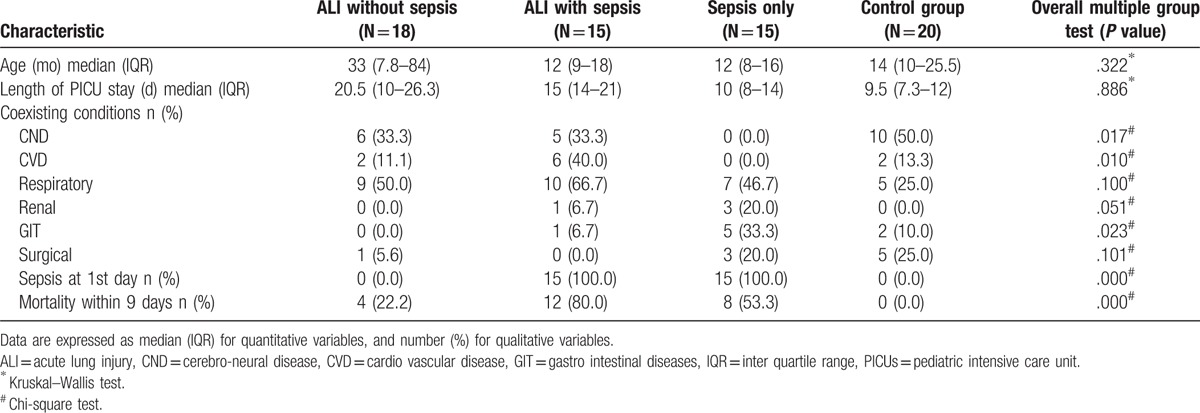
The severity of lung illness measured by lung score was significantly higher among the ALI with and without sepsis (P < .001). The severity of illness assessed by SOFA score was significantly higher among the ALI with sepsis than both ALI and Sepsis groups (P < .001). Meanwhile, the severity of lung pathology assessed by PaO2/FiO2 score was significantly higher among sepsis and control groups; than both ALI groups with and without sepsis (P < .001) (Table 2). Mortality rate was significantly higher among ALI with sepsis group (80%) followed by sepsis group (53.3%) (P < .001) (Table 1).
Table 2.
Baseline respiratory parameters and sRAGE levels among the study groups.
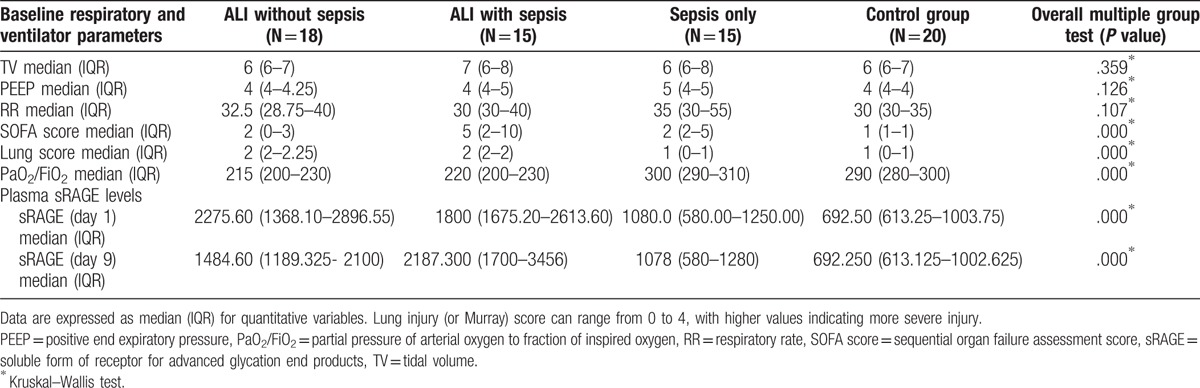
On day 1, plasma sRAGE levels were significantly higher in plasma from patients with ALI regardless of associated sepsis (P < .001). The highest plasma sRAGE levels were in the ALI without sepsis group (Table 2).
While in day 9, the reverse occurred, plasma sRAGE levels were significantly higher in the ALI with sepsis group more than ALI without sepsis (P < .001) (Table 2).
Box and whisker plots showed the evolution of sRAGE levels over time, wherein ALI without sepsis group, plasma sRAGE levels decreased in a significant manner from baseline on day 1 to day 9 (P = .014) (Fig. 1A and B)
Figure 1.
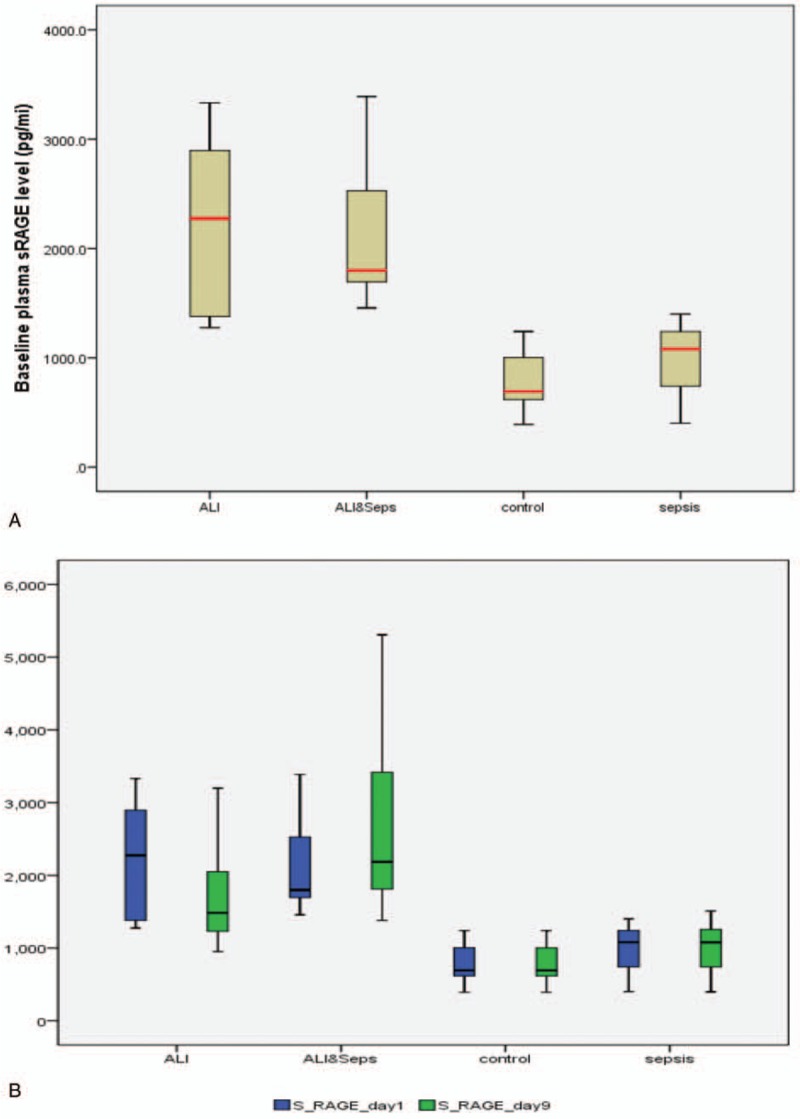
A: Box and whisker plots of baseline form of sRAGE Levels. B: Box and whisker plots of evolution of sRAGE levels over time in patients with acute lung injury (ALI group), ALI + sepsis (ALI + sepsis group), (sepsis group), and mechanically ventilated patients without ALI or sepsis (control group). Boxes show medians with inter-quartile ranges. ALI = acute lung injury, sRAGE = soluble form of receptor for advanced glycation end products.
Scatter plot diagram showed a positive, moderate, and significant linear relationship between sRAGE and lung score (r = 0.632; P < .001). While negative correlation between sRAGE and PaO2/FiO2 ratio was observed (r = –0.685; P < .001) (Fig. 2). ROC curve analysis revealed the significant predictive ability (P < .001) of the relative concentration of plasma sRAGE levels at day 1 (95% confidence interval [CI], 1–1) and day 9 (95% CI, 0.968–1) to differentiate between the presence and absence of ALI in patients with sepsis (Fig. 3). Survival analysis illustrated that mortality pattern among ALI with sepsis group and in the sepsis group was significantly higher compared with control and ALI group (Log Rank = 23.027; P = .000) (Fig. 4).
Figure 2.
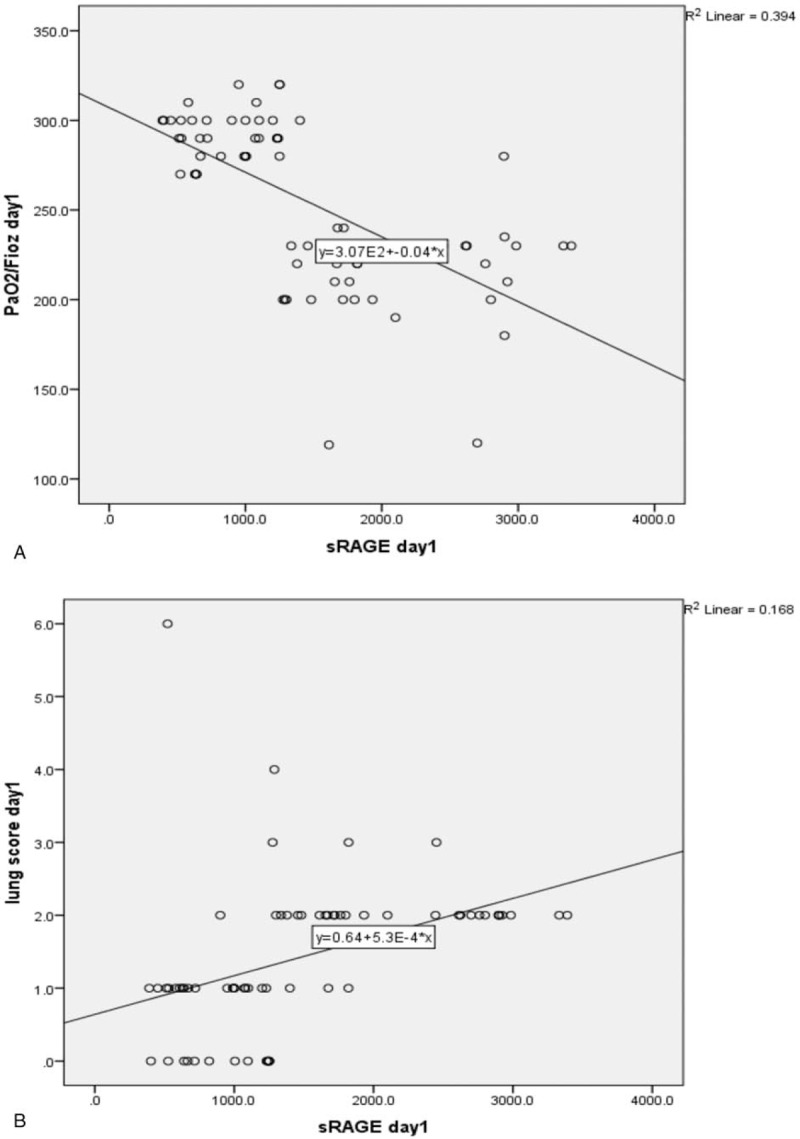
A: PaO2/FiO2 ratio versus plasma (sRAGE) level (pg/mL) in acute lung injury patients with and without sepsis (Spearman's rho correlation coefficient = –0.685; P ≤ .001). B: Lung injury score versus plasma sRAGE levels (pg/mL) in acute lung injury with and without sepsis and control. (Spearman's rho correlation coefficient = 0.632; P ≤ .001). PaO2/FiO2 = partial pressure of arterial oxygen to fraction of inspired oxygen, sRAGE = soluble form of receptor for advanced glycation end products.
Figure 3.
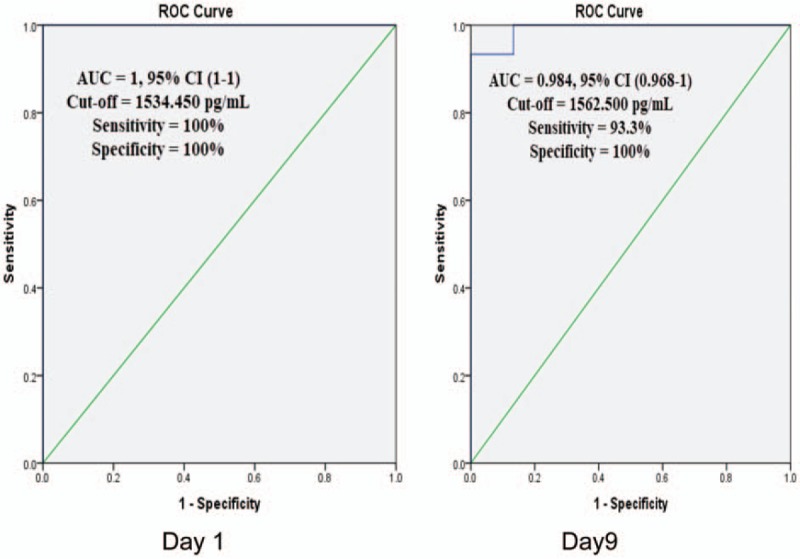
ROC curve: Receiver-operating characteristic curve of baseline plasma sRAGE levels in differentiating between the presence and absence of ALI, in day1 and day 9. ALI = acute lung injury, sRAGE = soluble form of receptor for advanced glycation end products.
Figure 4.
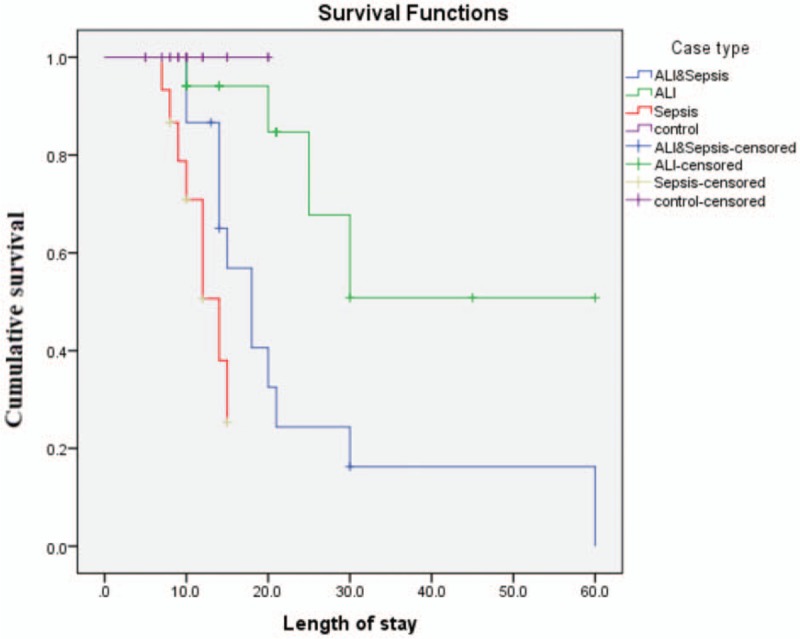
Kaplan–Meier curve: Cumulative survival curve of patients relative to case type. Log rank (Mantel–Cox) = 23.027; P ≤ .001).
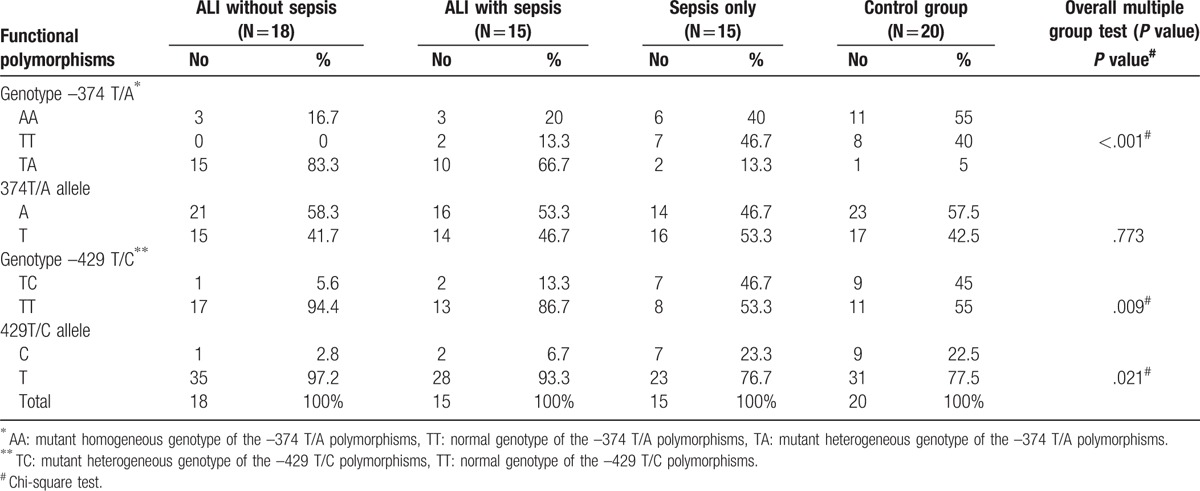
Distribution of genotypes of the main functional polymorphisms of sRAGE genes among the 4 study categories revealed that in the functional polymorphism –374T/A, TA genotype (mutant heterogeneous genotype) was significantly the main component (83.3%) of the ALI group and 66.7% of the ALI with sepsis group (P < .001). Whereas in the functional polymorphism –429T/C, TT genotype (normal genotype) was significantly the main component (94.4%) of the ALI group and 86.7% of the ALI with sepsis group (P = .009) (Table 3).
Table 3.
Distribution of the genotypes of the main functional polymorphisms of sRAGE genes among the 4 study groups.
4. Discussion
The current study was done to demonstrate the importance of diagnostic role of sRAGE for ALI pediatric patients admitted to PICU, meanwhile measuring the baseline level of sRAGE, and monitoring it over time will identify patients at risk of developing ALI. Thus, starting early therapy which could markedly affect their prognosis and survival.[16] Several studies support the evidence that cellular damage is sustained by RAGE-mediated pathogenesis, which eventually results in increased sRAGE generation and release.[16,17]
Many studies mentioned the fact that the primary source of plasma sRAGE in ALI patients is alveolar type 1 cells.[18] In the current study, higher levels of plasma sRAGE in the first day (baseline) following admission among ALI patients with and without sepsis may reflect higher endogenous protective response. This goes in accordance with a similar study done by Yehya et al[19], where sRAGE was one of the biomarkers which reflected the endothelial and alveolar epithelial dysfunction underlying PARDS.
When comparing day 1 to day 9; plasma sRAGE levels decreased in a significant manner. This goes in accordance with another study conducted in general ICU of Clermont-Ferrand University Hospital, France; which demonstrated that plasma sRAGE levels decreased over time and was associated with decreasing ALI severity evidenced by PaO2/FiO2 and lung injury score.[20]
The severity of pulmonary tissue injury assessed by lung injury score was significantly higher among the ALI regardless of sepsis. As acute hypoxia which is a major clinical criterion in ALI cases is manifested by high lung and low PaO2/FiO2 score, where both denotes worse lung condition.[1,2]
Meanwhile; plasma sRAGE levels were positively correlated with lung injury score but had a negative relation with the PaO2/FiO2 score. This finding goes in agreement with a recent study which showed that plasma sRAGE, correlated with worsening PALICC oxygenation categories.[19] Such findings support an alveolar epithelial source of sRAGE during ALI. Its decrease over time, along with the improvement in clinical and radiological parameters denotes resolution of the alveolar epithelium.
Also, the study showed that lung oxygenation measured by PaO2/FiO2 score was significantly higher among sepsis and control groups. Since the main pathology isn’t in the lung but related to other coexisting conditions. Nevertheless, the nonpulmonary sources of sRAGE particularly sepsis remains a perplexing factor when evaluating plasma levels in ALI patients.[21] Patients who suffered from sepsis only, showed no difference in plasma sRAGE over time, also after 9 days from PICU admission where the higher level of plasma sRAGE was in the group of ALI with sepsis. This goes with the results of other studies which showed a correlation between organ failures and sRAGE in septic, critically ill patients and coincides with Jabaudon et al,[20] who emphasized that sepsis alone, in the absence of ALI, does not result in increased sRAGE levels. Elevation of sRAGE in patients with ALI associated with sepsis is a specific indication of ALI.[22] This goes with the studies which proved that RAGE is implicated in leukocyte recruitment, which is the main event in sepsis patient. In the study done by Chavakis et al,[23] demonstrated that RAGE is a central player in inflammation. RAGE serves as a novel counter-receptor for the leukocyte integrin Mac-1, which is plunged in recruitment of leukocytes.
The significant predictive ability of the relative concentration of plasma sRAGE levels at day 1 and day 9 to differentiate between the presence and absence of ALI was illustrated by ROC curve analysis. The cut-off values of sRAGE in the current study were mostly different from cut-off values in other studies.[20] Such differences are attributed to statistical parameters that must be fulfilled before comparing cut-offs points from different populations in different studies bearing in mind that a derivation cohort will show biomarker test characteristics better than a validation cohort. Also, the difference in sample size may play a role. There is no uniform figure that can be applied across studies. It is generally agreed that well-powered association studies are based on hundreds of samples.[24]
Analysis of survival curve assessing cumulative survival of the study participants provided an evidence that non survivors were higher among ALI with sepsis patients compared with other groups. This coincides with a recent study showing that non-survivors patients who suffered from PARDS (a subcategory of PALI) had worse organ failures score.[19] Furthermore, Calfee et al,[5] highlighted that the baseline plasma sRAGE was strongly related to the clinical outcomes in the ALI patients. This points out to a direct association between higher baseline plasma sRAGE and increased rate of mortality and decreased ventilator-free and organ failure—free days.
The novel aspect of the current study is exploring the relationship between RAGE genetic polymorphisms and acute lung injury. In the current study, all cases and controls were of the same race belonging to Eastern Mediterranean region. The TA (mutant heterogeneous genotype) and the TT (normal genotype) were significantly the main components in most of the ALI with and without sepsis. This suggests the probability of linking those genotypes with high expression of sRAGE among ALI patients. Therefore, we can assume that these 2 RAGE polymorphisms determine sRAGE production within the complex RAGE pathway, resulting in not only different individuals’ susceptibility but also different population susceptibility to RAGE-mediated pathogenesis.[8] It is important to bear in mind that genetic factors are variable particularly when comparing results from racially different populations. With this information, population-based studies should be initiated to determine the real predictive value of these biomarkers.[25]
In the study done by You et al[26]; who investigated the existence of an association among 3 polymorphisms (–374T/A, –429T/C, and G82S) of the RAGE gene with the risk of chronic obstructive pulmonary disease (COPD) among Chinese population; results revealed that the genetic variant of the RAGE gene (G82S) is associated with increased risk of developing COPD. The identification of genetic factors associated with susceptibility, severity, and outcome/recovery of ALI constitutes a necessary step toward personalized treatment. Because the variation in recovery between ALI patients whether during the acute or the subacute phase may be related partially to the underlying genetic characteristics of the individual patient.[27]
4.1. Limitation of the study
Generalization of the findings of this study is hindered by the relatively small sample size and relatively short study duration. In addition to the limited number of patients who suffered only from ALI, since many patients were excluded due to their comorbid conditions like diabetes, and cardiac conditions. High costs of the kits used to assess biomarkers and genotypes.
5. Conclusion
The study revealed that plasma level of sRAGE was significantly higher in plasma from patients with ALI regardless of associated sepsis.
A positive, significant linear relationship between sRAGE and lung score was detected. Also, the study revealed the predictive ability of the concentration of plasma sRAGE over time.
Therefore, it is of great importance to monitor the levels sRAGE to identify patients at risk and to start early treatment for better survival.
Studies showed that treatment of RAGE-mediated inflammatory damage by therapy which can block the ligand pathways must be preceded by identifying biomarkers to monitor these critical pathways.[28,29]
Acknowledgments
The authors are thankful to all the nursing staff in the PICUs who helped the researchers during the data collection process. The research team also thanks all the patients’ relatives.
Footnotes
Abbreviations: AA = mutant homogeneous genotype of the –374 T/A polymorphisms, ALI = acute lung injury, ARDS = acute respiratory distress syndrome, BAL = broncho-alveolar lavage, COPD = chronic obstructive pulmonary disease, CUPH = Cairo University Pediatric Hospital, ELISA = enzyme-linked immunosorbent assay, ICU = intensive care units, PALI = pediatric acute lung injury, PALICC = Pediatric Acute Lung Injury Consensus Conference, PaO2/FiO2 = partial pressure of arterial oxygen to fraction of inspired oxygen, PARDS = pediatric acute respiratory distress syndrome, PCR = polymerase chain reaction, PEEP = positive end expiratory pressure, PICUs = pediatric intensive care units, RAGE = receptor for advanced glycation end products, ROC = receiver operating characteristics, RR = respiratory rate, SIRS = systemic inflammatory response syndrome, SNP = single nucleotide polymorphism, SOFA = sequential organ failure assessment, SPSS = Statistical Package for Social Science, sRAGE = soluble form of receptor for advanced glycation end products , TA = mutant heterogeneous genotype of the –374 T/A polymorphisms, TC = mutant heterogeneous genotype of the –429 T/C polymorphisms, TT = normal genotype of the –374 T/A polymorphisms, TV = tidal volume.
Authorship contribution: Each author has made a substantial contribution to the following:
JRL: the conception and design of the study, acquisition of data, drafting the article, or revising it critically for important intellectual content, final approval of the version to be submitted and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
SKI: acquisition of data, drafting the article, and final approval of the version to be submitted.
HMS: acquisition of data, drafting the article, and final approval of the version to be submitted.
MMI: acquisition of data, drafting the article, and final approval of the version to be submitted.
SAMA: analysis and interpretation of data, drafting the article, and final approval of the version to be submitted.
MRS: analysis and interpretation of data, drafting the article, and final approval of the version to be submitted.
AAA: acquisition of laboratory data, drafting the article, and final approval of the version to be submitted.
HA: acquisition of laboratory data, drafting the article, and final approval of the version to be submitted.
Source of funding: This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
There are no conflicts of interest.
References
- [1].Barreira ER, Munoz GO, Cavalheiro PO, et al. Epidemiology and outcomes of acute respiratory distress syndrome in children according to the Berlin definition: a multicenter prospective study. Critical Care Med 2015;43:947–53. [DOI] [PubMed] [Google Scholar]
- [2].Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–93. [DOI] [PubMed] [Google Scholar]
- [3].Vallyathan V, Repine JE. Vallyathan V, Castranova V, Shi X. Oxygen/nitrogen radicals: lung injury and disease (Lung biology in health and disease): acute respiratory distress syndrome and oxidative stress. Mechanisms of Disease Development and Opportunities for Antioxidant Prevention. Vol 187. New York: Marcel Dekker; 2004. 393–412. [Google Scholar]
- [4].Children Hospital Association [Internet]: Quality and Safety in Children Health Conference. Pediatric Sepsis Old Controversies, Contemporary Data, and New Metrics; 2015. Available at: http://childrenshospitals.net/quality15. Accessed July 26, 2017.
- [5].Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end-products and clinical outcomes in acute lung injury. Thorax 2008;63:1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schmidt AM, Yan SD, Yan SF, et al. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001;108:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Narvaez-Rivera RM, Rendon A, Salinas-Carmona MC, et al. Soluble RAGE as a severity marker in community acquired pneumonia associated sepsis. BMC Infect Dis 2012;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Santilli F, Vazzana N, Bucciarelli LG, et al. Soluble forms of RAGE in human diseases: clinical and therapeutical implications. Curr Med Chem 2009;16:940–52. [DOI] [PubMed] [Google Scholar]
- [9].Gao L, Barnes KC. Recent advances in genetic predisposition to clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol 2009;296:L713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bernard GR, Artigas A, Brigham KL, et al. Roger Spragg and the consensus committee. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149(3 Pt 1):818–24. [DOI] [PubMed] [Google Scholar]
- [11].Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- [12].Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2015;16:428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–74. Internet Resources Bibliographic Links. [PubMed] [Google Scholar]
- [14].Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care 2010;14:207.Published online 2010 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hudson BI, Stickland MH, Futers TS, et al. Effects of novel polymorphisms in the RAGE gene on transcriptional regulation and their association with diabetic retinopathy. Diabetes 2001;50:1505–11. [DOI] [PubMed] [Google Scholar]
- [16].Mukherjee TK, Mukhopadhyay S, Hoidal JR. The implication of receptor for advanced glycation end product (RAGE) in pulmonary health and pathophysiology. Respir Physiol Neurobiol 2008;162:210–5. [DOI] [PubMed] [Google Scholar]
- [17].Zhang H, Tasaka S, Shiraishi Y, et al. Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am J Respir Crit Care Med 2008;178:356–62. [DOI] [PubMed] [Google Scholar]
- [18].Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 2006;173:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yehya N, Thomas NJ, Meyer NJ, et al. Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Intensive Care Med 2016;42:1137–45. [DOI] [PubMed] [Google Scholar]
- [20].Jabaudon M, Futier E, Roszyk L, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med 2011;39:480–8. [DOI] [PubMed] [Google Scholar]
- [21].Bopp C, Hofer S, Weitz J, et al. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res 2008;147:79–83. [DOI] [PubMed] [Google Scholar]
- [22].Matsumoto H, Matsumoto N, Ogura H, et al. The clinical significance of circulating soluble RAGE in patients with severe sepsis. J Trauma Acute Care Surg 2015;78:1086–93. [DOI] [PubMed] [Google Scholar]
- [23].Chavakis T, Bierhaus A, Al-Fakhri N, et al. The pattern recognition receptor (RAGE) is a counter receptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med 2003;198:1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schaid DJ. Power and sample size for testing associations of haplotypes with complex traits. Ann Hum Genet 2006;70(Pt 1):116–30. [DOI] [PubMed] [Google Scholar]
- [25].Christie JD, Ma SF, Aplenc R, et al. Variation in the myosin light chain kinase gene is associated with the development of acute lung injury after major trauma. Crit Care Med 2008;36:2794–800. [DOI] [PubMed] [Google Scholar]
- [26].Li Y, Yang C, Ma G, et al. Association of polymorphisms of the receptor for advanced glycation end products gene with COPD in the Chinese population. DNA Cell Biol 2014;33:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].MacLaren R, Stringer KA. Emerging role of anticoagulants and fibrinolytic in the treatment of acute respiratory distress syndrome. Pharmacotherapy 2007;27:860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yehya N, Topjian AA, Thomas NJ, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med 2014;15:e147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med 2015;43:937–46. [DOI] [PubMed] [Google Scholar]


