Abstract
Few studies have investigated the relative safety of prescribing isometric exercise (IE) to reduce resting blood pressure (BP). This study aimed to ascertain the safety of the hemodynamic response during an IE wall squat protocol.
Twenty-six hypertensive (BP of 120–139 mm Hg systolic and/or 80–90 mm Hg diastolic) males (45 ± 8 years; 1.78 ± 0.07 m; 89.7 ± 12.3 kg; mean ± SD), visited the laboratory on 2 separate occasions. Heart rate (HR) and BP were measured at rest and continuously throughout exercise. In visit 1, participants completed a continuous incremental isometric wall squat exercise test, starting at 135° of knee flexion, decreasing by 10° every 2 minutes until 95° (final stage). Exercise was terminated upon completion of the test or volitional fatigue. The relationship between knee joint angle and mean HR was used to calculate the participant-specific knee joint angle required to elicit a target HR of 95% HRpeak. This angle was used to determine exercise intensity for a wall squat training session consisting of 4 × 2 minute bouts (visit 2).
Systolic BPs during the exercise test and training were 173 ± 21 mm Hg and 171 ± 19 mm Hg, respectively, (P > .05) and were positively related (r = 0.73, P < .05) with ratio limits of agreement (LoA) of 0.995 ×/÷ 1.077. Diastolic BPs were 116 ± 14 mm Hg and 113 ± 11 mm Hg, respectively, (P > .05) and were positively related (r = 0.42, P < .05) with ratio LoA of 0.99 ×/÷ 1.107. No participant recorded a systolic BP > 250 mm Hg. Diastolic BP values > 115 mm Hg were recorded in 12 participants during the incremental test and 6 participants during the training session. Peak rate pressure product was 20681 ± 3911 mm Hg bpm during the IE test and was lower (18074 ± 3209 mm Hg bpm) during the IE session (P = .002). No adverse effects were reported.
Based on the current ACSM guidelines for aerobic exercise termination, systolic BP does not reach the upper limit during IE in this population. Diastolic BP exceeds 115 mm Hg in some during the IE protocol, which may suggest the need to individualise IE training prescription in some with suboptimal BP control. Future research is required to ascertain if IE requires modified BP termination guidelines.
Keywords: exercise training, incremental test, isometric, squat, static
1. Introduction
The concept of performing regular moderate to vigorous intensity exercise for cardiovascular health has traditionally tended to take the form of aerobic exercise such as walking, jogging, cycling, or swimming. More recently, dynamic resistance exercise has been promoted in combination with aerobic training with the primary aim of maintaining/improving muscular fitness.[1] Exercise recommendations for those with controlled hypertension and no other overt cardiovascular disease or renal complications remain essentially the same.[2,3] However, some argue that resistance exercise in hypertensive populations should be avoided, since the blood pressure (BP) response may exceed safe limits.[4] Currently, there are no definitive position statement guidelines for the use of isometric exercise (IE), a specific form of resistance training, in relation to any adult population.
However, a growing body of evidence clearly demonstrates that isometric exercise training (IET) is capable of lowering resting BP[5–7] equally in both normotensive males and females[8] and also in those with suboptimal BP.[9] Indeed, in a recent meta-analysis[10] IET produced greater reductions in systolic and diastolic BP (10.9 mm Hg and 6.2 mm Hg, respectively) compared to aerobic and dynamic resistance exercise training (3.5 and 1.8 mm Hg in systolic BP and 2.5 and 3.2 mm Hg in diastolic BP, respectively; with no significant differences between these individually or when performed in combination upon resting BP). This capacity for IET to lower resting BP to a greater extent than other modes of exercise training has subsequently been reaffirmed by Inder et al.[11] To contextualise these findings, a 10 mm Hg and 5 mm Hg reduction in systolic BP and diastolic BP, respectively, is associated with a 40% lower risk of stroke mortality and 30% lower risk of mortality from ischaemic heart disease or other vascular causes.[12]
Additionally, IET has long been shown to be comparable to dynamic resistance training exercise in terms of the development of muscle hypertrophy,[13] maximal tetanic tension and peak rate of tension[14] and muscular/strength endurance.[15] Furthermore the benefits of IET in terms of its potential ease of access and use (c.f. Wiles et al).,[16] especially for those with co-morbidities that may restrict movement capacity, mean that it could provide a viable option to those unable or unwilling to participate in more traditional forms of exercise, but who would benefit from regular exercise involvement.
Despite this, there remains a continued reluctance amongst those responsible for public health recommendations for physical activity to promote the use of IE as a viable alternative to dynamic forms of exercise regardless of BP status.[3] Although a number of reasons may be responsible for this, one of the most striking acute physiological responses associated with IE is an exaggerated pressor response when compared to dynamic exercise of similar intensity.[17] This response may present a safety concern for the prescription of IE, especially for those with suboptimal BP control.[18] Whilst it has been shown that the acute rate of rise in cardiovascular variables such as BP, is proportional to the relative IE intensity and duration of the isometric contraction,[19,20] it is also clear that the exact nature of the cardiovascular response is also affected by other program variables that define the IE protocol; not least the mode of exercise used, for example, handgrip or leg extension exercise (hence muscle mass involved) and also the rest periods between bouts and the number of bouts involved. Upon examination of the literature it is evident that there are many unquantified IE protocols in terms of cardiovascular response currently being used in this area of research.[21] It is suggested that the dearth of published data quantifying the comparative BP and heart rate (HR) responses to the majority of IE protocols available only adds to the uncertainty of their relative safety when utilized in different populations.[22] Indeed, it is argued that the ability to accurately predict the expected cardiovascular response is fundamental for scientifically sound exercise prescription, especially in higher risk participants.
In an attempt to provide a clear evidence-based IET prescription, we recently demonstrated that an incremental isometric wall squat exercise (IWSE) test provides a reliable means of prescribing and monitoring IE intensity.[23] When using this protocol, individual exercise intensity during 4 × 2 minute IWSE bouts separated by 2 minutes recovery (composing a single IE session), resulted in mean systolic and diastolic blood pressure values during the final 30 seconds of each bout staying within current ACSM exercise termination guidelines[24] in normotensive participants. The use of this IET protocol in the home was also shown to result in statistically significant reductions in resting BP in healthy males.[25] However, there is little published data regarding the circulatory and BP responses during IE in those with elevated blood pressure.[22] Thus, whilst evidence suggests that IET has the potential to result in even greater reductions in resting BP in hypertensive populations compared to healthy participants,[9,11] only one previous paper has attempted to quantify the hemodynamic response during a specific IET protocol in medicated hypertensive patients.[26] However, hemodynamic responses were not recorded continuously and transitory measures may not adequately capture the true pressor response to IE. At present, there are no published data to support the safety of IET prescription in hypertensive populations free from the confounding effects of pharmacological intervention (or those newly diagnosed with hypertension who choose not to embark on a lifetime of medication) who potentially stand to benefit most from incorporating IET into their daily management regimen. It is crucial to provide an evidence-based approach to further promote the prescription of IET in these populations. Therefore, the aim of this study was to ascertain the safety of the cardiovascular pressor response during the recently validated IWSE protocol[23] in a hypertensive population.
2. Methods
Twenty-six physically inactive (<2.5 MET-h/week)[27] hypertensive males (45 ± 8 years; 1.78 ± 0.07 m; 89.7 ± 12.3 kg; mean ± SD) volunteered to take part in the study. All participants were nonmedicated, nonsmokers, and had a mean systolic BP of ≥120 and ≤140 mm Hg and/or diastolic BP of ≥80 and ≤90 mm Hg.[28] Inclusion in the study was subject to a normal cardiovascular examination and 12-lead electrocardiogram, determined by a consultant cardiologist and the satisfactory completion of a PAR-Q health and medical questionnaire. This investigation conformed to the Declaration of Helsinki and received institutional ethical approval. All participants provided signed informed consent before testing.
2.1. Experimental procedures
Prior to recruitment volunteers completed a 24 hour ambulatory blood pressure recording (Welch Allyn ABPM 6100, Welch Allyn, NY) to confirm hypertension. Systolic BP (sBP), diastolic BP (dBP), mean BP (mBP), and HR were measured every 20-minutes between 6:00 AM and 10:00 PM and every 30-minutes in the remaining time period. Those who were classified as hypertensive, using the criteria presented by Whelton et al[28] were then invited to take part in the second phase of the study. Participants attended the laboratory twice, with each visit separated by 48 hours and were required to fast for at least 4 hours prior to each laboratory visit, and abstain from caffeine and alcohol for 24 hours before each visit. All participants were required to maintain their normal circadian and dietary patterns and attend the laboratory at the same time of day.
2.2. Haemodynamic assessment
During both laboratory visits, HR and BP were recorded using the task force monitor (TFM), which is a validated noninvasive monitoring system.[29] Continuous beat-to-beat measurement of sBP, mBP, and dBP was recorded by use of the vascular unloading technique at the proximal limb of the index or middle finger, which was automatically corrected to oscillometric BP values obtained at the brachial artery of the contralateral arm. HR was recorded through a 6 channel electrocardiogram. Following 15 minutes of supine rest, baseline hemodynamic function was recorded continuously for 5 minutes. All measures were then recorded continuously throughout each stage of the incremental IE test, and during each 2 minute interval of the IE session. Hemodynamic parameters were then recorded during a 5 minute recovery period in the supine position immediately following the IE session. Intervention marks enable the separation of the cumulative data into independent stages of the IE session. Intervention marks were set at baseline, at each 2 minute exercise period and in recovery. All biological signals were recorded with a sample frequency of 1000 Hz and 16-bit resolution. This method of continuous hemodynamic recording has been previously utilised during isometric exercise.[30] Rate pressure product (HR × sBP), which is an index of myocardial oxygen demand (MVO2) was also calculated.[31]
2.3. Visit 1
2.3.1. Incremental isometric wall squat test
All participants then performed an incremental isometric wall squat test until competition of the protocol or volitional exhaustion. The test consisted of 5 consecutive 2-minute stages, and knee joint angle was manipulated to increase exercise intensity at each stage, which has been described in detail previously.[25] Knee joint angle, measured using a modified clinical goniometer, was established at 135° and participants were guided by the experimenter to reduce the angle by 10° every 2 minutes (125°, 115°, 105°, and 95°) (Fig. 1). Participants were required to rest their back against a fixed wall with their feet parallel, shoulder width apart, and their hands by their side. Participants were instructed to lower their back down a solid wall, and make small adjustments to their feet position until the required knee joint angle was reached whilst maintaining a vertical lower leg—confirmed at each stage using the spirit level attached to the stationary arm of the goniometer. To help ensure reliability between visits, each participants feet position was measured from the back of the left heel to the wall and their back position was measured as the distance from the ground to the coccyx, which was the lowest point of contact that the participants back had with the wall. Participants were not permitted to stand or rest between angles.
Figure 1.
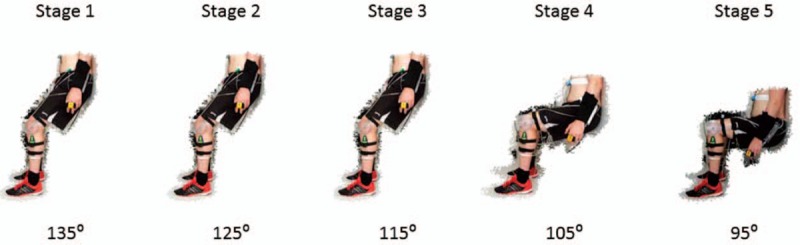
Knee joint angles used during the incremental isometric exercise test.
Prior research has demonstrated that knee joint angle produced an inverse curvilinear relationship with HR.[32] As such, following the incremental IWSE test, knee joint angle was plotted against mean HR for the last 30 seconds of each stage and the inverse curvilinear relationship produced was used to calculate each participants knee joint training angle that would elicit a target training HR of 95% peak HR (HRpeak—defined as the mean HR of the final 30 seconds achieved during the incremental test) as used in prior research.[25]
2.4. Visit 2
2.4.1. Isometric exercise session
The second visit took place a minimum of 48 hours after the first visit, and participants performed an IE session. Exercise intensity was prescribed as the knee joint angle that would elicit a target of 95% HRpeak in the incremental IWSE test (mean 106 ± 7 °). Based on the fact that knee joint angle produced linear relationships with both the feet (r = −1.00; P < .05) and back (r = 0.99; P < .05) positions,[25] measurements of heel and coccyx positioning, taken during the incremental test were used to give an indication of the positioning required to recreate the desired angle, which was confirmed using a goniometer as described above. Participants then completed an initial 10 second isometric wall squat exercise to ensure that the actual knee joint angle matched the prescribed angle. If the measured knee joint angle deviated from that prescribed their position was adjusted accordingly. The IE session then consisted of 4, 2 minute wall squats each separated by 2 minutes of recovery (see Fig. 2).
Figure 2.
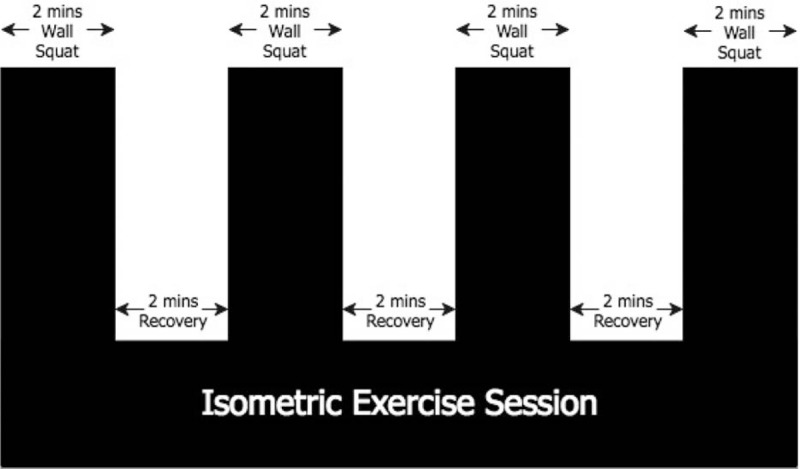
A schematic of the isometric exercise session.
2.5. Statistics
Unless otherwise stated, continuous variables are expressed as mean ± standard deviation. All data were analyzed using the statistical package for social sciences (SPSS 22 release version for Windows; SPSS Inc., Chicago IL). A paired samples t-test was used to assess BP changes from rest and differences between BP responses during the IE test and IE session. Peak BP was determined as the mean of the final 30 seconds during each continuous exercise period. Data were correlated using Pearson's correlation, and where there were repeated observations the technique outlined by Bland and Altman[33] was used. The ratio limits of agreement (LoA) between the 2 sessions was assessed according to Bland and Altman.[34] A P value of < .05 was regarded as statistically significant.
3. Results
Resting values for sBP, dBP, and mBP were 132 ± 6 mm Hg, 76 ± 8 mm Hg, and 94 ± 9 mm Hg, respectively. During the IE test, the peak BP values increased to 173 ± 21 mm Hg for sBP (P < .05) (range 139–211 mm Hg), 116 ± 14, mm Hg for dBP (P < .05) (range 85–127 mm Hg) and 140 ± 18 mm Hg for mBP (P < .05) (range 102–168 mm Hg). The IE test values were not significantly different from values obtained in the IE session for all parameters (sBP 171 ± 19 mm Hg, [range 140–210 mm Hg] dBP 113 ± 11 mm Hg [range 89–137 mm Hg] or mBP 135 ± 17 mm Hg [range 108–158 mm Hg], P > .05 in all cases). There were no statistically significant relationships between resting BP values and peak IE session responses for sBP (r = 0.31, P > .05), dBP (r = 0.36, P > .05) or mBP (r = 0.27, P > .05).
No participant in either the IE test or the IE session recorded sBP values > 250 mm Hg. Diastolic BP values > 115 mm Hg were recorded in 12 participants during the incremental IE test and in 6 participants during the IE session.
Ratio LoA between the BP values obtained during the IE test and the IE session were calculated to be 0.995 ×/÷ 1.077 for sBP, 0.99 ×/÷ 1.107 for dBP, and 0.985 ×/÷ 1.119 for mBP. Individual resting, IE test and IE session peak responses are shown in Figure 3A–C.
Figure 3.
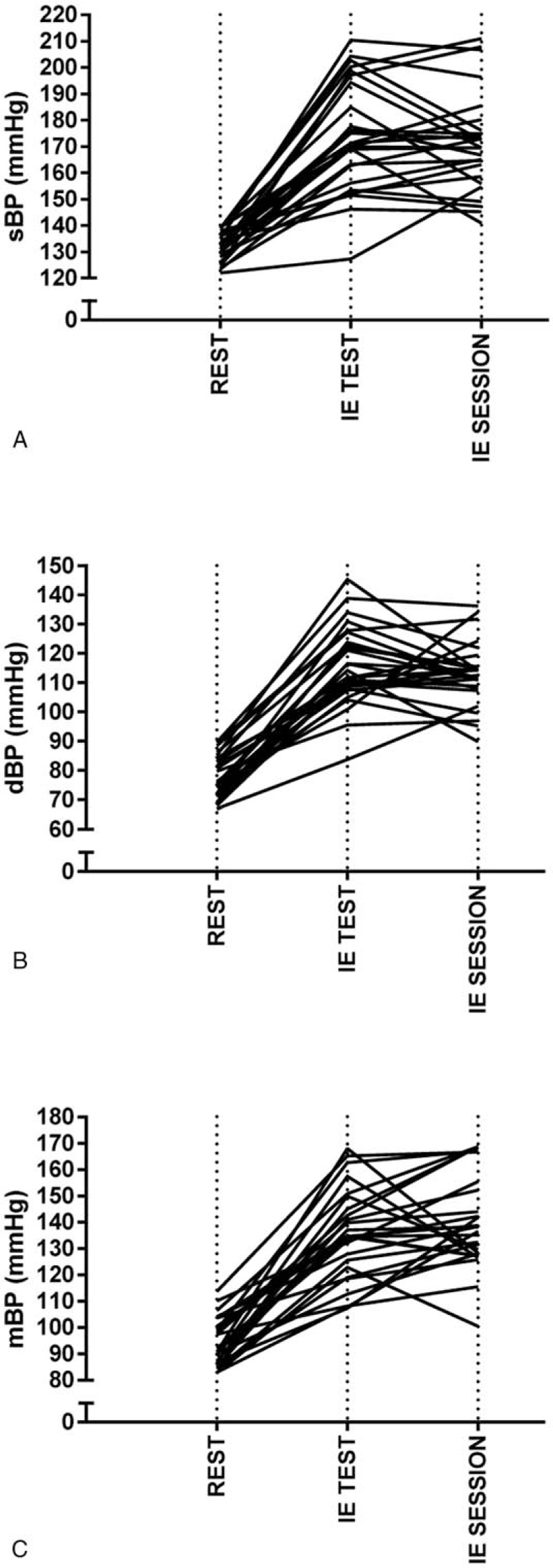
Individual resting, IE test (peak) and IE session (peak) responses for sBP in graph (A), dBP in graph (B), and mBP in graph (C). dBP = diastolic blood pressure, IE = isometric exercise, mBP = mean blood pressure, sBP = systolic blood pressure.
Analysis of the 4 IE session exercise bouts demonstrated significant increases in peak sBP as the bouts progressed from 1–4 (P < .05). For peak dBP, a rise of 2 mm Hg was not statistically significant (P = .37) between bouts 1 and 2; however, significant increases between bout 2–3 and bout 3 to 4 were found for peak dBP (P < .05). The individual response data demonstrated that in bout 1 and bout 2, 1 participant had a peak dBP reading over 115 mm Hg, and during bouts 3 and 4, 6 participants had peak dBP values above 115 mm Hg. Peak mBP responses were similar to dBP, a 3 mm Hg rise in mBP was not statistically significant between bouts 1 and 2 (P = .11), however significant increases were observed between bout 2–3 and bout 3–4 (P < .05). The bout data from the IE session is presented in Figure 4A–C.
Figure 4.
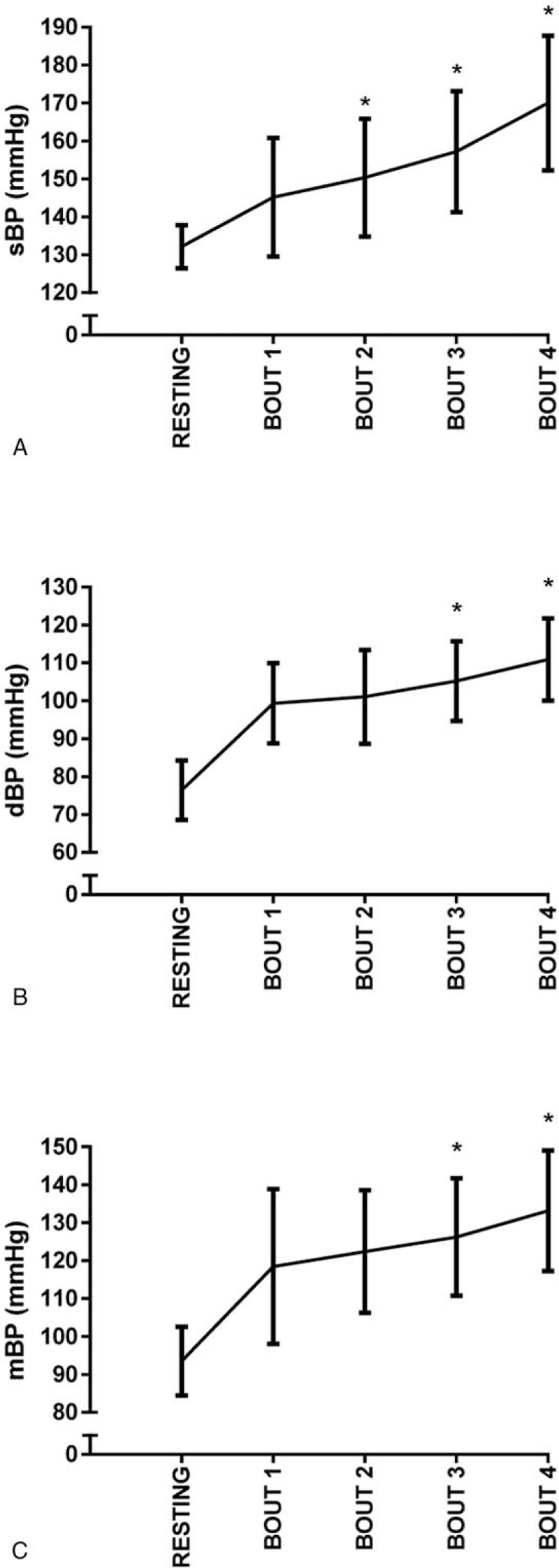
Bout data from the IE session is presented for peak sBP in graph (A), peak dBP in graph (B), and peak mBP in graph (C). dBP = diastolic blood pressure, IE = isometric exercise, mBP = mean blood pressure, sBP = systolic blood pressure.
The relationship between HR and the BP responses during exercise was assessed using correlations for repeated observations across the IE test and the IE session. There were strong positive relationships between HR and sBP (r = 0.81, P < .05), dBP (r = 0.89, P < .05), and mBP (r = 0.86, P < .05) during the incremental IE test. Using the same analysis technique, the relationship between HR and BP responses was explored during the 4 bouts of the IE session. This analysis revealed HR significantly increased between each bout, from 89 b min−1 during bout 1, to 94 b min−1, 101 b min−1 and 105 b min−1 in bouts 2, 3, 4. Moderate relationships between the HR and BP responses were observed for this analysis (sBP r = 0.51, dBP r = 0.47, and mBP r = 0.51, all P < .05). Resting values for RPP were 8071 ± 1332 mm Hg bpm and 8088 ± 1294 mm Hg bpm, prior to the IE test and the IE session respectively. During the IE test, the peak RPP value was recorded as 20681 ± 3911 mm Hg bpm; however, the peak value during the IE session was significantly lower (18074 ± 3209 mm Hg bpm) in comparison (P = .002). During the IE session RPP significantly increased between each exercise bout, from 12919 ± 2452 mm Hg bpm during bout 1, to 14086 ± 2957 mm Hg bpm, 15836 ± 3032 mm Hg bpm and 17859 ± 3037 mm Hg bpm in bouts 2, 3, 4, respectively.
4. Discussion
A substantial number of research studies now demonstrate that IET is an effective method to reduce resting BP,[35] which is increasingly associated with numerous CVD risk factors as it rises above optimal.[36] To date, a large proportion of these IET studies have used extremely low risk normotensive participants; however, it is suggested that the logical progression of this research is to establish IET as a prophylactic intervention for those at risk of developing hypertension and ultimately as a nonpharmacological treatment option for those already suffering from hypertension. Whilst a small number of studies have successfully used IET to lower resting BP in such populations,[6,7,9,37–40] it is argued that ideally the ability to accurately predict the hemodynamic response to IET is required, particularly in higher risk populations. The use of an evidence-based approach should allow for safer IET prescription in these individuals. This is the first study to assess and evaluate the acute hemodynamic response to an IET protocol in unmedicated asymptomatic participants with suboptimal BP regulation and to address the safety of performing IE in this specific population.
Although relatively rare, an acute bout of strenuous physical activity has been known to trigger cardiovascular events in selected individuals.[2] Indeed, exercise intensity, which determines the hemodynamic response and myocardial oxygen consumption (MVO2) is known to affect the risk of physical exertion.[31] The RPP, calculated as HR × sBP, provides a good index of MVO2 during upright exercise regardless of distinct differences in the proportional rise in these variables during aerobic and IE.[41] As such, Pescatello et al[2] suggest that an excessive sBP response to exercise may contribute to ischaemic cardiac events leading to myocardial infarction, or possibly even a stroke. However, it should be noted that the HR response during IE is considerably lower than the ACSM exercise test[24] attainment of 85% predicted maximum HR (149 b min−1 vs 105 b min−1 for our population). Furthermore when compared to previous work the RPP and by implication MVO2, are shown to be considerably lower during IE compared to maximal aerobic exercise,[42] adding further support to its safety. Indeed, the relative safety of the isometric wall squat exercise protocol is further contextualized when the highest RPP reported in this manuscript (20681 ± 3911 mm Hg bpm) is compared to that reported (27,729 ± 5018 mm Hg bpm) in high risk patients referred for clinical exercise testing for the evaluation of ischaemic heart disease.[43]
In the absence of any published data to establish hemodynamic response limits during IE, it has been necessary to refer to existing guidelines established for aerobic exercise testing. When the BP data are compared to the current ACSM guidelines for aerobic exercise termination,[24] sBP does not reach the upper limit during IE in this population. Diastolic BP briefly exceeds 115 mm Hg in some participants during these exercise protocols. In light of new ACSM pre-participation health screening recommendations,[44] which remove any recommendation for preparticipation medical evaluation in those with less than 2 CVD risk factors, it is suggested that the use of evidence-based exercise protocols improves the likelihood that those with any risk factors will have a safer exercise experience. Whilst the current data indicate that it is impossible to go so far as to be able to predict the magnitude of BP response during IET from resting values, the results do provide the capacity to identify those at greater risk of exceeding ACSM guidelines during IET based upon their peak BP during the incremental IE test administered under qualified supervision. Indeed, using the ratio LoA calculated from these data, peak BP values of 235 mm Hg for systolic and 106 mm Hg for diastolic during the incremental IE test would put a participant at risk of going over 250 systolic or 115 diastolic during a subsequent IE session. However, taking this evidence based approach would mean that once these BP limits have been exceeded the individual would then perform their first training session under supervision for confirmation and possible adjustment before commencing IET unsupervised in the home.
It is evident that regardless of the actual exercise used, for example, handgrip or leg extension, the most widely used IET protocol consists of 4 sets of 2 minute contractions performed using a fixed workload with each set separated by a fixed recovery period—with 2 minutes being commonly used.[21] Indeed, the use of this “standard” protocol thrice weekly with the isometric wall squat exercise has recently been shown to effectively reduce resting BP in healthy normotensive participants following 4 weeks of IET.[25] However, the current data suggest that the use of a standardized protocol, despite workload being determined according to relative exercise intensity, may not always be the most appropriate approach to use in terms of helping to ensure safety in higher risk participants. Indeed, examination of the individual response data reveals that one participant had a peak dBP reading over 115 mm Hg in bouts 1 and 2, and that 6 participants had peak dBP values above 115 mm Hg during bouts 3 and 4. Assuming that the ACSM guidelines for exercise termination are accepted as being applicable to those performing IE, this finding would support some adjustment to the initial exercise prescription. Moreover, as this is the first paper to assess and evaluate the hemodynamic responses of hypertensive participants to isometric (wall squat) exercise using this standard protocol, it is feasible to suggest that similar responses might be expected regardless of the IE used, although this requires evidence for confirmation. However, until this is available, a more evidence based approach is highly recommended when prescribing unsupervised IET to those with suboptimal BP control. Under these circumstances an “individualized” IET prescription would be provided for higher risk participants exhibiting this magnitude of dBP response. Thus, in terms of practical application, the practitioner might manipulate one or more acute programme variables in order to keep exercising BP below the 115 mm Hg guideline. For example, it is evident from the training session data presented in Figure 4 that the fourth bout of IE resulted in the greatest rise in all BP components. Therefore, reducing the number of bouts and possibly increasing the number of IET sessions per week would effectively reduce the intensity per session keeping dBP within an acceptable range, while maintaining overall IET volume per week. Alternatively, a drop-off in intensity could be employed to reduce the BP response in bouts subsequent to an acceptable response. For those practitioners wishing to use the IWSE protocol, this would necessitate increasing wall squat angle by 10°.[32] Finally, since the overall intensity of an IET session is also determined by the length of recovery between bouts, the practitioner may simply decide to implement a progressive increase in the duration of each rest period. However, further research is required to determine the effect that alterations in these acute programme variables may have upon the cardiovascular adaptations previously shown to be elicited following this type of training (c.f. Wiles et al).[5]
Notwithstanding this, the relevance of the ACSM exercise testing termination guidelines, which were originally developed for aerobic exercise testing, to those performing IE remains unclear. From a general perspective Pescatello et al[2] note that: these values were arbitrarily established by clinicians, no data exist to support these end points, and there are virtually no reports of hypertensive related cardiovascular implications that have resulted when participants have exceeded these levels. The latter being confirmed in this IE study, where participants did not report any adverse symptoms, such as shortness of breath, dizziness, chest pain, or light headedness at any point during or following the incremental test or exercise training session. It is also pertinent to point out that these guidelines were originally developed when one-off BP measurement was the norm, before the advent of automated online monitoring which provides a much higher density of data. As such, the use of continuous beat-to-beat BP measurement means these high values are picked up, whereas with one-off measurements there will be an inevitable regression to the mean. Indeed, the limitations associated with one off BP measurement are probably reflected in the much lower BP values reported by Araujo et al.[26] Therefore in reality, it is likely that exercising individuals have always had much higher peak BP values, but these were just not previously detected.
Furthermore, despite limited similarities such as an increase in RPP, there are obvious and distinct differences between IE and aerobic exercise being mainly related to the specific nature of a static contraction, which results in a sustained mechanical compression of the active muscle vasculature[45] and in turn an exaggerated pressor response. Indeed, it has been suggested that increased subendocardial perfusion secondary to the elevated dBP may actually reduce the risk of ischaemic cardiac responses during this type of exercise.[46] Furthermore, the magnitude of dBP response observed in the present study, which as indicated earlier might also be representative of previous IE interventions using the same standard protocol, may even act as a specific stimulus for the BP adaptations reported. Indeed, the physiological benefits following a single IE session including improved cardiac autonomic modulation and baroreceptor reflex sensitivity[30] and cardiac mechanical responses[47] have been recently reported. However, despite the potential importance of the dBP rise seen during IE, it is suggested that there will still be an adverse dBP threshold specifically pertinent to participants with suboptimal BP and as such a safe limit needs to be ascertained when prescribing this type of exercise in higher risk populations.
One final aspect to consider is that since IE is normally prescribed using a standardized formulae of 4 × 2 minute bouts, this means that the resultant cardiovascular stress is experienced for a much shorter period of time compared to current exercise recommendations.[48] Moreover, the current data demonstrate that where dBP does exceed ACSM guidelines, it occurs briefly in the final 30 seconds of an exercise bout. In support of earlier statements there is no evidence from the few available published studies that this transient response is associated with an increase in risk for acute cardiovascular events.[22] Therefore future research is required to ascertain if IE requires modified BP termination guidelines.
This relatively small sample sized study is limited to male Caucasian nonmedicated hypertensive participants. Therefore, the application to female and other ethnic populations remains to be explored. Whilst the protocol only utilised the results of a single incremental isometric exercise test and a single isometric exercise training session at the participant prescribed knee joint training angle, pervious research has demonstrated this protocol to be reliable.[49] Although the BP reduction capability of this protocol has been established in a normotensive population,[23] the application of this protocol as a nonpharmacological exercise training intervention for the management of BP in a population with hypertension is currently being investigated. Notwithstanding the fact that this protocol is readily available and does not require any specialist facility, in higher risk populations, the authors would recommend an initial supervised session following the incremental isometric exercise test to confirm appropriate hemodynamic responses and exercise competence (as with any form of novel exercise intervention).
5. Conclusion
Rate pressure product and by implication MVO2, remain considerably lower during IE compared to maximal aerobic exercise despite hypertensive blood pressure status. Based on the current ACSM guidelines for aerobic exercise termination, systolic BP does not reach the upper limit during IE in this population. Diastolic BP exceeds 115 mm Hg in some participants during these exercise protocols. These findings suggest that any individual with higher than normal BP who exceeds peak values of 235 mm Hg for systolic and 106 mm Hg for diastolic during the incremental IE test are at increased risk of exceeding the ACSM BP thresholds during their subsequent IE training prescription. As such, a more individualized IE prescription may be required for a small number of individuals. However, future research is required to ascertain if IE requires modified BP termination guidelines.
Footnotes
Abbreviations: BP = blood pressure, CVD = cardiovascular disease, dBP = diastolic blood pressure, HR = heart rate, IE = isometric exercise, IET = isomeric exercise training, IWSE = isometric wall squat exercise, LoA = limits of agreement, mBP = mean blood pressure, MVO2 = myocardial oxygen demand, RPP = rate pressure product, sBP = systolic blood pressure, TFM = task force monitor.
The authors have no conflicts of interest to disclose.
References
- [1].Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. [DOI] [PubMed] [Google Scholar]
- [2].Pescatello LS, Franklin BA, Fagard R, et al. Exercise and hypertension. Med Sci Sports Exerc 2004;36:533–53. [DOI] [PubMed] [Google Scholar]
- [3].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–219. [DOI] [PubMed] [Google Scholar]
- [4].Wallace JP. Exercise in hypertension: a clinical review. Sports Med 2003;33:585–98. [DOI] [PubMed] [Google Scholar]
- [5].Wiles JD, Coleman DA, Swaine IL. The effects of performing isometric training at two exercise intensities in healthy young males. Eur J Appl Physiol 2010;108:419–28. [DOI] [PubMed] [Google Scholar]
- [6].Baross AW, Wiles JD, Swaine IL. Effects of the intensity of leg isometric training on the vasculature of trained and untrained limbs and resting blood pressure in middle-aged men. Int J Vasc Med 2012;2012:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Badrov MB, Bartol CL, DiBartolomeo MA, et al. Effects of isometric handgrip training dose on resting blood pressure and resistance vessel endothelial function in normotensive women. Eur J Appl Physiol 2013;113:2091–100. [DOI] [PubMed] [Google Scholar]
- [8].Badrov MB, Freeman SR, Zokvic MA, et al. Isometric exercise training lowers resting blood pressure and improves local brachial artery flow-mediated dilation equally in men and women. Eur J Appl Physiol 2016;116:1289–96. [DOI] [PubMed] [Google Scholar]
- [9].Millar PJ, Levy AS, McGowan CL, et al. Isometric handgrip training lowers blood pressure and increases heart rate complexity in medicated hypertensive patients. Scand J Med Sci Sports 2013;23:620–6. [DOI] [PubMed] [Google Scholar]
- [10].Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Inder JD, Carlson DJ, Dieberg G, et al. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res 2016;39:88–94. [DOI] [PubMed] [Google Scholar]
- [12].Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- [13].Wernbom M, Augustsson J, Thomeé R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med 2007;37:225–64. [DOI] [PubMed] [Google Scholar]
- [14].Duchateau J, Hainaut K. Isometric or dynamic training: differential effects on mechanical properties of a human muscle. J Appl Physiol Respir Environ Exerc Physiol 1984;56:296–301. [DOI] [PubMed] [Google Scholar]
- [15].Komi PV, Viitasalo JT, Rauramaa R, et al. Effect of isometric strength training of mechanical, electrical, and metabolic aspects of muscle function. Eur J Appl Physiol Occup Physiol 1978;40:45–55. [DOI] [PubMed] [Google Scholar]
- [16].Wiles JD, Coleman D, Dunford M, et al. A novel method for the performance of isometric exercise in the home. J Sports Sci 2005;23:795–803. [DOI] [PubMed] [Google Scholar]
- [17].Rowell LB. Human Cardiovascular Control. USA: Oxford University Press; 1993. [Google Scholar]
- [18].Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007;25:1105–87. [DOI] [PubMed] [Google Scholar]
- [19].MacDougall JD, Tuxen D, Sale DG, et al. Arterial blood pressure response to heavy resistance exericse. J Appl Physiol 1984;58:785–90. [DOI] [PubMed] [Google Scholar]
- [20].Seals DR, Enoka RM. Sympathetic activation is associated with increases in EMG during fatiguing exercise. J Appl Physiol 1989;66:88–95. [DOI] [PubMed] [Google Scholar]
- [21].Millar PJ, McGowan CL, Cornelissen VA, et al. Evidence for the role of isometric exercise training in reducing blood pressure: potential mechanisms and future directions. Sports Med 2014;44:345–56. [DOI] [PubMed] [Google Scholar]
- [22].Brook RD, Appel LJ, Rubenfire M, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension 2013;61:1360–83. [DOI] [PubMed] [Google Scholar]
- [23].Wiles JD, Goldring N, O’Driscoll JM, et al. An alternative approach to isometric exercise training prescription for cardiovascular health. Translat J ACSM 2018;3:10–8. [Google Scholar]
- [24].Riebe D, Ehrman JK, Liguori G, et al. ACSM's Guidelines for Exercise Testing and Prescription. 10 ed.Philadelphia: Wolters Kluwer; 2017. [Google Scholar]
- [25].Wiles JD, Goldring N, Coleman D. Home-based isometric exercise training induced reductions resting blood pressure. Eur J Appl Physiol 2017;117:83–93. [DOI] [PubMed] [Google Scholar]
- [26].Araujo CG, Duarte CV, Goncalves Fde A, et al. Hemodynamic responses to an isometric handgrip training protocol. Arq Bras Cardiol 2011;97:413–9. [DOI] [PubMed] [Google Scholar]
- [27].Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016;388:1302–10. [DOI] [PubMed] [Google Scholar]
- [28].Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017;doi.org/10.1161/HYP.0000000000000065. [Google Scholar]
- [29].Fortin J, Haitchi G, Bojic A, et al. Validation and Verification of the Task Force Monitor® Results of Clinical Studies for F DA 510(k) n°: K014063. 2001:1–7. [Google Scholar]
- [30].Taylor KA, Wiles JD, Coleman DD, et al. Continuous cardiac autonomic and haemodynamic responses to isometric exercise. Med Sci Sports Exerc 2017;49:1511–9. [DOI] [PubMed] [Google Scholar]
- [31].Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training. A scientific statement from the American Heart Association. Circulation 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- [32].Goldring N, Wiles JD, Coleman D. The effects of isometric wall squat exercise on heart rate and blood pressure in a normotensive population. J Sports Sci 2014;32:129–36. [DOI] [PubMed] [Google Scholar]
- [33].Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1--Correlation within subjects. Brit Med J 1995;310:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- [35].Carlson DJ, Dieberg G, Hess NC, et al. Isometric exercise training for blood pressure management: A systematic review and meta-analysis. Mayo Clin Proc 2014;89:327–34. [DOI] [PubMed] [Google Scholar]
- [36].Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291–7. [DOI] [PubMed] [Google Scholar]
- [37].Wiley RL, Dunn CL, Cox RH, et al. Isometric exercise training lowers resting blood pressure. Med Sci Sports Exerc 1992;24:749–54. [PubMed] [Google Scholar]
- [38].Peters PG, Alessio HM, Hagerman AE, et al. Short-term isometric exercise reduces systolic blood pressure in hypertensive adults: possible role of reactive oxygen species. Int J Cardiol 2006;110:199–205. [DOI] [PubMed] [Google Scholar]
- [39].McGowan CL, Levy AS, McCartney N, et al. Isometric handgrip training does not improve flow-mediated dilation in subjects with normal blood pressure. Clin Sci 2007;112:403–9. [DOI] [PubMed] [Google Scholar]
- [40].Taylor AC, McCartney N, Kamath MV, et al. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc 2003;35:251–6. [DOI] [PubMed] [Google Scholar]
- [41].Nelson RR, Gobel FL, Jorgensen CR, et al. Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation 1974;50:1179–89. [DOI] [PubMed] [Google Scholar]
- [42].Simonson SR, Wyatt F. The rate pressure product is greater during supine cycle ergometry than during treadmill running. Biol Sport 2003;20:3–14. [Google Scholar]
- [43].Pinkstaff S, Peberdy MA, Kontos MC, et al. Quantifying exertion level during exercise stress testing using percentage of age-predicted maximal heart rate, rate pressure product, and perceived exertion. Mayo Clin Proc 2010;85:1095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Thompson PD, Arena R, Riebe D, et al. ACSM's new preparticipation health screening recommendations from ACSM's guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep 2013;12:215–7. [DOI] [PubMed] [Google Scholar]
- [45].Chrysant SG. Current evidence on the hemodynamic and blood pressure effects of isometric exercise in normotensive and hypertensive persons. J Clin Hypertens 2010;12:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].DeBusk R, Pitts W, Haskell W, et al. Comparison of cardiovascular responses to static-dynamic effort and dynamic effort alone in patients with chronic ischemic heart disease. Circulation 1979;59:977–84. [DOI] [PubMed] [Google Scholar]
- [47].O’Driscoll JM, Taylor KA, Wiles JD, et al. Acute cardiac functional and mechanical responses to isometric exercise in prehypertensive males. Physiol Rep 2017;5:e13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].World Health Organisation. Global Recommendations on Physical Activity for Health. Geneva:2010. [PubMed] [Google Scholar]
- [49].Wiles JD, Allum SR, Coleman DA, et al. The relationships between exercise intensity, heart rate, and blood pressure during an incremental isometric exercise test. J Sports Sci 2008;26:155–62. [DOI] [PubMed] [Google Scholar]


