Abstract
This study aims to evaluate the application of multimodal intraoperative monitoring (MIOM) in surgical treatment for spine burst fracture and dislocation (SBFD) patients.
Eleven patients who underwent posterior reduction and instrumentation (PRI) for SBFD from June 2014 to July 2016 were included into the study. The function of the spinal cord was monitored by MIOM. The muscle strength of the lower extremities and American Spinal Injury Association (ASIA) scores were, respectively, evaluated (before surgery, and at 1, 3, 6, and 12 months after surgery). Furthermore, the extent of reduction was also assessed.
Muscle strength recovery, ASIA score changes, and the extent of reduction were correlated with MIOM results. Among the 11 patients who received surgery under MIOM, 8 patients with negative MIOM results during the operation did not demonstrate neurological deterioration postoperatively and exhibited improvements in ASIA scores during follow-ups. However, among the 3 patients who encountered MIOM events (case 4, 7, and 8), 2 patients avoided nerve lesion and 1 patient suffered from neurologic deterioration postoperatively.
The application of MIOM technology during PRI surgery may detect spinal cord impairment at the early stage, and operative schemes can be modified before permanent nerve compromise is triggered by surgical manipulation.
Keywords: burst fracture, intraoperative monitoring, neurological lesion, spinal cord
1. Introduction
Spine burst fracture and dislocation (SBFD) is a common fracture that frequently results from high energy trauma. Posterior surgery is one of the most common surgical approaches in the treatment of SBFD,[1–3] and posterior reduction and instrumentation (PRI) has been successfully applied to spine unstable burst fractures, as described by Dai et al[4] and Verlaan.[5] However, fracture reduction and screw implantation are the 2 major high risk manipulations during surgery, which may result in neurological deterioration. Mechanical damage due to stretching of the nerve fibers during the reduction of the dislocation may lead to catastrophic neurological impairment. In addition, literatures have reported that the incidence of pedicle screw misplacement ranged within 20% to 30%, and 1% of which suffered from neurological damage that could bring about serious consequences such as paralysis.[6,7] Therefore, it is relatively indispensable for surgeons to clearly understand the real-time feedback of nerve function during the critical phases of surgical procedures. Kothbauer and Deletis.[8] and Hu et al[9] reported that intraoperative monitoring could be perfectly applied in spinal surgery, including scoliosis correction, intramedullary tumor resection, and lumbosacral spinal canal surgery, and that spinal cord monitoring technology can provide valuable eletrophysiological signals. From the perspective of the authors of the present study, MIOM technology could also be applied on SBFD patients in PRI surgery to avoid spinal cord compromise, such as the monitoring of the integrity of the spinal cord pathway, especially when reduction and screw implantation are carried out. The aim of the present study was to estimate the feasibility of MIOM in surgical treatment for SBFD, and provide clinical experience.
2. Material and methods
2.1. Patients
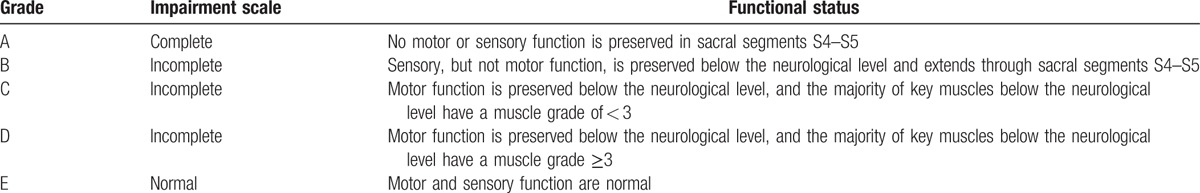
A total of 11 consecutive patients who underwent emergency PRI surgery under MIOM at our institution between June 2014 and July 2016 were included into the present study. All patients were diagnosed with fresh burst fracture dislocation, which occurred at T4 in 1 patient, T5 in 1 patient, T11 in 3 patients, T12 in 3 patients, L1 in 2 patients, and L2 in 1 patient. Among these patients, 1 patient presented with normal neurological function, 8 patients had incomplete paralysis, and 2 patients suffered from complete paralysis (Tables 1 and 2). All patients underwent x-rays, computer tomograph scans and MRI examinations preoperatively. The grades of spinal cord function were, respectively, evaluated (before surgery. and at 1, 3, 6, and 12 months after surgery; Table 3), according to the American Spinal Injury Association (ASIA, Table 4) impairment scale system. Muscle strength, including the quadriceps, anterior tibialis, peroneus longus, gastrocnemius and soleus, were graded according to the Medical Research Council scale.[10]
Table 1.
Patient characteristics.
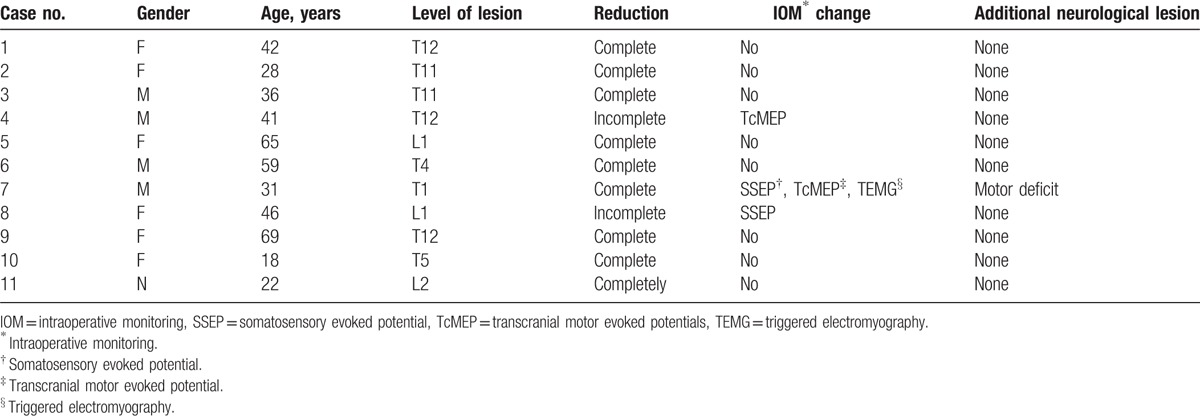
Table 2.
Risk factors of iatrogenic spinal cord injury.
Table 3.
Preoperative and postoperative American Spinal Injury Association (ASIA) grades.
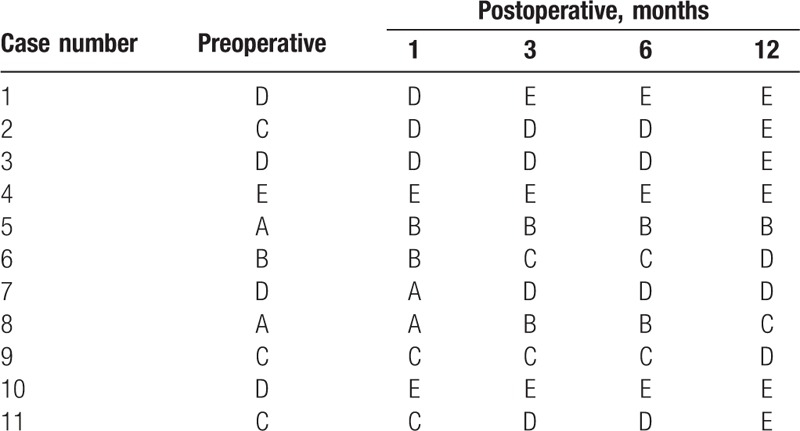
Table 4.
American Spinal Injury Association (ASIA) impairment scale.
2.2. Anesthesia
General anesthesia with intubation was achieved using propofol (200 μg/kg), fentanyl (250 μg), and midazolam (2 mg) for all patients. In addition, propofol (0.2–0.5 mg/kg per hour) was constantly infused for maintaining anesthesia. Short-acting muscle relaxants with succinylcholine (1 mg/kg) were only provided during induction and intubation.
2.3. MIOM techniques
MIOM, including somatosensory evoked potentials (SSEPs), transcranial motor evoked potentials (TcMEP), and triggered electromyography (TEMG), was conducted using the Nicolet Endeavor CR IOM system.
The bilaterally posterior tibia nerves were stimulated by SSEP at the ankle with an intensity of 16 to 40 mA, a rate of 2. 9 Hz, and duration of 0.1 ms. SSEP was recorded from the needle electrodes, which were placed on the scalp at locations C3 and C4, and Cz was referenced to Fpz.
The TcMEP stimulating electrodes were placed on the scalp at locations C1 and C2. A stimulation intensity that ranged within 100 to 400 V was presented to the scalp at an interstimulus interval of 2 ms for duration of 0.1 ms. TcMEP was recorded from needle electrodes that were placed on the muscles, including the bilateral vastus lateralis, tibialis anterior, gastrocnemius, and abductor halluces.
TEMG was carried out with the stimulation of the head of screws that were previously implanted. Cathodic electrical stimulation was delivered through a brass electrode, which was referenced to a needle type anode positioned in the paraspinal muscle, with a stimulation intensity of 8.2 mA, a frequency of 2.5 Hz, and duration of 0.3 ms.
2.4. Evaluations
The baselines of SSEP and TcMEP were measured after the induction of anesthesia, but before the surgical operation. The latency and amplitude of SSEP was continuously monitored, and TEMG was generally performed after each screw insertion, as required by the surgeon intraoperatively.
An abnormal SSEP was defined as a prolonged latency of more than 10% or a peak-to-peak amplitude decline of more than 50%, when compared to baseline. An abnormal TcMEP was defined as a TcMEP amplitude decrease of more than 50%. A normal TEMG response, which was described by Isley et al[11] as the absence of an electrical response on stimulation at 8.2 mA, indicated that the pedicle screw was not in contact with the spinal cord or nerve root. Otherwise, the screw was regarded as in contact with the adjacent neurological structure.
3. Results
MIOM was applied to 11 SBFD patients who received PRI surgery. Ten patients did not exhibit additional neurological deficits, while 1 patient (case 7) suffered from iatrogenic neurological impairment postoperatively. Furthermore, 9 patients had complete reduction and 2 patients had incomplete reduction. Moreover, MIOM events occurred in 3 patients (case 4, 7, and 8). The details are presented as follows:
Case 4: A 41-year-old male presented with T12 burst fracture–dislocation and normal neurological function (Fig. 1). PRI was performed under MIOM. During the operation, TcMEP amplitude decreased to 25% of baseline when the dislocated vertebra was corrected to its anatomical position. The surgeon was informed, and further corrective operation was suspended. Then, signal changes were resumed (Fig. 2). Fixation was maintained with a minor degree of correction than that designed. Postoperative neurological function was normal.
Figure 1.
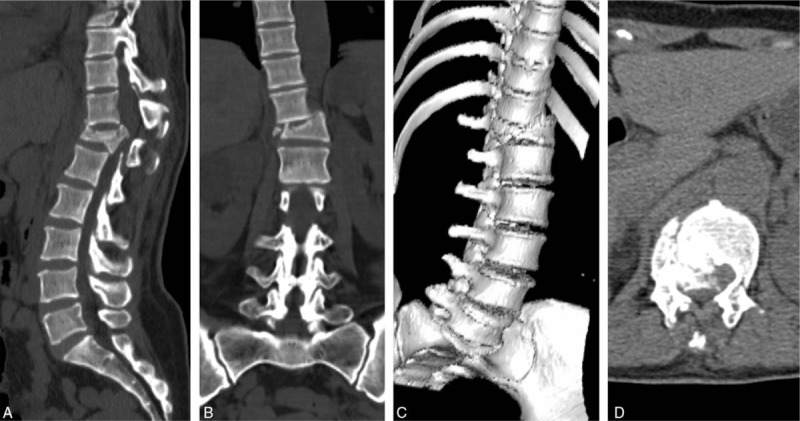
A 41-year-old male who suffered from T12 burst fracture–dislocation received emergency PRI surgery. (A) Sagittal CT image. (B) Coronal CT image. (C) 3D reconstruction of CT image. (D) Axial CT image. CT = computer tomograph.
Figure 2.
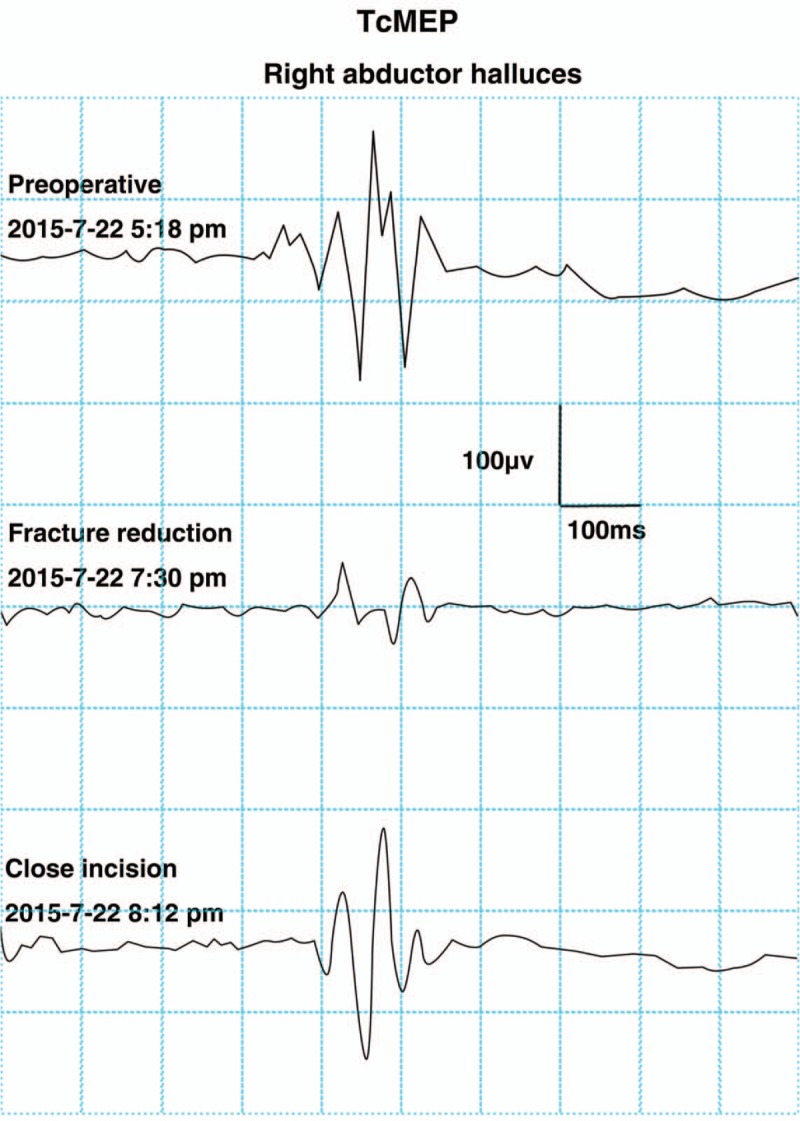
A typical TcMEP change associated with surgical manipulation. The amplitude of TcMEP decreased to 25% of baseline during surgery when the reduction was performed. The change was reversed by appropriate measures and returned to baseline at end of the surgery. No additional postoperative neurological injury was observed. TcMEP = transcranial motor evoked potentials.
Case 7: A 31-year-old female presented with minor motor and sensory dysfunction related to T11 burst fracture. The preoperative muscle strength of both lower extremities was grade IV. After placing the last screw, TEMG response, which was elicited with extremely low current (<8.2 mA), was positive, and the amplitudes of SSEP and TcMEP rapidly decreased. After excluding for interfering factors, the operation was suspended in time. The responsible screw was immediately removed, the SSEP returned to approximately 80% of the baseline, but TcMEP continued to decline to more than 50% at the end of the surgery. After the operation, the patient was incapable of moving her right lower extremity, of which muscle strength was grade 0. After a month of rehabilitation exercise, she had grade II muscle strength of the right lower extremity. One and a half month later, she had good motor function recovery, which presented with grade IV.
Case 8: A 46-year-old female demonstrated complete paralysis caused by severe L1 burst fracture. Preoperative lower extremity muscle strength was grade 0. MIOM was initially attempted using SSEP, TcMEP, and TEMG, but TcMEP and TEMG could not be elicited. Ultimately, only SSEP was utilized. The SSEP amplitude decreased to approximately 50% of baseline when the investigators attempted to completely correct the fracture dislocation. The surgery was suspended and the recordings spontaneously improved. The instrumentation was implanted with incomplete reduction. The amplitude of SSEP returned to baseline at the end of the surgery.
4. Discussion
SBFD is a common spinal injury that often results from high-energy trauma. The principle for treating SBFD mainly includes the decompression of neural structures, the correction of angular deformities, the restoration of vertebral body height, and the obtainment of stable fixation.[4,5,12–14] Surgery for SBFD remains technically demanding due to the potential risk of postoperative neurological deterioration such as sensory loss, lower limb weakness, and even paralysis. At present, MIOM has been widely utilized to prevent neurological compromise in scoliosis correction[15] and intramedullary tumor resection surgery.[16,17] MIOM data has been trusted by various authors.[8,9,15–19] However, no studies have focused on the intraoperative spinal cord function detected by MIOM during the reduction of the fracture and dislocation. Therefore, the present study was conducted to estimate the feasibility of MIOM on SBFD patients in PRI surgery.
SSEP, TcMEP, and TEMG are the 3 common modalities of neurophysiological monitoring, and each has its advantages and shortcomings. The combination of these 3 models can enable the comprehensive intraoperative monitoring of spinal cord injury, and further minimize the occurrence of false positive and false negative events.[20] Typically, SSEP monitoring has been utilized for continuous detection, while TcMEP and TEMG recording has been used intermittently. SSEP can only monitor the integrity of sensory pathways. Thus, injury to the motor pathways would be missed and may result in postoperative motor deficit.[21] On the other hand, TcMEP and TEMG could help in perfectly offsetting this shortage. SSEP and TcMEP have important roles in the procedure of fracture reduction. Meanwhile, TEMG plays a crucial role in identifying the position of the pedicle screw. Therefore, these 3 modalities were chosen for spinal cord monitoring in PRI surgery.
SBFD can be extremely unstable injuries that always need operative management.[1,2,14,22] However, the reduction of the dislocation may cause stretch injury to the spinal cord and inferior clinical outcomes. Therefore, it is critical for the surgical team to take measures to prevent this impending neurologic injury as soon as possible. In the present study, 2 (case 4 and 8) MIOM events were observed in the fracture-reduction process. The surgeon considered that the decrease in amplitudes were associated with the corresponding operation manipulation. After the exclusion of systemic or anesthetic factors, the corresponding actions were immediately taken, including the suspension of further reduction, flushing the dura with warm normal saline to wash out the blocking potassium,[17] intravenous methylprednisolone therapy, and the elevation of mean arterial pressure to improve regional spinal cord perfusion. No additional postoperative deficits were observed. We attribute this early detection of neurological deficit to the utilization of MIOM.
Instrumentation with the screw–rod system has been successfully applied in SBFD patients for the stabilization of the spinal column.[22] However, instrumentation in unstable spine fractures remains challenging due to unstable segments, destroyed anatomical landmarks, and discrepancies in anatomic orientation.[23] Furthermore, the misplacement of pedicle screws can result in a high incidence of neurological complications.[24] Esses et al[25] reported that 4.7% of patients experienced nerve root lesions postoperatively. Jahangiri et al[26] suggested that the sensitivity of MIOM in detecting malpositioned screws depends on the frequency and accuracy of data collection. In the present series, case 7 encountered neurological complications caused by pedicle screw misplacement, which was early detected by TEMG, SSEP, and TcMEP. The misplaced screw may lead to canal narrowing and spinal cord compression, and delayed intervention may result in serious consequences. It is paramount to collect TcMEP and TEMG immediately after the implantation of each screw, in order to timely detect the spinal cord injury. In a study conducted by Dimar et al[27], the spinal canals of 42 rats were placed with spacers. These rats were divided into 5 groups, and the decompression time was prolonged by 0, 2, 6, 24, and 72 hours. Neurological function recovery was evaluated by transcranial magnetic motor evoked potentials and serial basso beattie bresnahan motor scores. The results of this study suggested that early decompression is beneficial to the recovery of spinal cord injury. Furthermore, neurological outcomes were related to the time length of spinal cord compression. The longer the compression, the worse the result was. In addition, other studies[28,29] in humans also demonstrated that spinal cord contusion and canal narrowing may benefit from early decompression. In the present study, it was concluded that MIOM can reflect the poor function of the spinal cord as soon as possible, alert surgeons to immediately take actions, and ultimately minimize the lesion degree of the spinal cord. In the present study, a patient (case 7) with motor deficit was able to receive great help from MIOM. It was suspected that if MIOM was not carried out during the procedure, more severe or irreversible spinal cord injury might have occurred.
The present data demonstrates that the use of MIOM in PRI surgery could promptly detect iatrogenic neurological injury. Therefore, rapid response by appropriate intraoperative interventions can be taken to minimize the injury. Second, stable MIOM recordings encourage surgeons to reduce the dislocation, even when the anatomical situation is extremely difficult.
Although positive results were achieved in the present study, this study has a few limitations that should be mentioned. First, 2 patients (case 5 and 8) presented with complete paralysis and nontypical SSEP was elicited during surgery. Furthermore, MIOM was not able to identify the motor pathway injury caused by the surgical operation. Second, when using TEMG to determine the position of the screw, only the nerve dysfunction could be detected, and other injuries such as vascular injury could not be found. Therefore, patients with complete paralysis should be given more attention to prevent iatrogenic lesion, and it is necessary to verify the position of the pedicle screws by x-ray fluoroscopy intraoperatively. Further large-scale patients of spine injury should be included to evaluate the effectiveness of MIOM technology in PRI surgery for SBFD patients.
5. Conclusions
The application of MIOM technology during PRI surgery may detect spinal cord impairment at the early stage, and operative schemes can be modified before the permanent neurological compromise is triggered by surgical manipulation.
Acknowledgments
We gratefully acknowledge the cooperation of the doctors and nurses in the operating room.
Footnotes
Abbreviations: ASIA = American Spinal Injury Association, BBB = basso beattie bresnahan, CT = computer tomograph, ISCI = iatrogenic spinal cord injury, MIOM = multimodal intraoperative monitoring, PRI = posterior reduction and instrumentation, SBFD = spine burst fracture and dislocation, SSEPs = somatosensory evoked potentials, TcMEP = transcranial motor evoked potentials, TEMG = triggered electromyography.
TY and YW contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Zhang L, Wang J, Feng X, et al. Traumatic burst fracture and dislocation of the lumbar spine with an intact neurologic function. Spine J 2016;16:e47–8. [DOI] [PubMed] [Google Scholar]
- [2].Liu Z, Li Z, Xing D, et al. Two different surgery approaches for treatment of thoracolumbar fracture. Int J Clin Exp Med 2015;8:22425–9. [PMC free article] [PubMed] [Google Scholar]
- [3].Padalkar P, Virani N, Kathare A. Posterior reconstruction of vertebral body using expandable cage for L5 burst fracture dislocation: case report. J Orthop Case Rep 2014;4:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dai LY, Jiang SD, Wang XY, et al. A review of the management of thoracolumbar burst fractures. Surg Neurol 2007;67:221–31. discussion 231. [DOI] [PubMed] [Google Scholar]
- [5].Verlaan JJ. Stabilizing osteoporotic thoracolumbar fractures through an anterior or posterior approach: what works best? Spine J 2013;13:1733–5. [DOI] [PubMed] [Google Scholar]
- [6].Upendra BN, Meena D, Chowdhury B, et al. Outcome-based classification for assessment of thoracic pedicular screw placement. Spine 2008;33:384–90. [DOI] [PubMed] [Google Scholar]
- [7].Di Silvestre M, Parisini P, Lolli F, et al. Complications of thoracic pedicle screws in scoliosis treatment. Spine 2007;32:1655–61. [DOI] [PubMed] [Google Scholar]
- [8].Kothbauer KF, Deletis V. Intraoperative neurophysiology of the conus medullaris and cauda equina. Childs Nerv Syst 2010;26:247–53. [DOI] [PubMed] [Google Scholar]
- [9].Hu Y, Luk KD, Lu WW, et al. Application of time-frequency analysis to somatosensory evoked potential for intraoperative spinal cord monitoring. J Neurol Neurosurg Psychiatry 2003;74:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Martin EG, Lovett RW. A method of testing muscular strength in infantile paralysis. J Am Med Assoc 1915;lxv:1512–3. [Google Scholar]
- [11].Isley MR, Pearlman RC, Wadsworth JS. Recent advances in intraoperative neuromonitoring of spinal cord function: pedicle screw stimulation techniques. Am J Electroneurodiagn Technol 1997;37:93–126. [Google Scholar]
- [12].Briem D, Lehmann W, Ruecker AH, et al. Factors influencing the quality of life after burst fractures of the thoracolumbar transition. Arch Orthop Trauma Surg 2004;124:461–8. [DOI] [PubMed] [Google Scholar]
- [13].Alvine GF, Swain JM, Asher MA, et al. Treatment of thoracolumbar burst fractures with variable screw placement or Isola instrumentation and arthrodesis: case series and literature review. J Spinal Disord Tech 2004;17:251–64. [DOI] [PubMed] [Google Scholar]
- [14].Qian Y, Jin C, He L, et al. Anterior reconstruction via a relatively noninvasive retroperitoneal approach for lumbar burst fracture. Orthop Surg 2015;7:185–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thirumala PD, Huang J, Thiagarajan K, et al. Diagnostic accuracy of combined multimodality SSEP and TcMEP intraoperative monitoring in patients with idiopathic scoliosis. Spine 2016;41:E1177–84. [DOI] [PubMed] [Google Scholar]
- [16].Novak K, Widhalm G, de Camargo AB, et al. The value of intraoperative motor evoked potential monitoring during surgical intervention for thoracic idiopathic spinal cord herniation. J Neurosurg Spine 2012;16:114–26. [DOI] [PubMed] [Google Scholar]
- [17].Forster MT, Marquardt G, Seifert V, et al. Spinal cord tumor surgery—importance of continuous intraoperative neurophysiological monitoring after tumor resection. Spine 2012;37:E1001–8. [DOI] [PubMed] [Google Scholar]
- [18].Jin SH, Chung CK, Kim CH, et al. Multimodal intraoperative monitoring during intramedullary spinal cord tumor surgery. Acta Neurochir 2015;157:2149–55. [DOI] [PubMed] [Google Scholar]
- [19].Kothbauer KF. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin 2007;37:407–14. [DOI] [PubMed] [Google Scholar]
- [20].Pencovich N, Korn A, Constantini S. Intraoperative neurophysiologic monitoring during syringomyelia surgery: lessons from a series of 13 patients. Acta Neurochir 2013;155:785–91. discussion 791. [DOI] [PubMed] [Google Scholar]
- [21].Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus 1998;4:e1. [DOI] [PubMed] [Google Scholar]
- [22].Okten AI, Gezercan Y, Ozsoy KM, et al. Results of treatment of unstable thoracolumbar burst fractures using pedicle instrumentation with and without fracture-level screws. Acta Neurochir 2015;157:831–6. [DOI] [PubMed] [Google Scholar]
- [23].Lee CY, Wu MH, Li YY, et al. Intraoperative computed tomography navigation for transpedicular screw fixation to treat unstable thoracic and lumbar spine fractures: clinical analysis of a case series (CARE-compliant). Medicine 2015;94:e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matsuzaki H, Tokuhashi Y, Matsumoto F, et al. Problems and solutions of pedicle screw plate fixation of lumbar spine. Spine 1990;15:1159–65. [DOI] [PubMed] [Google Scholar]
- [25].Esses SI, Sachs BL, Dreyzin V. Complications associated with the technique of pedicle screw fixation. A selected survey of ABS members. Spine 1993;18:2231–8. discussion 2238-2239. [DOI] [PubMed] [Google Scholar]
- [26].Jahangiri FR, Sheryar M, Al Behairy Y. Early detection of pedicle screw-related spinal cord injury by continuous intraoperative neurophysiological monitoring (IONM). Neurodiagn J 2014;54:323–37. [DOI] [PubMed] [Google Scholar]
- [27].Dimar JR2nd, Glassman SD, Raque GH, et al. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine 1999;24:1623–33. [DOI] [PubMed] [Google Scholar]
- [28].Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am 1995;77:1042–9. [DOI] [PubMed] [Google Scholar]
- [29].Marshall LF, Knowlton S, Garfin SR, et al. Deterioration following spinal cord injury. A multicenter study. J Neurosurg 1987;66:400–4. [DOI] [PubMed] [Google Scholar]
- [30].Alcanyis-Alberola M, Giner-Pascual M, Salinas-Huertas S, et al. Iatrogenic spinal cord injury: an observational study. Spinal Cord 2011;49:1188–92. [DOI] [PubMed] [Google Scholar]
- [31].Chen Q, Li F, Wu W. Risk factors of iatrogenic spinal cord injury in spinal surgery: a multicenter retrospective study. Int J Neurosci 2012;122:606–10. [DOI] [PubMed] [Google Scholar]


