Abstract
The present study was conducted to explore the association of endocytoscopy (EC) classification with microscopic inflammatory features of ulcerative colitis (UC) and disease relapse.
EC was performed for mild-to-moderate UC 32 cases from January 2010 to August 2016. EC appearance was stratified into 4 categories: EC-A, regular arrangement of round to oval pits; EC-B, irregular arrangement with/without enlarged spaces between regular pits; EC-C, deformed pits with distorted crypt lumen which are unordered in arrangement but not disrupted; and EC-D, disruptive or disappeared pits. We evaluated the association of EC classification with Mayo endoscopic subscores (MES) and the clinically active state. Microscopic features including the severity in mucosal inflammatory infiltrates the presence of crypt abscess and goblet cell depletion were assessed by an experienced pathologist who was blinded to clinical and endoscopic information. Clinical follow-up was provided for treating 22 UC patients more than 60 months after EC.
There were 15 cases in EC-A, 8 in EC-B, 5 in EC-C, and 4 in EC-D. Interobserver agreement was excellent with κ value of 0.77. There were 13 patients in active disease stage, while 19 in remission. Each EC-A case was in clinically remission stage, while all the EC-C and EC-D cases were in the active stage. There were 4 and 4 EC-B cases in remission and active stage, respectively. The EC-A group consisted of 11 MES0 and 4 MES1 cases, whereas the EC-B group consisted of 2 MES0 and 6 MES1 cases. There were no cases of MES0 in the EC-C and -D groups. The EC stratification was significantly associated with pathognomonic microscopic features for UC. There were significant differences in the remission rate among the EC groups. None had relapse in the EC-A group during the follow-up period.
EC stratification could be predictive for relapse in UC. Moreover, EC is reliable to assess UC specific microscopic features.
Keywords: crypt abscess, endocytoscopy, relapse, ulcerative colitis
1. Introduction
The diagnosis of ulcerative colitis (UC) is based on incorporating clinical, laboratory, radiological, endoscopic, and histolopathogical findings. Assessment of UC activity is essential to determine extent and severity of the disease for optimized therapeutic strategy.[1] Severity of endoscopic lesions in UC predicts more aggressive clinical courses and increased rates of surgery.[2] Again, histopathological findings play an important role in clinical management predicting disease relapse.[3] Histological assessment of UC is inseparable from colonoscopic investigation and biopsy in current clinical practice.[4]
More recently, confocal laser endomicroscopy (CLE) and endocytoscopy (EC) represent emerging endoscopic techniques allowing for in vivo histopathological diagnosis of diverse gastrointestinal diseases during ongoing endoscopy.[5,6] Several studies assessed the virtual assessment of inflammation activity using CLE or EC.[7–12] The inflammatory assessment included crypt architectures, cellular infiltration and microvascular structures. Nevertheless, there is little information on usefulness of these novel endoscopic modalities to predict the clinical course of UC.[13] Using EC, we proposed a simple grading system depending on the alterations in crypt microstructures in rectal mucosa of UC patients. The current study suggests the classification can predict subsequent relapse of UC.
2. Methods
Between January 2010 and August 2016, consecutive patients who were diagnosed as having UC, who visited the outpatient clinic of Tottori University Hospital and Nagasaki University Hospital, were enrolled in this study. Exclusion criteria were defined as follows; inability to provide written informed consent, severe coagulopathy, cirrhosis, impaired renal dysfunction, pregnancy or breast feeding, active gastrointestinal bleeding, or known allergy to methylene blue or crystal violet. The UC patients with aggressive endoscopic disease were excluded from the study, as EC was not feasible in this setting due to excessive exudates.[9] The diagnosis of UC was based on established clinical, radiological, endoscopic and histopathological findings. We assessed clinical symptoms with respect to rectal bleeding and stool frequency and measured blood markers at the time of EC observation and of each follow-up. Such blood markers as peripheral leukocyte and red blood cell and platelet counts, hemoglobin concentration, hematocrit, serological levels of C-reactive protein and albumin and erythrocyte sedimentation rate were measured at each time point. Liver and kidney function tests were also assessed periodically. The study was approved by the Tottori University Hospital (#1512A094) and Nagasaki University Ethics Committee (#14120111), and it was conducted in accordance with the Helsinki Declaration.
The endoscopic system included a light source and a processor (Olympus Optical Co., Tokyo, Japan), and EC (Olympus). We did not perform the preassessment step endoscopy, since EC used in this study was the integrated-type thin endoscope which can be switched from conventional to magnifying views in anytime via pushing a button located at the top of endoscope. After endoscopic insertion, luminal lavage was conducted with water including dimethicone and pronase thoroughly to remove sticky mucus and residue. The disease activity was assessed by Mayo endoscopic subscore (MES),[14] which is most frequently used for macroscopic assessment of ulcerative colitis and in recent international clinical trials.[15–17] The MES grades the severity from 0 to 3, 0 for no signs of inflammation, 1 for mild disease (erythema, decreased vascular pattern and mild friability), 2 for moderate decrease (marked erythema, absent vascular pattern, friability and erosions), and 3 for severe disease (spontaneous bleeding and ulceration). Endoscopic remission was defined as MES0 or MES1 whereas relapse was defined as MES2 or MES3 even following the remission with MES0 or MES1. Following the standard observation, EC with 0.05% crystal violet and 1% methylene blue staining were conducted in rectal mucosa. Two of the 3 endoscopists (H.I., Hajime Isomoto; R.U., Ryohei Uehara; and N.U., Naoki Ueda) attended the same EC procedure. By the experienced eodoscopist, H.I., EC findings were reviewed and all items relevant to the pathological features of UC were collected. The grading system thereby created and set by H.I. was called EC classification. Then, EC findings were reviewed and classified by H.I. and N.U. upon reaching agreement. There were 4 EC classifications as follows: EC-A, regular arrangement of round to oval pits (Fig. 1A); EC-B, irregular arrangement with/without enlarged spaces between regular pits (Fig. 1B); EC-C, deformed pits with distorted crypt lumen which are unordered in arrangement but not disrupted (Fig. 1C); and EC-D, disruptive or disappeared pits (Fig. 1D). In the end, targeted biopsy sampling was performed for histopathological evaluation in 22 cases.
Figure 1.
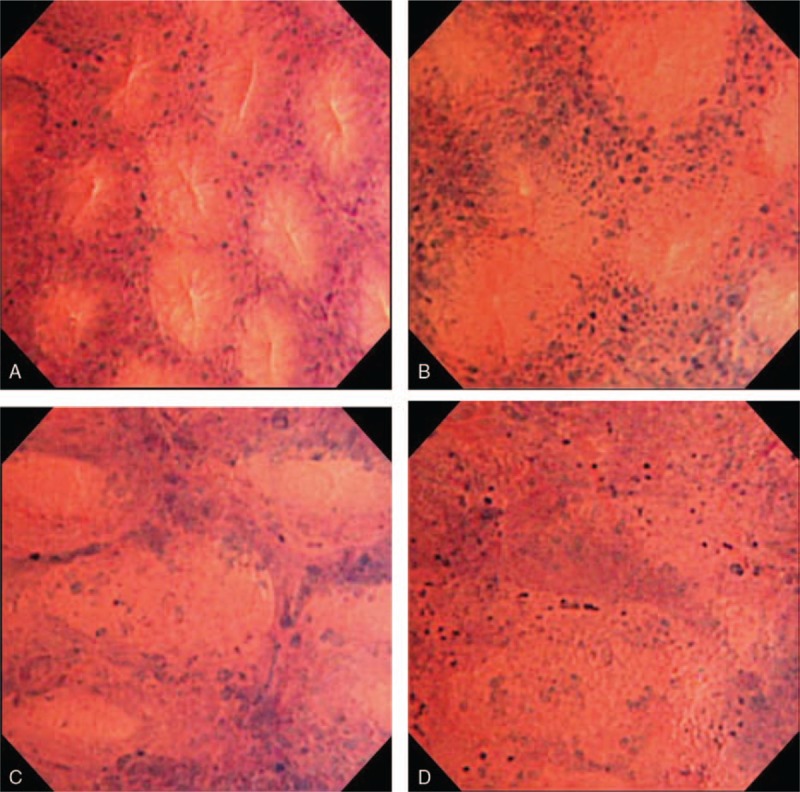
Endocytoscopy (EC) findings were classified into 4 types as follows: EC-A, regular arrangement of round to oval pits (A); EC-B, irregular arrangement with/without enlarged spaces between regular pits (B); EC-C, deformed pits with distorted crypt lumen which are unordered in arrangement but not disrupted (C); and EC-D, disruptive or disappeared pits (D). EC = endocytoscopy, EC-A = regular arrangement of round to oval pits, EC-B = irregular arrangement with/without enlarged spaces between regular pits, EC-C = deformed pits with distorted crypt lumen which are unordered in arrangement but not disrupted, EC-D = disruptive or disappeared pits.
Biopsy specimens were fixed with 10% formalin and embedded in paraffin, and sections were stained with hematoxylin and eosin. The pathologist (T.H.) who was blinded to clinical and endoscopic information evaluated the slices for histopathological assessment of inflammation in UC with special reference to the severity in mucosal inflammatory infiltrates and the presence of crypt abscess and goblet cell depletion.
EC appearance was compared to clinicopathological characteristics including disease activity and extension and clinical course. All the patients were followed monthly or bimonthly. The relapse was defined as described previously.[13]
Statistical analyses were performed using Fisher's exact test, the χ2 test, Student's t-test, the Mann–Whitney U test, and the Kruskal–Wallis test as appropriate. Comparison of the percentage of patients in remission on the basis of the EC classification was made using the Kaplan–Meier method and analyzed by log-rank test. A P value <.05 was accepted as statistically significant. The κ value was used to estimate interobserver variations in the EC classification between the endoscopists. Agreement was considered poor (κ <0.4), good (κ 0.4–0.75) and excellent (κ >0.75).[8]
3. Results
EC was conducted for mild to moderate UC 32 cases from January 2010 to August 2016. They consisted of 24 men and 8 women, and mean age at entry was 41.4 years (range, 20–77). The median duration of UC was 8.3 years ranging 0 to 22. They included 12 pan-colitis, 8 left-sided colitis, and 12 proctitis type. There were 13 patients in active disease stage, while 19 in remission, with being 30 cases with relapse-remitting and 2 cases with first attack type of disease. There were 15 cases in EC-A, 8 in EC-B, 5 in EC-C, and 4 in EC-D. The interobserver agreement for the EC stratification between H.I. and N.U. was excellent with the κ value of 0.77. Each EC-A case was in remission stage, while all the EC-C and EC-D cases were in active stage. There were 4 and 4 EC-B cases in remission and active stage, respectively. The EC-A cases were divided into MES0 (n = 11) and MES1 (n = 4) endoscopic grade, while the EC-B cases were into MES0 (n = 2) and MES1 (n = 6). There were no cases of MES0 in the EC-C and -D group. The differences reached statistical significance (P < .01). The other clinical parameters did not show significant correlation with the EC classification. The treatment method at the time of EC observation, it was divided into 5-aminosalicylic acid (5-ASA) alone, steroid, azathioprine, steroid + azathioprine, antitumor necrosis factor (TNF)-α antibody preparation, tacrolimus, and no treatment. We summarized the association of different therapy regimens with MES and EC classifications in Table 1. UC patients who were prescribed more intensive treatments including anti-TNF-α antibody and tacrolimus and steroid tended to have the MES1 despite statistical insignificance. The correlation between the different therapies and EC classifications appeared still poor.
Table 1.
Summary of relationship between ulcerative colitis treatment, Mayo endoscopic subscore (MES), and endocytoscopy (EC) classification.
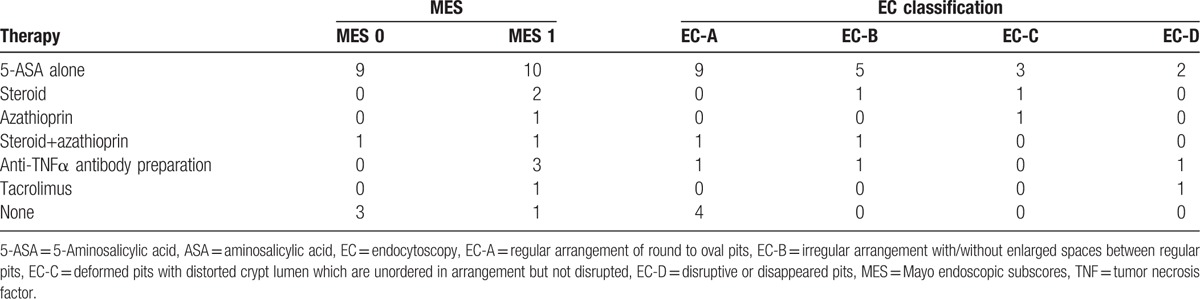
Table 2 showed the correlations between the EC classification and the histopathological findings specific for the disease. The differences in the presence of severe mucosal inflammation, crypt abscess and goblet cell depletion among the EC classifications reached statistical significance for each. That is, in the EC-A group, almost all except one patients had no pathognomonic features. On the other hand, all the EC-D patients had severe mucosal inflammation, crypt abscess and goblet cell depletion. Again, there were substantially close correspondences in glandular architectures between the EC and histopathological view as shown in Figures 2–4.
Table 2.
Endocytoscopy (EC) classification and pathognomonic histological features of ulcerative colitis.
Figure 2.
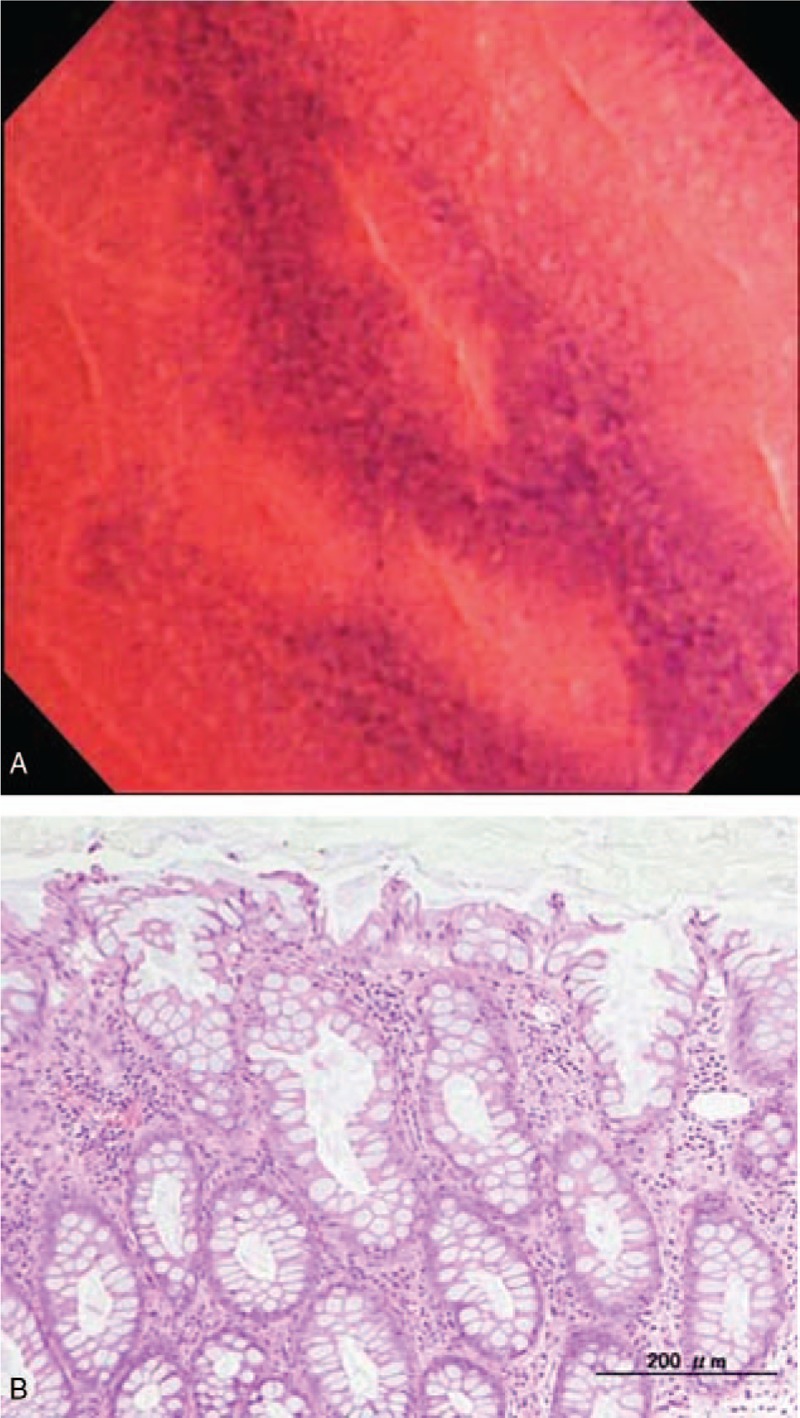
EC shows regular arrangement of even oval pits, as classified into EC-A (A). The targeted biopsy specimen shows regular arrangement of crypts containing substantial goblet cells with nominal acute inflammation (B). EC = endocytoscopy, EC-A = regular arrangement of round to oval pits.
Figure 4.
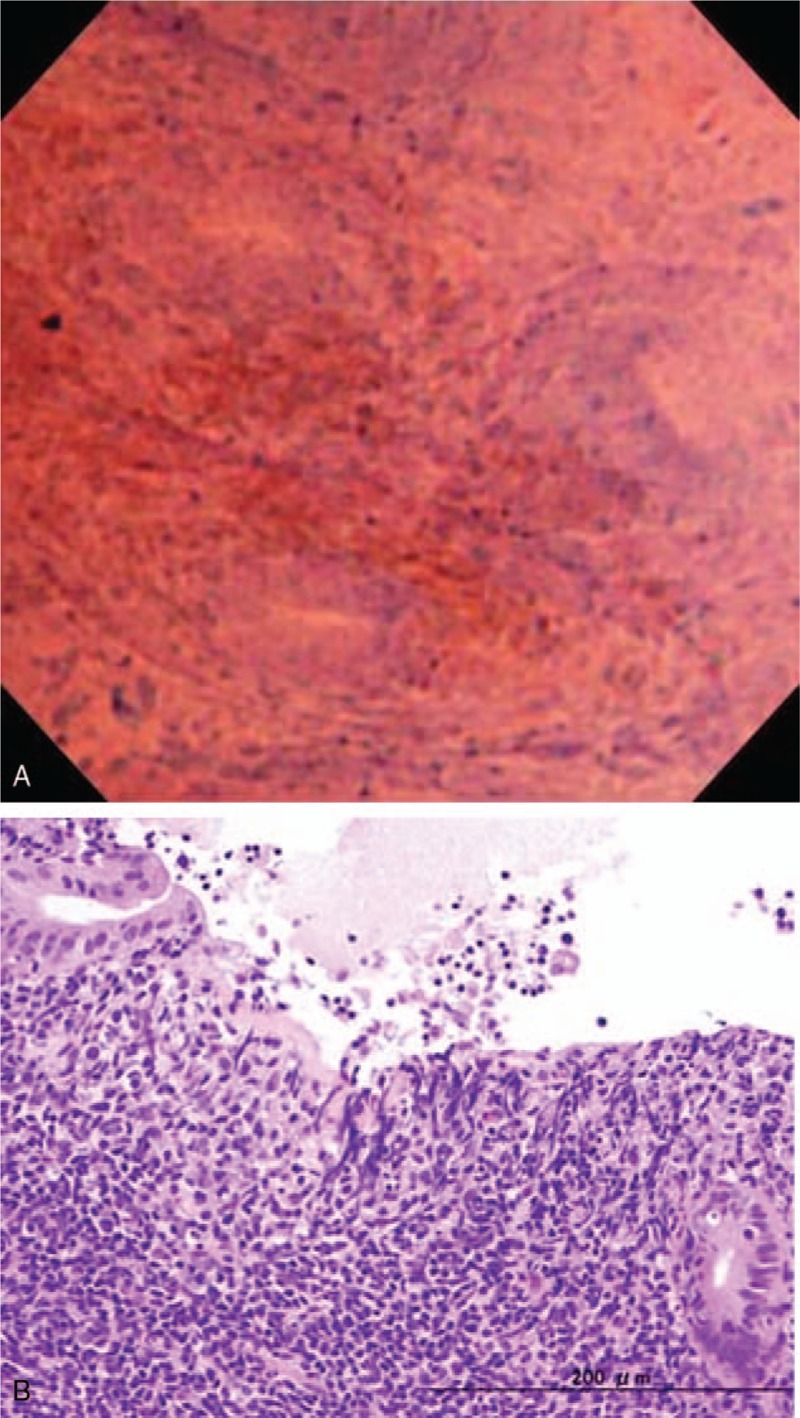
EC shows highly disruption of pits (A). Histopathology using the targeted biopsy specimen shows erosion and severe mucosal inflammation (B). EC = endocytoscopy.
Figure 3.
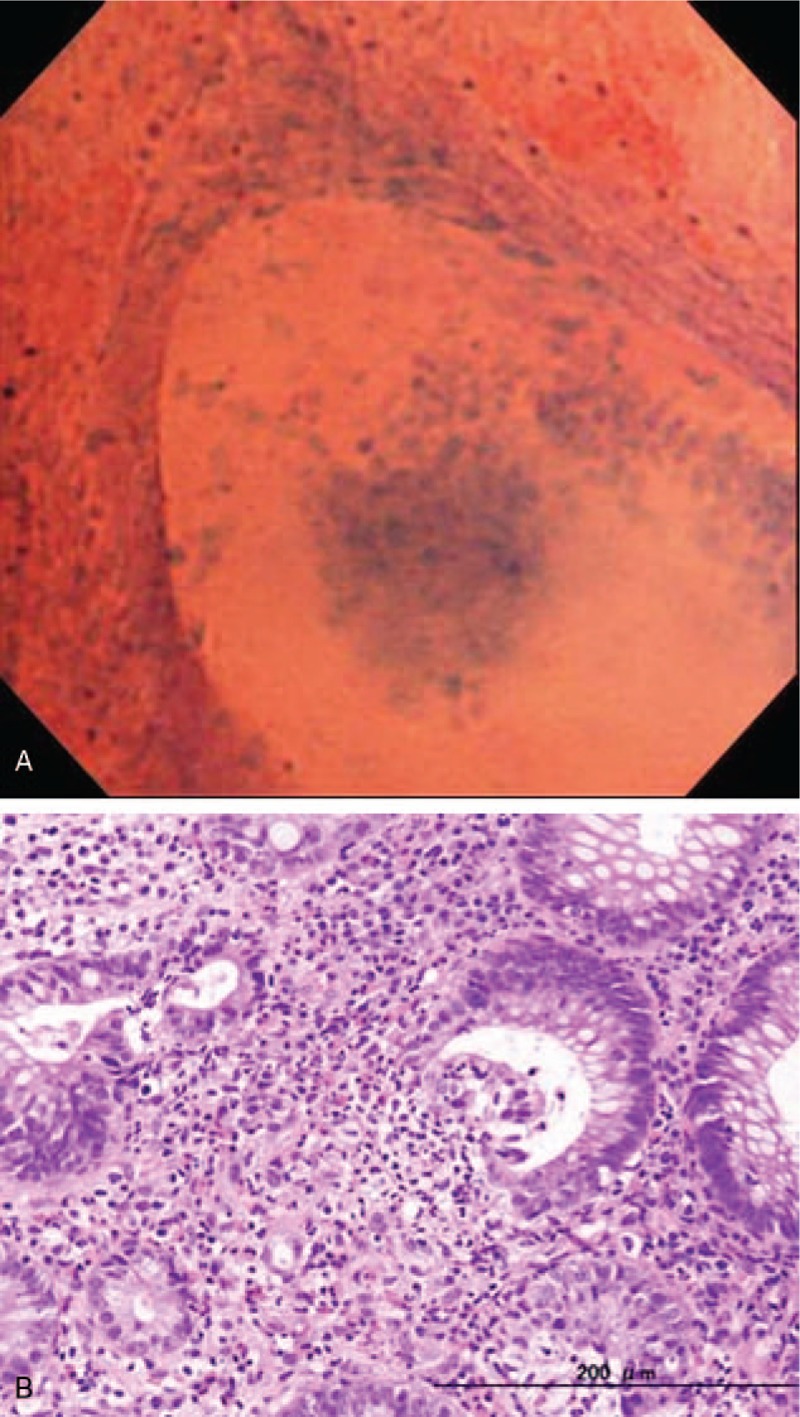
EC shows the irregularly dilated pits and intraglandular infiltration of inflammatory cell components (A). Histopathology using the targeted biopsy specimen shows crypt abscess formation within the dilated crypt (B).
Endoscopy was performed in case of relapse or once in 2 years for UC-associated cancer surveillance (median 0.5 per year; mean 0.7 per year, range 0.3–2.5 per year). Twenty-two in the 32 UC patients were followed-up more than 60 months. There were 9 cases in EC-A, 4 in EC-B, 5 in EC-C, and 4 in EC-D. There were significant differences in the percentages of the remission among the EC classifications (P < .005, Fig. 5). There were 2 (50%) recurrent cases among in the EC-B group; 2 (40%) recurrent cases in the EC-C group, and 3 (75%) recurrent cases in the EC-D group during the follow-up period (median, 521 days). On the contrary, none had relapse in the EC-A group during the follow-up period.
Figure 5.
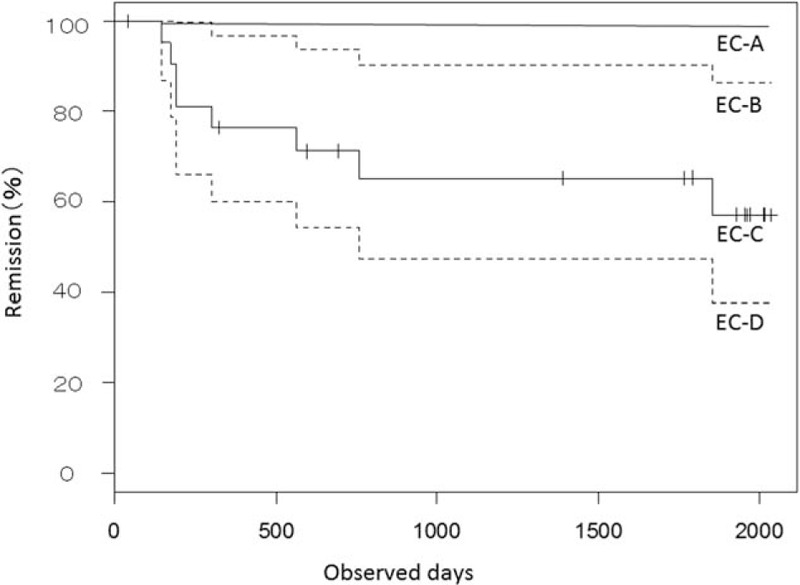
Comparison of the percentage of patients in remission on the basis of the endocytoscopic classification using the Kaplan–Meier method. There are significant differences in the percentages of the remission of UC among the EC classifications. EC = endocytoscopy, UC = ulcerative colitis.
4. Discussion
We documented here the simple stratification for endoscytoscopic findings in UC patients. The interobserver agreement for the EC stratification was comparable to the prior studies via CLE or EC.[8,11] Currently, there have been several stratification systems for UC using the ultramagnified endoscopic modalities.[8,11,13] Bessho et al established the EC scoring system consisting of shape of crypts, distance between crypts and small vessels with acceptable interobserver agreement.[8] However, it was difficult to identify microvessels after staining with methylene blue and crystal violet during EC in our experience. Instead, CLE using fluorescein could be reliably evaluate the microvascular alterations in the gut mucosa of UC patients.[11,13] Our 4-grade stratification system based on cryptal architectures can be simple and easy to learn for endoscopists beginning to use EC. Nevertheless, EC has potential limitations related to additional processes of washing, staining, and imaging.[9]
In this study, the EC classification was significantly associated with MES under standard colonoscopic observation. In addition, there were significant correlation between the disease activity and the EC classification. Increased percentages of active stage were seen in paralleled with the more aggressive stratification. Bessho et al[11] reported significant association of the EC scores with the endoscopic grades but not with clinical activity. We also assessed clinical symptoms and inflammatory markers at each follow-up point. Achieving clinical remission without mucosal healing, however, does not always lead to preventing relapse.[16,17] Inflammatory markers were not increased in our study cohort as reported in prior studies, which showed that inflammatory markers were not correlated with macroscopic findings and could not contribute to prediction of the disease relapse in UC.[18] Based on these reasons, we depended on endoscopic assessment for the disease severity. The discrepancy, however, might be attributed to sample size, bias in case enrolment and difference in the evaluation system. Thus, there were limitations including a relatively small number of patients who were heterogeneous in terms of medication.
The differences in the presence of severe mucosal inflammation, crypt abscess, and goblet cell depletion among the EC classifications reached statistical significance for each. The final diagnosis and grading of inflammatory activity in inflammatory bowel disease (IBD) is still predominantly based on histopathological analysis.[3,4,9] Nevertheless, the preparation of tissue section and ensuing histopathological examination usually take considerable time. Due to sampling errors, histopathological assessment is not representative of the whole inflamed area. In addition, the possible risk of physical bleeding may give reason for the alternative methods for nondestructive in vivo imaging modalities.[9] In this respect, EC and CLE provide real-time microscopic images in vivo during on-going endoscopy.[5,6] Using CLE, more than half of UC patients with normal endoscopy had evidence of acute inflammation histologically, but the assessment of mucosal inflammatory infiltrates seems difficult when using CLE.[8] In contrast, Neumann et al[9] showed the possibility to reliably distinguish even single inflammatory cells by EC with acceptable accuracy via the probe-based EC at a magnification capability of 1390-fold.
Staining with methylene blue and crystal violet could visualize nuclei of not only the epithelia but also inflammatory cells in the mucosa of UC patients even at the 450-fold magnification via the integrated prototype EC employed here, as seen in other sites of the alimentary tract.[19,20] Then, EC enabled real-time color identification of inflammatory cell infiltrates within the colorectal mucosa and visualization of crypt abscess formation. In this regard, we have documented that EC identified disease-specific histology including inter-glandular infiltration of smaller cell components and the lymphoepithelial origin in the case of mucosa-associated lymphoid tissue lymphoma of the stomach.[19] On the other hand, the most common used fluorescent agent, fluorescein, does not enable nuclear visualization via CLE. In addition, CLE images are black-and-white pictures only, and hence, it is currently not possible to differentiate inflammatory cells.[8]
Of clinical significance, the EC stratification can predict the disease relapse in UC patients. Kiesslich et al[13] reported that increased cell shedding with fluorescein leakage identified by CLE was associated with relapse of IBD within 12 months. Nowadays, it is well recognized that mucosal healing has been shown to reduce the likelihood of clinical relapse, reduce the risk of future surgeries and reduce hospitalizations.[1,21] Severity of endoscopic lesions in both UC and Crohn's disease predicts more aggressive clinical courses and increased rates of surgery.[1,2,21] Nevertheless, no validated endoscopic definition of mucosal healing exists in IBD, as the current scoring systems may be too complex or rigid to be readily adopted into clinical practice.[21] Riley et al[3] reported increased risk of relapse among UC patients with histological disease activity despite symptomatic and endoscopic remission. These data suggest that macroscopic healing may not be sufficient to accurately predict long-term disease outcomes. In turn, adamantly mucosal healing could be sustained in UC patients in the EC-A classification where the even crypt arrangement and regular size and configuration of pits were confirmed. The data from this pilot study provides insights to enable the design of further clinical trials to assess whether EC can predict the mucosal healing, eventually leading to long-lasting remission in UC.
In conclusion, EC is reliable for the assessment of microscopic inflammatory features of UC, in particular crypt abscess, culminating in vivo virtual histopathology during on-going endoscopic examination. The EC stratification based on crypt architectures in UC patients can predict the disease relapse.
Footnotes
Abbreviations: 5-ASA = 5-Aminosalicylic acid, CLE = confocal laser endomicroscopy, EC = endocytoscopy, IBD = inflammatory bowel disease, MES = Mayo endoscopic subscores, TNF = tumor necrosis factor, UC = ulcerative colitis.
The authors have no conflicts of interest to disclose.
References
- [1].Travis SP, Higgins PD, Orchard T, et al. Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther 2011;34:113–24. [DOI] [PubMed] [Google Scholar]
- [2].Carbonnel F, Lavergne A, Lémann M, et al. Colonoscopy of acute colitis. A safe and reliable tool for assessment of severity. Dig Dis Sci 1994;39:1550–7. [DOI] [PubMed] [Google Scholar]
- [3].Riley SA, Mani V, Goodman MJ, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut 1991;32:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Salvatori F, Siciliano S, Maione F, et al. Confocal laser endomicroscopy in the study of colonic mucosa in IBD patients: a review. Gastroenterol Res Pract 2012;2012:525098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wallace MB, Kiesslich R. Advances in endoscopic imaging of colorectal neoplasia. Gastroenterology 2010;138:2140–50. [DOI] [PubMed] [Google Scholar]
- [6].Neumann H, Fuchs FS, Vieth M, et al. Review article: in vivo imaging by endocytoscopy. Aliment Pharmacol Ther 2011;33:1183–93. [DOI] [PubMed] [Google Scholar]
- [7].Watanabe O, Ando T, Maeda O, et al. Confocal endomicroscopy in patients with ulcerative colitis. J Gastroenterol Hepatol 2008;23(Suppl 2):S286–290. [DOI] [PubMed] [Google Scholar]
- [8].Li CQ, Xie XJ, Yu T, et al. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol 2010;105:1391–6. [DOI] [PubMed] [Google Scholar]
- [9].Neumann H, Vieth M, Neurath MF, et al. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: a pilot study. Inflamm Bowel Dis 2013;19:356–62. [DOI] [PubMed] [Google Scholar]
- [10].Gheorghe C, Cotruta B, Iacob R, et al. Endomicroscopy for assessing mucosal healing in patients with ulcerative colitis. J Gastrointestin Liver Dis 2011;20:423–6. [PubMed] [Google Scholar]
- [11].Bessho R, Kanai T, Hosoe N, et al. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J Gastroenterol 2011;46:1197–202. [DOI] [PubMed] [Google Scholar]
- [12].Thekkek N, Muldoon T, Polydorides AD, et al. Vital-dye enhanced fluorescence imaging of GI mucosa: metaplasia, neoplasia, inflammation. Gastrointest Endosc 2012;75:877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012;61:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- [15].Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- [16].Walsh A, Palmer R, Travis S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin N Am 2014;24:367–78. [DOI] [PubMed] [Google Scholar]
- [17].Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501–23. [DOI] [PubMed] [Google Scholar]
- [18].Mohammed N, Subramanian V. Clinical relevance of endoscopic assessment of inflammation in ulcerative colitis: Can endoscopic evaluation predict outcomes? World J Gastroenterol 2016;22:9324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Isomoto H, Ishii H, Matsushima K, et al. Gastrointestinal: novel endocytoscopic findings of gastric low-grade mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol 2012;27:1535. [DOI] [PubMed] [Google Scholar]
- [20].Minami H, Yamaguchi N, Matsushima K, et al. Improvement of endocytoscopic findings after per oral endoscopic myotomy (POEM) in esophageal achalasia; does POEM reduce the risk of developing esophageal carcinoma? Per oral endoscopic myotomy, endocytoscopy and carcinogenesis. BMC Gastroenterol 2013;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ha C, Kornbluth A. Mucosal healing in inflammatory bowel disease: where do we stand? Curr Gastroenterol Rep 2010;12:471–8. [DOI] [PubMed] [Google Scholar]


