Abstract
Objectives
To assess the clinical value of 18F-flurodeoxyglucose (FDG) positron emission tomography (PET) in a prospective cohort of patients with large-vessel vasculitis (LVV) and disease comparators.
Methods
Patients with Takayasu’s arteritis (TAK) and giant cell arteritis (GCA) were studied, along with a comparator group consisting of patients with hyperlipidemia, diseases that mimic LVV, and healthy controls. Participants underwent clinical evaluation and FDG-PET imaging, and patients with LVV underwent serial imaging at six-month intervals. Performance characteristics of FDG-PET interpretation to differentiate clinically active LVV from disease comparators and from clinical remission were calculated. A qualitative summary score (PETVAS) based on global arterial FDG uptake was used to study associations between PET activity and clinical characteristics and to predict future relapse.
Results
170 FDG-PET scans were performed in 115 participants (LVV=56; comparators=59). FDG-PET differentiated patients with clinically active LVV and disease comparators with a sensitivity=85% (95%CI: 69–94%) and specificity=83% (95%CI: 71–91%). FDG-PET scans were interpreted as active vasculitis in most patients with LVV in clinical remission (41 of 71, 58%). Clinical disease activity status, disease duration, body mass index, and glucocorticoid use were independently associated with PET scan activity. Among patients who underwent PET during clinical remission, future clinical relapse was more common in patients with a high versus low PETVAS (45% versus 11%, p=0.03) over a median follow-up of 15 months.
Conclusions
FDG-PET provides information about vascular inflammation that is complimentary to, and unique from, clinical assessment in LVV. FDG-PET scan activity during clinical remission was associated with future clinical relapse.
Keywords: vasculitis, large-vessel vasculitis, giant cell arteritis, Takayasu’s arteritis, positron emission tomography, fluorodeoxyglucose, vascular inflammation
Positron emission tomography (PET) to detect regional distribution of 18F-flurodeoxyglucose (FDG) is an established form of molecular imaging currently used to detect/diagnose disease and monitor treatment response in oncologic diseases[1]. Activated inflammatory cells, including macrophages, exhibit increased glycolytic activity and thus accumulate FDG similar to certain tumor cells, making FDG-PET a potentially useful modality to assess the extent of inflammation in tissue[2]. However, the use of FDG-PET to diagnose and monitor inflammatory diseases remains controversial[3].
Giant cell arteritis (GCA) and Takayasu’s arteritis (TAK) are the two major forms of large vessel vasculitis (LVV), defined by inflammation of the aorta and primary branches[4]. Clinical assessment of disease activity in LVV can be challenging, thus posing a barrier to effective monitoring and treatment[5]. Patients with LVV can develop new vascular lesions during periods of apparent sustained clinical remission and with normal inflammatory markers; temporal artery biopsies performed during clinical remission can demonstrate ongoing active vasculitis[6, 7]. Angiography is often recommended to complement clinical assessment; however, there are no guidelines for using imaging for assessment of LVV, and angiography may not be adequately sensitive to detect vascular inflammation prior to the onset of vessel wall damage[8]. FDG-PET can detect metabolic activity from leukocytes infiltrating the walls of large arteries and may, therefore, be more sensitive than angiography to monitor vascular inflammation in LVV[9].
While several studies have examined the potential of molecular imaging in LVV, the role of FDG-PET to detect vascular inflammation, monitor disease activity over time, and predict clinical outcomes remains unclear. Most studies suggest that FDG-PET can detect vascular inflammation in LVV; however, these studies are mostly retrospective and involve healthy subjects or patients with cancer as comparators rather than patients with other conditions that clinically resemble LVV[10–17]. The performance characteristics of FDG-PET in LVV should be determined against an appropriate comparator population for whom the test would be clinically indicated to rule out vasculitis. Additionally, most studies of FDG-PET in LVV have focused on the time of initial diagnosis, and the utility of FDG-PET to monitor disease activity or predict relapse is uncertain due to limited imaging-based assessments at later time points in the disease.
The objective of this study was to assess the role of FDG-PET as an imaging biomarker in a prospective, longitudinal cohort of patients with LVV and a composite comparator group comprised mainly of patients with diseases that mimic LVV.
METHODS
Study population
Patients ≥ 5 years of age were recruited into a prospective, observational cohort at the National Institutes of Health (NIH) in Bethesda, MD, USA (NCT02257866). All patients with LVV fulfilled the 1990 American College of Rheumatology (ACR) Classification Criteria for Takayasu’s arteritis or modified 1990 ACR Criteria for giant cell arteritis[18, 19]. Patients with LVV were enrolled at various stages during the disease, but initial imaging studies were preferentially performed during periods of clinically active disease or when taking < 10mg daily prednisone during clinical remission.
A composite disease comparator group was studied, consisting of patients with hyperlipidemia, patients referred to the NIH for possible LVV who were subsequently diagnosed with a different condition (LVV mimics), and healthy subjects. The hyperlipidemia group was recruited from a parallel study (NCT01212900) as part of a larger investigation of atherosclerosis imaging related to statin treatment[20]. Patients with hyperlipidemia were > 55 years of age and required therapy with a statin. This group was chosen because atherosclerosis is a known radiographic mimic of LVV. Patients referred to the NIH for possible LVV who were determined to have another form of systemic inflammatory disease (e.g. polyarteritis nodosa) or a non-inflammatory vasculopathy (e.g. fibromuscular dysplasia) were included as LVV mimics. This group underwent repeat clinical assessment at 6-months to insure diagnostic accuracy. A group of healthy adult subjects was recruited across the age spectrum of TAK and GCA to serve as additional comparators.
Clinical Assessment and Definitions of Disease Activity
All patients were clinically evaluated and imaged at the NIH Clinical Center, and patients with LVV underwent repeat imaging studies and clinical assessments at 6 month intervals. Outside physician records were obtained throughout the study period and reviewed. All clinical assessments were performed by the investigative study team within 24 hours prior to imaging assessment. Physician-determined disease activity status was based upon clinical history, physical examination, and laboratory assessments and was recorded prior to conducting imaging studies. Active disease was defined as presence at the time of assessment of any clinical disease feature directly attributed to vasculitis. Chronic fatigue or elevated acute phase reactants in the absence of clinical symptoms were not considered evidence of active disease. Remission was defined as the absence of any clinical symptoms directly attributable to vasculitis. A disease relapse was defined as a recurrence of clinical disease activity after a period of remission necessitating an increase in prednisone dose of ≥ 10mg per day and/or addition of a glucocorticoid-sparing therapy. Relapse was determined by the investigative study team while blinded to imaging findings. No clinical care or treatment decisions were made by the investigative team.
FDG-PET Imaging Protocol
PET studies were performed at each study visit. Subject preparation included instructions to consume a carbohydrate-sparse meal on the day prior, and to fast on the day of imaging. Patients ≥ 18 years of age underwent FDG-PET-CT of the torso. At 2-hours uptake time, image acquisition commenced with a Siemens Biograph mCT (Siemens Medical Solutions, Erlangen, Germany). Image reconstruction employed CT attenuation correction and iterative reconstruction (24 iterations, 3 subsets, 256 matrix, 1.2 zoom, 1.5mm slice thickness, time-of-flight, point spread function correction, no post reconstruction filtering). To minimize radiation exposure, patients <18 years of age underwent whole body FDG-PET-MR with a Siemens Biograph mMR (Siemens Medical Solutions, Erlangen, Germany). PET-MR reconstruction employed MR-based attenuation correction, and iterative reconstruction (172 matrix, 1.2 zoom, 2.03mm slice thickness, point spread function correction, 4.0 Gaussian post reconstruction filtering). Adult patients received a fixed dose of FDG (10mCi) and pediatric patients received a weight-based dose (0.1mCi/kg).
Nuclear Medicine Physician Interpretation of FDG-PET Findings
Two nuclear medicine physicians (MA, CC) interpreted all PET scans included in this study blinded to clinical data and to each other’s assessment. PET interpretation determined whether the findings were consistent with active or inactive vasculitis based upon assessment of vascular FDG uptake. Discrepancies between the readers were adjudicated ≥ 2 weeks after the initial assessment. During adjudication, the readers were blinded to results from the initial assessment, and the final impression of imaging activity was determined by consensus review.
Qualitative Assessment of FDG Uptake in Arterial Territories and Creation of PETVAS
A single nuclear medicine physician (MA) performed qualitative assessment of FDG uptake in 4 segments of the aorta (ascending, arch, descending thoracic, and abdominal) and in 11 branch arteries (innominate, carotids, subclavians, axillaries, iliacs, and femorals). The degree of arterial uptake was visually assessed relative to liver uptake as: 0 = no uptake; 1 = less than liver; 2 = same as liver; 3 = greater than liver[12, 21]. Only FDG-PET-CT scans were considered for qualitative review. To assess the qualitative burden of arterial FDG uptake across multiple arterial regions, a summary score (hereafter termed PET Vascular Activity Score - PETVAS) was created by adding the qualitative scores in specific arterial territories where the mean qualitative scores were significantly higher in LVV versus comparators (see Supplemental Table 1 for calculation of PETVAS).
Statistical Analysis
An overview of analyses presented in this study is as follows: 1) calculation of performance characteristics of FDG-PET in patients with LVV versus comparators; 2) calculation of performance characteristics of FDG-PET in patients with clinically active LVV versus clinical remission; 3) determination of clinical variables associated with PET scan activity; 4) comparison of qualitative FDG uptake in specific arterial territories in patients with LVV and comparators; 5) correlation of PETVAS score with clinical features of disease and laboratory tests; and 6) a subgroup analysis to determine if PET scans performed during clinical remission predict future clinical relapse.
Characteristics of FDG-PET interpretation (sensitivity, specificity, receiver operating characteristic (ROC) curve) were calculated. Overall image interpretation, rather than qualitative assessment, was considered the gold standard for determining PET scan activity. Interobserver agreement of PET interpretation was assessed by the kappa statistic. Mixed effects logistic regression, adjusting for within-patient correlated data as a random effect, was performed to study the associations between nuclear medicine interpretation of active vasculitis on the PET scan (outcome measure) and the following predictor variables: clinical disease activity status, disease duration since the time of initial symptom onset, body mass index, form of LVV, current use of glucocorticoid-sparing therapy, age, daily prednisone dose, sex, erythrocyte sedimentation rate, and serum levels of fibrinogen, endothelin 1, and C-reactive protein. Only variables with p<0.2 in univariable analyses were included in the multivariable model. Correlation between PETVAS and selected clinical variables in patients with LVV stratified by clinical disease activity status was assessed using the Spearman correlation coefficient. For the correlation analyses, repeated measures were averaged over successive visits if there was no change in clinical status. Differences between distributions of continuous data were compared using a two-tailed student t-test or Mann-Whitney U test for pairwise comparisons and one-way analysis of variance or Kruskal Wallis with post-hoc testing for comparisons across multiple groups, as appropriate. Fisher’s exact test was used to assess differences in categorical data. A p value <0.05 defined statistical significance.
Ethics and Informed Consent
All patients provided written informed consent. An institutional review board and radiation safety committee at the NIH approved the research.
RESULTS
Study Population
From September 2014 through February 2017, a total of 115 participants were recruited into the study. There were 56 patients with LVV (GCA = 30; TAK = 26) and 59 disease comparators (hyperlipidemia = 35; LVV mimic = 17; healthy control = 7). A total of 170 FDG-PET scans were performed, including 111 scans in patients with LVV and 59 scans in the disease comparator group. Among patients with LVV, 35 patients (63%) underwent at least one follow up PET scan over a mean follow up of 6.3 months (± 6.7 months). Demographic characteristics of the study participants are presented in Table 1. Additional clinical features of the patients with LVV stratified by vasculitis type and disease activity status are presented in Supplemental Table 2. Clinical features of disease activity are listed in Supplemental Table 3. Diagnoses in the LVV mimic group are provided in Supplemental Table 4. Five children with TAK and 3 children with LVV mimic conditions were included in this study.
Table 1.
Study Population Baseline Characteristics
| Large Vessel Vasculitis | Comparator Groups | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| GCA | TAK | Total | Hyper-lipidemia | LVV Mimic | Healthy | Total | |
| Patients (n) | 30 | 26 | 56 | 35 | 17 | 7 | 59 |
| PET Scans (n) | 67 | 44 | 111 | 35 | 17 | 7 | 59 |
| Age Years (sd) | 68.5 | 31.4 | 51.0 | 63.6 | 44.6 | 49.3 | 56.5 |
| (9.1) | (10.8) | (21.5) | (5.7) | (24.5) | (20.7) | (17.4) | |
| Sex Female (%) | 21 | 18 | 39 | 11 | 11 | 5 | 27 |
| (70.0) | (69.2) | (69.6) | (31.4) | (64.7) | (71.4) | (45.8) | |
| Body Mass Index kg/m2 (sd) | 27.8 | 27.6 | 27.7 | 28.1 | 26.7 | 30.3 | 28.0 |
| (5.7) | (8.8) | (7.3) | (4.5) | (7.7) | (7.7) | (6.0) | |
| Disease Duration Years (sd) | 2.6 | 12.5 | 6.9 | NA | NA | NA | NA |
| (2.7) | (10.9) | (8.9) | |||||
| No. of abnormal FDG-PET Scans (%) | 49 | 26 | 75 | 6 | 3 | 1 | 10 |
| (73.1) | (59.1) | (67.6) | (17.1) | (17.6) | (14.3) | (16.9) | |
GCA=giant cell arteritis; TAK=Takayasu’s arteritis; LVV=large vessel vasculitis; n=number; sd=standard deviation; NA=not applicable.
Performance Characteristics of FDG-PET
There was excellent agreement between the two independent nuclear medicine physicians regarding whether PET scan findings demonstrated active vasculitis (kappa=0.84; 95% Confidence Interval 0.75–0.94). 75 of 111 scans (68%) performed in patients with LVV were interpreted as consistent with active vasculitis (Table 1). Fewer scans performed in the disease comparators were interpreted as active vasculitis (10 out of 59 scans, 17%).
Results from the adjudicated interpretation of FDG-PET scans are displayed in Table 2. The proportion of patients who had a PET scan interpreted as active vasculitis were: clinically active LVV = 34/40; LVV in remission = 41/71; and comparators = 10/59. The performance characteristics of FDG-PET to differentiate between patients with clinically active LVV and disease comparators were sensitivity=85% (95% confidence interval (CI) 69–94%) and specificity=83% (95% CI 71–91%). The specificity of FDG-PET to differentiate between patients with clinically active LVV and patients with LVV in clinical remission was 42% (95% CI 31–55%). Representative FDG-PET scans and clinical descriptions are provided in Supplemental Figures 1–4.
TABLE 2.
Qualitative Impression of PET Scans
| Clinically Active LVV | Clinical Remission LVV | Comparator | Total | |
|---|---|---|---|---|
| PET Scan | ||||
| Active Vasculitis | 34 | 41 | 10 | 85 |
| PET Scan | ||||
| No Vasculitis | 6 | 30 | 49 | 85 |
| Total | 40 | 71 | 59 | 170 |
PET = positron emission tomography; LVV = large-vessel vasculitis
Clinical Predictors of FDG-PET Activity
Regression modeling was used to determine the clinical variables associated with interpretation of PET scan activity. In a multivariable model, clinically active disease, shorter disease duration, lower body mass index, and lower amounts of daily prednisone use were independently associated with increased odds of the PET scan being interpreted as active vasculitis (Table 3). Acute phase reactants, age, sex, type of vasculitis (GCA vs TAK), and use of glucocorticoid-sparing therapies were not significantly associated with physician interpretation of PET scan activity. Effect estimates for type of vasculitis and prednisone use varied considerably between the univariable and multivariable models, indicating that the association between these variables and PET scan activity was strongly influenced by other predictor variables within the multivariable model.
TABLE 3.
Variables Associated with PET Scan Interpretation of Active Vasculitis
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Disease Activity Active vs Remission | 4.63 (1.62 – 13.32) | <0.01 | 16.14 (3.14–83.03) | <0.01 |
| Disease Duration (per year) | 0.99 (0.98–0.99) | <0.01 | 0.99 (0.98–0.99) | 0.01 |
| BMI (kg/m2) | 0.91 (0.85–0.97) | <0.01 | 0.89 (0.82–0.97) | 0.03 |
| Vasculitis TAK vs GCA | 0.42 (0.18–0.96) | 0.04 | 9.24 (0.43–196.58) | 0.20 |
| Immune Med Yes vs No | 2.33 (0.93–5.83) | 0.07 | 2.30 (0.65–8.18) | 0.31 |
| Age (per year) | 1.02 (0.99–1.04) | 0.13 | 1.06 (0.99–1.13) | 0.14 |
| Prednisone (mg/day) | 0.98 (0.95–1.01) | 0.16 | 0.94 (0.90–0.99) | 0.03 |
| Sex Male vs Female | 1.40 (0.57–3.46) | 0.47 | Not included in multivariable analysis | |
| Fibrinogen (mg/dL) | 1.00 (0.99–1.01) | 0.51 | ||
| ESR (mm/hr) | 1.01 (0.98–1.03) | 0.64 | ||
| Endothelin 1 (pg/mL) | 0.98 (0.88–1.09) | 0.68 | ||
| CRP (mg/mL) | 1.01 (0.98–1.02) | 0.89 | ||
OR = odds ratio; CI = confidence interval; BMI = body mass index; TAK = Takayasu’s arteritis; GCA = giant cell arteritis; Immune Med = glucocorticoid-sparing medication; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
Qualitative Assessment of Global Arterial FDG Uptake (PETVAS)
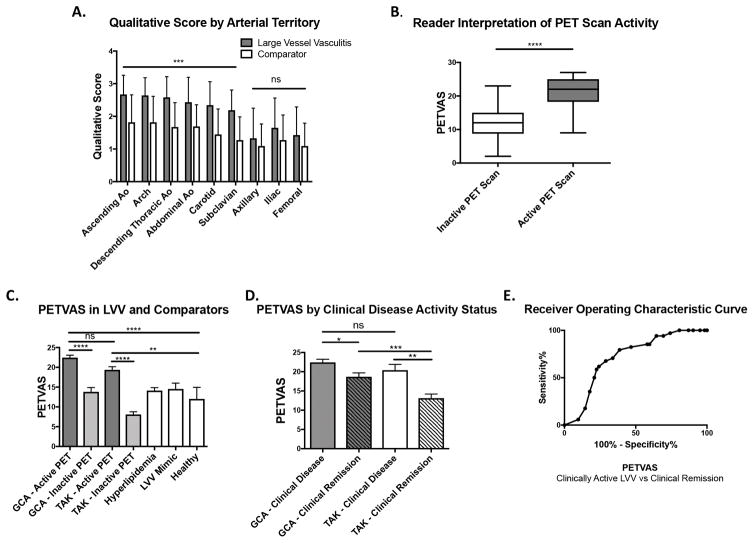
Distribution of arterial FDG uptake on a qualitative score of 0–3, with higher scores indicating increased uptake, are shown in Figure 1A. There was significantly increased FDG uptake in the ascending aorta, aortic arch, descending thoracic aorta, abdominal aorta, carotid and subclavian arteries for patients with LVV versus disease comparators (p<0.001). There were no differences in arterial FDG uptake in the axillary, iliac, and femoral arteries between patients with LVV and comparators. Statistical comparisons between qualitative scores in arterial territories among the two forms of vasculitis and disease comparators are listed in Supplemental Table 5. Notably, significantly more FDG uptake was observed in the abdominal aorta, axillary, and iliofemoral arteries in GCA compared to TAK, with no differences in FDG uptake in the thoracic aorta, carotid, and subclavian arteries between these diseases.
Figure 1.
Qualitative Summary Score of Global Arterial FDG Uptake (PETVAS). A, Mean qualitative scores by arterial region in patients with large vessel vasculitis (LVV) versus a composite disease comparator group. B, Comparison of mean summary score of FDG uptake in 9 arterial territories (PETVAS) in patients with a PET scan interpreted as inactive versus active vasculitis. C, Comparison of PETVAS in patients with giant cell arteritis (GCA), Takayasu’s arteritis (TAK), and disease comparator groups. D, Comparison of PETVAS in patients with GCA and TAK stratified by clinically determined disease activity status. E, Receiver Operating Characteristic Curve of the performance characteristics of PETVAS to differentiate clinically active LVV from LVV in clinical remission. Results in A and B are analyzed by two-tailed student t test. Results in C and D are presented as means with standard error margin and analyzed by one-way ANOVA with post hoc Tukey’s multiple comparison’s test. * p <0.05; ** p<0.01; *** p<0.001; **** p<0.0001. ns = not significant.
The mean PETVAS was significantly higher in scans visually interpreted as active versus inactive vasculitis (21.5 versus 12.2, p<0.0001), indicating that a global pattern of FDG uptake tended to result in an interpretation of active vasculitis (Figure 1B). PETVAS was significantly higher in patients with GCA or TAK who had visual evidence of active vasculitis on PET compared to scores from any of the specific disease comparator groups (Figure 1C). PETVAS was significantly higher in both GCA and TAK during periods of clinically-determined active disease versus clinical remission (Figure 1D). Among patients with LVV considered by the clinical investigator to be in clinical remission, higher PETVAS was observed in GCA compared to TAK. An ROC curve indicated fair performance characteristics (area under the curve = 0.72) of PETVAS to distinguish between clinically active LVV and remission (Figure 1E) with a cut-point of ≥ 20 yielding a sensitivity of 68% (95%CI: 50 – 83%) and a specificity of 71% (95%CI: 58 – 82%).
PETVAS Associations During Active Disease and Clinical Remission
To test whether factors associated with the degree of arterial FDG uptake differ in patients during periods of active clinical disease versus clinical remission, associations between PETVAS and specific clinical features were studied in patients with LVV stratified by clinical disease activity status. Among patients with clinically active disease (Table 4), there were moderate positive correlations between specific acute phase reactants (ESR, CRP, fibrinogen) and PETVAS (r=0.36–0.52). Amount of daily prednisone use was inversely associated with arterial FDG uptake, while age and body mass index were not associated with FDG uptake. A different pattern of associations was observed in patients with LVV during clinical remission: age was positively and BMI negatively associated with PETVAS, while acute phase reactants and daily prednisone use were not significantly associated with PETVAS.
TABLE 4.
Correlations Between Summary Score of Arterial FDG Uptake (PETVAS) and Clinical Features in Patients with Large Vessel Vasculitis
| Clinically Active LVV | Clinical Remission LVV | |||
|---|---|---|---|---|
|
| ||||
| Spearman r (95% CI) | P value | Spearman r (95% CI) | P value | |
| ESR (mm/hr) | 0.37 (0.03 to 0.64) | 0.03 | −0.07 (−0.32 to 0.19) | 0.57 |
| CRP (mg/mL) | 0.36 (0.02 to 0.63) | 0.03 | −0.12 (−0.36 to 0.14) | 0.37 |
| Fibrinogen (mg/dL) | 0.52 (0.18 to 0.75) | 0.004 | 0.05 (−0.22 to 0.32) | 0.70 |
| Endothelin-1 (pg/mL) | −0.18 (−0.53 to 0.22) | 0.36 | −0.10 (−0.38 to 0.20) | 0.50 |
| Prednisone (mg/day) | −0.36 (−0.62 to −0.01) | 0.04 | −0.24 (−0.47 to 0) | 0.06 |
| Age (years) | 0.11 (−0.25 to 0.44) | 0.55 | 0.37 (0.13 to 0.57) | 0.003 |
| BMI (kg/m2) | −0.19 (−0.50 to 0.17) | 0.28 | −0.26 (−0.49 to −0.01) | 0.04 |
LVV = large vessel vasculitis; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; BMI = body mass index.
Value of FDG-PET to Predict Future Clinical Relapse in LVV
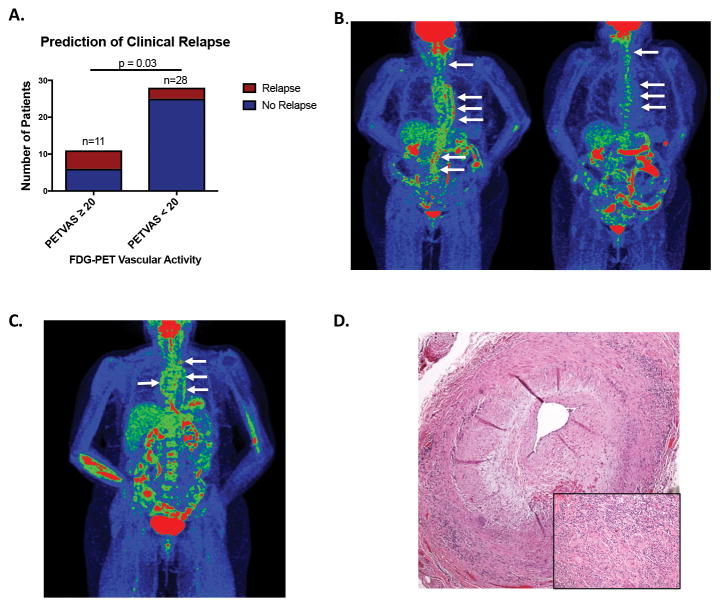
The value of PET scan findings to predict future clinical events was studied. Thirty-nine patients with LVV who underwent an FDG-PET-CT scan during a period of clinical remission and had at least 3 months of clinical follow up were selected within the cohort. Eight of these patients experienced a clinical relapse over a median follow up of 15 months. Based upon a PETVAS threshold of 20, which optimally differentiated patients with clinically active LVV from those in clinical remission (Figure 1E), patients were categorized during clinical remission as having a high amount of arterial FDG uptake (PETVAS ≥ 20) or a lower amount of arterial FDG uptake (PETVAS < 20). Significantly more patients with PETVAS ≥ 20 experienced future clinical relapse compared to patients with PETVAS < 20 (45% vs 11%, p=0.03) (Figure 2A). Clinical characteristics of these patients are shown in Supplemental Table 6. The mean daily prednisone dose in both groups was ≤ 5mg at the time of imaging. Patients with PETVAS ≥ 20 were more likely to have GCA than TAK, had significantly shorter disease duration, and were less likely to be taking glucocorticoid-sparing therapies compared to patients with PETVAS < 20 (p<0.05). Representative PET scan images from two patients with a high PETVAS during clinical remission who subsequently experienced a clinical relapse are shown in Figures 2B, 2C. A temporal artery biopsy was performed in one patient during a period of apparent clinical remission when the FDG-PET scan findings were interpreted as active vasculitis (PETVAS=27). The biopsy was diagnostic for active vasculitis, revealing transmural inflammation and giant cells (Figure 2D).
Figure 2.
Clinical value of FDG-PET findings. A, Global summary score of arterial FDG (PETVAS) calculated in patients with large vessel vasculitis (LVV) during clinical remission predicts future clinical relapse. Patients with high PETVAS (≥ 20) were significantly more likely to relapse than patients with lower PETVAS (< 20) [6/11 (45%) vs 3/28 (15%); p=0.03]. B, PET-CT obtained in a patient with giant cell arteritis during clinical remission (disease duration=3 years, taking prednisone 2.5mg every other day) shows severe FDG uptake throughout the aorta and branch vessels (left panel, white arrows). Patient experienced disease relapse (mesenteric ischemia, fatigue, newly elevated acute phase reactants) one month after the baseline scan and was treated with methotrexate and tapered glucocorticoids with improvement of arterial FDG uptake (right panel, white arrows). C, Patient with GCA in apparent clinical remission (disease duration = 1 year, taking prednisone 7mg per day) had severe FDG arterial uptake in the aorta and branch vessels with normal inflammatory markers. Upon further reduction of daily prednisone to 3mg per day over the subsequent 6 months, the patient experienced clinical relapse (polymyalgia rheumatica, fatigue, elevated acute phase reactants) requiring additional therapy. D, A temporal artery biopsy performed in a patient with GCA during clinical remission with FDG-PET CT suggestive of active vasculitis demonstrated transmural inflammation with giant cells (inset).
DISCUSSION
This study demonstrates that FDG-PET provides information about vascular inflammation that is at times contradictory to clinical assessment in LVV. While most patients with clinically active vasculitis had FDG-PET scan findings that demonstrate concordant vascular inflammation, the majority of patients with LVV in clinical remission also had FDG-PET scan findings interpreted by independent nuclear medicine physicians as active vasculitis. In the absence of contemporaneous histologic samples, it is unclear whether increased metabolic activity in the vascular wall detected in patients with LVV during clinical remission represents subclinical vasculitis[22], a secondary process such as vascular remodeling, hypoxia[23], atherosclerosis[2], or a combination of these factors. While FDG uptake correlates with histological macrophage density in animal models of atherosclerosis[24], altered metabolism in endothelial or smooth muscle cells secondary to vascular remodeling rather than active inflammation could also contribute to PET scan activity observed within the arterial wall in patients with LVV during clinical remission.
Despite the lack of histologic confirmation, results from this study strongly suggest that subclinical vascular inflammation is likely a major contributor to the varying degree of arterial FDG uptake observed during clinical remission. Patients with LVV who had a high global burden of arterial FDG uptake during clinical remission were at risk for future clinical relapse over a median of 15-months follow-up time. Histiologic evidence of vasculitis was demonstrated in a temporal artery biopsy from a patient in apparent clinical remission with a PET scan indicating active vasculitis (Figure 2D). The amount of vascular FDG uptake detected during clinical remission was reduced over time in response to treatment (e.g. Figure 2B). These findings raise important questions about how to define disease activity and remission in LVV and support prior autopsy studies which demonstrate ongoing vascular inflammation in patients with LVV who were otherwise in clinical remission at the time of death[25, 26].
Several studies have shown that FDG-PET can detect vascular inflammation in LVV. A recent meta-analysis of 8 studies examining the performance of FDG-PET to detect vasculitis in LVV[10, 12–17, 21] reported a pooled sensitivity of 76% (69–82%) and specificity of 93% (89–96%)[27]. These findings differ slightly from the performance characteristics in this study to differentiate active LVV from disease comparators (sensitivity 85%, specificity 83%). Some technique differences from other studies exist. To be consistent with the recommended procedure for atherosclerosis imaging[28], FDG-PET-CT images were obtained in the current study at a delayed uptake time of 2 hours compared to a 1-hour uptake time used in all prior studies. Delay in the time interval from injection of FDG to image acquisition can increase the sensitivity to detect FDG uptake in the arterial wall by allowing more time for distribution of FDG into tissue with concomitant elimination from the blood pool. Selection of a comparator population from diseases that resemble LVV rather than healthy controls and patients with cancer, as done in prior studies, likely contributes to lower specificity observed in this study. Notably, 17% of subjects in the disease comparator group had FDG-PET scan findings interpreted as active vasculitis, emphasizing that a diagnosis of LVV should not be made solely based on FDG-PET findings.
FDG-PET performed poorly as a biomarker of disease activity in LVV against a clinical reference standard, as most patients in clinical remission had PET scan findings interpreted as ongoing vascular inflammation. There is minimal data on FDG-PET findings during clinical remission in GCA, with one study noting increased arterial uptake in a sizable portion of patients during clinical remission[29]. Several studies suggest a stronger correlation between FDG uptake and clinical disease activity in TAK compared to GCA[9]. However, in a recent meta-analysis, vascular FDG uptake did not consistently correlate with disease activity in TAK and there was considerable heterogeneity in 7 studies[30–36] with a pooled sensitivity of 86% (78–93%) and specificity of 73% (63–81%)[37]. In the present study, 48% of PET scans performed in patients with TAK and 62% of PET scans performed in patients with GCA during clinical remission were interpreted as active vasculitis (Supplemental Table 2), and the global burden of arterial uptake during clinical remission was significantly higher in patients with GCA compared to patients with TAK (Figure 1D). These findings corroborate prior reports that PET scan activity does not correlate with clinical disease assessment in LVV and highlight a greater discordance between clinical and imaging assessment in patients with GCA compared to TAK.
Associations between clinical features and arterial FDG uptake suggest that additional factors beyond subclinical vasculitis also contribute to vascular FDG uptake during clinical remission in LVV. In studies of atherosclerosis, age and BMI are positively associated with arterial FDG uptake; however, the results are not uniform among these studies [38]. In this study, age was positively, but only modestly, correlated with the degree of vascular uptake in patients with LVV during clinical remission and not during active disease. A greater burden of arterial uptake during clinical remission was observed in patients with GCA, who are by disease definition older, compared to TAK. These findings suggest that atherosclerosis contributes in part to vascular FDG uptake in LVV during clinical remission.
Disease duration was significantly shorter in patients with GCA compared to TAK in this study, which may further explain the differences observed in arterial uptake between these diseases during remission. In multivariable regression models, disease duration, and not age or type of vasculitis, was significantly and inversely associated with PET scan activity. Patients with GCA had a higher burden of vascular PET scan activity during remission compared to patients with TAK, potentially because patients with GCA in remission were assessed closer to the time of initial diagnosis than patients with TAK. Furthermore, the inverse association between disease duration and vascular uptake in clinical remission suggests that the burden of vascular inflammation decreases over time in LVV, an unexpected finding if vascular uptake during remission was primarily driven by atherosclerosis or accrued vascular damage.
Results from prior studies on the value of FDG-PET scan to predict future clinical relapse have been inconclusive. One prior study failed to demonstrate predictive value of FDG-PET in 35 patients with GCA; however, all patients in that study were studied early in the course of disease (3 and 6 months after diagnosis) and were receiving moderate doses of glucocorticoids at the time of imaging which could dampen the predictive value of PET[29] given that increasing prednisone dose is negatively associated with PET scan activity (Table 3). A recent study demonstrated prognostic value of FDG-PET at the time of diagnosis in patients imaged before starting glucocorticoid therapy[39]. In the current study, the burden of arterial uptake during clinical remission predicted future relapse in patients who were assessed on average several years into the course of disease while taking minimal doses of glucocorticoids. While a PETVAS threshold of 20 was useful to predict future clinical relapse, a threshold score to define PET scan activity was not proposed in this study. A substantial number of PET scans with PETVAS < 20 were interpreted by independent readers as demonstrating active vasculitis, particularly in scans where there was intense FDG uptake confined to focal arterial territories.
This study has some important potential limitations to consider. This was a single-center study subject to assessment bias from the participating investigators. However, inter-rater agreement for independent and blinded PET scan interpretation was excellent. Physician interpretation and qualitative metrics, rather than semiquantitative values, e.g. standardized uptake values, were used to assess arterial FDG uptake. The standardization of semiquantitative values to study vascular inflammation is of recent interest[28] and should be the subject of future investigations[9]. Patients with LVV could be enrolled at any time in the disease course, limiting the ability to assess performance characteristics of FDG-PET at the time of diagnosis. Whether FDG-PET findings predicted angiographic progression of disease was not assessed.
This study has several important strengths. The study was prospective, larger than prior studies, contained both patients with GCA and TAK enabling comparative assessment across different forms of vasculitis, included children and a composite group of comparator diseases with vasculopathy, and introduced a new qualitative summary score of arterial FDG uptake (PETVAS). Unlike prior studies which have created a summary score based on qualitative FDG uptake in specific arterial territories to represent global burden of inflammation[29], the arterial territories included in PETVAS were determined by a data-driven approach comparing differences in qualitative scores between patients with LVV and a relevant comparator group.
In conclusion, findings from this study demonstrate that advanced imaging techniques provide information about disease activity that is complimentary to, and unique from, clinical assessment. This study provides novel, prospective evidence about the potential value of FDG-PET scans in patients with LVV who are assessed months to years into the course of disease. While serial monitoring of patients with FDG-PET may identify vascular abnormalities in patients with LVV otherwise in apparent clinical remission, use of FDG-PET to monitor vascular inflammation in routine clinical practice is not currently advisable. The value of FDG-PET later in the course of disease needs to be tested and validated in additional independent cohorts of patients with LVV who are monitored over longer periods of observation, ideally starting at the time of initial diagnosis, to determine whether PET abnormalities during clinical remission are associated with poor long-term clinical outcomes, including vascular progression of disease. However, this study provides preliminary evidence that FDG-PET performed in patients with LVV during established clinical remission can identify subsets of patients at risk for future clinical relapse. This study also demonstrates that simple metrics of arterial FDG uptake are useful to quantify the burden of vascular abnormalities on PET scans. Whether such assessments could function as a useful outcome measure to monitor vascular inflammation should be tested in clinical trials. Prior histopathologic studies demonstrated a disconnect between clinical assessment and vascular disease activity in LVV; this study provides additional imaging-based data about how to define disease activity in these complex conditions.
Supplementary Material
Acknowledgments
Financial supports of conflicts disclosure:
This research was supported through the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
References
- 1.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354(5):496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum D, Millon A, Fayad ZA. Molecular imaging in atherosclerosis: FDG PET. Curr Atheroscler Rep. 2012;14(5):429–37. doi: 10.1007/s11883-012-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamashita H, Kubota K, Mimori A. Clinical value of whole-body PET/CT in patients with active rheumatic diseases. Arthritis Res Ther. 2014;16(5):423. doi: 10.1186/s13075-014-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 5.Direskeneli H, Aydin SZ, Kermani TA, Matteson EL, Boers M, Herlyn K, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol. 2011;38(7):1471–9. doi: 10.3899/jrheum.110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Maleszewski JJ, Younge BR, Fritzlen JT, Hunder GG, Goronzy JJ, Warrington KJ, et al. Clinical and pathological evolution of giant cell arteritis: a prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod Pathol. 2017 doi: 10.1038/modpathol.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68(3):318–23. doi: 10.1136/ard.2008.088351. [DOI] [PubMed] [Google Scholar]
- 9.Danve A, O’Dell J. The Role of 18F Fluorodeoxyglucose Positron Emission Tomography Scanning in the Diagnosis and Management of Systemic Vasculitis. Int J Rheum Dis. 2015;18(7):714–24. doi: 10.1111/1756-185X.12713. [DOI] [PubMed] [Google Scholar]
- 10.Besson FL, de Boysson H, Parienti JJ, Bouvard G, Bienvenu B, Agostini D. Towards an optimal semiquantitative approach in giant cell arteritis: an (18)F-FDG PET/CT case-control study. Eur J Nucl Med Mol Imaging. 2014;41(1):155–66. doi: 10.1007/s00259-013-2545-1. [DOI] [PubMed] [Google Scholar]
- 11.Papathanasiou ND, Du Y, Menezes LJ, Almuhaideb A, Shastry M, Beynon H, et al. 18F-Fludeoxyglucose PET/CT in the evaluation of large-vessel vasculitis: diagnostic performance and correlation with clinical and laboratory parameters. Br J Radiol. 2012;85(1014):e188–94. doi: 10.1259/bjr/16422950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter MA, Melzer RA, Schindler C, Muller-Brand J, Tyndall A, Nitzsche EU. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32(6):674–81. doi: 10.1007/s00259-004-1757-9. [DOI] [PubMed] [Google Scholar]
- 13.Henes JC, Muller M, Krieger J, Balletshofer B, Pfannenberg AC, Kanz L, et al. [18F] FDG-PET/CT as a new and sensitive imaging method for the diagnosis of large vessel vasculitis. Clin Exp Rheumatol. 2008;26(3 Suppl 49):S47–52. [PubMed] [Google Scholar]
- 14.Lehmann P, Buchtala S, Achajew N, Haerle P, Ehrenstein B, Lighvani H, et al. 18F-FDG PET as a diagnostic procedure in large vessel vasculitis-a controlled, blinded re-examination of routine PET scans. Clin Rheumatol. 2011;30(1):37–42. doi: 10.1007/s10067-010-1598-9. [DOI] [PubMed] [Google Scholar]
- 15.Prieto-Gonzalez S, Depetris M, Garcia-Martinez A, Espigol-Frigole G, Tavera-Bahillo I, Corbera-Bellata M, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis. 2014;73(7):1388–92. doi: 10.1136/annrheumdis-2013-204572. [DOI] [PubMed] [Google Scholar]
- 16.Hautzel H, Sander O, Heinzel A, Schneider M, Muller HW. Assessment of large-vessel involvement in giant cell arteritis with 18F-FDG PET: introducing an ROC-analysis-based cutoff ratio. J Nucl Med. 2008;49(7):1107–13. doi: 10.2967/jnumed.108.051920. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs M, Briel M, Daikeler T, Walker UA, Rasch H, Berg S, et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39(2):344–53. doi: 10.1007/s00259-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 18.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 19.Grayson PC, Maksimowicz-McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S, et al. Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheum Dis. 2012;71(8):1329–34. doi: 10.1136/annrheumdis-2011-200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandfort V, Lai S, Ahlman MA, Mallek M, Liu S, Sibley CT, et al. Obesity Is Associated With Progression of Atherosclerosis During Statin Treatment. J Am Heart Assoc. 2016;5(7) doi: 10.1161/JAHA.116.003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meller J, Strutz F, Siefker U, Scheel A, Sahlmann CO, Lehmann K, et al. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003;30(5):730–6. doi: 10.1007/s00259-003-1144-y. [DOI] [PubMed] [Google Scholar]
- 22.Newman KA, Ahlman MA, Hughes M, Malayeri AA, Pratt D, Grayson PC. Diagnosis of Giant Cell Arteritis in an Asymptomatic Patient. Arthritis Rheumatol. 2016;68(5):1135. doi: 10.1002/art.39517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, et al. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58(6):603–14. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 24.Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J Nucl Med. 2009;50(6):959–65. doi: 10.2967/jnumed.108.060749. [DOI] [PubMed] [Google Scholar]
- 25.Ostberg G. Morphological changes in the large arteries in polymyalgia arteritica. Acta Med Scand Suppl. 1972;533:135–59. [PubMed] [Google Scholar]
- 26.Unizony S, Arias-Urdaneta L, Miloslavsky E, Arvikar S, Khosroshahi A, Keroack B, et al. Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res (Hoboken) 2012;64(11):1720–9. doi: 10.1002/acr.21750. [DOI] [PubMed] [Google Scholar]
- 27.Lee YH, Choi SJ, Ji JD, Song GG. Diagnostic accuracy of 18F-FDG PET or PET/CT for large vessel vasculitis: A meta-analysis. Z Rheumatol. 2016;75(9):924–31. doi: 10.1007/s00393-015-1674-2. [DOI] [PubMed] [Google Scholar]
- 28.Bucerius J, Hyafil F, Verberne HJ, Slart RH, Lindner O, Sciagra R, et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging. 2016;43(4):780–92. doi: 10.1007/s00259-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55(1):131–7. doi: 10.1002/art.21699. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y, Ishii K, Oda K, Nariai T, Tanaka Y, Ishiwata K, et al. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med. 2005;46(6):917–22. [PubMed] [Google Scholar]
- 31.Webb M, Chambers A, AAL-N, Mason JC, Maudlin L, Rahman L, et al. The role of 18F-FDG PET in characterising disease activity in Takayasu arteritis. Eur J Nucl Med Mol Imaging. 2004;31(5):627–34. doi: 10.1007/s00259-003-1429-1. [DOI] [PubMed] [Google Scholar]
- 32.Lee SG, Ryu JS, Kim HO, Oh JS, Kim YG, Lee CK, et al. Evaluation of disease activity using F-18 FDG PET-CT in patients with Takayasu arteritis. Clin Nucl Med. 2009;34(11):749–52. doi: 10.1097/RLU.0b013e3181b7db09. [DOI] [PubMed] [Google Scholar]
- 33.Arnaud L, Haroche J, Malek Z, Archambaud F, Gambotti L, Grimon G, et al. Is (18)F-fluorodeoxyglucose positron emission tomography scanning a reliable way to assess disease activity in Takayasu arteritis? Arthritis Rheum. 2009;60(4):1193–200. doi: 10.1002/art.24416. [DOI] [PubMed] [Google Scholar]
- 34.Lee KH, Cho A, Choi YJ, Lee SW, Ha YJ, Jung SJ, et al. The role of (18) F-fluorodeoxyglucose-positron emission tomography in the assessment of disease activity in patients with takayasu arteritis. Arthritis Rheum. 2012;64(3):866–75. doi: 10.1002/art.33413. [DOI] [PubMed] [Google Scholar]
- 35.Tezuka D, Haraguchi G, Ishihara T, Ohigashi H, Inagaki H, Suzuki J, et al. Role of FDG PET-CT in Takayasu arteritis: sensitive detection of recurrences. JACC Cardiovasc Imaging. 2012;5(4):422–9. doi: 10.1016/j.jcmg.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Karapolat I, Kalfa M, Keser G, Yalcin M, Inal V, Kumanlioglu K, et al. Comparison of F18-FDG PET/CT findings with current clinical disease status in patients with Takayasu’s arteritis. Clin Exp Rheumatol. 2013;31(1 Suppl 75):S15–21. [PubMed] [Google Scholar]
- 37.Soussan M, Nicolas P, Schramm C, Katsahian S, Pop G, Fain O, et al. Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine (Baltimore) 2015;94(14):e622. doi: 10.1097/MD.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheikine Y, Akram K. FDG-PET imaging of atherosclerosis: Do we know what we see? Atherosclerosis. 2010;211(2):371–80. doi: 10.1016/j.atherosclerosis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Dellavedova L, Carletto M, Faggioli P, Sciascera A, Del Sole A, Mazzone A, et al. The prognostic value of baseline (18)F-FDG PET/CT in steroid-naive large-vessel vasculitis: introduction of volume-based parameters. Eur J Nucl Med Mol Imaging. 2016;43(2):340–8. doi: 10.1007/s00259-015-3148-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.