Abstract
In the developing retina, as in other regions of the CNS, neural progenitors give rise to individual cell types during discrete temporal windows. Pax6 is expressed in retinal progenitor cells (RPCs) throughout the course of retinogenesis, and has been shown to be required during early retinogenesis for generation of most early-born cell types. In this study, we examined the function of Pax6 in postnatal mouse retinal development. We found that Pax6 is essential for the generation of late-born interneurons, while inhibiting photoreceptor differentiation. Generation of bipolar interneurons requires Pax6 expression in RPCs, while Pax6 is required for the generation of glycinergic, but not for GABAergic or non-GABAergic-non-glycinergic (nGnG) amacrine cell subtypes. In contrast, overexpression of either full-length Pax6 or its 5a isoform in RPCs induces formation of cells with nGnG amacrine features, and suppresses generation of other inner retinal cell types. Moreover, overexpression of both Pax6 variants prevents photoreceptor differentiation, most likely by inhibiting Crx expression. Taken together, these data show that Pax6 acts in RPCs to control differentiation of multiple late-born neuronal cell types.
Introduction
The developing vertebrate retina is an excellent model for unraveling the mechanisms by which the remarkable diverse cell types of the adult central nervous system (CNS) are generated from the seemingly homogeneous pool of multipotent neural progenitors found in the embryo. The mature vertebrate retina is composed of six major types of neurons and one type of glial cell (Müller glia), which constitute three cell layers: retinal ganglion cells in the ganglion cell layer (GCL); horizontal, amacrine and bipolar interneurons, and Müller glial cells in the inner nuclear layer (INL); cone and rod photoreceptors in the photoreceptor layer or the outer nuclear layer (ONL) (Dowling, 1987; Wassle and Boycott, 1991). During retinogenesis, these seven cell types arise from a common population of retinal progenitor cells (RPCs) in an evolutionarily conserved temporal order, although the duration of differentiation and the ratio of mature cell types vary considerably among different species (Harman and Beazley, 1987; Rapaport et al., 2004; Young, 1985). The early-born retinal cell types are the retinal ganglion cells, cone photoreceptors, GABAergic amacrine cells and horizontal interneurons. Late-born cell types consist of the bipolar cells, the glycinergic and non-GABAergic non-glycinergic (nGnG) amacrines, and the Müller glia (Cherry et al., 2009; Kay et al., 2011). Rod photoreceptors, which comprise the majority of cells in the mouse retina, are born throughout the period of retinal neurogenesis, with their generation peaking in the first days of life (Carter-Dawson and LaVail, 1979; Morrow et al., 1998; Young, 1985). The current model for retinogenesis suggests that RPCs undergo a series of gradual and unidirectional changes in their competence to give rise to different retinal cell types. Current results suggest that this competence depends on intrinsic differences among RPCs that change over time, as well as stochastic effects that are at least partly mediated by extrinsic cues such as the Notch/Delta signaling (reviewed in (Cepko, 2014; Xiang, 2013)).
The paired and homeodomain transcription factor (TF) Pax6 is important for normal development of the CNS and pancreas. Moreover, it is required for eye formation in all animal phyla investigated to date, and is necessary and in some cases also sufficient to induce formation of a diverse set of ocular cell types (reviewed in (Cvekl and Ashery-Padan, 2014; Shaham et al., 2012). Removal of Pax6 from early RPCs using the αCre-transgenic line revealed a cryptic divergence of RPCs into two qualitatively different progenitor pools. In more peripheral RPCs, Pax6 prevents premature activation of photoreceptor differentiation by inhibiting expression of the homeodomain TF Crx, which is required for terminal differentiation and survival of photoreceptors and is one of the earliest selective markers of photoreceptor precursors (Chen et al., 1997; Furukawa et al., 1997). More centrally, Pax6 ablation leads to the exclusive generation of GABA+ amacrine interneurons, with no other retinal cell types formed (Marquardt et al., 2001; Oron-Karni et al., 2008).
Several alternatively spliced retinal isoforms of Pax6 have been identified, and three of these have been functionally investigated: the canonical variant that encodes both paired domain and homeodomain, the Pax6(5a) splicing variant that includes intron 5a within the paired domain, and an isoform that lacks the paired domain entirely (Pax6ΔPD) (Shaham et al., 2012). The expression patterns of these Pax6 isoforms, along with the phenotypes of human patients carrying mutations in splicing sites and misexpression studies in avian embryos (Azuma et al., 2005; Hanson et al., 1999; Lakowski et al., 2007; Singh et al., 2002; Vincent et al., 2004), all suggest that these isoforms of Pax6 play distinct roles in retinal development. Despite progress in our understanding of how Pax6 regulates early retinal development, we know very little about how Pax6 functions during later stages of retinogenesis.
Here, we conducted gain- and loss-of-function analyses of Pax6 in postnatal mouse retina to directly address its roles in late RPCs. Pax6 was found to be essential for generating late-born glycinergic amacrine cells, along with most bipolar cell subtypes. Furthermore, as is the case in the early peripheral retina, Pax6 actively repressed expression of Crx. Overexpression of Pax6 greatly increased the fraction of amacrine cells expressing the nGnG subtype marker Satb2, suppressed generation of both glycinergic amacrine cells and bipolar interneurons, and disrupted rod photoreceptor morphogenesis. This study provides novel insight into the gene-regulatory networks controlled by Pax6 in postnatal retina, and demonstrates both parallels and differences between its function in early and late stages of retinogenesis.
Materials and Methods
Mouse lines
The Pax6lox allele contains loxPs flanking the initiator ATG and exons 4–6 encoding the paired domain (Ashery-Padan et al., 2000). The Pax6lox/+ were bread with ICR for 10 generations and then bread to obtain Pax6lox/lox mice employed in the study as compared with pups of ICR mice. All animal work was conducted according to national and international guidelines and all efforts were made to minimize suffering. The protocol was approved by the Tel Aviv University institutional animal care and use committee (IACUC permit: M08092).
Immunofluorescence
Immunofluorescence analysis was performed as described previously (Farhy et al., 2013). The following primary antibodies were used: rabbit anti-Ccnd3 (1:100, SC-182, Santa Cruz), rabbit anti-Crx (1:400; kind gift from T. Furukawa), goat anti-GFP (1:1000; 600-101-215, Rockland), rabbit anti-GFP (1:500; A6455, Invitrogen), mouse anti-Pax6 (monoclonal, 1:25; SC-32766, Santa Cruz), rabbit anti-Pax6 (polyclonal, 1:400; Prb-278p, Covance), PNA (1:200, FL-1071, Vector), rabbit anti-recoverin (1:1000; AB5585, Millipore), rat anti-rhodopsin (1:1000; kind gift from M. Applebury), mouse anti-Satb2 (1:100; AB51502, Abcam), rabbit anti-Sox2 (1:500, AB5603, Chemicon), mouse anti-syntaxin (HPC-1, 1:400; S0644, Sigma), sheep anti-Vsx2 (1:1000; X1180P, Exalpha).
Misexpression constructs, in vivo electroporation and statistical analyses
The following plasmids were used for electroporation: pCAG-Cre (Addgene repository ID: 13775 (Matsuda and Cepko, 2007)), pCALNL-GFP (Addgene repository ID: 13770 (Matsuda and Cepko, 2007)), pCAG-GFP (Addgene repository ID: 11150 (Matsuda and Cepko, 2004)). Pax6 constructs were cloned into pCAG-GFP plasmid.
In vivo injection of expression constructs and electroporation were performed on male and female pups as previously described (Matsuda and Cepko, 2004, 2007) to deliver 0.3 µl of 5 µg/µl DNA solution to the subretinal space of the developing mouse eye.
The average number of GFP+ cells expressing each indicated marker was calculated for each eye, and at least 3 eyes were analyzed per genotype. To compare differences between control and experimental values for statistical significance, Student’s two-tailed t-test was used for measurements based on categorical data (i.e., percentage of electroporated cells belonging to a given cell class).
Results
Inactivation of Pax6 in the postnatal retina leads to changes in the fate of late-born inner nuclear layer retinal cell types
To determine the roles of Pax6 in late RPCs, we generated mosaic loss-of-function mutations in Pax6 in neonatal (postnatal day 0, P0) retinas, and traced the fate of Pax6-deficient cells. This was performed by co-electroporating vectors expressing Cre recombinase (pCAG-Cre) and a Cre-inducible GFP reporter (pCALNL-GFP; Fig. 1A (Matsuda and Cepko, 2007)) into the retinas of Pax6lox/lox (termed Pax6lox/lox;Crelate) and control wild-type (ICR, termed Pax6+/+;Crelate) neonate mice. Retinas were analyzed at P14, when cell specification is complete (Young, 1985). Pax6 deletion in Cre-GFP-expressing cells was verified by co-immunostaining with an antibody against GFP and a monoclonal antibody against the N-terminus of Pax6 protein, which includes the region of Pax6 that is deleted following Cre activation (Ashery-Padan et al., 2000; Raviv et al., 2014). GFP+ cells were detected in the INL and the ONL, but not in the GCL of both Pax6+/+;Crelate (Fig. 1B) and Pax6lox/lox;Crelate (Fig. 1D) retinas, corresponding with the reports that ganglion cells and displaced amacrine cells are born earlier than INL amacrine cells, and mostly before P0 (**Voinescu et al., 2009; Young, 1985). Pax6 was detected in the GFP+ cells in the INL but not in the Pax6lox/lox;Crelate retina,, confirming the efficacy of Pax6 deletion (Fig. 1B,B',D,D'). The loss of Pax6 in late RPCs did not result in significant changes in the proportion of cells that resided in the INL or ONL, although there was a small increase in the percentage of GFP+ cells in the ONL of Pax6lox/lox;Crelate (75.2 ± 2.7%) compared to controls (69.0 ± 4.3%), and a corresponding decrease in the proportion of GFP+ cells in the INL from 31.0 ± 4.3% in controls to 24.8 ± 2.7% in the Pax6lox/lox;Crelate retinas (Fig. 1F). We did not observe an obvious change in photoreceptor morphology or localization following deletion of Pax6 (Fig. 1C,E), and the majority of GFP positive cells in the ONL were positive for the rod marker rhodoposin and negative to the cone marker PNA (Figure S1).
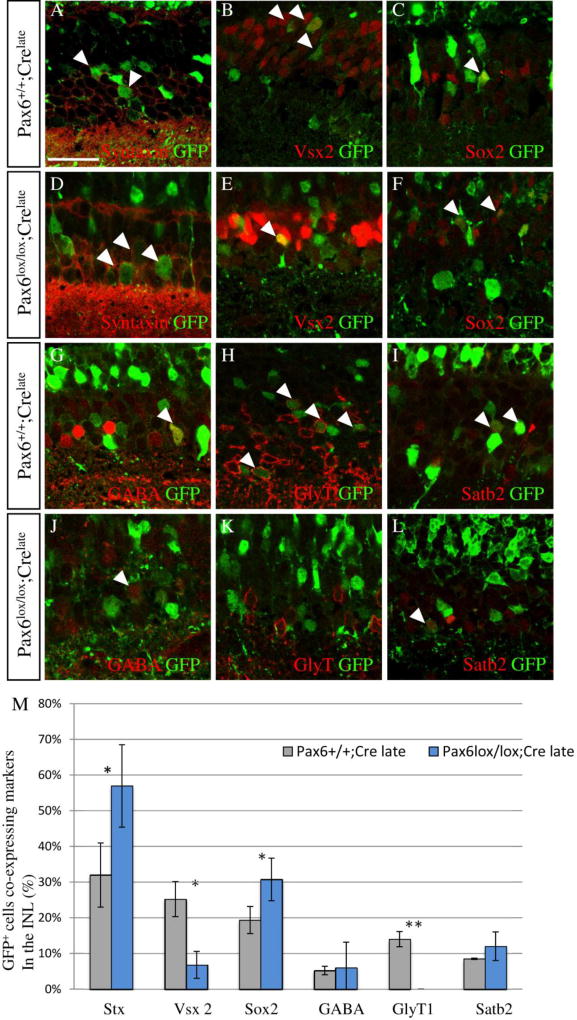
Fig. 1. Deletion of Pax6 from late RPCs does not alter layer localization of the retinal cells.
(A) Schematic representation of the constructs employed for gene mutation and lineage tracing. Double-immunolabeling with monoclonal Pax6 antibody and GFP antibody of P14 control (Pax6+/+;Crelate; B,B',C) and Pax6 mutant cells (Pax6lox/lox;Crelate; D, D', E). In the control, Pax6 co-localizes with amacrine cells in the inner part of the inner nuclear layer (INL) (white arrowheads, B'), whereas there is no co-localization of Pax6 and GFP+ cells in the INL of Pax6lox/lox;Crelate confirming deletion of Pax6 (empty arrowheads, D'). Photoreceptor morphology was viewed by the GFP distribution in the sparsely labeled Pax6+/+;Crelate (C) and Pax6lox/lox;Crelate (E) cells. (F) Quantification of the proportion of GFP+ cells within the INL and outer nuclear layer (ONL). The proportion of GFP+ cells in each layer was similar between control and Pax6 mutant cells (P = 0.11, N = 3). Scale bar = 100 µm for B,D, and 25 µm for B',C,D',E.
We next characterized the differences in cellular composition of INL lineages in Pax6lox/lox;Crelate as compared to control Pax6+/+;Crelate (Fig. 2) by co-immunostaining analysis using the following cell type-specific markers: amacrine cell-specific syntaxin (Fig. 2A,D), bipolar cell-specific Vsx2 (Fig. 2B,E), and Müller glial-enriched Sox2, when expressed in cells with radial morphology (Fig. 2C,F). Intriguingly, we detected a fourfold decrease in the proportion of GFP+;Vsx2+ bipolar interneurons from 25.2 ± 4.9% of all GFP+ cells in the INL in controls to 6.8 ± 3.8% in Pax6lox/lox;Crelate retinas (Fig. 2M, P = 0.008). This implies that Pax6 is required in late-stage RPCs for the generation of most bipolar cells. In contrast to the reduction in bipolar cells, we detected an increase in the proportion of GFP+;syntaxin+ amacrine cells, from 32.0 ± 9% in Pax6+/+;Crelate to 57.0 ± 11.6% in Pax6lox/lox;Crelate (Fig. 2M, P = 0.04487). We also observed an elevation in the proportion of GFP+;Sox2+ cells with radial morphology from 19.4 ± 3.8% in the Pax6+/+;Crelate to 30.8 ± 6.0% in Pax6lox/lox;Crelate, suggesting an increase in Müller glia (Fig. 2M; P = 0.046). Thus, during late retinogenesis, Pax6 is required for the generation of most bipolar cells and it restricts the generation of glia and late-born amacrine cells, similar to its role in early stages of retinogenesis (Marquardt et al., 2001).
Fig. 2. Pax6 in late RPCs is required for generation of bipolar and glycinergic amacrine cells.
Double-immunolabeling with antibodies against GFP and cell-type-specific markers of electroporated retinas: syntaxin for amacrine interneurons (A,D), Vsx2 for bipolar interneurons (B,E), Sox2 for Müller glia (C,F), GABA for GABAergic amacrines (G,J), glycine transporter 1 (GlyT1) for glycinergic amacrines (H,K), Satb2 for nGnG amacrines (I,L). Arrowheads – co-localized cells. (M) Quantification of the number of marker-positive cells out of total GFP+ cells in the inner nuclear layer (INL) reveals an increase in amacrine cells (P = 0.045), a decrease in bipolar cells (P = 0.008), and an increase in Müller glia (P = 0.046); Stx, syntaxin. The glycinergic amacrine subclass is not detected in the mutant cells, whereas there was no significant change in the proportion of GABAergic (P = 0.2) or nGnG (P = 0.27) amacrines, N = 3 for all samples. Scale bar = 25 µm.
Pax6 is required for the generation of glycinergic amacrine cells
Inactivation of Pax6 in early-stage RPCs results in the excess generation of GABAergic amacrine cells, but blocks the formation of glycinergic and nGnG cells (Marquardt et al., 2001; Farhy et al., 2013). These latter two amacrine cell-types are born during late stages of retinogenesis (Cherry et al., 2009; Kay et al., 2011) and their absence following early loss of Pax6 could be secondary to the early depletion of RPCs. We therefore aimed to characterize the amacrine subclasses generated upon late inactivation of Pax6 in the Pax6lox/lox;Crelate as compared with control Pax6+/+;Crelate by immunostaining analysis to the following cell type-specific markers: GABA for the GABAergic subclass (Fig. 2G,J), GlyT1 for the glycinergic subclass (Fig. 2H,K) and Satb2 for nGnG cells (Fig. 2I,L). Quantitative analysis (Fig. 2M) revealed a complete absence of GlyT1+ cells among the GFP+ Pax6 mutant cells in the INL, whereas in controls, 14.3 ± 2.1% of all GFP+ cells in the INL were GlyT1+ amacrines (P = 0.0013; Fig. 2H,K,M). This was accompanied by a slight, though nonsignificant, increase in the proportion of GABA+ (8.1 ± 2.7%) and Satb2+ (12.0 ± 4.0%) cells in Pax6lox/lox;Crelate retinas compared to Pax6+/+;Crelate retinas (5.2 ± 1.2% and 8.5 ± 0.2%, respectively). It thus seems that some of the syntaxin+Pax6− cells in the Pax6lox/lox;Crelate do not express molecular markers of any of the major amacrine subclasses. Based on these findings, we conclude that during late retinogenesis, Pax6 is essential for differentiation of the glycinergic amacrine cells.
Overexpression of Pax6 variants alters amacrine subtype specification and disrupts rod photoreceptor differentiation
These loss-of-function studies revealed that Pax6 is required in the postnatal retina for generation of bipolar cells, constrains amacrine and glia cell generation, and controls amacrine cell subtype specification. To further investigate the ability of major Pax6 isoforms to control cell-fate specification during late stages of retinogenesis, we electroporated bicistronic constructs (Fig. 3A) expressing the three different variants of Pax6: canonical full-length Pax6 (pCAG-Pax6-GFP), the Pax6(5a) splice variant (pCAG-Pax6(5a)-GFP) or Pax6 lacking the paired domain (pCAG-Pax6ΔPD-GFP). As a control, we electroporated the pCAG plasmid expressing GFP alone (pCAG-GFP) into the P0 retina. Pax6 misexpression was confirmed 5 days after the electroporation (P5) by double-labeling with anti-GFP antibody and a polyclonal anti-Pax6 antibody that detects all three Pax6 isoforms tested (Fig. 3B–E, red). At P14, as expected, GFP+ cells were detected in the ONL and INL of electroporated retinas, but not in the GCL (Fig. 3F–I). Quantification of GFP+ cells in each layer (Fig. 3J) revealed a small but significant increase in the percentage of cells in the INL of pCAG-Pax6-GFP (32.1 ± 3.1%, P = 0.034) and pCAG-Pax6(5a)-GFP (33.2 ± 2.3%, P = 0.019) retinas compared to pCAG-GFP control retinas (26.0 ± 3.3%). The distribution of GFP following misexpression of pCAG-Pax6ΔPD-GFP did not significantly differ from the control (30.0 ± 1.8%, P = 0.085).
Fig. 3. Overexpression of Pax6, Pax6(5a), and Pax6ΔPD in the postnatal retina.
(A) Schematic representation of the constructs. Pax6/Pax6(5a)/Pax6ΔPD sequence was placed next to the CMV enhancer and chicken β-actin fusion promoter (CAG) connected to the GFP coding sequence via internal ribosome entry site(IRES) or pCAG-GFP for control. (B–E) Immunostaining with Pax6 and GFP of P5 control and electroporated Pax6/Pax6(5a)/Pax6ΔPD-overexpressing retinas. In pCAG-GFP controls, Pax6 was only detected in the inner part of the inner nuclear layer (INL) in agreement with its wild-type expression pattern; in pCAG-Pax6-GFP, pCAG-Pax6(5a)-GFP and pCAG-Pax6ΔPD-GFP, Pax6 expression was detected along with GFP in the INL and outer nuclear layer (ONL) indicating persistent expression of Pax6 in the electroporated cells. (F–I) Immunostaining with anti-GFP antibody of electroporated retinas at P14. (J) Quantification of the distribution of electroporated cells within retinal layers. There was a small but significant increase in the percentage of GFP+ cells in the INL relative to the ONL of Pax6-misexpressing retinas compared to control (P = 0.034). Misexpression of Pax6(5a) led to a similar elevation, whereas distribution of GFP upon misexpression of Pax6ΔPD did not significantly differ from the control (P = 0.085). Scale bar = 100 µm.
We next performed a quantitative analysis of the INL cell types generated from the electroporated cells (Fig. 4, Fig. S2). Of the GFP+ cells in the INL of retinas electroporated with the pCAG-GFP control plasmid, 51.7 ± 4.5% co-expressed syntaxin, 20.4 ± 2.7% co-expressed Vsx2 and 14.9 ± 3.1% both co-expressed Sox2 and displayed radial morphology (Fig. 4, Fig. S2). In contrast, most of the GFP+ cells in the INL of retinas electroporated with pCAG-Pax6-GFP acquired amacrine-like morphology and expressed syntaxin (92 ± 3.1%), while only 1.6 ± 2.8% and 1.0 ± 0.9% of GFP+ cells expressed Vsx2 and Sox2, respectively (Fig. 4, Fig. S2). Similar results were documented by labeling with the Müller glia marker Ccnd3 (Figure S3), thus Sox2 labeling faithfully monitors Muller Glia. Overexpression of Pax6(5a) also increased the proportion of syntaxin+ amacrine cells (81.3 ± 2.2%), and reduced the fraction of those expressing Vsx2 and Sox2 (0% and 4.2 ± 4.0%, respectively) (Fig. 4, Fig. S2). Overexpression of Pax6ΔPD, however, had only a minor effect on cell composition in the INL, with 63 ± 7.8% of GFP+ cells expressing syntaxin amacrine, 12.9 ± 5.6% expressing Vsx2, and 5.7 ± 1.4% expressing Sox2 (Fig. 4, Fig. S2). Thus, both Pax6 and Pax6(5a) misexpression in late RPCs results in the generation of amacrine-like cells at the expense of the bipolar and Müller glia cells.
Fig. 4. Pax6 and Pax6(5a) overexpression leads to exclusive generation of nGnG amacrine cells at the expense of other late inner nuclear layer (INL) cell types.
The percentage cells that were positive for cell-specific markers out of all GFP+ cells in the INL was calculated (Table 1). Almost all amacrine cells generated upon Pax6 and Pax6(5a) overexpression became nGnG amacrines. Overexpression of Pax6ΔPD led to a slight elevation in the generation of glycinergic amacrines compared to controls but did not induce nGnGs.
Pax6 and Pax6(5a) overexpression promotes the generation of non-glycinergic and non-GABAergic amacrine cells
Since full-length Pax6 and Pax6(5a) overexpression resulted in a marked increase in amacrine cells at the expense of other INL cell types, we next examined the amacrine subclasses that were generated following Pax6 or Pax6(5a) overexpression. In pCAG-GFP-electroporated retina, the proportion of GABA+ was 3.6 ± 1.5%, GlyT was 25 ± 5.1% and Satb2 was 15.03 ± 8.3%. In contrast, in the pCAG-Pax6-GFP-electroporated retinas, we observed a near-complete loss of glycinergic (Fig. 4, Fig. S1) and GABAergic amacrine markers (0.5 ± 1.0%, Fig. 4, Fig. S1). In contrast, we observed a dramatic increase in the fraction of cells expressing the nGnG marker Satb2 (90.5 ± 4.1% of all GFP+ cells in the INL, Fig. 4; S1) (Kay et al., 2011). Thus, while full-length Pax6 overexpression promotes the generation of amacrine cells at the expense of other late-born INL lineages, it also suppresses the differentiation of GABAergic and glycinergic amacrine cells, but not of the nGnG amacrine subtype. Overexpression of the Pax6(5a) isoform also led to increased generation of Satb2+ amacrine cells at the expense of the other amacrine cell types: 61.3 ± 8.2% Satb2+, 0.7 ± 1.5% GlyT+, and 0.7 ± 1.7% GABA+ (Fig. 4, Fig. S1). Thus, while Pax6 and Pax6(5a) overexpression promotes the generation of amacrine cells at the expense of other late-born INL lineages, both also suppress the differentiation of GABAergic and glycinergic amacrine cells. Interestingly, overexpression of Pax6ΔPD gave rise to more glycinergic amacrine cells than in controls (37.0 ± 0.47% of all GFP+ cells in the INL, P = 0.019, Fig. 4; S2,T).
Pax6 suppresses normal photoreceptor differentiation by inhibiting Crx
Overexpression of full-length Pax6 and Pax6(5a) in late RPCs only slightly decreased the overall number of GFP+ cells in the ONL, but dramatically altered their morphology and location within the ONL relative to both controls and Pax6ΔPD (Fig. 3). To quantify the distribution of GFP+ cells within the ONL of Pax6/Pax6(5a)/Pax6ΔPD-and control-electroporated retinas, we split the width of the ONL into inner ONL (IONL) and outer ONL (OONL) halves, and quantified the percentage of GFP+ cells in these layers out of the total number of GFP+ cells in the ONL (Fig. 5A), as previously described (Rapicavoli et al., 2011). While 63.0 ± 4.3% of photoreceptor cell bodies in controls were localized to the IONL and the remaining 37.1% to the OONL, only 17.8±7.8% of cells overexpressing full-length Pax6 were located in the IONL (P = 0.00024). Misexpression of Pax6(5a) led to a similar distribution: 79.1 ± 5.5% of ONL GFP+ cells were located in the OONL (P = 0.00003), whereas Pax6ΔPD misexpression did not alter cell body location within the ONL (Fig. 5A).
Fig. 5. Photoreceptor differentiation is disrupted upon Pax6 and Pax6(5a) misexpression.
(A) The width of the outer nuclear layer (ONL) was divided into two halves: the outer ONL (OONL) and inner ONL (IONL) and the percentage of cell bodies residing in each half are presented in the graph. (B–E) Double-immunostaining with Crx and GFP of electroporated retinas. In pCAG-GFP-electroporated retinas, Crx is detected in the GFP+ cells (B). The cells that misexpress Pax6 (C) or Pax6(5a) (D) but not Pax6ΔPD (E) show reduced Crx expression. (F–K) Double-immunostaining with antibodies to GFP and Crx (F–I), recoverin (G–J) and rhodopsin (H,K). The corresponding red channel is shown in I'-K'. The scale bar = 100 µm in B–E, 25 µm in F–K'.
Pax6 has been shown to suppress the expression of the cone/rod-specific homeobox TF Crx in early RPCs (Klimova and Kozmik, 2014; Oron-Karni et al., 2008). Crx knockout mice do not develop photoreceptor outer segments, and lack fully functional rod and cone activity as assayed by electroretinogram (ERG) (Chen et al., 1997; Furukawa et al., 1997). We examined Crx expression in control and full-length Pax6/Pax6(5a)/Pax6ΔPD-misexpressing retinas. Crx was detected in all photoreceptors, co-localizing with the GFP-expressing cells in control (Fig. 5B,F) and Pax6ΔPD-overexpressing cells (Fig. 5E), but not in photoreceptor cells overexpressing full-length Pax6 (Fig. 5C,I, I') or Pax6(5a) (Fig. 5D). In addition to a change in localization of the photoreceptor cell bodies within the ONL, the outer segments of the photoreceptor overexpressing full-length Pax6 and Pax6(5a) were reduced or completely absent (Fig. 5C,D). Outer segment photoreceptors overexpressing Pax6ΔPD (Fig. 5E) were similar in morphology to controls (Fig. 5B).
To investigate changes in gene expression induced by full-length Pax6 misexpression, we examined the expression of recoverin and rhodopsin, which are two key genes in the rod-photoreceptor-specification pathway downstream of Crx (Chen et al., 1997; Furukawa et al., 1997). Expression of both recoverin and rhodopsin was markedly downregulated compared to the control (Fig. 5G,H) upon full-length Pax6 misexpression (Fig. 5J,J',K,K') and did not label with PNA (Fig. S4), suggesting that they are neither rods nor cones. Considering all of the above, overexpression of Pax6 in late retinal progenitors prevents normal differentiation of photoreceptor precursors.
Crx downregulation alone may account for the observed photoreceptor phenotype following Pax6 misexpression. However, Pax6 may also prevent acquisition of the photoreceptor fate by a Crx-independent mechanism. To distinguish between these two possibilities, we conducted a rescue experiment in which Pax6 (pCAG-Pax6-GFP) was co-electroporated with Crx (pCAG-Crx-GFP) (Fig. 6). Co-expression of Crx and Pax6 was confirmed by immunohistochemistry (Fig. 6E). Whereas there was a shift in the location of GFP+ cell bodies toward the apical region of the ONL upon Pax6 misexpression (Figs. 5, 6B,C), photoreceptor cells co-expressing Crx and Pax6 showed primarily basal localization (Fig. 6F,G) and seemingly normal outer segments (Fig. 6F,G). To quantify this, we measured the lengths of outer segments from photoreceptors misexpressing Pax6 (Pax6+), Pax6+Crx+ and pCAG-GFP (Fig. 6H). Misexpression of Pax6 with CRX in the photoreceptor layer led to an increase in OS length from 3.5 ± 0.9 µm in pCAG-Pax6-GFP photoreceptors, to 10.1 ± 1.6 µm in pCAG-Pax6-GFP/ pCAG-Crx-GFP, which was similar in length to pCAG-GFP-electroporated photoreceptor OSs (11.3 ± 1.4 µm; Fig. 6H). Thus, photoreceptor cell body localization and outer segment length were restored upon co-expression of both Pax6 and Crx in RPCs. This finding implies that disruption of photoreceptor differentiation, in terms of cellular localization and outer segment length, induced by Pax6 misexpression is mediated by inhibition of Crx.
Fig. 6. Co-electroporation of Crx rescues phenotypes caused by Pax6 misexpression in photoreceptor precursors.
(A) Schematic representation of the pCAG-Crx construct. (B–D) ONL cells misexpressing Pax6 or (E–G) both Pax6 and Crx. (B, E) Double-immunostaining of Pax6 and Crx shows co-localization in Pax6+Crx+-electroporated photoreceptors (E) and downregulation of Crx in Pax6-electroporated photoreceptors (B). (C,F) Double-labeling of GFP and Pax6 shows reduced outer segments in photoreceptors misexpressing Pax6 (C) and normal appearance of Pax6+ photoreceptors rescued by co-electroporation with Crx (F). (D,G) Labeling with GFP and DAPI. (H) Average outer segment length was calculated following misexpression of Pax6, Pax6 and Crx, or GFP only. Co-expression of Pax6 and Crx seems to restore the length of the outer segments (P = 0.0006, N = 4). Scale bar = 25 µm
Discussion
This study reveals lineage specific roles of Pax6 in late-born retinal cell-types. The development of all late-born cell types was affected by conditional loss of function of Pax6 in late-stage RPCs: Pax6 was required for generation of bipolar and glycinergic amacrine cells, whereas both generation and differentiation of rods was inhibited by Pax6. Pax6-dependent inhibition of rod differentiation was mediated by repression of Crx expression, and required the paired domain of Pax6. Interestingly, while loss of Pax6 function increased the total number of amacrine cells, as measured by the number of INL cells expressing the amacrine marker syntaxin, it selectively disrupted the development of late-born glycinergic amacrine cells. Misexpression of full-length Pax6 and the Pax6(5a) variant greatly increased the fraction of syntaxin-positive amacrine-like cells at the expense of bipolar cell and Müller glia. Virtually all of these syntaxin-positive cells expressed Satb2, a marker of nGnG amacrine cells. These findings demonstrate both the essential and complex role of Pax6 in regulating the differentiation of late-born retinal cell types.
Pax6 regulates the balance of late-born retinal cell types
The fate of a RPC that exits the cell cycle is dependent on the balance between the factors promoting and inhibiting that fate, as was elegantly demonstrated for the binary fate decision between rods and bipolar interneurons, where regulatory interactions of Blimp1, RORb and Otx2 define the correct ratio of rods and bipolar cells (Wang et al., 2014). Postmitotic rod, bipolar, and Müller glial precursor cells do not express Pax6, and it is therefore likely that the fourfold reduction in bipolar cells, and the corresponding increase in the number of rods, Müller glia and amacrine cells, result from disrupted Pax6 action in late-stage RPCs. RPC-specific expression of Vsx2, which is essential for both RPC proliferation and generation of bipolar cells (Elshatory et al., 2007; Green et al., 2003), is dependent on Pax6 (Farhy et al., 2013). Pax6 may thus act in late-stage RPCs to sustain expression of Vsx2, and render these cells competent to generate bipolar cells. However, overexpression of full-length Pax6 and Pax6(5a) potentially inhibits bipolar cell generation, implying that elevated levels may drive expression of other transcription factors that promote amacrine specification while simultaneously repressing bipolar cell formation.
Together with our finding that both overexpression and loss of function of Pax6 lead to an increased number syntaxin-positive amacrine cells, these results imply that both upregulation and downregulation of Pax6 activity can produce similar developmental phenotypes. Interestingly, a similar phenotype has been observed following both knockdown and overexpression of the homeodomain transcription factor Six3 in postnatal retina, where both decreasing and increasing Six3 expression suppresses bipolar cell formation (Rapicavoli, et al. 2011). Tight control of expression and/or activity levels of RPC-expressed TFs may thus be critically important for generation of retinal cell types in normal physiological ratios.
In mice, the role of Pax6 in photoreceptor development is complex, and heavily dependent on spatial and temporal context. During early stages of retinogenesis, systemic or conditional loss of Pax6 using mRx-Cre results in elevated expression of Crx in most mutant cells. However, these precursors do not give rise to mature photoreceptors (Klimova and Kozmik, 2014; Oron-Karni et al., 2008). Similarly, when Pax6 was conditionally deleted from RPCs at the optic cup stage using the αCre-transgenic line, two distinct effects were observed: the less mature RPCs located at the optic cup periphery upregulated Crx, yet failed to differentiate, whereas more centrally located RPCs did not express Crx, but instead differentiated into GABAergic amacrine-like interneurons (Oron-Karni et al., 2008). In both regions, Pax6 was essential for photoreceptor differentiation, but Pax6-dependent inhibition of Crx expression seemed to be restricted to the earlier-stage RPCs (Oron-Karni et al., 2008).
Several findings presented in the current study also provide strong evidence for Pax6-dependent inhibition of Crx expression in late RPCs. The conditional deletion of Pax6 in late-stage RPCs yielded more rod photoreceptors than in controls, based on the increased number of GFP+ cells in the ONL of Pax6lox/lox;Crelate and Pax6+/+;Crelate retinas (Fig. 1). This effect is fairly modest, possibly because deletion of Pax6 induced by electroporation of pCAG-Cre into late-stage RPCs will in some cases only occur after cells have exited the cell cycle, and thus may no longer express Pax6. Overexpression of both full-length Pax6 and Pax6(5a) resulted in loss of expression of Crx, recoverin and rhodopsin. The cell bodies of ONL cells overexpressing either Pax6 and Pax6(5a) were displaced apically, and exhibited reduction or loss of outer segments (Figs. 3, 5), further confirming that Pax6 downregulation is necessary for normal rod photoreceptor differentiation. The finding that co-expression of Pax6 with Crx rescues both normal distribution of photoreceptor cell bodies within the ONL and the length of the outer segments of the Pax6+/Crx+ cells further suggests that Pax6 suppresses photoreceptor differentiation primarily through inhibition of Crx expression. Notably, Pax6ΔPD misexpression did not cause any detectable change in the photoreceptor layer compared to control electroporation (Fig. 5). This suggests that an intact paired domain is required for the ability of Pax6 to suppress photoreceptor differentiation. In addition, these disruptions in photoreceptor differentiation phenocopy the effects of overexpression of the transcription factor Six3, which is expressed selectively in RPCs and amacrine cells (Rapicavoli, et al. 2011). This suggests that direct suppression of photoreceptor differentiation may be a more general property of RPC-expressed TFs whose expression is sustained in inner retinal cell types.
Pax6’s roles in the regulation of the proliferation and survival of neural progenitors, including RPCs, is complex and context dependent (reviewed in (Manuel et al., 2015)). The early inactivation of Pax6 disrupted the proliferation of the RPCs more severely in the distal than in the central retina (Oron-Karni et al., 2008), whereas the mutant post-mitotic precursors maintain the expression of Ccnd1 and Ccnd2 (Farhy et al., 2013). Recent studies suggest that several subpopulations of RPCs are biased towards producing specific cell types at higher ratios than other RPCs (Wang et al., 2014). It is possible that altering the Pax6 levels will have different effects on the proliferation of different RPC subpopulations, leading to alterations in the cell-type proportions of the retina. Pax6 was also shown to be involved in regulating the survival of neurons and neuronal progenitors, since overexpression of Pax6 in the developing cortex promoted the apoptosis of specific cortical progenitor populations but not others (Berger et al., 2007), whereas Pax6 inactivation in dopaminergic neurons of the olfactory bulb also led to their apoptosis (Ninkovic et al., 2010). Our initial analysis did not reveal an increase in the number of cleaved caspase 3 positive cells in our gain- or loss-of-function models at P5 (data not shown); however, this does not exclude the effects on specific lineages or at other stages. Further studies, employing lineage-specific Cre lines, should be conducted in order to determine how Pax6 levels impact the survival, proliferation, and differentiation of the specific retinal lineages and eventually, the cell-type composition of the retina.
Roles of Pax6 in the generation of amacrine cells
The gene-regulatory network controlling amacrine cell specification and differentiation is partially understood. Foxn4 acts in early-stage RPCs in conjunction with Rorb to activate expression of Ptf1a, which functions upstream of Tfap2a/b to drive amacrine cell specification (Jin et al., 2015; Liu et al., 2013). Downstream of these events, other factors act to control the specification of amacrine cell subtype. In previous work, the TF Barhl2 was identified as a key regulator that confers the identity of glycinergic amacrine cells. Barhl2 was expressed in all syntaxin+ amacrine cells, yet its forced expression in late-stage RPCs specifically promoted the differentiation of glycinergic amacrine cells, whereas a dominant-negative form of Barhl2 had the opposite effect (Ding et al., 2009; Mo et al., 2004). We did not detect a marked loss of Barhl2+ in Pax6-deficient cells (data not shown), suggesting that Pax6 functions in parallel to Barhl2 in conferring a glycinergic fate.
Consistent with our findings that Pax6 is necessary for the generation of late-born glycinergic amacrine cells, overexpression of full-length Pax6 led to almost exclusive generation of cells that exhibited many amacrine features, including interneuron morphology, localization in the inner INL, synaptic arbors into the inner plexiform layer, and expression of syntaxin (Fig. S2). However, virtually all of these cells expressed Satb2, a TF that is specific to nGnG amacrine cells, and did not express either GABA or the synaptic glycine transporter (Fig. 4, Fig. S2). In the normal retina, nGnG amacrine cells are a subpopulation with unknown function, and comprise 15% of total amacrine cells (Kay et al., 2011). These narrow-field nGnG amacrines have dendrites confined to sublaminae S1 to S3 of the inner plexiform layer (Kay et al., 2011). Similarly, the amacrine cells that are generated following Pax6 overexpression project prominently in sublaminae S1 and S3, but not between them. However, they seem to be wide-field rather than narrow-field (Fig. 3G). This is similar to the phenotype seen following overexpression of NeuroD class bHLH factors in postnatal retina (Cherry et al., 2011), and raises the possibility that Pax6 may directly or indirectly activate expression of these genes. In addition, previous studies have revealed that Pax6 directly regulates expression of genes encoding cell-adhesion molecules such as L1, Ncad, and NCAM (Rungger-Brandle et al., 2010). These differences in dendritic arbor size and morphology could thus also be mediated by Pax6-dependent activation of expression of cell-adhesion molecules.
Our findings demonstrate that Pax6 activates the expression of Satb2, which is essential for nGnG amacrine cell formation. This regulation may also be relevant to the function of Pax6 in early retinogenesis, as Satb2 is not expressed following Pax6 mutation in the early optic cup (not shown). Moreover, in the developing telencephalon, loss of Pax6 results in reduced Satb2 expression, whereas Pax6 overexpression increases Satb2 expression (Holm et al., 2007). This regulatory relationship may thus be more broadly conserved in other CNS regions. Interestingly, in the current study, Pax6 overexpression did not lead to the appearance of syntaxin+ or Satb2+ cells outside the INL, unlike what is seen following Satb2 misexpression (Kay et al., 2011).
Previous studies have attempted to shed some light on the roles of Pax6 in late retinogenesis. Hatakeyama et al. misexpressed Pax6 in retinal explants at 17.5 days of embryonic age (E17.5) using a viral vector, which promoted the generation of INL cells. These INL cells were morphologically immature and did not express PKCa (bipolar marker), calbindin (horizontal and amacrine marker), glutamine synthetase or cyclin D3 (glial markers). It is worth noting that they did not test syntaxin, GABA or GlyT expression, and nGnG had not yet been identified (Hatakeyama et al., 2001). Furthermore, misexpression of Pax6 in combination with the bHLH factor NeuroD4 led to increased generation of both amacrines (calbindin+, syntaxin+) and horizontal cells (calbindin+), and co-expression of NeuroD1 and Pax6 increased amacrine cell generation, suggesting that combinatorial interactions among individual bHLH and homeobox genes are important for retinal cell-type specification (Inoue et al., 2002). While those studies reported no generation of syntaxin+ amacrine cells upon Pax6 misexpression alone, in our experimental model, most of the electroporated cells in the INL were syntaxin+ as well as Satb2+. This might be due to differences in context, timing or gene dosage, with viral transduction of E17.5 retinal explants used in those earlier studies (Hatakeyama et al., 2001; Inoue et al., 2002), in contrast to in-vivo electroporation of the plasmid vector in P0 retina in the current study, which targets progenitors and possibly also post-mitotic precursors. Moreover, at E17.5, expression of NeuroD1 and NeuroD4 are lower than at P0 (Blackshaw et al., 2004; Morrow et al., 1999). Little is known about the direct transcriptional targets and biochemical partners of Pax6 in RPCs at either E17.5 or P0.5, owing to the cellular heterogeneity of the retinal cells that express Pax6 at both stages. Biochemical analysis of isolated RPCs from different stages of retinal development, in combination with genetic studies employing RPC-specific conditional Cre lines will ultimately enable a detailed mechanistic analysis of how Pax6 function is regulated during the course of retinogenesis.
Alternative splicing isoforms of Pax6 and their functions in late retinogenesis
The DNA binding specificity of Pax6 is mediated by the combinatory activity of several DNA binding domains (Jun and Desplan, 1996). In the developing cortex the PD seems to be necessary and sufficient for different aspects of telencephalic development, whereas mutations in the HD only seem to have minor effects (Haubst et al., 2004). Similarly, in the late stages of retinogenesis the misexpression of PD resulted in the reduction of bipolar cells and Muller glia as well as the abnormal differentiation of photoreceptors and amacrines, whereas the misexpression of Pax6ΔPD seemed to increase the number of Glycinergic interneurons but did not abrogate the differentiation of the late-born cell types.
The PD itself has two DNA binding domains: the PAI and RED subdomains, which can function either together or independently. The two variants, Pax6(5a) and Pax6, differ in their binding specificity due to alternative splicing, leading to the insertion of 14 amino acids into the PAI subdomain (Czerny et al., 1993; Duncan et al., 1998; Epstein et al., 1994). Previous studies showed that Pax6 and Pax6(5a) share some of their functions, whereas other functions differ (Azuma et al., 2005; Haubst et al., 2004; Walcher et al., 2013). Our finding that the misexpression of Pax6 and Pax65a in late RPCS gave rise to a seemingly similar phenotype of an elevated number of abnormal, amacrine-like cells and altered the differentiation of photoreceptors points to shared targets for the two variants. It is also important to consider that the Pax6 gene is auto regulated. Specifically, Pax6(5a) was shown to activate Pax6 (Pinson et al., 2006). Thus, the misexpression of one variant may alter the expression and endogenous Pax6 variants, which eventually will result in a similar outcome. Future studies, using single-cell sequencing, are required to reveal differences in the activity and to expose the possible regulatory interactions between Pax6 and Pax6(5a) proteins during retinal development.
Supplementary Material
Fig S1. Rhodopsin is detected in the Pax6 mutant cells.
(A–H) Double-immunostaining with GFP and the rod marker rhodopsin of Pax6+/+ (A–D) and Pax6lox/lox (E–H) retinas electroporated with pCAG-Cre and pCALNL-GFP reporter. The green (GFP, A, E), red (Rhodopsin, B, F), blue (DAPI, C, G) channels are presented. Double positive cells are marked by white arrows. (I–P) Double-immunostaining with GFP and the cone marker PNA of Pax6+/+ (I–L) and Pax6lox/lox (M–P) retinas electroporated with pCAG-Cre pCALNL-GFP. The green (GFP, I, M), red (PNA, J, N), blue (DAPI, K, O) channels are presented. Scale bar = 20 µm.
Fig. S2. Pax6 and Pax6(5a) miss-expression leads to exclusive generation of nGnG amacrine cells at the expense of other late-born interneurons types. Double-immunostaining with GFP and cell-type-specific markers of electroporated retinas: syntaxin for amacrine interneurons (A–D), Vsx2 for bipolar interneurons (E–H), Sox2 for Müller glia (I–L), GABA for GABAergic amacrines (M–P), glycine transporter 1 (GlyT) for glycinergic amacrines (Q–T), Satb2 for the nGnG amacrines (U–X). Arrowheads point to co-localized cells. Immunostaining shows elevation in syntaxin and Satb2 and reduction in all other markers in pCAG-Pax6-GFP and pCAG-Pax6(5a)-GFP compared to the pCAG-GFP control and pCAG-Pax6ΔPD-GFP retinas. Quantification is shown in Figure 4. The scale bar = 25 µm.
Fig. S3. Changes in the number of Ccnd3+ Müller glia upon Pax6 overexpression. Double-immunostaining with GFP and Ccnd3 of retinae electroporated with pCAG-GFP (A–D) and pCAG-Pax6-GFP (E–H). The green (GFP, A, E), red (Ccnd3, B, F), blue (DAPI, C, G) channels are shown. The number of cells positive for both Ccnd3 and GFP was quantified (I). The number of GFP+ cells co-expressing Ccnd3 was significantly higher in the retians electroporated with the pCAG-GFP control plasmid than in the retinas that were electroporated with pCAG-Pax6-GFP plasmid (P=0.03, N=3 for both genotypes). Scale bar = 20 µm.
Fig. S4. PNA does overlap with the cells that miss express Pax6 in the ONL Double-immunostaining with GFP and the cone marker PNA of retinas electroporated with pCAG-GFP (A–D) and pCAG-Pax6-GFP (E–H). The green (GFP, A, E), red (PNA, B, F), blue (DAPI, C, G) channels are shown. Scale bar = 20 µm.
Table 1. Summary of cell-type quantification for Fig. 4.
The counts were conducted on at least 3 eyes, and at least 3 sections per eye. The P-values are for each Pax6 variant compared to control pCAG-GFP electroporation.
| Marker | pCAG-GFP | pCAG-Pax6 | pCAG-Pax6(5a) | pCAG-Pax6ΔPD | |
|---|---|---|---|---|---|
| Syntaxin | 51.7 | 92.0 | 81.3 | 63.7 | average |
| 4.5 | 3.1 | 2.2 | 8.0 | SD | |
| 0.00024 | 0.00341 | 0.05526 | P-value | ||
| *** | ** | Ns | significance | ||
| Vsx2 | 20.3 | 1.63 | 0.0 | 12.9 | average |
| 2.7 | 2.8 | 0.0 | 5.6 | SD | |
| 0.00048 | 0.0053 | 0.11 | P-value | ||
| *** | ** | Ns | significance | ||
| Sox2 | 14.9 | 1.0 | 4.1 | 5.7 | average |
| 3.1 | 0.9 | 4.0 | 1.4 | SD | |
| 0.0057 | 0.024 | 0.022 | P-value | ||
| ** | * | * | significance | ||
| GABA | 3.6 | 0.5 | 0.7 | 1.4 | average |
| 1.5 | 1.0 | 1.7 | 1.2 | SD | |
| 0.0041 | 0.014 | 0.068 | P-value | ||
| ** | * | Ns | significance | ||
| GlyT1 | 25.3 | 0 | 0.7 | 37.0 | average |
| 5.1 | 0 | 1.6 | 0.47 | SD | |
| 0 | 0.0014 | 0.019 | P-value | ||
| ** | ** | * | significance | ||
| Satb2 | 15.0 | 92.2 | 61.2 | 8.9 | average |
| 8.3 | 3.7 | 8.2 | 2.5 | SD | |
| 0.00067 | 0.0024 | 0.33 | P-value | ||
| *** | ** | Ns | significance |
Highlights.
Pax6’s roles in the differentiation of late-born retinal cell types are uncovered.
Pax6 is required for the generation of bipolar and glycinergic amacrine cells.
Pax6 inhibit rod differentiation and promote generation of amacrine-like cells.
The paired domain of Pax6 is required for the inhibition of rod differentiation.
Pax6 inhibits rod differentiation through the repression of Crx.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma N, Tadokoro K, Asaka A, Yamada M, Yamaguchi Y, Handa H, Matsushima S, Watanabe T, Kohsaka S, Kida Y, Shiraishi T, Ogura T, Shimamura K, Nakafuku M. The Pax6 isoform bearing an alternative spliced exon promotes the development of the neural retinal structure. Hum Mol Genet. 2005;14:735–745. doi: 10.1093/hmg/ddi069. [DOI] [PubMed] [Google Scholar]
- Berger J, Berger S, Tuoc TC, D'Amelio M, Cecconi F, Gorski JA, Jones KR, Gruss P, Stoykova A. Conditional activation of Pax6 in the developing cortex of transgenic mice causes progenitor apoptosis. Development. 2007;134:1311–1322. doi: 10.1242/dev.02809. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, Yung R, Asch E, Ohno-Machado L, Wong WH, Cepko CL. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188:263–272. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci. 2014;15:615–627. doi: 10.1038/nrn3767. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- Cherry TJ, Trimarchi JM, Stadler MB, Cepko CL. Development and diversification of retinal amacrine interneurons at single cell resolution. Proc Natl Acad Sci U S A. 2009;106:9495–9500. doi: 10.1073/pnas.0903264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry TJ, Wang S, Bormuth I, Schwab M, Olson J, Cepko CL. NeuroD factors regulate cell fate and neurite stratification in the developing retina. J Neurosci. 2011;31:7365–7379. doi: 10.1523/JNEUROSCI.2555-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Ashery-Padan R. The cellular and molecular mechanisms of vertebrate lens development. Development. 2014;141:4432–4447. doi: 10.1242/dev.107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny T, Schaffner G, Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993;7:2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- Ding Q, Chen H, Xie X, Libby RT, Tian N, Gan L. BARHL2 differentially regulates the development of retinal amacrine and ganglion neurons. J Neurosci. 2009;29:3992–4003. doi: 10.1523/JNEUROSCI.5237-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. The Retina: An Approachable Part of the Brain. Belknap Press of Harvard, University Press; Cambridge, MA: 1987. [Google Scholar]
- Duncan MK, Haynes JI, 2nd, Cvekl A, Piatigorsky J. Dual roles for Pax-6: a transcriptional repressor of lens fiber cell-specific beta-crystallin genes. Mol Cell Biol. 1998;18:5579–5586. doi: 10.1128/mcb.18.9.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci. 2007;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JA, Glaser T, Cai J, Jepeal L, Walton DS, Maas RL. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 1994;8:2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- Farhy C, Elgart M, Shapira Z, Oron-Karni V, Yaron O, Menuchin Y, Rechavi G, Ashery-Padan R. Pax6 is required for normal cell-cycle exit and the differentiation kinetics of retinal progenitor cells. PLoS One. 2013;8:e76489. doi: 10.1371/journal.pone.0076489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Green ES, Stubbs JL, Levine EM. Genetic rescue of cell number in a mouse model of microphthalmia: interactions between Chx10 and G1-phase cell cycle regulators. Development. 2003;130:539–552. doi: 10.1242/dev.00275. [DOI] [PubMed] [Google Scholar]
- Hanson I, Churchill A, Love J, Axton R, Moore T, Clarke M, Meire F, van Heyningen V. Missense mutations in the most ancient residues of the PAX6 paired domain underlie a spectrum of human congenital eye malformations. Hum Mol Genet. 1999;8:165–172. doi: 10.1093/hmg/8.2.165. [DOI] [PubMed] [Google Scholar]
- Harman AM, Beazley LD. Patterns of cytogenesis in the developing retina of the wallaby Setonix brachyurus. Anat Embryol (Berl) 1987;177:123–130. doi: 10.1007/BF00572536. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Tomita K, Inoue T, Kageyama R. Roles of homeobox and bHLH genes in specification of a retinal cell type. Development. 2001;128:1313–1322. doi: 10.1242/dev.128.8.1313. [DOI] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Gotz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–6140. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Holm PC, Mader MT, Haubst N, Wizenmann A, Sigvardsson M, Gotz M. Loss- and gain-of-function analyses reveal targets of Pax6 in the developing mouse telencephalon. Mol Cell Neurosci. 2007;34:99–119. doi: 10.1016/j.mcn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Jin K, Jiang H, Xiao D, Zou M, Zhu J, Xiang M. Tfap2a and 2b act downstream of Ptf1a to promote amacrine cell differentiation during retinogenesis. Mol Brain. 2015;8:28. doi: 10.1186/s13041-015-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S, Desplan C. Cooperative interactions between paired domain and homeodomain. Development. 1996;122:2639–2650. doi: 10.1242/dev.122.9.2639. [DOI] [PubMed] [Google Scholar]
- Kay JN, Voinescu PE, Chu MW, Sanes JR. Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nat Neurosci. 2011;14:965–972. doi: 10.1038/nn.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimova L, Kozmik Z. Stage-dependent requirement of neuroretinal Pax6 for lens and retina development. Development. 2014;141:1292–1302. doi: 10.1242/dev.098822. [DOI] [PubMed] [Google Scholar]
- Lakowski J, Majumder A, Lauderdale JD. Mechanisms controlling Pax6 isoform expression in the retina have been conserved between teleosts and mammals. Dev Biol. 2007;307:498–520. doi: 10.1016/j.ydbio.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Liu H, Kim SY, Fu Y, Wu X, Ng L, Swaroop A, Forrest D. An isoform of retinoid-related orphan receptor beta directs differentiation of retinal amacrine and horizontal interneurons. Nat Commun. 2013;4:1813. doi: 10.1038/ncomms2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel MN, Mi D, Mason JO, Price DJ. Regulation of cerebral cortical neurogenesis by the Pax6 transcription factor. Frontiers in cellular neuroscience. 2015;9:70. doi: 10.3389/fncel.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci U S A. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci U S A. 2007;104:1027–1032. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Li S, Yang X, Xiang M. Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development. 2004;131:1607–1618. doi: 10.1242/dev.01071. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Cepko CL. Vertebrate photoreceptor cell development and disease. Trends Cell Biol. 1998;8:353–358. doi: 10.1016/s0962-8924(98)01341-5. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Pinto L, Petricca S, Lepier A, Sun J, Rieger MA, Schroeder T, Cvekl A, Favor J, Gotz M. The transcription factor Pax6 regulates survival of dopaminergic olfactory bulb neurons via crystallin alphaA. Neuron. 2010;68:682–694. doi: 10.1016/j.neuron.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron-Karni V, Farhy C, Elgart M, Marquardt T, Remizova L, Yaron O, Xie Q, Cvekl A, Ashery-Padan R. Dual requirement for Pax6 in retinal progenitor cells. Development. 2008;135:4037–4047. doi: 10.1242/dev.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson J, Simpson TI, Mason JO, Price DJ. Positive autoregulation of the transcription factor Pax6 in response to increased levels of either of its major isoforms, Pax6 or Pax6(5a), in cultured cells. BMC Dev Biol. 2006;6:25. doi: 10.1186/1471-213X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- Rapicavoli NA, Poth EM, Zhu H, Blackshaw S. The long noncoding RNA Six3OS acts in trans to regulate retinal development by modulating Six3 activity. Neural Dev. 2011;6:32. doi: 10.1186/1749-8104-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv S, Bharti K, Rencus-Lazar S, Cohen-Tayar Y, Schyr R, Evantal N, Meshorer E, Zilberberg A, Idelson M, Reubinoff B, Grebe R, Rosin-Arbesfeld R, Lauderdale J, Lutty G, Arnheiter H, Ashery-Padan R. PAX6 regulates melanogenesis in the retinal pigmented epithelium through feed-forward regulatory interactions with MITF. PLoS Genet. 2014;10:e1004360. doi: 10.1371/journal.pgen.1004360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungger-Brandle E, Ripperger JA, Steiner K, Conti A, Stieger A, Soltanieh S, Rungger D. Retinal patterning by Pax6-dependent cell adhesion molecules. Dev Neurobiol. 2010;70:764–780. doi: 10.1002/dneu.20816. [DOI] [PubMed] [Google Scholar]
- Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: a multi-level regulator of ocular development. Prog Retin Eye Res. 2012;31:351–376. doi: 10.1016/j.preteyeres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Singh S, Mishra R, Arango NA, Deng JM, Behringer RR, Saunders GF. Iris hypoplasia in mice that lack the alternatively spliced Pax6(5a) isoform. Proc Natl Acad Sci U S A. 2002;99:6812–6815. doi: 10.1073/pnas.102691299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MC, Gallai R, Olivier D, Speeg-Schatz C, Flament J, Calvas P, Dollfus H. Variable phenotype related to a novel PAX 6 mutation (IVS4+5G>C) in a family presenting congenital nystagmus and foveal hypoplasia. Am J Ophthalmol. 2004;138:1016–1021. doi: 10.1016/j.ajo.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Walcher T, Xie Q, Sun J, Irmler M, Beckers J, Ozturk T, Niessing D, Stoykova A, Cvekl A, Ninkovic J, Gotz M. Functional dissection of the paired domain of Pax6 reveals molecular mechanisms of coordinating neurogenesis and proliferation. Development. 2013;140:1123–1136. doi: 10.1242/dev.082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sengel C, Emerson MM, Cepko CL. A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev Cell. 2014;30:513–527. doi: 10.1016/j.devcel.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Xiang M. Intrinsic control of mammalian retinogenesis. Cell Mol Life Sci. 2013;70:2519–2532. doi: 10.1007/s00018-012-1183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Rhodopsin is detected in the Pax6 mutant cells.
(A–H) Double-immunostaining with GFP and the rod marker rhodopsin of Pax6+/+ (A–D) and Pax6lox/lox (E–H) retinas electroporated with pCAG-Cre and pCALNL-GFP reporter. The green (GFP, A, E), red (Rhodopsin, B, F), blue (DAPI, C, G) channels are presented. Double positive cells are marked by white arrows. (I–P) Double-immunostaining with GFP and the cone marker PNA of Pax6+/+ (I–L) and Pax6lox/lox (M–P) retinas electroporated with pCAG-Cre pCALNL-GFP. The green (GFP, I, M), red (PNA, J, N), blue (DAPI, K, O) channels are presented. Scale bar = 20 µm.
Fig. S2. Pax6 and Pax6(5a) miss-expression leads to exclusive generation of nGnG amacrine cells at the expense of other late-born interneurons types. Double-immunostaining with GFP and cell-type-specific markers of electroporated retinas: syntaxin for amacrine interneurons (A–D), Vsx2 for bipolar interneurons (E–H), Sox2 for Müller glia (I–L), GABA for GABAergic amacrines (M–P), glycine transporter 1 (GlyT) for glycinergic amacrines (Q–T), Satb2 for the nGnG amacrines (U–X). Arrowheads point to co-localized cells. Immunostaining shows elevation in syntaxin and Satb2 and reduction in all other markers in pCAG-Pax6-GFP and pCAG-Pax6(5a)-GFP compared to the pCAG-GFP control and pCAG-Pax6ΔPD-GFP retinas. Quantification is shown in Figure 4. The scale bar = 25 µm.
Fig. S3. Changes in the number of Ccnd3+ Müller glia upon Pax6 overexpression. Double-immunostaining with GFP and Ccnd3 of retinae electroporated with pCAG-GFP (A–D) and pCAG-Pax6-GFP (E–H). The green (GFP, A, E), red (Ccnd3, B, F), blue (DAPI, C, G) channels are shown. The number of cells positive for both Ccnd3 and GFP was quantified (I). The number of GFP+ cells co-expressing Ccnd3 was significantly higher in the retians electroporated with the pCAG-GFP control plasmid than in the retinas that were electroporated with pCAG-Pax6-GFP plasmid (P=0.03, N=3 for both genotypes). Scale bar = 20 µm.
Fig. S4. PNA does overlap with the cells that miss express Pax6 in the ONL Double-immunostaining with GFP and the cone marker PNA of retinas electroporated with pCAG-GFP (A–D) and pCAG-Pax6-GFP (E–H). The green (GFP, A, E), red (PNA, B, F), blue (DAPI, C, G) channels are shown. Scale bar = 20 µm.