Abstract
Dietary factors, including sugar-sweetened beverages, may have adverse effects on fertility. Sugar-sweetened beverages have been associated with poor semen quality in cross-sectional studies, and female soda intake has been associated with lower fecundability in some, but not all, studies. We evaluated the association of female and male sugar-sweetened beverage intake with fecundability among 3828 women planning pregnancy and 1045 of their male partners in a North American prospective cohort study. We followed participants enrolled between June 2013 and May 2017 until pregnancy or for up to twelve menstrual cycles. Eligible women were aged 21–45 years (male partners ≥21), attempting conception for ≤6 cycles, and not using fertility treatments. Participants completed a comprehensive baseline questionnaire, including questions on soda (sugar-sweetened and diet), fruit juice, energy, and sports drink consumption during the previous 4 weeks. We estimated time-to-pregnancy from follow-up questionnaires completed every 2 months by the female partner. We calculated adjusted fecundability ratios (FR) and 95% confidence intervals (CIs) according to intake of sugar-sweetened beverages using proportional probabilities regression. Both female and male intakes of sugar-sweetened beverages were associated with reduced fecundability (FR= 0.81; 95% CI: 0.70, 0.94 and 0.78; 95% CI: 0.63, 0.95 for ≥ 7 sugar-sweetened beverages per week compared with none, for females and males, respectively). Fecundability was further reduced among those who drank ≥7 servings per week of sugar-sweetened sodas (FR= 0.75, 95% CI: 0.59, 0.95 for females and 0.67, 95% CI: 0.51, 0.89 for males). Diet soda had little association with fecundability.
Keywords: fertility, fecundability, sodas, preconception cohort
Introduction
Approximately 10%–15% of North American couples experience infertility, defined as the inability to conceive after 12 or more months of attempting pregnancy.1 Both female and male factors contribute to infertility, with estimates of 39% of cases due to a female factor alone, 20% to a male factor, 33% to both male and female factors, and 8% with unknown cause.2 Thus, identifying modifiable factors in both partners that can improve fertility (e.g., diet) could help couples avoid expensive and stressful fertility treatments.
The amount of added sugar in the American diet increased by 19% between 1970 and 2005.3 Much of this increase stems from consumption of sugar-sweetened beverages, which on average supply 33% of total added sugar intake.3 Sodas are the largest component of sugar-sweetened beverages in the United States (U.S.).4 U.S. intake of high fructose corn syrup (HFCS), the most common sweetener in sodas, has increased substantially since 1970.5 Intake of HFCS in Canada has also increased since 1994,6 but levels of other added sugars have declined over the last few decades and Canadians consume 50% less soda than U.S. citizens.7 Soda consumption has been linked to weight gain and type 2 diabetes,8,9 but few studies have investigated consumption of soda, either sugar-sweetened or diet, in relation to female or male fertility. Higher intake of sugar-sweetened beverages has been associated with earlier menarche10 and elevated follicular estradiol, but not with anovulation.11 Sugar-sweetened beverages are important contributors to high glycemic load diets,12 which in turn have been related to increased risk of ovulatory infertility.13 Findings for female soda consumption and fertility are mixed14–17 but, with the exception of a recent study in an infertility clinic population,18 most studies were designed primarily to evaluate caffeine, and have not comprehensively evaluated sugar-sweetened beverages or diet sodas and fertility. In males, three cross-sectional studies reported an association between soda consumption and poor semen quality,19–21 but semen quality may be a poor proxy for male fecundity.22. Here, we use data from a prospective cohort study of North American pregnancy planners to examine the association between female and male preconception sugar-sweetened beverage consumption and fecundability.
Methods
Study population
PRESTO is an ongoing internet-based cohort study of pregnancy planners from the U.S. and Canada that began enrollment in June 2013. Couples are eligible if trying to conceive and not using contraception or fertility treatments. Female participants are aged 21–45 years; male partners are aged ≥21 years. The study methods are described elsewhere.23 Briefly, female participants complete a comprehensive web-based baseline questionnaire and are encouraged to invite their male partner to complete a one-time baseline questionnaire. Ten days after completing the baseline questionnaire, participants complete a separate food frequency questionnaire (FFQ) developed by the National Cancer Institute.24,25 Women complete follow-up questionnaires every 8 weeks up to 12 months or until conception, whichever comes first. Of the 4856 women enrolled through May 2017, we excluded 830 who had been attempting conception >6 cycles at entry, 155 with insufficient/implausible dates of last menstrual period (LMP), and 43 pregnant at study entry, leaving 3828 women in analyses of sugar-sweetened beverage intake by women. A total of 2126 of these women (56%) invited their male partners, of whom 1045 (49%) enrolled and were included in analyses of intake of sugar-sweetened beverages by males. PRESTO was approved by the Institutional Review Board at Boston Medical Center and all participants provided informed consent.
Assessment of sugar-sweetened beverage intake
On the baseline questionnaire, female and male participants reported their average soda consumption (number of 12-ounce servings per week) in the past month, selecting from a list of popular sodas. Responses to an open-ended category for “other soda” were categorized as sugar-sweetened or diet. Total sugar-sweetened soda intake was calculated by summing the number of 12-ounce servings of Coca-Cola, Pepsi, Mountain Dew, Dr. Pepper, Sprite, Fanta, and ‘other’ sugar-sweetened sodas. Total diet soda represented the sum of the number of 12-ounce servings of Fresca, Diet Coca-Cola, Diet Pepsi, Diet Mountain Dew, Diet Dr. Pepper, Coke Zero, Zevia, and ‘other’ diet sodas. In addition, participants reported consumption of popular energy drinks (e.g., Red Bull, Monster, Five-Hour Energy, Rockstar, Full Throttle, Sobe No Fear, Amp, Sobe Adrenaline Rush, Tab Energy, and Monster XXL). They also answered an open-ended question on other types of sugar-sweetened energy drinks. For each positive response, they were asked to report the number of cans/ bottles consumed per week. Consumption of sugar-sweetened sports drinks (e.g., Gatorade) and fruit juices was elicited as part of a list of other beverages asking: “Did you drink any of the following beverages in the past month?”
Assessment of time-to-pregnancy
We estimated time-to-pregnancy (TTP) using data from the female questionnaires (screening, baseline, and follow-up). Women reported their pregnancy attempt time (in months and menstrual cycles) at baseline. Those with regular menstrual cycles were asked their usual menstrual cycle length. For women with irregular cycles, we estimated cycle length based on date of LMP at baseline and on prospectively-reported LMP dates during follow-up. We estimated TTP in discrete cycles as follows: [(menstrual cycles of attempt time at baseline) + [(LMP date from most recent follow-up questionnaire - date of baseline questionnaire)/cycle length] + 1].26 Participants who did not complete any follow-ups (n=443 females and 49 males) were assigned one cycle of observation; their outcome information was imputed (see multiple imputation procedures below).
Assessment of covariates
The female and male baseline questionnaires collected data on age, race/ethnicity, education, income, reproductive history, cigarette smoking, caffeine and alcohol intake, sleep duration, perceived stress using the 10-item Perceived Stress Scale,27 depressive symptoms using the Major Depression Inventory,28,29 height, weight, physical activity, intercourse frequency and use of methods to improve chances of conception (e.g., charting cycles, monitoring cervical mucus). Body mass index was calculated as weight (kg) divided by height (m2). Total metabolic equivalents (METs) of physical activity were calculated by multiplying the average number of hours per week engaging in various activities by metabolic equivalents estimated from the Compendium of Physical Activities.30,31 Female dietary variables (i.e., total caloric intake and Healthy Eating Index (HEI) score) were collected on the FFQ.32
Data analysis
Analyses were run separately among the 3828 female and the 1045 male participants. Average weekly intake of sugar-sweetened and diet sodas, sugar-sweetened energy drinks, sports drinks, and fruit juices was categorized into 0, 1, 2–6, and ≥7 servings per week and also modeled continuously using restricted cubic splines.33 Total sugar-sweetened beverage consumption was calculated by summing the weekly servings from all such beverages.
Couples contributed menstrual cycles to follow-up until pregnancy, initiation of fertility treatment, cessation of pregnancy attempts, withdrawal, loss to follow-up, or completion of 12 cycles, whichever came first. To account for variation in time trying to conceive at study entry (range: 0–6 cycles) and avoid left truncation bias,34 we analyzed observed cycles at risk using the Anderson-Gill data structure.35 We used proportional probabilities regression to estimate fecundability ratios (FRs), defined as the cycle-specific probability of conception comparing the exposed with unexposed.36
We controlled for variables hypothesized to be associated with sugar-sweetened beverage consumption and subfertility. Female beverage models were adjusted for the following female variables from the baseline questionnaire: age (<25, 25–29, 30–34, ≥35 years), race/ethnicity (white/non-Hispanic vs. not), education (<college degree, college graduate, graduate school), annual household income (<$50,000, $50,000–$99,999, $100,000–$149,999, ≥$150,000), smoking history (current regular, current occasional, former, never), BMI (continuous and categorical: <25, 25–29, 30–34, ≥35 kg/m2), physical activity (<10, 10–19, 20–39, ≥40 MET-hours/week), caffeine intake (<100, 100–199, 200–299, ≥300 mg/day), alcohol intake (0, 1–6, 7–13, ≥14 drinks/week), sleep duration (<7, 7–8, ≥9 hours/night), perceived stress scale score (<10, 10–19, 20–29, ≥30), intercourse frequency (<1, 1, 2–3, ≥4 times/week) and doing something to improve chances of conception (yes, no). In a sub-analysis, we also controlled for Healthy Eating Index and total energy intake calculated from the female FFQ. Male beverage models were adjusted for male and female age (<30, 30–34, ≥35 years) and male and female BMI (continuous and categorical), as well as male versions of all variables listed above. Sugar-sweetened and diet sodas were mutually adjusted, and estimates for all other individual beverages (energy drinks, sports drinks and juice) were adjusted for intake of sugar-sweetened sodas.
In the subset of 1045 couples with information from both males and females, we mutually adjusted female and male total sugar-sweetened beverage intake and sugar-sweetened soda intake (0, 1, 2–6, ≥7 servings/week). Among the 161 couples with complete FFQ data, we computed Pearson correlation coefficients between female and male Healthy Eating Index.
We evaluated female and male intake of sugar-sweetened beverages jointly to look for evidence of synergistic effects. We also assessed whether associations between sugar-sweetened beverage consumption and fecundability varied by attempt time at study entry (<3 vs. 3–6 cycles), female age (<30 vs ≥30 years), and female and male BMI (<25 vs. ≥25 kg/m2).
Missing data ranged from 0% (female age, intercourse frequency) to 2.5% (household income). Because the male FFQ was not implemented until November 2015, only 161 males have completed it, limiting our ability to control for additional dietary variables in males. We used multiple imputation to impute missing covariate values.37 Analyses were conducted using SAS software (Version 9.4, SAS Institute, Cary, NC).38
Results
Among the 3828 women included in the study, 330 (8.6%) initiated fertility treatment and 824 (22%) were lost to follow-up before completion of 12 cycles. Among the 2674 couples who completed the study, 2276 (85%) became pregnant and 398 (10%) were censored at 12 cycles. Men consumed an average of 2.4 sugar-sweetened sodas (standard deviation (SD): ±13.6), 1.6 diet sodas (SD: ±4.4), 1.0 glasses of fruit juice (SD: ±2.3), 0.5 servings of energy drinks (SD: ±1.9), and 0.5 sports drinks per week (SD: ±1.6 servings). Women consumed an average of 1.3 sugar-sweetened sodas (SD: ±3.6), 1.2 diet sodas (SD: ±3.9), 0.9 glasses of fruit juice (SD: ±2.1), 0.2 servings of energy drinks (SD: ±0.9), and 0.3 sports drinks (SD: ±1.7). Most men consumed only one type of sugar-sweetened beverages (39%), 27% consumed two types, 13% consumed three types, and 2.2% consumed more than three types; 19% consumed no sugar-sweetened beverages. A similar pattern was found for females (31% consumed none, 41% consumed one, 21% consumed two, 5.4% consumed three, and 1.2% consumed more than 3). Individual beverages were not strongly correlated with each other: the strongest correlation among males was between sugar-sweetened sodas and energy drinks (Pearson’s correlation coefficient (r)=0.31). The strongest correlation among females was between energy drinks and sports drinks (r=0.22). Male and female beverage intakes were not strongly correlated (sugar-sweetened beverages: r=0.21; sugar-sweetened sodas: r=0.27). The average Healthy Eating Index score for females was 67.9 (range: 24.3–92.4). We found moderate correlation between male and female Healthy Eating Index score (r=0.39) among the 161 couples with both FFQs.
Women who consumed more sugar-sweetened beverages were slightly younger and were more likely to be non-white (Table 1). Participants who drank more sugar-sweetened beverages were more likely to smoke, have a higher BMI, lower physical activity, lower Healthy Eating Index scores and higher caloric intake, and lower education and household income. Intercourse frequency and using methods to improve conception were similar across categories of sugar-sweetened beverage consumption.
Table 1.
Baseline characteristics of 3,828 female and 1,045 male pregnancy planners in relation to sugar-sweetened beverage consumption.
| Characteristica | Female sugar-sweetened beverage consumption (drinks/week) | Male sugar-sweetened beverage consumption (drinks/week) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0 | 1 | 2–6 | ≥7 | 0 | 1 | 2–6 | ≥7 | |
| Number of subjects | 1752 | 542 | 1071 | 463 | 332 | 134 | 342 | 237 |
| Age (mean, years) | 30.7 | 30.1 | 29.5 | 28.6 | 31.9 | 32.2 | 31.6 | 31.1 |
| Partner’s age (mean, years) | 32.0 | 32.1 | 31.9 | 31.6 | 30.0 | 30.2 | 30.1 | 29.4 |
| Healthy Eating Index score (mean)b | 70.3 | 68.3 | 66.0 | 61.0 | 66.8 | 62.8 | 62.6 | 55.4 |
| Partner’s SSB intake (mean, drinks/week)c | 2.7 | 4.2 | 5.3 | 10.1 | 0.9 | 1.2 | 2.1 | 3.8 |
| Alcohol intake (mean, drinks/week) | 3.4 | 3.5 | 3.4 | 2.7 | 6.0 | 5.8 | 6.3 | 6.8 |
| Caffeine intake (mean, mg/day) | 120.3 | 112.2 | 110.5 | 142.5 | 181.4 | 160.1 | 165.9 | 191.0 |
| BMI (mean, kg/m2) | 26.1 | 26.8 | 27.3 | 28.2 | 27.2 | 26.7 | 27.6 | 28.6 |
| Partner’s BMI (mean, kg/m2) | 27.6 | 28.0 | 28.1 | 28.3 | 25.6 | 26.1 | 26.4 | 28.0 |
| Physical activity (mean, MET-hours/week) | 37.9 | 33.7 | 34.8 | 30.7 | 34.7 | 35.4 | 34.1 | 29.7 |
| Total caloric intake (mean, kcal/day)† | 1498 | 1573 | 1635 | 1852 | 1571 | 1712 | 1753 | 1793 |
| PSS-10 score (mean) | 15.1 | 15.5 | 16.0 | 17.1 | 14.1 | 13.4 | 14.8 | 15.1 |
| MDI score (mean) | 8.7 | 9.8 | 10.3 | 12.9 | 8.9 | 8.4 | 9.0 | 9.9 |
| Ever pregnant/ever impregnated partner (%) | 42 | 50 | 50 | 56 | 43 | 39 | 40 | 53 |
| Parous (%) | 26 | 31 | 30 | 38 | -- | -- | -- | -- |
| Sleep duration <7 hours/night (%) | 20 | 19 | 25 | 35 | 30 | 23 | 32 | 44 |
| Intercourse frequency <1 time/week (%) | 20 | 23 | 20 | 22 | 18 | 18 | 23 | 20 |
| Doing something to improve chances (%) | 77 | 74 | 73 | 71 | 77 | 79 | 72 | 79 |
All characteristics are adjusted for age at baseline.
Among 2,518 females and 161 males who completed the Food frequency questionnaire.
Among 1,045 couples where both partners participated.
SSB=sugar-sweetened beverages
BMI=body mass index
MET=metabolic equivalent
MDI=Major Depression Inventory
Female SSB and Fecundability
Compared with no consumption of sugar-sweetened beverages, female consumption of ≥7 servings per week was associated with decreased fecundability after adjustment for potential confounders (FR=0.81; 95% CI: 0.70, 0.94) but there was little association with fewer than seven servings per week (Table 2). Female consumption of sugar-sweetened sodas, a subset of all SSB, was also associated with reduced fecundability, with FRs of 0.87 (95% CI: 0.77, 0.98) and 0.75 (95% CI: 0.59, 0.95) for 2–6 and ≥7 servings per week vs. none, respectively. There was little association between diet soda consumption and fecundability (Table 2). Female consumption of energy drinks was associated with reduced fecundability (FR=0.61, 95% CI: 0.29, 1.32 for ≥7 servings vs. none), but these estimates were imprecise. Fruit juice intake was not appreciably associated with fecundability and fecundability ratio estimates for intake of sports drinks did not follow a dose–response pattern and were imprecise. Among the subset of 2518 women who filled out an FFQ, adjustment for Healthy Eating Index and total energy intake had little effect on the estimates (FR for ≥7 sugar-sweetened sodas=0.66; 95% CI: 0.47, 0.91). Among the subset of females with male partners in the study, adjustment for male sugar-sweetened beverage intake also had little effect (data not shown).
Table 2.
Sugar-sweetened beverage consumption by females and fecundability.
| Beverage (servings/week) | No. of pregnancies | No. of cycles | Unadjusted FR (95% CI) | Adjusteda FR (95% CI) | Adjustedb FR (95% CI) |
|---|---|---|---|---|---|
| Sugar-sweetened beverages (total) | |||||
| 0 | 1083 | 6975 | 1.0 (Ref) | 1.0 (Ref) | -- |
| 1 | 323 | 2120 | 0.99 (0.89, 1.1) | 1.0 (0.91, 1.1) | -- |
| 2–6 | 592 | 4137 | 0.96 (0.87, 1.1) | 1.0 (0.92, 1.1) | -- |
| ≥7 | 219 | 2036 | 0.72 (0.62, 0.83) | 0.81 (0.70, 0.94) | -- |
| Sugar-sweetened sodas | |||||
| 0 | 1604 | 10141 | 1.0 (Ref) | 1.0 (Ref) | 1.00 (Ref) |
| 1 | 257 | 1981 | 0.86 (0.76, 0.97) | 0.89 (0.79, 1.0) | 0.88 (0.78, 1.00) |
| 2–6 | 278 | 2341 | 0.80 (0.71, 0.90) | 0.87 (0.77, 0.98) | 0.86 (0.76, 0.98) |
| ≥7 | 78 | 805 | 0.64 (0.51, 0.81) | 0.75 (0.59, 0.95) | 0.74 (0.59, 0.94) |
| Diet sodas | |||||
| 0 | 1612 | 10956 | 1.0 (Ref) | 1.0 (Ref) | 1.00 (Ref) |
| 1 | 222 | 1310 | 1.1 (1.0, 1.3) | 1.1 (0.95, 1.2) | 1.06 (0.93, 1.20) |
| 2–6 | 270 | 2101 | 0.91 (0.81, 1.0) | 0.95 (0.84, 1.1) | 0.92 (0.81, 1.05) |
| ≥7 | 113 | 901 | 0.94 (0.78, 1.1) | 1.0 (0.83, 1.2) | 0.97 (0.79, 1.18) |
| Sugar-sweetened energy drinks | |||||
| 0 | 2062 | 14094 | 1.0 (Ref) | 1.0 (Ref) | 1.00 (Ref) |
| 1 | 93 | 683 | 0.97 (0.79, 1.2) | 0.99 (0.81, 1.2) | 1.00 (0.81, 1.23) |
| 2–6 | 55 | 403 | 1.0 (0.78, 1.3) | 1.1 (0.84, 1.4) | 1.09 (0.84, 1.41) |
| ≥7 | 7 | 88 | 0.55 (0.26, 1.2) | 0.61 (0.29, 1.3) | 0.59 (0.28, 1.28) |
| Fruit juices | |||||
| 0 | 1591 | 10898 | 1.0 (Ref) | 1.0 (Ref) | 1.00 (Ref) |
| 1 | 169 | 1066 | 1.1 (0.91, 1.2) | 1.1 (0.94, 1.3) | 1.10 (0.95, 1.28) |
| 2–6 | 386 | 2748 | 0.97 (0.87, 1.1) | 1.0 (0.90, 1.1) | 1.02 (0.92, 1.14) |
| ≥7 | 71 | 556 | 0.86 (0.69, 1.1) | 0.91 (0.73, 1.1) | 0.94 (0.75, 1.18) |
| Sports drinks | |||||
| 0 | 2028 | 13836 | 1.0 (Ref) | 1.0 (Ref) | 1.00 (Ref) |
| 1 | 111 | 655 | 1.1 (0.96, 1.4) | 1.2 (1.0, 1.4) | 1.22 (1.02, 1.45) |
| 2–6 | 68 | 683 | 0.71 (0.57, 0.90) | 0.78 (0.62, 0.98) | 0.81 (0.65, 1.02) |
| ≥7 | 10 | 94 | 0.74 (0.42, 1.3) | 0.86 (0.49, 1.5) | 0.88 (0.50, 1.55) |
Adjusted for female age, race/ethnicity, education, income, smoking history, BMI, physical activity, caffeine intake, alcohol intake, sleep duration, PSS-10 score, intercourse frequency, and doing something to improve chances of conception.
Additionally adjusted for diet soda intake (for sugar-sweetened soda models), or for sugar-sweetened soda intake (for all other models).
BMI=body mass index
PSS=Perceived Stress Score
Male SSB and Fecundability
Consumption of sugar-sweetened beverages by males was associated with reduced fecundability (FRs =0.85 (95% CI: 0.72, 1.01) and 0.78 (95% CI: 0.63, 0.96) for two to six and more than seven servings per week vs. none, respectively). Lower fecundability was also seen among male consumers of sugar-sweetened sodas (FR=0.67 (95% CI: 0.51, 0.89) but was not evident for diet sodas (FR=0.96 (95% CI: 0.72, 1.22) for at least seven servings per week vs. none (Table 3). The largest reduction in fecundability was seen in men who consumed seven or more energy drinks per week (FR=0.42; 95% CI: 0.20, 0.90). Men who consumed the most fruit juices and sports drinks appeared to have slightly higher fecundability, but estimates were imprecise. Controlling for female intake of the same beverages had little effect on the results; for example, the FR for male intake of ≥7 sugar-sweetened sodas increased from 0.67 to 0.72, after controlling for female intake. We also adjusted the male models for female Healthy Eating Index and total energy intake which produced similar results (data not shown).
Table 3.
Sugar-sweetened beverage consumption by males and fecundability.
| Beverage (servings/week) | No. of pregnancies | No. of cycles | Unadjusted FR (95% CI) | Adjusteda FR (95% CI) | Adjustedb FR (95% CI) | Adjustedc FR (95% CI) |
|---|---|---|---|---|---|---|
| Sugar-sweetened beverages (total) | ||||||
| 0 | 226 | 1243 | 1.0 (Ref) | 1.0 (Ref) | -- | 1.0 (Ref) |
| 1 | 90 | 516 | 0.99 (0.80, 1.2) | 0.97 (0.78, 1.2) | -- | 0.97 (0.77, 1.2) |
| 2–6 | 222 | 1463 | 0.87 (0.74, 1.0) | 0.85 (0.72, 1.0) | -- | 0.87 (0.73, 1.0) |
| ≥7 | 134 | 1075 | 0.76 (0.63, 0.93) | 0.78 (0.63, 0.96) | -- | 0.83 (0.67, 1.0) |
| Sugar-sweetened sodas | ||||||
| 0 | 375 | 2115 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 1 | 115 | 652 | 1.0 (0.85, 1.2) | 0.97 (0.80, 1.2) | 0.96 (0.79, 1.2) | 0.96 (0.79, 1.2) |
| 2–6 | 125 | 988 | 0.77 (0.64, 0.93) | 0.76 (0.63, 0.93) | 0.76 (0.62, 0.92) | 0.79 (0.64, 0.96) |
| ≥7 | 57 | 542 | 0.66 (0.51, 0.86) | 0.67 (0.51, 0.89) | 0.67 (0.50, 0.89) | 0.72 (0.54, 0.96) |
| Diet sodas | ||||||
| 0 | 473 | 3048 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 1 | 61 | 344 | 1.2 (0.90, 1.5) | 1.1 (0.87, 1.4) | 1.1 (0.83, 1.4) | 1.1 (0.82, 1.4) |
| 2–6 | 91 | 583 | 0.99 (0.81, 1.2) | 1.0 (0.82, 1.3) | 1.0 (0.80, 1.3) | 1.0 (0.79, 1.3) |
| ≥7 | 47 | 322 | 0.93 (0.71, 1.2) | 0.96 (0.72, 1.3) | 0.90 (0.67, 1.2) | 0.93 (0.68, 1.3) |
| Sugar-sweetened energy drinks | ||||||
| 0 | 565 | 3515 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 1 | 53 | 349 | 0.94 (0.73, 1.2) | 0.94 (0.72, 1.2) | 0.95 (0.73, 1.2) | 0.97 (0.74, 1.3) |
| 2–6 | 47 | 307 | 0.95 (0.72, 1.3) | 0.96 (0.71, 1.3) | 0.97 (0.72, 1.3) | 1.01 (0.74, 1.4) |
| ≥7 | 7 | 126 | 0.42 (0.21, 0.87) | 0.42 (0.20, 0.90) | 0.44 (0.21, 0.94) | 0.44 (0.20, 0.93) |
| Fruit juices | ||||||
| 0 | 472 | 3072 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 1 | 47 | 296 | 0.97 (0.74, 1.3) | 1.0 (0.78, 1.4) | 1.1 (0.81, 1.4) | 1.1 (0.82, 1.5) |
| 2–6 | 125 | 758 | 1.0 (0.87, 1.3) | 1.0 (0.86, 1.3) | 1.1 (0.89, 1.3) | 1.1 (0.88, 1.3) |
| ≥7 | 28 | 171 | 1.2 (0.82, 1.6) | 1.2 (0.86, 1.8) | 1.3 (0.87, 1.8) | 1.2 (0.85, 1.8) |
| Sports drinks | ||||||
| 0 | 557 | 3509 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 1 | 50 | 333 | 0.93 (0.71, 1.2) | 0.89 (0.68, 1.2) | 0.92 (0.70, 1.2) | 0.93 (0.71, 1.2) |
| 2–6 | 57 | 405 | 0.90 (0.70, 1.2) | 0.92 (0.71, 1.2) | 0.97 (0.74, 1.3) | 1.0 (0.77, 1.3) |
| ≥7 | 8 | 50 | 1.2 (0.61, 2.1) | 1.20 (0.64, 2.3) | 1.3 (0.71, 2.5) | 1.5 (0.78, 2.8) |
Adjusted for male age, education, smoking, BMI, race/ethnicity, alcohol intake, caffeine intake, PSS-10 score, physical activity, and female age, body mass index, total household income, intercourse frequency, and use of methods to improve chances of conception.
Additionally adjusted for daily servings of diet soda (for sugar-sweetened soda model) or daily servings of sugar-sweetened soda (all other models).
Additionally adjusted for female beverage intake.
BMI=body mass index
PSS=Perceived Stress Score
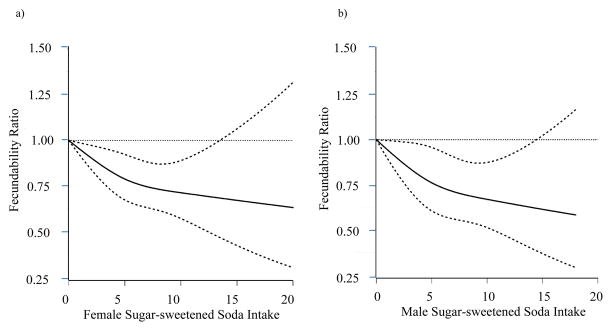
Table 4 presents the results for the joint effects of male and female sugar-sweetened beverage and sugar-sweetened soda consumption. Compared with the referent category (both partners drink <1 beverage per week), all other combinations showed reduced fecundability. In general the strongest effects were seen among couples where both partners had high intake but the effects were approximately additive, with little indication of any interaction.39 Figures 1 a and b present results of the analyses using restricted cubic splines for female and male sugar-sweetened soda consumption. Both splines show a decline in fecundability with increasing intake.
Table 4.
Female and male sugar-sweetened beverage consumption and fecundability.
| Beverage (servings/week) | No. of pregnancies | No. of cycles | Unadjusted FR (95% CI) | Adjusteda FR (95% CI) |
|---|---|---|---|---|
| Sugar-sweetened beverages | ||||
| Female ≤1/week, male ≤1/week | 255 | 1392 | 1.0 (Ref) | 1.0 (Ref) |
| Female ≤1/week, male 2–6/week | 138 | 773 | 0.97 (0.81, 1.2) | 0.95 (0.78, 1.2) |
| Female ≤1/week, male ≥7/week | 61 | 462 | 0.78 (0.60, 1.0) | 0.77 (0.59, 1.0) |
| Female 2–6/week, male ≤1/week | 51 | 302 | 0.95 (0.73, 1.3) | 1.0 (0.75, 1.3) |
| Female 2–6/week, male 2–6/week | 75 | 570 | 0.77 (0.61, 0.97) | 0.76 (0.59, 0.98) |
| Female 2–6/week, male ≥7/week | 49 | 355 | 0.85 (0.64, 1.1) | 0.91 (0.67, 1.2) |
| Female ≥7/week, male ≤1/week | 10 | 65 | 0.78 (0.44, 1.4) | 0.71 (0.40, 1.3) |
| Female ≥7/week, male 2–6/week | 9 | 120 | 0.46 (0.23, 0.92) | 0.50 (0.24, 1.0) |
| Female ≥7/week, male ≥7/week | 24 | 258 | 0.57 (0.38, 0.85) | 0.61 (0.39, 0.94) |
| Sugar-sweetened sodas | ||||
| Female ≤1/week, male ≤1/week | 457 | 2494 | 1.0 (Ref) | 1.0 (Ref) |
| Female ≤1/week, male 2–6/week | 100 | 704 | 0.83 (0.68, 1.0) | 0.81 (0.66, 1.0) |
| Female ≤1/week, male ≥7/week | 30 | 291 | 0.61 (0.42, 0.87) | 0.57 (0.39, 0.83) |
| Female 2–6/week, male ≤1/week | 31 | 232 | 0.78 (0.56, 1.1) | 0.79 (0.55, 1.1) |
| Female 2–6/week, male 2–6/week | 21 | 247 | 0.55 (0.38, 0.83) | 0.57 (0.37, 0.88) |
| Female 2–6/week, male ≥7/week | 21 | 178 | 0.76 (0.50, 1.2) | 0.88 (0.56, 1.4) |
| Female ≥7/week, male ≤1/week | 2 | 41 | 0.29 (0.07, 1.1) | 0.30 (0.08, 1.2) |
| Female ≥7/week, male 2–6/week | 4 | 37 | 0.45 (0.12, 1.7) | 0.41 (0.10, 1.7) |
| Female ≥7/week, male ≥7/week | 6 | 73 | 0.48 (0.21, 1.1) | 0.49 (0.20, 1.2) |
Adjusted for male and female age, BMI, education, race/ethnicity, smoking, physical activity, caffeine intake, alcohol intake, sleep duration, PSS-10 score, annual household income, intercourse frequency, and doing something to improve chances of conception.
BMI=body mass index
PSS=Perceived Stress Score
Figure 1.
Association between female (a) and male (b) sugar-sweetened soda intake and fecundability, fitted by restricted cubic splines, PRESTO, 2013–2017. The reference level for the FR is 0 sugar-sweetened sodas/week. The curve for female sugar-sweetened soda intake and fecundability is adjusted for female age, race/ethnicity, education, income, smoking history, BMI, physical activity, caffeine intake, sleep duration, PSS-10 score, intercourse frequency, and doing something to improve chances of conception. The curve for male sugar-sweetened soda intake and fecundability is adjusted for male and female age, male race/ethnicity, male and female BMI, education, income, and smoking. The splines are trimmed at the 99th percentile and have three knots at 2, 5, and 10 sugar-sweetened sodas/week.
Associations were stronger among couples trying for fewer than three cycles at entry, but were absent among couples who had been trying for three to six cycles at entry (eTable 1). Associations did not vary appreciably across strata of female age (data not shown). Among females, results were similar across BMI strata (eTable 2). Among overweight and obese males (BMI≥25 kg/m2), who constituted 65% of our study population, consumption of ≥7 servings of sugar-sweetened soda per week was strongly associated with reduced fecundability compared with no consumption (FR=0.60; 95% CI: 0.44, 0.83), whereas among normal weight males the FR was 0.89 (95% CI: 0.50, 1.58). We found little change when sugar-sweetened and diet sodas were mutually adjusted for each other, or when other beverage types were adjusted for consumption of sugar-sweetened sodas. As a sensitivity analysis to evaluate possible residual confounding by caffeine, we compared FRs for caffeinated and decaffeinated sodas; both groups comprise a mixture of sugar-sweetened and diet sodas. FRs for caffeinated and decaffeinated sodas were similar (data not shown).
Discussion
In this preconception cohort study of North American pregnancy planners, both female and male consumption of any sugar-sweetened beverages were associated with reduced fecundability. The associations were driven mainly by intake of sugar-sweetened sodas. Energy drink consumption also was associated with reduced fecundability, but numbers were small. We found little consistent evidence for associations between consumption of diet soda, sport drinks, or fruit juice and fecundability. Adjustment for caffeine intake had little effect on our results. Similarly, adjustment for female and male BMI had minimal effects, but the association between intake of sugar-sweetened soda and fecundability was stronger among overweight and obese men.
To our knowledge, only three studies have evaluated female soda intake and fecundability. A study of 221 pregnancy planners in North Carolina reported a 50% reduction in fecundability associated with one caffeinated soft drink per day;14 similarly, a large Danish preconception cohort study found that sweetened sodas were associated with reduced fecundability (FRs=0.91 for less than one, 0.72 for one, and 0.58 for two sugar-sweetened sodas/day) whereas results for diet soda were inconsistent (FRs=0.97 for less than one, 1.07 for one, and 0.79 for two diet sodas/day, respectively).16 A previous analysis of PRESTO data found little association between female caffeinated sodas and fecundability; non-caffeinated sodas were not evaluated.40 Sugar-sweetened and diet sodas were associated with a higher risk of ovulatory infertility in the Nurses’ Health Study, but neither caffeine nor fructose content explained these findings.15 A recent study conducted among 340 women receiving in vitro fertilization found that those who drank sugar-sweetened sodas and energy drinks had fewer oocytes retrieved and lower fertilization rates compared with women who did not drink these beverages.18
No prior prospective studies to our knowledge have evaluated male soda consumption and fecundability. A Canadian retrospective cohort study of 1277 couples found little association between colas (diet and regular combined) and fecundability.41 Three cross-sectional studies of sugar-sweetened beverages in relation to semen quality have been conducted.19–21 A study of 2544 Danish males found poorer semen quality among men who drank more than fourteen 0.5 liter bottles of cola per week compared with men who did not drink colas; findings were not explained by caffeine content.20 A study among 189 U.S. men aged 18–22 years found that progressive sperm motility, but not count, concentration, or morphology, was reduced by 9.8 percent (95% CI: 1.9, 17.8) in the highest (≥1.3 12-ounce servings) compared with the lowest quartile (≤0.2 servings) of daily sugar-sweetened beverage intake.19 A Chinese study reported a dose-response relation between colas and lower semen volume.21 Studies have indicated that male caffeine intake may reduce fertility; 40,42,43 among these is an earlier report from PRESTO (n=662 males),17 which found that male consumption of ≥300 milligrams of caffeine per day was associated with slightly lower fecundability. Coffee (≥2 cups per day), caffeinated sodas (≥1 can per day), and energy drinks (≥1 serving per day), but not black or green tea, were each associated with lower fecundability. We were unable to evaluate sugar-sweetened, non-caffeinated beverages separately in the current study because of small numbers. Adjustment for total caffeine intake had virtually no effect on our estimates for sugar-sweetened beverages and thus caffeine is unlikely to explain our findings. In addition, when we compared effects of caffeinated with non-caffeinated sodas, results were similar, suggesting that caffeine is not responsible for the observed reductions in fecundability.
We evaluated exposure information reported at baseline, introducing potential for misclassification if beverage consumption changed over time. Because we provided a list of individual beverages for sodas and energy drinks, overestimation of these specific beverages may have occurred. Conversely, other types of sugar-sweetened beverages not on our list may not have been captured (e.g., we did not distinguish between sweetened and unsweetened tea and we only gave examples of two brands of sports drinks instead of providing a comprehensive list. In addition, the category ‘fruit juice’ likely includes both 100% juice and juice ‘drinks’ with added sugars. Any misclassification is likely to be non-differential across time-to-pregnancy categories, however, because beverage consumption was assessed prospectively before the occurrence of infertility. Furthermore, evidence that associations were stronger among couples who had been trying to conceive for fewer than 3 cycles at study entry argues against reverse causation.
Strengths of PRESTO include its prospective design and a geographically dispersed study population.23 Male participation was optional, but we were still able to enroll a large number of male partners, and fecundability was similar among males who participated and those who did not (eFigure 1). Most participants enrolled near the beginning of their pregnancy attempt (66% within the first 3 cycles), reducing the potential for selection bias and reverse causation. However, we lacked information on male dietary factors that may have confounded the observed associations, such as total energy intake and consumption of high glycemic or high-fat diets. When we controlled for female Healthy Eating Index score (which was moderately correlated with male scores), we found no material effect on our results for either male or female intake of sugar-sweetened beverages. Nevertheless, if a dietary pattern or food item among males was positively associated with both sugar-sweetened beverage consumption and subfertility, confounding would have exaggerated the inverse association between sugar-sweetened beverages and fecundability.
Critics occasionally have questioned whether Internet-based studies are prone to selection bias. The selection issues related to internet volunteers, however, applies more generally. Many cohort studies, including all randomized trials, enroll volunteers, and some randomized trials recruit via the Internet. Regardless of the method used to recruit volunteers for a cohort study, recruitment of volunteers should not affect validity of study results based on internal comparisons, unless the relation between the study factors differed between those who volunteer and those who do not, which seems unlikely.44 We see no reason that the relation between intake of sugar-sweetened beverages and fecundability would differ between Internet-recruited volunteers and non-participants. Furthermore, our study45 and others46,47 have shown that even when participation at cohort entry differs by characteristics such as age, parity, or smoking, measures of association are not biased due to self-selection.
A possible mechanism for an association between sugar-sweetened beverage consumption and fertility includes increased insulin resistance, leading to oxidative stress,19,48 which may deleteriously affect semen quality,49 or ovulatory function.50 Among women with polycystic ovary syndrome, who have high rates of insulin resistance, anovulation and infertility, diets with a low glycemic load improve menstrual cycle regularity and insulin resistance.51 Moreover, the type of sweetener found in sodas may matter. Most sodas in North America are sweetened with HFCS and have higher fructose to glucose ratios than other sugar-sweetened beverages.52 HFCS has been associated with insulin resistance53 and fatty liver.54 A small cross-over study found altered fatty acid synthesis among men consuming fructose, compared with glucose or sucrose,55 and fatty acid composition may affect semen quality.56 Sugar-sweetened soda, but not diet soda or fruit juice, was linked with shorter telomeres in a cross-sectional study of 5000 individuals.57 Shorter telomeres indicate faster cellular aging58 and have been associated with reduced male fertility59, poorer embryo quality and lower success of in vitro fertilization.60 Exposure to bisphenol A, which is present in lining of soda cans61 and may impair male62 and female63 fertility, is an unlikely explanation of our findings because we saw no material association between diet sodas and fecundability.
In summary, we found an association between female and male consumption of sugar-sweetened beverages and reduced fecundability, which appeared to be driven mainly by sugar-sweetened sodas, the most commonly consumed sugar-sweetened beverages. We also found a large reduction in fecundability among both females and males who consumed more than one sugar-sweetened energy drink per day, but these results were based on small numbers. Given the high levels of sugar-sweetened beverages consumed by reproductive-aged couples in North America, these findings could have important public health implications.
Supplementary Material
Acknowledgments
Sources of funding: This research was supported by NICHD (R21-HD072326, R01-HD086742, R03-HD090315, and T32-HD052458).
We acknowledge the contributions of PRESTO participants and staff. We thank Mr. Michael Bairos for technical support in developing the study’s web-based infrastructure and Drs. Amy Subar and Ken Bishop for assistance with the National Cancer Institute Food Frequency Questionnaire.
Footnotes
The authors declare that they have no conflict of interest. The computing code and de-identified data are available by contacting the first or last author.
References
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–1331. e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Hum Reprod. 1991;6(6):811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J American Heart Association Nutrition Committee of the Council on Nutrition PA, Metabolism, the Council on E, Prevention. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–20. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 4.Han E, Powell LM. Consumption patterns of sugar-sweetened beverages in the United States. J Acad Nutr Diet. 2013;113(1):43–53. doi: 10.1016/j.jand.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 6.Barlow P, McKee M, Basu S, Stuckler D. Impact of the North American Free Trade Agreement on high-fructose corn syrup supply in Canada: a natural experiment using synthetic control methods. CMAJ. 2017;189(26):E881–E887. doi: 10.1503/cmaj.161152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisbois TD, Marsden SL, Anderson GH, Sievenpiper JL. Estimated intakes and sources of total and added sugars in the Canadian diet. Nutrients. 2014;6(5):1899–912. doi: 10.3390/nu6051899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97(4):667–75. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–88. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carwile JL, Willett WC, Spiegelman D, Hertzmark E, Rich-Edwards J, Frazier AL, Michels KB. Sugar-sweetened beverage consumption and age at menarche in a prospective study of US girls. Hum Reprod. 2015;30(3):675–83. doi: 10.1093/humrep/deu349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schliep KC, Schisterman EF, Mumford SL, Pollack AZ, Perkins NJ, Ye A, Zhang CJ, Stanford JB, Porucznik CA, Hammoud AO, Wactawski-Wende J. Energy-containing beverages: reproductive hormones and ovarian function in the BioCycle Study. Am J Clin Nutr. 2013;97(3):621–30. doi: 10.3945/ajcn.111.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010;100(1):47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur J Clin Nutr. 2009;63(1):78–86. doi: 10.1038/sj.ejcn.1602904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox AJ, Weinberg CR. Tea and fertility. Lancet. 1991;337(8750):1159–60. doi: 10.1016/0140-6736(91)92825-m. [DOI] [PubMed] [Google Scholar]
- 15.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Caffeinated and alcoholic beverage intake in relation to ovulatory disorder infertility. Epidemiology. 2009;20(3):374–81. doi: 10.1097/EDE.0b013e31819d68cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatch EE, Wise LA, Mikkelsen EM, Huybrechts KF, Riis A, Rothman KJ, Sorensen HT. Caffeine Intake and Time to Pregnancy in a Prospective Study of Danish Women. American Journal of Epidemiology. 2009;169:S22–S22. [Google Scholar]
- 17.Wesselink AK, Wise LA, Rothman KJ, Hahn KA, Mikkelsen EM, Mahalingaiah S, Hatch EE. Caffeine and caffeinated beverage consumption and fecundability in a preconception cohort. Reproductive Toxicology. 2016;62:39–45. doi: 10.1016/j.reprotox.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machtinger R, Gaskins AJ, Mansur A, Adir M, Racowsky C, Baccarelli AA, Hauser R, Chavarro JE. Association between preconception maternal beverage intake and in vitro fertilization outcomes. Fertil Steril. 2017;108(6):1026–1033. doi: 10.1016/j.fertnstert.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Mendiola J, Jorgensen N, Swan SH, Chavarro JE. Sugar-sweetened beverage intake in relation to semen quality and reproductive hormone levels in young men. Hum Reprod. 2014;29(7):1575–84. doi: 10.1093/humrep/deu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen TK, Swan SH, Skakkebaek NE, Rasmussen S, Jorgensen N. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol. 2010;171(8):883–91. doi: 10.1093/aje/kwq007. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Chen Q, Zhou N, Sun L, Bao H, Tan L, Chen H, Zhang G, Ling X, Huang L, Li L, Ma M, Yang H, Wang X, Zou P, Peng K, Liu K, Liu T, Cui Z, Liu J, Ao L, Zhou Z, Cao J. Lifestyles Associated With Human Semen Quality: Results From MARHCS Cohort Study in Chongqing, China. Medicine (Baltimore) 2015;94(28):e1166. doi: 10.1097/MD.0000000000001166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney A, Lynch CD, Kim S, Maisog JM, Gore-Langton R, Eisenberg ML, Chen Z. Semen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertil Steril. 2014;101(2):453–62. doi: 10.1016/j.fertnstert.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, Gruschow SM, Horgan CE, Wiley AS, Hahn KA, Sorensen HT, Hatch EE. Design and Conduct of an Internet-Based Preconception Cohort Study in North America: Pregnancy Study Online. Paediatr Perinat Epidemiol. 2015;29(4):360–71. doi: 10.1111/ppe.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative Validation of the Block, Willett, and National Cancer Institute Food Frequency Questionnaires : The Eating at America’s Table Study. American Journal of Epidemiology. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 25.Wise LA, Wesselink AK, Mikkelsen EM, Cueto H, Hahn KA, Rothman KJ, Tucker KL, Sorensen HT, Hatch EE. Dairy intake and fecundability in 2 preconception cohort studies. Am J Clin Nutr. 2017;105(1):100–110. doi: 10.3945/ajcn.116.138404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise LA, Mikkelsen EM, Rothman KJ, Riis AH, Sorensen HT, Huybrechts KF, Hatch EE. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol. 2011;174(6):701–9. doi: 10.1093/aje/kwr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983:385–396. [PubMed] [Google Scholar]
- 28.Bech P. Quality of life instruments in depression. Eur Psychiatry. 1997;12(4):194–8. doi: 10.1016/S0924-9338(97)89104-3. [DOI] [PubMed] [Google Scholar]
- 29.Olsen LR, Jensen DV, Noerholm V, Martiny K, Bech P. The internal and external validity of the Major Depression Inventory in measuring severity of depressive states. Psychol Med. 2003;33:351–356. doi: 10.1017/s0033291702006724. [DOI] [PubMed] [Google Scholar]
- 30.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 31.McKinnon CJ, Hatch EE, Rothman KJ, Mikkelsen EM, Wesselink AK, Hahn KA, Wise LA. Body mass index, physical activity and fecundability in a North American preconception cohort study. Fertil Steril. 2016;106(2):451–9. doi: 10.1016/j.fertnstert.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, Casavale KO, Carroll RJ. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014;144(3):399–407. doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 34.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013;27(5):491–502. doi: 10.1111/ppe.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Therneau TMGP. Modeling Survival Data: Extending the Cox Model. New York: Springer-Verlag; 2000. [Google Scholar]
- 36.Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129(5):1072–8. doi: 10.1093/oxfordjournals.aje.a115211. [DOI] [PubMed] [Google Scholar]
- 37.Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20(9–10):1541–9. doi: 10.1002/sim.689. [DOI] [PubMed] [Google Scholar]
- 38.Institute S. SAS/STAT 9.4 User’s Guide. Cary, NC: SAS Institute; 2014. [Google Scholar]
- 39.Knol MJ, VanderWeele TJ, Groenwold RH, Klungel OH, Rovers MM, Grobbee DE. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26(6):433–8. doi: 10.1007/s10654-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wesselink AK, Wise LA, Rothman KJ, Hahn KA, Mikkelsen EM, Mahalingaiah S, Hatch EE. Caffeine and caffeinated beverage consumption and fecundability in a preconception cohort. Reprod Toxicol. 2016;62:39–45. doi: 10.1016/j.reprotox.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtis KM, Savitz DA, Arbuckle TE. Effects of cigarette smoking, caffeine consumption, and alcohol intake on fecundability. Am J Epidemiol. 1997;146(1):32–41. doi: 10.1093/oxfordjournals.aje.a009189. [DOI] [PubMed] [Google Scholar]
- 42.Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Caffeine intake and fecundability: a follow-up study among 430 Danish couples planning their first pregnancy. Reprod Toxicol. 1998;12(3):289–95. doi: 10.1016/s0890-6238(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 43.Ricci E, Vigano P, Cipriani S, Somigliana E, Chiaffarino F, Bulfoni A, Parazzini F. Coffee and caffeine intake and male infertility: a systematic review. Nutr J. 2017;16(1):37. doi: 10.1186/s12937-017-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol. 2013;42(4):1012–4. doi: 10.1093/ije/dys223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatch EE, Hahn KA, Wise LA, Mikkelsen EM, Kumar R, Fox MP, Brooks DR, Riis AH, Sorensen HT, Rothman KJ. Evaluation of Selection Bias in an Internet-based Study of Pregnancy Planners. Epidemiology. 2016;27(1):98–104. doi: 10.1097/EDE.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006;17(4):413–8. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
- 47.Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 48.Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, Jacobs DR., Jr Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32(7):1302–7. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32(1):1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 51.Moran LJ, Ko H, Misso M, Marsh K, Noakes M, Talbot M, Frearson M, Thondan M, Stepto N, Teede HJ. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. Hum Reprod Update. 2013;19(5):432. doi: 10.1093/humupd/dmt015. [DOI] [PubMed] [Google Scholar]
- 52.Walker RW, Dumke KA, Goran MI. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition. 2014;30(7–8):928–35. doi: 10.1016/j.nut.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–22. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 54.Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;299(5):E685–94. doi: 10.1152/ajpendo.00283.2010. [DOI] [PubMed] [Google Scholar]
- 55.Hochuli M, Aeberli I, Weiss A, Hersberger M, Troxler H, Gerber PA, Spinas GA, Berneis K. Sugar-sweetened beverages with moderate amounts of fructose, but not sucrose, induce Fatty Acid synthesis in healthy young men: a randomized crossover study. J Clin Endocrinol Metab. 2014;99(6):2164–72. doi: 10.1210/jc.2013-3856. [DOI] [PubMed] [Google Scholar]
- 56.Esmaeili V, Shahverdi AH, Moghadasian MH, Alizadeh AR. Dietary fatty acids affect semen quality: a review. Andrology. 2015;3(3):450–61. doi: 10.1111/andr.12024. [DOI] [PubMed] [Google Scholar]
- 57.Leung CW, Laraia BA, Needham BL, Rehkopf DH, Adler NE, Lin J, Blackburn EH, Epel ES. Soda and cell aging: associations between sugar-sweetened beverage consumption and leukocyte telomere length in healthy adults from the National Health and Nutrition Examination Surveys. Am J Public Health. 2014;104(12):2425–31. doi: 10.2105/AJPH.2014.302151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revesz D, Milaneschi Y, Verhoeven JE, Penninx BW. Telomere length as a marker of cellular aging is associated with prevalence and progression of metabolic syndrome. J Clin Endocrinol Metab. 2014;99(12):4607–15. doi: 10.1210/jc.2014-1851. [DOI] [PubMed] [Google Scholar]
- 59.Ferlin A, Rampazzo E, Rocca MS, Keppel S, Frigo AC, De Rossi A, Foresta C. In young men sperm telomere length is related to sperm number and parental age. Hum Reprod. 2013;28(12):3370–6. doi: 10.1093/humrep/det392. [DOI] [PubMed] [Google Scholar]
- 60.Kalmbach KH, Fontes Antunes DM, Dracxler RC, Knier TW, Seth-Smith ML, Wang F, Liu L, Keefe DL. Telomeres and human reproduction. Fertil Steril. 2013;99(1):23–9. doi: 10.1016/j.fertnstert.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao XL, Corriveau J, Popovic S. Levels of bisphenol A in canned soft drink products in Canadian markets. J Agric Food Chem. 2009;57(4):1307–11. doi: 10.1021/jf803213g. [DOI] [PubMed] [Google Scholar]
- 62.Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Joensen UN, Main KM, Skakkebaek NE, Juul A, Jorgensen N, Andersson AM. Urinary bisphenol A levels in young men: association with reproductive hormones and semen quality. Environ Health Perspect. 2014;122(5):478–84. doi: 10.1289/ehp.1307309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caserta D, Di Segni N, Mallozzi M, Giovanale V, Mantovani A, Marci R, Moscarini M. Bisphenol A and the female reproductive tract: an overview of recent laboratory evidence and epidemiological studies. Reprod Biol Endocrinol. 2014;12:37. doi: 10.1186/1477-7827-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.