Abstract
G-protein coupled receptor (GPCR) heterodimerization has emerged as a means by which alternative signaling entities can be created; yet, how receptor heterodimers affect receptor pharmacology remains unknown. Previous observations suggested a biochemical antagonism between GPCRs, CXCR4 and CB2 (CNR2), where agonist-bound CXCR4 and agonist-bound CB2 formed a physiologically non-functional heterodimer on the membrane of cancer cells, inhibiting their metastatic potential in vitro. However, the reduced signaling entities responsible for the observed functional outputs remain elusive. This study now delineates the signaling mechanism whereby heterodimeric association between CXCR4 and CB2, induced by simultaneous agonist treatment, results in decreased CXCR4-mediated cell migration, invasion and adhesion through inhibition of the Gα13/RhoA signaling axis. Activation of CXCR4 by its cognate ligand, SDF1α, stimulates Gα13 (GNA13), and subsequently, the small GTPase RhoA which is required for directional cell migration and the metastatic potential of cancer cell. These studies in prostate cancer cells demonstrate decreased protein expression levels of Gα13 and RhoA upon simultaneous CXCR4/CB2 agonist stimulation. Furthermore, the agonist-induced heterodimer abrogated RhoA-mediated cytoskeletal rearrangement resulting in the attenuation of cell migration and invasion of an endothelial cell barrier. Lastly, a reduction was observed in the expression of integrin α5 (ITGA5) upon heterodimerization, supported by decreased cell adhesion to extracellular matrices in vitro. Taken together, the data identifies a novel pharmacological mechanism for the modulation of tumor cell migration and invasion in the context of metastatic disease.
Keywords: Ras homolog gene family member A (RhoA), G protein alpha-13 (Gα13), C-X-C chemokine receptor type 4 (CXCR-4), cannabinoid receptor type 2, migration
Introduction
G protein coupled receptors (GPCRs) represent a large family of cell surface signaling proteins that are involved in multiple physiological functions and diseases (1,2). In classical GPCR signaling, the binding of an agonist to a GPCR induces a conformational change of the receptor that catalyzes the exchange of guanosine diphosphate (GDP) from the Gα subunit for guanosine triphosphate (GTP), and the dissociation of Gα from Gβ subunits (2). Both Gα and Gβ subunit complexes stimulate several downstream effectors leading to diverse physiological outcomes (2,3). While the conventional theory of GPCR signaling has been that GPCRs can exist and function as monomers, homodimers or as larger oligomeric complexes, a number of GPCRs have been identified to form functionally relevant heterodimers after translation (4–7). GPCR heterodimerization has slowly been accepted as a means by which new signaling entities can be created to amplify or desensitize signaling mechanisms that would otherwise result from each individual receptor leading to diverse pharmacological outcomes (4). Therefore, GPCR heterodimers have emerged as novel targets for therapeutic development (3,4,7,8).
CXCR4 is a chemokine receptor, sub-class of GPCR, that is defined by its ability to induce directional migration of cells towards a chemotactic gradient (chemotaxis) (9–11). The role of CXCR4, and its natural ligand CXCL12, has expanded beyond leukocyte recruitment to include critical processes such as tissue remodeling, angiogenesis, hematopoiesis, cell proliferation, and most notably, cell migration (10,12). Its ability to regulate multiple functions over the lifespan of neoplastic and malignant cells makes CXCR4 a critical driver of tumorigenesis, the progression of cancer, and metastasis (9,10,12,13). Clinically, overexpression of CXCR4 correlates with increased tumor growth, invasion, angiogenesis, metastasis and therapeutic resistance while CXCR4 antagonism has been shown to sensitize cancer cells to cytotoxic drugs, reduce tumor growth and metastatic burden (10,14), thus making CXCR4 an attractive target for early and late-stage disease management.
The cannabinoids system consists of lipophilic cannabinoids (endocannabinoids and exocannabinoids), cannabinoid receptors (CB1 or CB2) and their enzymes for synthesis and degradation (15–18). Delta-9-tetrahydrocannabinol (Δ9-THC), the major psychoactive and immunomodulatory component of marijuana, is the most notable cannabinoid (18,19), and clinically, is used for its anti-palliative effects. However, emergent data demonstrates that cannabinoids possess anti-proliferative capabilities and regulate key cell signaling pathways that promote cell survival, invasion, angiogenesis and metastasis in various cancer models, propelling cannabinoids to the forefront of cancer therapeutics research (3,20).
Allosteric interactions within chemokine receptor heterodimers inhibit receptor activation in some instances, while other receptor pairings cause enhanced signaling and trigger entirely new responses downstream of receptor activation (3,21,22). For example, Décaillot et al. demonstrated that CXC chemokine receptors CXCR4 and CXCR7 form heterodimers that enhance cell migration in response to CXCL12 through recruitment of β-arrestin to the heterodimeric complex. Additionally, extensive evidence points to heterodimerization between chemokine receptors with non-chemokine GPCRs or non-GPCR receptors (21,22). In cancer, we (3) and our colleagues (5,6) demonstrated that CXCR4-mediated functions were abrogated by simultaneous, agonist induced heterodimerization of CXCR4 with GPCRs, such as we’ve demonstrated with CB2 (3), whereby heterodimers reduced cellular migration and the metastatic potential of tumor cells. However, the underlying signaling mechanisms governing the observed reduction in cellular migration was not elucidate.
The Rho-family of GTPases are small GTP-binding proteins that function as molecular switches controlling a wide spectrum of signal transduction pathways in eukaryotic cells (23–26). They regulate actin cytoskeleton dynamics, and modulate cell polarity, microtubule dynamics, membrane transport pathways and transcriptional activity (8,21,23–25). Expression of the GTPase RhoA is implicated in increased tumor aggressiveness in multiple cancer types, and loss of RhoA prevents proliferation and cellular migration both in vitro and metastasis in vivo (23). Furthermore, the CXCL12/CXCR4 signaling axis stimulates RhoA activation through Gα13, leading to phosphorylation of myosin light chain (MLC) by Rho-associated protein kinase (ROCK), which is required for directional cell migration, the promotion of invasive protrusions, and remodeling of the extracellular matrix (26–28).
We tested whether a physical heterodimeric association between CXCR4 and CB2 results in decreased CXCR4-mediated cell migration via reduction of the Gα13-RhoA signaling axis. First, we analyzed RhoA activation upon heterodimerization, and expression of downstream targets of RhoA signaling. Second, we investigated alterations to cellular invasion, adhesion and morphology as a result of reduced RhoA expression upon CXCR4/CB2 heterodimerization. Finally, to correlate the relationship between the effects of CXCR4 heterodimerization, reduced RhoA expression and the migratory ability of cancer cells, we for expression of metastatic markers. We observed that CXCR4/CB2 heterodimerization antagonized expression of Gα13, followed by reduced activity of RhoA and further downstream targets. The resultant signaling blockade interfered with cancer cells developing a metastatic phenotypic and functionally migrating through an endothelial cell layer in a RhoA-dependent manner. Altogether, our results further supports agonist-induced CXCR4/CB2 heterodimerization as an emerging approach to inhibiting the metastatic spread of cancer.
Materials and Methods
Cell lines and reagents
Human metastatic prostate cancer cell line (PC3), human metastatic breast cancer cell line (MDA-MB-231) and human embryonic kidney cells (HEK 293T) were purchased from American Type Culture Collection (ATCC). PC3 and MDA-MB-231 cells were grown in RPMI 1640 and HEK239T cells were grown in DMEM-F-12; all lines were supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids and 1% antibiotic/antimycotic in a humidified incubator (5% CO2) at 37°C. Human umbilical vein endothelial cells (HUVEC) were purchased from American Type Culture Collection (ATCC) and maintained in Endothelial Growth Medium-2 (Lonza; 2% FBS, human endothelial growth factor (hEGF), hydrocortisone, GA-1000 (gentamicin, amphotericin-B), human fibroblast growth factor-B (hFGF-B), R3-IGF-1, ascorbic acid and VEGF) in a humidified incubator (5% CO2) at 37°C. Cell lines were authenticated using a short tandem repeat (STR) DNA profiling by ATTC at time of purchase, and every 6 months thereafter. Mycoplasma was monitored using the MycoAlert Detection Kit (Lonza). Human CXCR4 agonist, CXCL12 (100ng/ml working conc.), was purchased from PeproTech, Inc. Human AM1241 ligand (CB2 agonist; 1μM) was purchased from Cayman Chemicals. The CXCR4 antagonist, AMD3100 (1μ/ml) and Rho-associated kinase inhibitor (ROCK), Y-27631 (10μM) were purchased from Sigma-Aldrich. Human CXCR4-siRNA and control-siRNA (60nM) was from Santa Cruz Biotechnology. Prior to treatment with agonists and antagonists, cells were serum-starved for 24 hours in media only (0% FBS, 0% non-essential amino acids and 0% antibiotic/antimycotic) for 24 hours in 5% CO2 at 37°C. Samples denoted as “control” or “untreated” received fresh media supplemented with corresponding vehicles (e.g. 0.1 %PBS/BSA, DMSO, etc.) for each agonist or antagonist.
RhoA activity assay
Activated RhoA was detected using a RhoA Activity Assay Kit (Cell Bio Labs, Inc.) per the manufacturer’s instructions. Briefly, 1.5×106 cells were cultured to 80% confluence then serum-starved for 24 hours. Cells were pretreated with antagonists for 1 hour prior to stimulation with various agonists for 1 minute 5% CO2 at 37°C. Cells were harvested as described by the kit, and 1mg of protein from each sample was immunoprecipitated with Rhotekin-RBD beads at 4°C overnight with gentle agitation. The beads were pelleted, washed thrice, and boiled in Laemmeli sample buffer for 5 minutes. Samples were separated by 15% SDS-PAGE, transferred to a PVDF membrane and blocked in 3% bovine serum albumin (BSA) in 1X TBST. Rho-A was detected by anti-RhoA antibody (1:100) in 3% BSA/TBST overnight at 4°C and harvested for Western blot analysis. RhoA activity was normalized to total RhoA protein expression via Western blot.
Short interfering RNA transfection
Transient transfection of CXCR4 specific human siRNA (Santa Cruz Biotechnology; sc-35421) was performed on PC3 cells using Lipofectamine 2000 (Invitrogen). Cells (1×106) were plated in 10%FBS/RPMI in 100mm culture dishes and then transfected with 60μM CXCR4-siRNA or scramble/control-siRNA (Santa Cruz Biotechnology) in Opti-Mem at 37ºC/5% CO2 for 12 hours. Cells recovered in 10%FBS/RPMI for 24 hours, followed by treatment with 100ng/mL CXCL12 and/or 1μM AM1241, and then were harvested for immunoprecipitation and/or western blot analysis.
Immunoprecipitation
One million five hundred (1.5×106) cells per sample were serum starved for 24 hours prior to pre-treatment with 1 μg/ml AMD3100 or 10μM Y-27632, followed by treatment with 100ng/ml CXCL12, 1μM AM1241, or CXCL12 and AM1241 simultaneously for 1 (PC3 and HEK 293) or 10 (MDA-MB-231) minutes. Cells were lysed in Rho A lysis buffer from above, sonicated, then followed by incubation on ice for 30 minutes. Lysates were centrifuged at max speed for 10 minutes at 4°C, then 500–750μg of protein from each sample was incubated with Gα13 antibody (1:100; Abcam) overnight at 4°C on a rocking platform. Protein A/G PLUS Agarose Beads (1:10, Santa Cruz Biotechnology) were added to each sample for 2 hours at 4°C with gentle agitation. Cell lysates were washed 2X in 1X PBS (max speed for 5 min at 4°C), boiled in Laemelli sample buffer for 5 mins, followed by 12% SDS-PAGE. Protein was transferred to a PVDF membrane, blocked in 5%milk/TBST, immunoblotted in 5%BSA/TBST with an anti-RhoA (1:1000; Cell Signaling). Primary antibodies were detected with corresponding HRP-conjugated secondary antibodies (1:1000; Jackson) and enhanced chemiluminescence (Luminata; Fisher Scientific).
Antibodies and western blotting
PDZ-RhoGEF and LKB1 antibodies were purchased from Santa Cruz; F-actin was purchased from Abcam; phosphorylated myosin light chain (pMLC), integrin αV and integrin β3 were purchased from Cell Signaling. Two million (2.0 X 106) cells per sample were serum starved for 24 hours prior to pre-treatment with 1 μg/ml AMD3100 or 10 μM Y-27632 followed by treatment with 100ng/ml CXCL12, 1μM AM1241, or CXCL12 and AM1241 simultaneously for various time points. Cells were lysed and sonicated in 1X Cell Signaling lysis buffer prior to incubation on ice for 30 minutes. Lysates were centrifuged at max speed for 10 minutes at 4°C, and then equal amounts of protein per sample were separated by SDS-PAGE and transferred to PVDF membrane. Protein bound membranes were blocked in 3% BSA/1XTBST, (1X TBST for pMLC), and subsequently incubated with primary antibodies: RhoA (1:500, Cell Signaling); F-actin (1:1000, Abcam); pMLC (1:500, Cell Signaling); LKB1 (1:100, Santa Cruz); integrin α5 (1:1000, Cell Signaling); integrin β3 (1:1000, Cell Signaling); total AKT (1:1000, Cell Signaling); or total ERK1/2 (1:1000, Cell Signaling) overnight at 4°C in 3% BSA/TBST; (1X TBST alone for pMLC). Primary antibodies were detected by HRP-conjugated secondary antibodies at 1:1000 diluted in 3% BSA/1X TBST (1X TBST alone for pMLC) and enhanced chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate or Lumniglo).
Filamentous (F)-actin immunocytochemistry
Cells were seeded on glass coverslips at a density of 200,000 cells/well and incubated for 72 hours at 5% CO2 at 37°C until they were approximately 80% confluent. Cells were then serum starved for 24 hours, then pre-treated with 1 μg/ml of AMD3100 or 10 μM of Y-27632 for 1 hour prior to treatment with 100ng/ml CXCL12 or 1μM AM1241 each alone, or combined CXCL12/AM1241 at 5% CO2 and 37°C in serum-free media for 90 minutes. Coverslips were rinsed twice with ice-cold 1X PBS, fixed with 4% paraformaldehyde for 10 minutes and washed again with 1X PBS. Cells were blocked with 1% BSA/TBST and incubated with FITC-conjugated phalloidin (100nM in 1X PBS, Enzo Life Science) to detect F-actin for 1 hour at room temperature with gentle agitation. Cells were then washed thrice with 1X PBS and counterstained with DAPI for nuclear visualization. Slides were visualized and images were captured using a Zeiss LSM 700 confocal microscope. Scale bar=50 μM.
Cell migration
MDA-MB-231 cells (1×106 per sample) were plated in p-100 tissue culture plates and serum-starved for 24 hours. Cells were detached with 1X Citric Saline, washed in 1X PBS, pelleted by centrifugation at 3000xg for 5 mins, and then counted to prepare 50,000 cells per sample in 150μl of media. Transwell inserts were prepared by pre-coating the outer membrane with 50μl rat tail collagen (50μg/ml in 0.02N acetic acid; BD Biosciences) for cell adherence to the transwell insert, and incubating at 4ºC overnight. The next day, the inner membrane was coated as above, and incubated for 1 hour at room temperature. Inserts were washed thrice in 1X PBS prior to use for assay. Treatment (100 ng/ml CXCL12 and 1μM AM1241 each or in combination, 1μg/ml AMD3100 or 10μM Y-27632) were calculated for a final concentration of 500μl each, but prepared in 350μl per sample, and placed into the bottom well of each chamber. Fifty thousand cells per sample were resuspended in 150μl of media and were seeded into the transwell insert of 24-well transmigration chambers (8μm pore; BD Falcon), bringing the total volume of each chamber to 500μl. To allow for migration, plates were incubated in 5% CO2 and 37°C for 6 hours, and the inner-upper chambers were cleaned with a cotton swab. Cells attached to the outer-upper chambers were fixed with 10% formalin for 15 minutes at room temperature, washed thrice in 1X PBS, stained with 0.05% crystal violet for 15 minutes, then washed 6 times in water. Five representative fields of each insert were counted under a light microscope, and the migration index was calculated and graphed as the x-fold change in migration observed over control cells or CXCL12-treated cells. Experiments were performed at least thrice, and significant significance was analyzed via GraphPad Prism (*p<0.05 and **p<0.001).
Transendothelial migration assay
Migration though an endothelial cell barrier was performed via a Transwell Migration Assay Kit (Cell Bio Labs, Inc.) per the manufacturer’s instructions. Briefly, 1×105 HUVECS per well were seeded inside of transwell inserts in 500μl complete EGM-2 media at 5% CO2 and 37°C for 72 hours to allow formation of a cell monolayer. In the interim, 1×106 of PC3, MDA-MB-231 or HEK293T cells were pre-incubated with 1X CytoTracker (kit provided) allowing each cell to become fluorescently labeled, then were pelleted and washed 3X in serum-free media and resuspended in 500μl of serum-free media. EGM-2 media was removed from inside each HUVEC transwell and replaced with 300μl each of PC3, MDA-MB-231 or HEK293T serum-free RPMI cell suspension. Transwell inserts were transferred to fresh plates containing serum-free media supplemented with combinations of 100ng/ml CXCL12, 1μM of AM1241, 1 μg/ml of AMD3100, and/or 10μM Y-27632 and the plate was incubated in 5% CO2 at 37°C for 22 hours. Fluorescently labeled cells that trans-migrated were lysed in 1X Cell Lysis Buffer (Cell Bio Labs, Inc.) and analyzed using a plate reader at OD 480nm/520nm. Experiment was repeated thrice, and statistical significance was analyzed via GraphPad Prism (*p<0.05 and **p<0.001).
Wound healing assay
One million cells per sample were seeded in a 6-well culture plate overnight, and then serum-starved for 24 hours. Cells were pre-treated with 1μg/ml of AMD3100 or 10 μM of Y-27632 for 1 hour, removed, then plates were stored on ice. One milliliter of 1X PBS was added to each well, a vertical wound was made across each well using a micropipette tip, and non-adherent cells were removed by washing with 1X PBS. Serum-free media with supplements of 100ng/ml CXCL12, and 1μM of AM1241 each or in combination, along with 1μg/ml AMD3100, or 10μM Y-27632. Wound closure was allowed for up to 6 hours at 5%CO2 and 37ºC, with pictures captured at 0, 2, 4, and 6 hours. The wound area of each sample was quantified using ImageJ software. Experiments were repeated thrice; statistical significance was analyzed via GraphPad Software (*p<0.05 and **p<0.001).
Extracellular matrix adhesion assay
The adhesion of cells to components of the extracellular matrix (ECM) was quantified using the CytoSelect Adhesion Assay (ECM Array, Colorimetric Format; Cell Bio Lab, Inc.). One million five hundred (1.5×106) cells per sample were seeded and grown to approximately 80% confluence. Cells were serum-starved for 24 hours and then harvested by 0.25% trypsin/EDTA for 5 minutes. Trypsin was removed after centrifugation (2000xg for 5 minutes) and cells were resuspended in serum-free RPMI at a concentration of 1.0×106 cells/ml. The cell suspension was pre-incubated 1μg/ml AMD3100 or 10μM Y-27632 in serum-free media containing for 1 hour in 5% CO2 at 37°C. Thereafter, additional treatments of 100 ng/ml of CXCL12 alone, 1μM of AM1241 each or in combination was added to cell suspension. One hundred fifty microliters of each cell suspension was plated onto a 48-well culture plate pre-coated with various ECM components (fibronectin, collagen I, and laminin I), then incubated for 2 hours in 5% CO2 at 37°C to allow attachment. Cells were washed carefully with 1X PBS to remove unbound cells, and adherent cells were incubated in 200μl of Cell Stain Solution for 10 minutes on an orbital shaker (Cell Bio Labs, Inc). Thereafter, each well was gently washed 4X with 500μl of double deionized water, followed by incubation in 200μl Extraction Solution (Cell Bio Labs, Inc.) for 10 minutes on an orbital shaker. Following extraction, 150μl of each sample was transferred to a 96-well plate and measured using a spectrophotometer at OD 560nm. Each experiment was repeated thrice and statistical significance via GraphPad Prism (*p<0.05 and **p<0.001).
Statistical analysis
Student’s t test, two-way ANOVA or Bonferroni’s Multiple Comparison Test were performed to determine statistically significant differences between groups. Statistical analysis was performed via GraphPad Prism.
Results
Simultaneous treatment with CXCL12 and AM1241 inhibits Gα13/PRG-mediated activation of RhoA
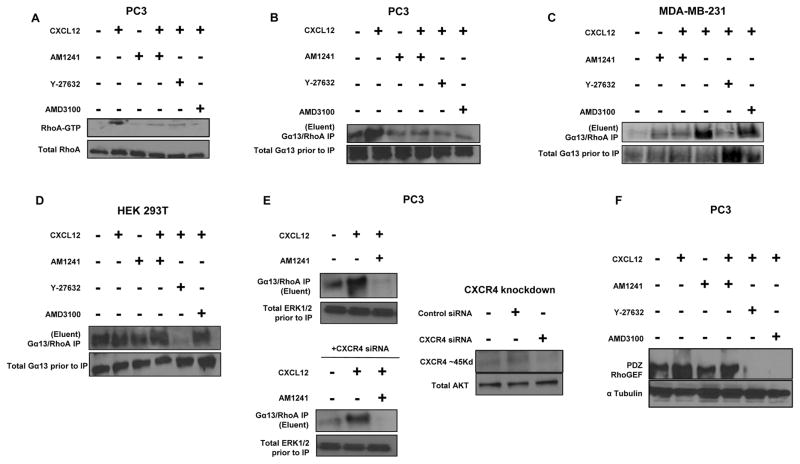
We’ve shown formation of a physical heterodimer between CXCR4 and CB2 upon simultaneous (combined) treatment with agonists CXCL12 and AM1241, respectively (3). This heterodimer between CXCR4 and CB2 impeded cellular migration; however, a mechanism was not defined. Independent studies have demonstrated that CXCR4-mediated migration occurs via the RhoA-PRG-Gα13 pathway (27,28). Therefore, we first investigated whether heterodimerization of CXCR4/CB2 decreased RhoA activation in the malignant human prostate cancer cells (PC3). Isolating active forms (GTP-bound) of RhoA with Rhotekin-glutathione beads demonstrated that CXCL12 caused an increase in expression of RhoA-GTP compared to control (untreated) cells (Fig. 1A). However, simultaneous treatment with CXCL12 and AM1241, which induced the heterodimer (3), resulted in decreased protein expression of RhoA-GTP, indicating a decrease in activation of RhoA compared to CXCL12-stimulated cells (Fig. 1A). The decrease in activated Rho-A expression levels was consistent in cells pretreated with AMD3100 (CXCR4 antagonist) or Y-27632 (RhoA/ROCK inhibitor) prior to treatment with CXCL12 (Fig. 1A).
FIGURE 1. CXCR4/CB2 heterodimerization attenuates Gα13/PRG-mediated RhoA activation in PC3 cells.
(A) Serum-starved PC3 cells were pre-treated for 1 hour with Y-27632 or AMD3100 followed by treatment with CXCL12 for 1 minute. Alternatively, PC3 cells were treated for 1 minute with CXCL12 or AM1241 alone, or CXCL12/AM1241 simultaneously. Lysates were incubated with Rhotekin-RBD beads prior to harvesting for immunoblotting for RhoA-GTP protein expression; total RhoA represents lysate prior to pull-down with Rhotekin-RBD beads. (B) PC3 cells, (C) MDA-MB-231 cells and (D) HEK 293T cells were treated as described in Methods, then lysates were incubated with anti-Gα13 antibody overnight at 4°C followed by standard immunoprecipitation techniques. Isolated protein lysates was immunoblotted for RhoA protein expression; input represents lysate prior to immunoprecipitation. (E) PC3 were transfected with 60μM human siRNA targeting CXCR4 or non-specific control siRNA for 12 hours, followed by recovery in 10%FBS/RPMI for 24 hours. Cells were then harvested for Gα13/RhoA immunoprecipitation or western blotting. Total ERK1/2 and AKT were used as: (i) loading input controls to demonstrate that siRNA was specific; and (ii) to demonstrate that equal amounts of protein were used for assay. (F) Serum-starved PC3 cells were treated as described above, and whole protein lysates were immunoblotted for PRG protein expression; α-tubulin was used as a loading control. Each experiment was performed at least twice. *p<0.05 and **p<0.001
Tan et al. demonstrated that CXCR4-mediated activation of RhoA, and resulting directional cellular migration, was specifically mediated by the small G protein Gα13 (27). Consistent with the pattern for RhoA activity, we observed that CXCL12 led to increased expression Gα13, as determined by expression of RhoA via Gα13/RhoA immune-complexes, compared to untreated cells in PC3 and the human breast cancer cell line, MDA-MB-231 (Figs. 1B and 1C). Conversely, simultaneous stimulation of PC3 or MDA-MB-231 cells with CXCL12 and AM1241 resulted in decreased expression of Gα13, which was consistent with expression levels of Gα13 in cells treated with AMD3100 or Y-27632 (Figs. 1B and 1C).
To support our premise that a dimer between CXCR4 and CB2 inhibits the action of CXCR4 to activate Gα13-RhoA, we used the human embryonic kidney cell line, HEK 293T, as we (3) and others have published is CXCR4-null. Pre-treatment with the RhoA/ROCK inhibitor Y-27632 visibly reduced Gα13/RhoA immune-complexes. However, RhoA protein levels displayed consistent expression among all other treatment groups, with no visible induction by CXCL12 (Fig. 1D); the basal level of Gα13-RhoA immune complexes could have been mediated by any other GPCR. Likewise, we used siRNA targeting CXCR4 to further show that the dimer between CXCR4 and CB2 has similar antagonistic effects as would a knockdown of the CXCR4 receptor (Fig. 1E). In PC3 cells, we observed reduction of Gα13/Rho A immune-complexes in CXCL12-treated cells transfected with CXCR4-siRNA compared to cells without siRNA. However, the reduction in Gα13/Rho A immune-complexes in cells was more robust in cells treated CXCL12 and AM1241 (both with and without siRNA), suggesting that the heterodimer is an efficient way to manage CXCR4 signaling (Fig. 1E).
In breast cancer cells stimulated with CXCL12, Struckhoff et al. demonstrated that PDZ-RhoGEF (PRG) guanine nucleotide exchange factor was required for RhoA-dependent cell migration (28). We, too, observed that CXCL12 increased PRG protein expression levels in PC3 cells (Fig. 1C). Contrastingly, when PC3 cells were treated with CXCL12 and AM1241 simultaneously, expression levels of PRG protein expression decreased compared to that of CXCL12 treatments, further supporting that agonist-induced heterodimerization is an effective mechanism to antagonize CXCR4-mediated signaling that leads to cell migration. As a control, PC3 cells treated with AMD3100 or Y-27632 virtually abolished PRG protein expression in the presence of CXCL12 (Fig. 1F), indicating that the observed results were specifically mediated by CXCR4-RhoA signaling. Collectively, coinciding with our previously observed decrease in cellular migration (3), an induced GPCR heterodimer between CXCR4 and CB2 antagonizes CXCR4-mediated cell migration via the RhoA-Gα13-PRG pathway.
Agonist-induced CXCR4/CB2 heterodimerization inhibits RhoA-mediated cytoskeleton reorganization and attenuates RhoA-dependent cell migration
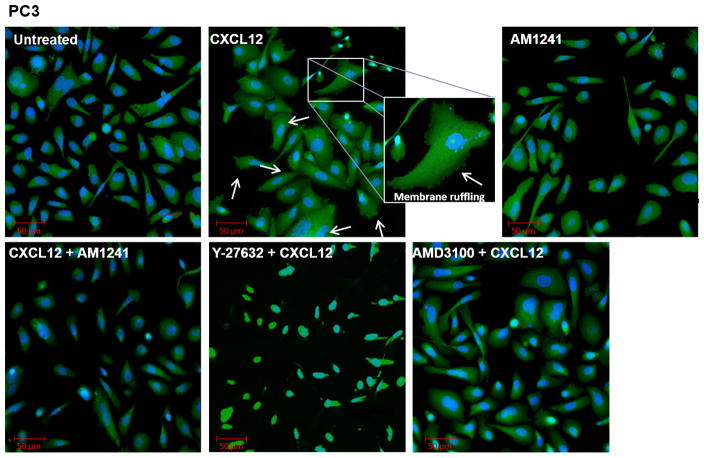
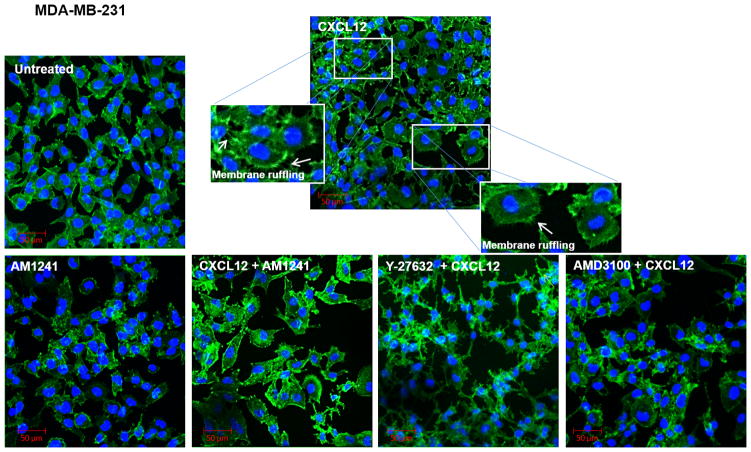
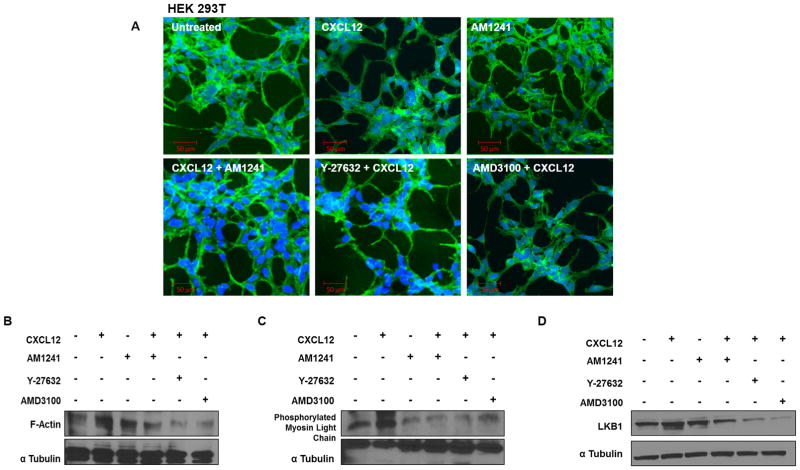
RhoA plays a critical role in cell migration through its intimate relationship with the actin cytoskeleton, which has long been implicated in generating the contractile forces needed for membrane blebbing, lamellae formation, and membrane ruffling at the leading edge and retraction of the trailing edge of migratory cells (29–32). To evaluate whether our induced heterodimer could inhibit RhoA-mediated changes in the actin cytoskeleton that are necessary for migration, we first examined phenotypic changes in filamentous actin (F-actin) in response to independent (CXCL12) and combinatory (CXCL12/AM1241) agonist stimulation in PC3, and MDA-MB-231 cell lines. CXCL12 produced expected pro-migratory alterations in cell morphology such as membrane ruffling, lamellae formation and membrane protrusions in cancer cell lines compared to untreated cells that remained characteristically smooth at the cell membrane (Figs. 2 and 3). However, cells simultaneously stimulated with CXCL12 and AM1241 also exhibited a smooth cell membrane, similar to untreated cells, and indicated little-to-no changes in cytoskeletal rearrangement; this same phenotype was observed for cells pre-treated with AMD3100 (Figs. 2 and 3). Contrastingly, pretreatment with Y-27632 resulted in apoptotic phenotypes with an overall decrease in volume of cell cytoplasm and membrane blebbing in PC3 and MDA-MB-231 cells (Figs. 2 and 3), which is confirmed in literature (33,34). In HEK 293T cells, as expected, we did not observe phenotypic changes in response to CXCL12, AM1241, or the agonist combined (Fig. 4A) as they are CXCR4-null.
FIGURE 2. Agonist-induced CXCR4/CB2 heterodimerization inhibits RhoA-mediated cytoskeleton reorganization.
Serum-starved PC3 cells were pre-treated for 1 hour with Y-27632 or AMD3100 prior to treatment with CXCL12 for 90 minutes. Subsequently, cells were treated for 90 minutes with CXCL12 or AM1241 alone, or CXCL12/AM1241 simultaneously. Samples were fixed and stained with FITC-phalloidin to visualize F-actin; nuclei were counter-stained with DAPI. Arrows highlight areas of lamellae formation and membrane ruffling.
FIGURE 3. Agonist-induced CXCR4/CB2 heterodimerization inhibits RhoA-mediated cytoskeleton reorganization.
Serum-starved MDA-MB-231 cells were pre-treated for 1 hour with Y-27632 or AMD3100 prior to treatment with CXCL12 for 90 minutes. Subsequently, cells were treated for 90 minutes with CXCL12 or AM1241 alone, or CXCL12/AM1241 simultaneously. Samples were fixed and stained with FITC-phalloidin to visualize F-actin; nuclei were counter-stained with DAPI. Arrows highlight areas of lamellae formation and membrane ruffling.
FIGURE 4. Agonist-induced CXCR4/CB2 heterodimerization inhibits RhoA-mediated cytoskeleton reorganization.
(A) Serum-starved HEK 293T cells were pre-treated for 1 hour with Y-27632 or AMD3100 prior to treatment with CXCL12 for 90 minutes. Subsequently, cells were treated for 90 minutes with CXCL12 or AM1241 alone, or CXCL12/AM1241 simultaneously. Samples were fixed and stained with FITC-phalloidin to visualize F-actin; nuclei were counter-stained with DAPI. Arrows highlight areas of lamellae formation and membrane ruffling. (B–D) Serum-starved PC3 cells were pre-treated with Y-27632 or AMD3100 followed by treatment with CXCL12, AM1241 or agonists simultaneously for 90 minutes. Whole protein lysates were analyzed for (B) F-actin, (C) pMLC or (D) LKB1. Each experiment was performed at least twice.
We examined protein expression levels of F-actin to support the phenotypic changes observed above where expression of F-actin in PC3 cells, simultaneously stimulated with CXCL12 and AM1241, was comparable to untreated cells, further supporting that the CXCR4/CB2 heterodimer prevents the pro-migratory phenotype necessary for cellular migration (Fig. 4B). Likewise, AMD3100 and Y-27632 displayed markedly reduced F-actin protein levels (Fig. 4B). RhoA regulates actomyosin contraction in part by stimulating phosphorylation of myosin light chain (p-MLC) (27,28,35) which is also required for phenotypic changes associated with cellular migration. Therefore, we determined whether agonist-induced CXCR4/CB2 heterodimerization led to downstream attenuation of p-MLC expression in PC3 cells. CXCL12 increased expression of p-MLC protein levels compared to the untreated control; however, protein levels of p-MLC in simultaneously treated samples were reduced compared to both untreated control and CXCL12-treated cells (Fig. 4C). Taken together, these results further support that an induced CXCR4/CB2 heterodimer effectively attenuates signaling pathways that leads to a migratory phenotype in cancer.
Failed directional migration is usually a consequence of loss of cell polarity as marked by decreased expression of human tumor suppressor and serine-threonine kinase LKB1 (29). Therefore, we harvested treated samples and immunoblotted for LKB1, an established regulator of RhoA-dependent cell polarity (30,31). Simultaneous stimulation of PC3 cells with CXCL12 and AM1241 decreased protein expression levels of LKB1 compared to untreated control and CXCL12, as did treatment with Y-27632 and AMD3100 (Fig. 4D), indicative of reduced polarity for migration.
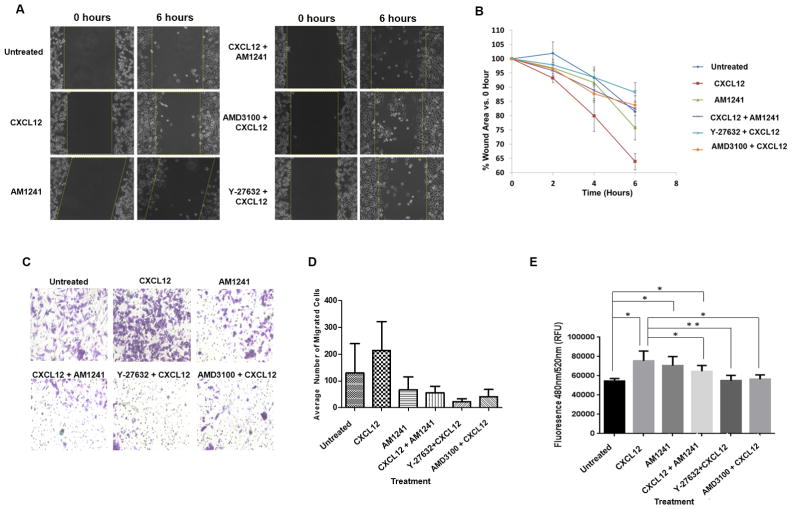
To attribute the reduction in RhoA-mediated signaling, as a result of heterodimerization, to migratory behavior and events (Figs. 5A and B), we observed that CXCL12 significantly stimulated wound closure of PC3 cells compared to untreated cells (Fig. 5B, p=0.0462). CXCL12 and AM1241 combined were deficient in stimulating migration across the wound area when compared to CXCL12 alone (Fig. 5A and B, p=0.042). Likewise, pre-treatment of cells with AMD3100 (p=0.0099) or Y-27632 decreased wound closure efficiency compared to CXCL12, lending more credence that GPCR heterodimerization attenuates RhoA signaling associated with cancer-progressing activities.
FIGURE 5. CXCR4/CB2 heterodimerization attenuates RhoA-dependent cell migration and wound closure.
(A–B) Serum-starved PC3 cells were pre-treated for 1 hour with Y-27632 or AMD3100 followed by treatment with CXCL12 and/or AM1241 for up to 6 hours as described in Methods. ImageJ was used to measure the width of each wound at 0 hour and 6 hour. Experiments were performed thrice. *p<0.05 and **p<0.001. (C–D) 5x104 cells each were seeded into the upper transwell chamber and allowed to migrate towards combinations of agonists and chemical inhibitors in the lower chamber for 6 hours at 37ºC, 5% CO2. Five fields of each transwell insert were randomly selected and counted for migrated cells at 10X magnification using a Zeiss Axiovert 200M light microscope, and results were analyzed via GraphPad Prism. Experiments were repeated thrice, and data represents the average of three independent experiments. (D) A graphical representation of total migrated cells. Data represents the average of 3 independent experiments; *p<0.05 and **p<0.001. (E) HUVECs were seeded in the upper chamber of transwell inserts allowing for the formation of a confluent monolayer. Fluorescently-labeled, serum-starved PC3 cells were added on top of HUVECs in the upper chamber, then combinations of agonists and chemical inhibitors were added to the lower (bottom) chambers followed by a 24 hour incubation. Fluorescent invasive cells were quantified at OD 480 nm/520 nm. Experiments were performed thrice *p<0.05 and **p<0.001.
Via simple transwell migration, CXCL12-treated MDA-MB-231 cells exhibited significantly increased migration compared to untreated migrating cells (p=0.043) (Figs. 5C and D). Likewise, CXCL12 and AM1241 combined exhibited significantly decreased migratory capacity compared to CXCL12 alone (p<0.0001) (Figs. 5C and D). Additionally, both Y-27632 (p<0.0001) and AMD3100 (p<0.0001) significantly reduced transmigration when compared to cells migrating towards CXCL12 alone (Figs. 5C and D). To effectively metastasize from a primary tumor site to a distal organs, aggressive cancer cells must demolish and migrate through an endothelial cell barrier, which is characteristic of intravasation and extravasation in vivo (25,32), and CXCL12-driven transendothelial migration of metastatic tumor cells has been shown to be Rho-dependent (20,33,34). As such, we observed that CXCL12 resulted in significantly increased transmigration compared to untreated transmigrating cells (p=0.0296) (Fig. 5E). Likewise, CXCL12 and AM1241 combined exhibited significantly decreased transendothelial migratory capacity compared to CXCL12 alone (p=0.0476) (Fig. 5E). Additionally, both Y-27632 (p=0.0054) and AMD3100 (p=0.0187) significantly reduced transmigration when compared to cells migrating towards CXCL12 alone (Fig. 5E).
Inhibition of CXCR4-mediated signaling via heterodimerization results in decreased integrin expression and RhoA-mediated adhesion to the extracellular matrix
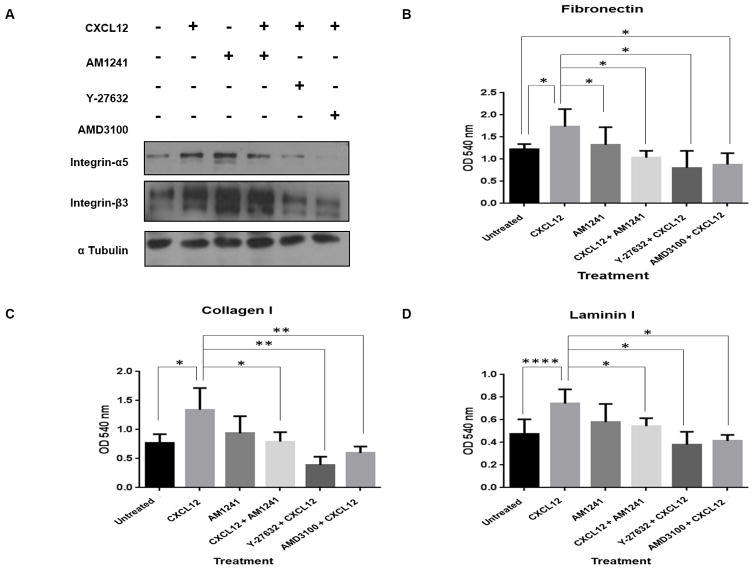
Rho GTPase molecules regulate cell adhesion (36,37) further implicating its role in tumor cell invasion and migration (26). Multiple studies report that CXCR4 signaling leads to enhanced expression of α5 integrin and β3 integrin, which trigger persistent adhesion of tumor cells to immobilized laminin, collagen and fibronectin (38). In PC3 cells, CXCL12 alone stimulated increased expression levels of α5 integrin compared to the untreated control, while AM1241 in combination with CXCL12 reduced expression (Fig. 6A). Although we observed an increase in β3 expression upon CXCL12 treatment, which aligns with observations in literature, heterodimerization of CXCR4 with CB2 caused no significantly observable reduction of β3 protein levels when compared to CXCL12; chemical inhibition with AMD3100 and Y-27632 resulted in reduced levels of both α5 integrin and β3 integrin (Fig. 6A).
FIGURE 6. Inhibition of CXCR4-mediated signaling via heterodimerization results in decreased integrin expression and RhoA-mediated adhesion to the extracellular matrix.
(A) Serum-starved PC3 cells were pre-treated for 1 hour with Y-27632 or AMD3100 followed by treatment with CXCL12 or AM1241 alone, or CXCL12/AM1241 simultaneously for 6 hours. Cell lysates were harvested for integrin α5 and integrin β3 protein expression; α-tubulin was used as a loading control. Experiment was performed at least twice. (B–D) Cells were serum-starved and pre-treated Y-27632 and AMD3100 for 1 hour prior to the addition of CXCL12 or AM1241 alone, or CXCL12/AM1241 simultaneously on matrix-coated plates for 2 hours, stained with Cell Stain Solution (Cell Bio Labs, Inc.), and then analyzed at OD 560nm. Each assay was performed at least thrice. *p<0.05 and **p<0.001.
Finally, we confirmed whether CXCR4/CB2 heterodimerization regulates adherence to components of the extracellular matrix (ECM). Using in vitro models of ECM components, we observed that CXCL12 led to a significant increase in adhesion to fibronectin (p=0.0484), collagen I (p=0.0287) and laminin I (p<0.0001) in PC3 cells when compared to untreated cells (Figs. 6B–6D). CXCL12 and AM1241 combined significantly reduced cellular adhesion to fibronectin (p=0.0171), collagen I (p=0.0354), and laminin I (p=0.0317) compared to CXCL12 alone; likewise, Y-27632 (fibronectin; p=0.0154), (collagen I; p=0.0031), and (laminin I; p=0.005), and AMD3100 (fibronectin; p=0.0322), (collagen I; p=0.0086) and (laminin I; p=0.0264), both yielded reduced adhesion of cells to ECM components (Figs. 6B–6D). These results suggest that a physical CXCR4/CB2 heterodimer is sufficient to decrease tumor cell adhesion, likely due to decreased expression of integrin alpha and subsequent inhibition of RhoA activation.
Discussion
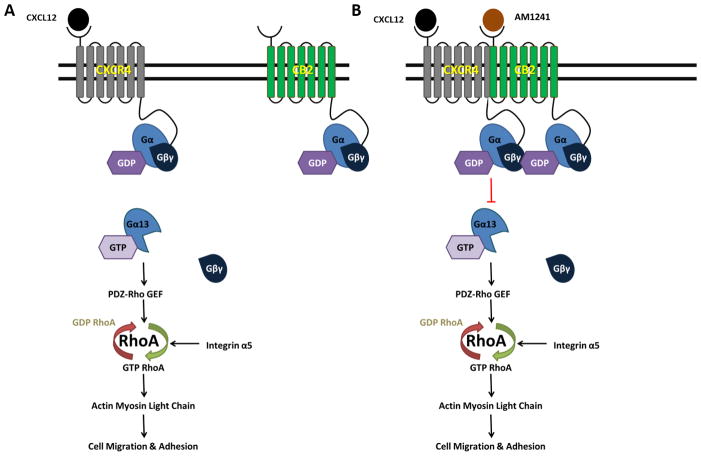
Our results provide mechanistic insight into our previous observation that a physical CXCR4/CB2 heterodimer reduces cell migration via antagonism of the Rho A pathway (Figs. 7A and 7B). CXCL12 binds to CXCR4 and activates RhoA specifically through activation of the small G protein, Gα13 (27), which leads to the subsequent activation of PRG, and is required for directional cell migration (35) (Fig. 7A). Once activated, RhoA induces changes in the actin cytoskeleton through phosphorylation of myosin light chain (26,27), increased F-actin protein levels and changes in integrin expression (39–41). Molecular signaling events initiated by the CXCL12/CXCR4 cascade enhances cellular functionality by increasing cell migration and adhesion, ultimately contributing to tumor progression (Fig. 7A). We demonstrated that the physical heterodimer between CXCR4/CB2 reduced Gα13 recruitment of PRG and subsequent RhoA activation (Fig. 7B). We also demonstrated that reduced migration, adhesion, and other coordinating events that encourage tumor cell migration was specifically attributed to the RhoA pathway as reduction upon heterodimerization was parallel to treatment with RhoA/ROCK inhibitor, Y-27632.
FIGURE 7. Simultaneous agonist-induced CXCR4/CB2 heterodimerization inhibits RhoA-mediated cell migration and adhesion.
(A) The binding of CXCL12 to its cognate receptor, CXCR4 initiates the activation of Gα13 and its dissociation from Gβγ subunits. Gα13 directly activates the DBl family RhoGEF, PDZ-RhoGEF (PRG), which subsequently activates the small GTPase, RhoA. The downstream target of RhoA, Rho-associated protein kinase (ROCK), phosphorylates myosin light chain (MLC) leading to directional cell migration, the formation of invasive protrusions including membrane ruffling, and increased adhesion to the extracellular matrix. Integrin signaling through an alternate pathway can modulate RhoA activity through activation of focal adhesion kinase 1 (FAK1) which activates RhoA through Ras protein-specific guanine nucleotide-releasing factor 1 (RASGRF1). (B) Agonist-induced heterodimerization of CXCR4 and CB2 inhibits Gα13 activation and dissociation from Gβγ subunits. As a result, PRG ineffectively activates RhoA, which in turn, fails to mediate phosphorylation of myosin light chain leading to abrogation of cell migration, invasion and adhesion. Signaling through alternate pathways, including integrin-mediated signaling, is insufficient to overcome inhibition caused by heterodimerization.
RhoGTPases are key regulators of cytoskeletal dynamics that lead to cell adhesion, polarity, and directional movement. Across several studies, CXCR4 was shown to induce cell migration via signaling through Rho GTPases, which therefore suggests this group of proteins as a treatment target for tumors that express CXCR4 (27,42). During cell movement, Rho is important for regulating the formation of contractile actin-myosin filaments and stress fibers, and maintaining focal adhesions at the rear of the migrating cells, whereas Rac is involved in forming actin-rich membrane ruffles, or lamellipodia formation, at the leading edge of the migrating cells, which is required to determine directional movement (42). Therefore, the collective events resulting in Rho GTPases represent a key regulatory event for the intentional chemotaxis of cancer cells (27). Our findings that heterodimerization reduced CXCR4-mediated RhoA signaling and phenotypic changes associated with cell migration may have broad implications as the CXCL12/CXCR4 signaling axis is critical to a successful metastatic tumor.
Receptor dimerization is becoming a key paradigm in GPCR biology and cancer therapeutics. It has been reported for most aspects of GPCR function: trafficking, internalization, and pharmacological antagonism and signal transduction (3,36,43–45). Although the functional consequences of receptor heterodimerization are still emerging, physical heterodimers amplify or antagonize signals that would otherwise result from each receptor individually, and change the signaling profile or direction to favor one downstream pathway over another (43,44). When CXCR4/CB2 heterodimerize, the receptor pair reduces signals that would come from CXCR4 alone, strongly supporting our global results that inhibiting the action of CXCR4 whether via: (i) induced receptor heterodimerization; (ii) chemical antagonism of CXCR4 with AMD3100; or (iii) reduced translation of CXCR4 protein via siRNA will reduce the propensity of CXCR4-mediated signaling to activate cell migration.
GPCR heterodimers also alter ligand selectivity where physical heterodimerization resulted in negative binding cooperativity - only one-chemokine ligand binds with high affinity to the receptor dimer pair (36–38). Considering the overwhelming clinical and social support for medical cannabinoids in cancer treatment, our current and previous (3) studies mechanistically demonstrate cannabinoid applications, and surely opens avenues for considering antagonizing CXCR4 via induced CXCR4/CB2 heterodimers to slow the metastatic process. What’s more, we use agonists, instead of antagonists, which currently result in severe immune dysfunction due to inhibition of CXCR4 (46). The established role of CXCR4 in metastasis and the broad role for cannabinoids in cancer metastasis treatment, pain management, palliative care, bioavailability and non-invasive administration (47,48) present this specific heterodimer as a target for metastasis intervention.
Implications.
This study investigates a signaling mechanism by which GPCR heterodimerization inhibits cancer cell migration.
Acknowledgments
Grant support: This work was supported by grant funding to C.V. Hinton from NIH/NIGMS (R01GM106020). Training support for students was provided, in part, by NIH/NIGMS 2R25GM060414 and NIH/NIHMD 4P20MD002285; confocal analyses was funded by Research Centers in Minority Institutions Program (RCMI)/NIMHD (5G12MD007590).
Footnotes
Disclosure of potential conflict of interest: The authors do not have any financial interests that could be perceived as being a conflict of interest.
References
- 1.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10(1):47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 2.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7(2):79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 3.Coke C, Scarlett K, Chetram M, Jones K, Sandifer B, Davis A, et al. Simultaneous Activation of Induced Heterodimerization between CXCR4 Chemokine Receptor and Cannabinoid Receptor 2 (CB2) Reveals a Mechanism for Regulation of Tumor Progression. Journal of Biological Chemistry. 2016;291(19):9991–10005. doi: 10.1074/jbc.M115.712661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacological reviews. 2005;57(3):289–98. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 5.Pello OM, Martinez-Munoz L, Parrillas V, Serrano A, Rodriguez-Frade JM, Jose Toro M, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. European Journal of Immunology. 2008;38(2):537–449. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- 6.Nasser M, Qamri Z, Deol Y, Smith D, Shilo K, Zou X, et al. Crosstalk between chemokine receptor CXCR4 and cannabinoid receptor CB2 in modulating breast cancer growth and invasion. PLoS One. 2011;6(9):e23901. doi: 10.1371/journal.pone.0023901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satake H, Matsubara S, Aoyama M, Kawada T, Sakai T. Functional Regulation and Implication for Biodiversity. Frontiers in Endocrinology. 2013;4:100. doi: 10.3389/fendo.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland SL. Are GPCRs still a source of new targets? Journal of biomolecular screening. 2013;18(9):947–66. doi: 10.1177/1087057113498418. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Advances in Cancer Research. 2014:31–82. doi: 10.1016/B978-0-12-411638-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 Entry Cofactor: Functional cDNA Cloning of a Seven-Transmembrane, G Protein-Coupled Receptor. Science. 1996;272(5263):872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Huang S, Peng S. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. International Journal of Oncology. 2005;27(5):1329–39. [PubMed] [Google Scholar]
- 13.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(11):2927–31. doi: 10.1158/1078-0432.ccr-09-2329. [DOI] [PubMed] [Google Scholar]
- 14.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(8):2074–80. doi: 10.1158/1078-0432.ccr-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackie K. Cannabinoid receptors: where they are and what they do. Journal of neuroendocrinology. 2008;20(Suppl 1):10–4. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological reviews. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological reviews. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 18.Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert reviews in molecular medicine. 2009;11:e3. doi: 10.1017/s1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarti B, Ravi J, Ganju RK. Cannabinoids as therapeutic agents in cancer: current status and future implications. Oncotarget. 2014;5(15):5852–72. doi: 10.18632/oncotarget.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14(3):171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Muñoz LM, Lucas P, Holgado BL, Barroso R, Vega B, Rodríguez-Frade JM, et al. Receptor oligomerization: A pivotal mechanism for regulating chemokine function. Pharmacology & Therapeutics. 2011;131(3):351–8. doi: 10.1016/j.pharmthera.2011.05.002. doi http://dx.doi.org/10.1016/j.pharmthera.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Stephens B, Handel TM. Chemokine receptor oligomerization and allostery. Progress in molecular biology and translational science. 2013;115:375–420. doi: 10.1016/b978-0-12-394587-7.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2(2):133–42. doi: 10.1038/nrc725. doi http://www.nature.com/nrc/journal/v2/n2/suppinfo/nrc725_S1.html. [DOI] [PubMed] [Google Scholar]
- 24.Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 2011;5(2):170–80. doi: 10.4161/cam.5.2.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–12. doi: 10.1016/j.ceb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582(14):2093–101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 27.Tan W, Martin D, Gutkind JS. The Galpha13-Rho signaling axis is required for SDF-1-induced migration through CXCR4. J Biol Chem. 2006;281(51):39542–9. doi: 10.1074/jbc.M609062200. [DOI] [PubMed] [Google Scholar]
- 28.Struckhoff AP, Vitko JR, Rana MK, Davis CT, Foderingham KE, Liu CH, et al. Dynamic regulation of ROCK in tumor cells controls CXCR4-driven adhesion events. J Cell Sci. 2010;123(Pt 3):401–12. doi: 10.1242/jcs.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan KT, Asokan SB, King SJ, Bo T, Dubose ES, Liu W, et al. LKB1 loss in melanoma disrupts directional migration toward extracellular matrix cues. J Cell Biol. 2014;207(2):299–315. doi: 10.1083/jcb.201404067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano A, Takashima S. LKB1 and AMP-activated protein kinase: regulators of cell polarity. Genes Cells. 2012;17(9):737–47. doi: 10.1111/j.1365-2443.2012.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Jin D, Durgan J, Hall A. LKB1 controls human bronchial epithelial morphogenesis through p114RhoGEF-dependent RhoA activation. Mol Cell Biol. 2013;33(14):2671–82. doi: 10.1128/MCB.00154-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, et al. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33(33):4203–12. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagi H, Tan W, Dillenburg-Pilla P, Armando S, Amornphimoltham P, Simaan M, et al. A synthetic biology approach reveals a CXCR4-G13-Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal. 2011;4(191):ra60. doi: 10.1126/scisignal.2002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reymond N, Riou P, Ridley AJ. Rho GTPases and cancer cell transendothelial migration. Methods Mol Biol. 2012;827:123–42. doi: 10.1007/978-1-61779-442-1_9. [DOI] [PubMed] [Google Scholar]
- 35.Struckhoff AP, Rana MK, Kher SS, Burow ME, Hagan JL, Del Valle L, et al. PDZ-RhoGEF is essential for CXCR4-driven breast tumor cell motility through spatial regulation of RhoA. J Cell Sci. 2013;126(Pt 19):4514–26. doi: 10.1242/jcs.132381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, Fernandez S, Martin de Ana A, Jones DR, et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001;20(10):2497–507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Asmar L, Springael JY, Ballet S, Andrieu EU, Vassart G, Parmentier M. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol Pharmacol. 2005;67(2):460–9. doi: 10.1124/mol.104.003624. [DOI] [PubMed] [Google Scholar]
- 38.Gillies K, Wertman J, Charette N, Dupre DJ. Anterograde trafficking of CXCR4 and CCR2 receptors in a prostate cancer cell line. Cell Physiol Biochem. 2013;32(1):74–85. doi: 10.1159/000350126. [DOI] [PubMed] [Google Scholar]
- 39.Lim SM, Trzeciakowski JP, Sreenivasappa H, Dangott LJ, Trache A. RhoA-induced cytoskeletal tension controls adaptive cellular remodeling to mechanical signaling. Integr Biol (Camb) 2012;4(6):615–27. doi: 10.1039/c2ib20008b. [DOI] [PubMed] [Google Scholar]
- 40.Marjoram RJ, Lessey EC, Burridge K. Regulation of RhoA activity by adhesion molecules and mechanotransduction. Curr Mol Med. 2014;14(2):199–208. doi: 10.2174/1566524014666140128104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engl T, Relja B, Marian D, Blumenberg C, Muller I, Beecken WD, et al. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 2006;8(4):290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azab AK, Azab F, Blotta S, Pitsillides CM, Thompson B, Runnels JM, et al. RhoA and Rac1 GTPases play major and differential roles in stromal cell-derived factor-1-induced cell adhesion and chemotaxis in multiple myeloma. Blood. 2009;114(3):619–29. doi: 10.1182/blood-2009-01-199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milligan G. G-protein-coupled receptor heterodimers: pharmacology, function and relevance to drug discovery. Drug Discov Today. 2006;11(11–12):541–9. doi: 10.1016/j.drudis.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5(1):30–4. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286(37):32188–97. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–72. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Percherancier Y, Berchiche YA, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, et al. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280(11):9895–903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 48.Ramos JA, Bianco FJ. The role of cannabinoids in prostate cancer: Basic science perspective and potential clinical applications. Indian J Urol. 2012;28(1):9–14. doi: 10.4103/0970-1591.94942. [DOI] [PMC free article] [PubMed] [Google Scholar]