Abstract
Prostate cancer afflicts 1 in 7 men and is the second leading cause of male cancer-related deaths in the United States. MicroRNAs (miRNAs), an extensive class of ~22 nucleotide non-coding RNAs, are often aberrantly expressed in tissues and fluids from prostate cancer patients but the mechanism of how specific miRNAs regulate prostate tumorigenesis and metastasis are poorly understood. Here, miR-888 was identified as a novel prostate factor that promotes proliferation and migration. miR-888 resides within a genomic cluster of 7 miRNA genes (mir-892c, mir-890, mir-888, mir-892a, mir-892b, mir-891b, mir-891a) on human chromosome Xq27.3. Moreover, as miR-888 also maps within HPCX1, a locus associated with susceptibility and/or hereditary prostate cancer, it was hypothesized that additional miRNA cluster members also play functional roles in the prostate. Expression analysis determined that cluster members were similarly elevated in metastatic PC3-ML prostate cells and their secreted exosomes, as well as enriched in expressed prostatic secretions (EPS) urine derived-exosomes obtained from clinical patients with high-grade prostate cancer. In vitro assays revealed that miR-888 cluster members selectively modulated PC3-derived and LNCaP cell proliferation, migration, invasion, and colony formation. Mouse xenograft studies verified miR-888 and miR-891a as pro-oncogenic factors that increased prostate tumor growth in vivo. Further analysis validated RBL1, KLF5, SMAD4 and TIMP2 as direct miR-888 targets and that TIMP2 is also co-regulated by miR-891a. This study provides the first comprehensive analysis of the entire miR-888 cluster and reveals biological insight.
Implications
This work reveals a complex non-coding RNA network in the prostate that could be developed as effective diagnostic and therapeutic tools for advanced prostate cancer.
Keywords: miR-888 cluster, microRNA, prostate cancer
Introduction
Prostate Cancer is a major health problem in the United States requiring more effective diagnostic and therapeutic tools. Among U.S. men, prostate cancer is the most common cause of non-skin cancer and the second leading cause of cancer-related deaths after lung cancer (1). Prostate cancer can be divided into two distinct clinical groups; indolent and advanced disease. No biomarker exists that can discriminate between indolent and high-grade prostate cancer or identify men earlier during the course of their disease who would benefit from aggressive multimodal treatment regimens (i.e., surgery, castration therapy, radiation). Clinicians also lack effective treatment options for advanced disease and metastatic prostate cancer remains incurable and lethal. Small non-coding microRNAs (miRNAs) such as miR-888 may play a direct role in converting slow-growing indolent tumor cells towards a more aggressive phenotype during prostate cancer progression (2,3).
MiRNAs are single-stranded non-coding RNAs of 18–22 nucleotides in length and constitute an important class of cellular regulators. These small RNAs generally block gene expression by binding to complementary sequences within their target protein-coding messenger RNAs (mRNAs) resulting in translational inhibition and/or mRNA degradation (4). However, there are a few instances where miRNAs can activate gene expression via epigenetic regulation of enhancer regions in the nucleus (5) or post-transcriptionally to induce protein translation (6). Over 2,500 miRNAs exist in the human genome (miRBase, release 21). Although the function of the majority of miRNAs is unknown, those characterized play essential roles in cell growth, differentiation, apoptosis, metabolism, and the immune response. It is therefore not surprising that miRNA misexpression correlates with a wide range of human cancers, including prostate cancer (2,7). Studies comparing miRNA expression in cell lines, tissue, blood, and urine from prostate cancer and non-cancer patients reveal unique patterns of miRNA deregulation that can discriminate for advanced prostate cancer, drug response, disease recurrence, and metastasis (2). Specific miRNA profiles observed in diseased patients likely reflect abnormalities in the underlying mechanistic pathways related to cancer progression.
Functional studies employing immortalized human cell lines and mouse cancer models show that subsets of miRNAs can directly impact prostate tumor initiation and progression by controlling cancer pathways, i.e., cell cycle progression, differentiation, angiogenesis and epithelial-to-mesenchymal transitions (EMT). A growing class of tumor suppressors characterized in the prostate include miR-15a/miR-16-1, miR-34, miR-143/miR-145 cluster, miR-200/miR-141 family, miR-203, miR-205, let-7 family, miR-101, miR-26, and miR-99 family. MiRNAs such as miR-34a, miR-200, miR-203, miR-205 and miR-29b act as prostate metastasis suppressors (2). Less is understood how miRNAs promote prostate tumor growth and progression to aggressive disease, although the pro-oncogenic miRNAs miR-21 and the miR-154-3p/miR-379/miR-409 cluster are likely important contributors (2).
Our laboratory identified miR-888 as a novel human oncogenic miRNA in the prostate. In a screen for miRNAs correlating with advanced prostate cancer, hsa-miR-888-5p (referred to as miR-888 in this study) was enriched in aggressive human prostate cancer cell lines and tumor specimens from prostate cancer patients (3). miR-888 was also elevated in prostatic fluids, termed EPS urine (Expressed Prostatic Secretions in post-DRE urine), from patients with high-grade prostate cancer compared to those with lower-grade disease and non-cancer patients. We postulated miR-888 induced prostate cancer progression. Indeed, miR-888 stimulated prostate cell proliferation, migration, and colony formation in vitro (3). Our study was the first functional analysis for miR-888 in any tissue. Elevated miR-888 expression in other human cancers has been documented. miR-888 is upregulated in human renal (8) and colon cancer (9), and in MCF-7 side population human breast cancer cells possessing cancer stem cell characteristics (10). Notably, miR-888 is elevated in endometrial cancers and particularly enriched in malignant mixed Mullerian tumors, a very aggressive endometrial disease with poor prognosis (11,12). miR-888 also induces breast cancer cell migration and invasion in vitro (13). These reports are consistent with our work characterizing the oncogenic role of miR-888 in the prostate and highlights its’ clinical potential.
miR-888 resides within a genomic cluster of 7 miRNA genes (mir-892c, mir-890, mir-888, mir-892a, mir-892b, mir-891b, mir-891a) located within a ~34-kb region on the minus strand of chromosome X (Xq27.3) (Fig. 1A). miR-888 and related miR-890/892a/892b/892c cluster members belong to the mammalian-conserved miR-743 family and show little sequence homology to the primate-specific miR-891 family, miR-891a and miR-891b (14). Genomic organization of the miR-888 cluster is conserved in primates (i.e., humans, rhesus macaque, chimpanzees, orangutans) (15). The miR-888 cluster was initially cloned from human epididymis tissue (16), and members are expressed throughout this organ as well as in the testis (12,17,18). Interestingly, the miR-888 cluster lies within HPCX1 (hereditary prostate cancer, X-linked 1, Xq27-28), a region associated with 16% of hereditary prostate cancer cases (19,20). Due to our work defining miR-888 as a novel oncogenic factor in the prostate (3), we hypothesized that additional miR-888 cluster members also regulate prostate cancer progression.
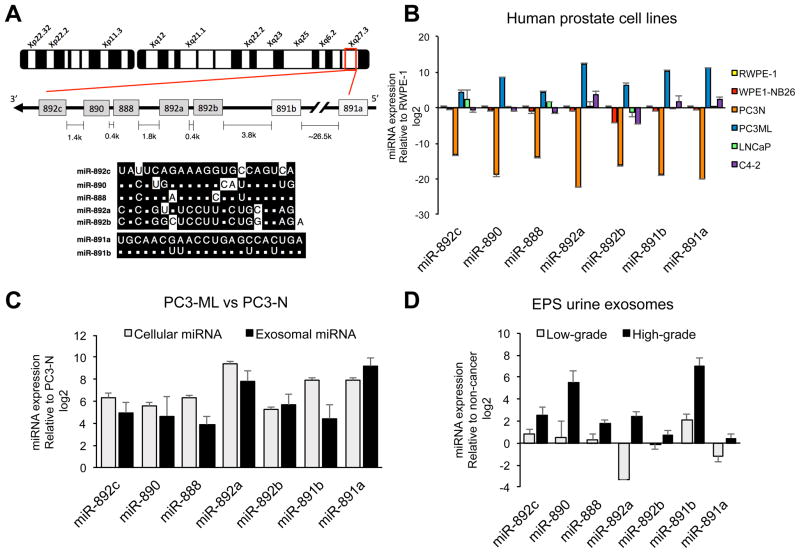
Figure 1. Enriched miR-888 cluster expression in metastatic PC3-ML prostate cells & their secreted exosomes, and in EPS urine exosomes from high-grade prostate cancer patients.
A, Schematic representation of the miR-888 cluster on human chromosome Xq27.3 and sequence homology shared between the miR-743 (grey) and the miR-891 (white) miRNA families. B, miR-888 cluster expression was measured using qRT-PCR on a panel of human prostate cell lines differing in their metastatic status and response to androgen. C, Comparison of miRNA levels by qRT-PCR in metastatic PC3-ML prostate cancer cells & exosomes relative to non-aggressive PC3-N; and D, in EPS urine-derived exosomes from high-grade and low-grade prostate cancer patients relative to non-cancer patients. Results were normalized using snoRNA RNU48 (cells) or U6 snRNA (exosomes).
In this work, we performed the first comprehensive analysis of the miR-888 cluster and focused on their role in prostate cancer using a combination of in vitro and in vivo assays. We found that miR-888 cluster member expression was similarly enriched in aggressive castration-resistant human prostate cancer cells and exosomes, as well as EPS urine derived-exosomes obtained from patients with high-grade prostate disease. Select cluster members modulated proliferation, migration, invasion, and colony formation. Mouse xenograft experiments verified miR-888 and miR-891a as bona fide pro-oncogenic factors that accelerated prostate tumor growth in vivo. This study indicates that a complex non-coding RNA regulatory network exists in the prostate and could be targeted as clinical tools for aggressive prostate disease.
Materials and Methods
Cell Lines and Cell Culture
PC3-ML and PC3-N (gift from Dr. M. Stearns, authenticated as described (21)) were used up to passage 18 and grown in DMEM with 10% fetal bovine serum (FBS, Gibco) and 1% antibiotic-antimycotic (Gibco). LNCaP (American Type Culture Collection) and C4-2 cells (gift from Dr. E. Keller) were grown in RPMI-1640 with 10% FBS and 1% antibiotic-antimycotic. RWPE-1 and WPE1-NB26 cells (American Type Culture Collection) were grown in Keratinocyte serum free media (Gibco) with 1% antibiotic-antimycotic, 0.05 mg/mL bovine pituitary extract and 5 ng/mL recombinant epidermal growth factor growth factor. All cells were incubated at 37 °C with 5% CO2. LNCaP, C4-2, RWPE-1, and WPE1-NB26 cells were used at low passages, authenticated using short tandem repeat analysis, and mycoplasma tested by ATCC.
EPS Urine Specimens
EPS urine patient specimens were collected by a clinician and centrifuged at 2500 rpm for 15 min to obtain the EPS urine supernatant fraction, as previously described (3). Exosomes from the EPS urine supernatant were isolated using a precipitation reagent (Ymirite) and total RNA was generated by Ymir Genomics LLC. EPS urine specimens were pooled prior to RNA isolation based on clinical grade: non-cancer (15 patients), low-grade cancer (Gleason 6, 16 patients), and high-grade cancer (Gleason 8 and 9, 9 patients). De-identified EPS urine specimens were obtained from patients with written informed consent and housed in the biorepository at the Leroy T. Canoles Jr. Cancer Research Center under protocols approved by the EVMS Institutional Review Board (IRB) and in accordance with ethical and NIH guidelines and HIPAA regulations.
Exosome Isolation
PC3-N and PC3-ML cells were cultured in serum-free DMEM medium for 48 h. Collected culture media was centrifuged at 2,000 × g for 10 min at 4°C. The supernatant was passed through a 0.22 μM filter and ultracentrifuged twice at 100,000 × g for 1.5 h at 4°C. Exosome pellets were resuspended in PBS. Exosome size and concentration was obtained using a NanoSight NS300 (Malvern) and total protein quantified (Pierce Micro BCA Protein Assay Kit). Exosomes were imaged using electron microscopy. Briefly, samples (7 μl) were loaded onto glow discharged grids, blotted, and negatively stained with 2% (wt/vol) uranyl acetate. EM grids were imaged using theJEOL JEM1200EX II electron microscope equipped with 11 megapixel AMT digital camera. Images were taken at an accelerating voltage of 100 kV, and a nominal magnification of 30,000x.
RNA Expression using TaqMan qRT-PCR
MiRNA expression was measured by qRT-PCR with individual TaqMan MicroRNA Assays (Applied Biosystems) (Suppl. Table S1) on total RNA using the mirVana miRNA Isolation Kit (Ambion). Reverse transcription was performed on 35 ng total RNA using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) and miRNA-specific stem-loop primers (Applied Biosystems) on a Veriti 96-well thermal cycler (Applied Biosystems). qRT-PCR was performed on a StepOnePlus Real-Time PCR System (Applied Biosystems), as previously described (3). For messenger RNA (mRNA) expression, 1 ug total RNA was reverse transcribed to cDNA using the High Capacity RNA-to-cDNA Kit (Applied Biosystems) and 100 ng cDNA was used per qRT-PCR reaction with TaqMan Gene Expression Assays (Applied Biosystems). MiRNA expression was normalized using snoRNA RNU48 (cells) or U6 snRNA (exosomes). mRNA expression was normalized using human 18S rRNA. Results were quantified in triplicate and relative fold-change was calculated by the delta delta Ct method.
Cell Culture Transfection
PC3-N and LNCaP cells were reverse transfected with siPORT NeoFX Transfection Agent (Ambion) and 50 nM mirVana miRNA mimics (Ambion) or 50 nM mirVana Negative Control No. 1 mimics (Cat. #4464058, Ambion). To repress miRNA activity, PC3-ML cells were transfected with DharmaFECT Transfection Reagent 2 (Dharmacon) and 25 nM miRIDIAN MicroRNA Hairpin Inhibitors (Dharmacon) or 25 nM miRIDIAN Negative Control No. 1 (IN-001005-01-05, Dharmacon) (Suppl. Table S1). Negative control mimics and inhibitors were referred to as SCR (scrambled) controls. Cells were reverse transfected using Lipofectamine 3000 (Invitrogen) with pCMV6-Entry-TrueORF vectors for RBL1 and TIMP2 (Origene) to overexpress these proteins or reverse transfected using siPORT NeoFX Transfection Agent (Ambion) and 20 nM Silencer Select Pre-Designed siRNAs for RBL1, TIMP2 and Negative Control No. 1 (s11854, s656; 4390843 respectively, Ambion) to block their gene expression.
Lentiviral microRNA vector infected cell lines
To generate stable miRNA overexpression cell lines, PC3-N and LNCaP cells were infected with shMIMIC lentivirus microRNA vectors (MOI 5, pSMART hCMV, Dharmacon) to either overexpress hsa-miR-888-5p (V2SMHS07_23787, TurboRFP), hsa-miR-891a-5p (V2SMHS07_28407, TurboRFP), or a non-targeting Scrambled (SCR) Negative Control (S-005000-01, TurboGFP-NTC) mimics for 24 h in DMEM culture medium supplemented with 0.8 μg/ml Hexadimethrine bromide. Infected cells were puromycin selected (0.5 μg/ml) for two weeks in DMEM with 10% FBS and 1% antibiotic-antimycotic. A Zeiss AxioObserver A1 inverted fluorescence microscope visualized enrichment of RFP (miR-888 or miR-891a vectors) or GFP (negative control vector) fluorescent cells following puromycin selection.
Cell Proliferation WST-1 Assays
Four hours prior to the 24 h, 48 h, and 72 h time points, WST-1 reagent (Roche) was added to the cells. Absorbance was measured with a spectrophotometer (Synergy HT, BioTek) at 450 nm test and 620 nm reference wavelengths. Results were quantified in triplicate.
Migration and Invasion Boyden Chamber Assays
Cells were added to the upper portion of a 24-well chamber and allowed to traverse across a 0.8 μM polycarbonate insert toward a chemoattractant (10% FBS) for 24 h, to analyze migration (Corning BioCoat Control Inserts) or invasion (Corning Matrigel Invasion Chambers). Cells failing to traverse the insert were mechanically removed and remaining cells were methanol-fixed and stained with 1% crystal violet. Wells were destained with 10% acetic acid, 30% methanol. Results were quantified in triplicate using a Synergy HT spectrophotometer (BioTek, 595 nm wavelength).
Soft Agar Assays
A “bottom plug” (0.8% agarose in 0.5× cell medium) was added to each well of a 24-well plate. 500 cells incorporated into a “top plug” (0.35% agarose in 0.6× cell medium) were added to these wells. After 14 days, cells were ethanol-fixed and stained with 0.05% crystal violet. Colonies were counted with a Zeiss AxioObserver A1 inverted microscope. Pictures were taken at randomly selected fields with an upright Zeiss Stereo Discovery.V12 at 25× magnification.
Mouse Xenograft Tumor Model
1 × 106 PC3-N cells (in 100 μL PBS) infected with shMIMIC Lentiviral microRNA vectors (miR-888 or miR-891a) were mixed 1:1 v/v with Matrigel Basement Membrane Matrix (Corning) and injected subcutaneously into the right flanks of male NOD/SCID mice (Charles River). 1 × 106 PC3-N cells (in 100 μL PBS) infected with shMIMIC Lentiviral Non-targeting Negative Control vector (SCR) mixed 1:1 v/v with Matrigel were injected into the left flank of the same mice. Tumor size was recorded weekly with digital calipers. Tumor volume was calculated using the ellipsoidal formula 1/2 (length × width2), based on the largest longitudinal (length) and transverse (width) diameters. Mice were sacrificed 5 weeks post-injection. Dissected tumors were processed for RNA and protein analysis. Animal procedures were approved by the EVMS Institutional Animal Care and Use Committee and performed in accordance with institutional policies and NIH guidelines.
Western Blot Analysis
Protein lysates were prepared using RIPA Lysis Buffer 10× (EMD Millipore) with protease inhibitors. 40 μg of lysate was resolved on a 4–12% Bis-Tris Protein Gel (NuPAGE Novex, Invitrogen) and transferred to Immobilon-FL PVDF membranes (EMD Millipore). Membranes were blocked with Odyssey Blocking Buffer (Li-Cor) for 1 h and probed with primary antibodies against SMAD4 (1:500, Santa Cruz sc-7154), TIMP2 (1:1000, Abcam ab38975), RBL1 (1:200, Santa Cruz sc-318), KLF5 (1:200, Abcam ab24331), Calnexin (1:2000, BD Biosciences 610523), exosome markers CD9 (1:200, Santa Cruz sc-59140), TSG101 (1:1000, BD Biosciences 612696), Alix (1:1000. Cell Signaling CS2171), or GAPDH (1:5000, Abcam ab8245). Blots were incubated with secondary goat anti-mouse or goat anti-rabbit antibodies (1:5000, Li-Cor). Protein expression was normalized to GAPDH levels using an Odyssey scanner and software (Li-Cor).
Luciferase Assays
3′ untranslated regions (3′UTR) of KLF5, RBL1, SMAD4, and TIMP2 containing predicted miR-888 binding sites were cloned by PCR (Suppl. Table S2) and ligated into luciferase reporter vector psiCHECK-2 (Promega) downstream of the Renilla luciferase translational stop codon. psiCHECK2 vector also contained a firefly luciferase cassette to normalize Renilla luciferase expression. PC3-N cells stably overexpressing miR-888 or scrambled (SCR) control mimics using lentiviral vectors (described above) were co-transfected with the 3′UTR luciferase reporter construct using Lipofectamine 2000 Reagent (Invitrogen). After 48 h, Dual-Glo Luciferase Reagent was added to each well and measured for Firefly luminescence with a GloMax 96 Microplate Luminometer (Promega). Stop & Go Reagent was subsequently added to the same wells and Renilla luminescence was measured.
Statistical Analysis
Experimental data represented at least 2 independent trials performed in triplicate and error bars depicted standard deviation (SD). Results were analyzed using unpaired two-tailed t-test. Statistical tests were performed with GraphPad Prism or Microsoft Excel software. Statistically significant P values were set at *p < 0.05 and **p<0.001.
Results
miR-888 cluster expression correlated with advanced prostate cancer
Our lab reported that hsa-miR-888-5p (referred to here as miR-888) was differentially elevated in metastatic PC3-ML cells and EPS urine supernatant from high-grade prostate cancer patients (3). We hypothesized that additional members of the miR-888 cluster would exhibit similar expression patterns to miR-888 in the prostate. Expression of the entire miR-888 cluster consisting of hsa-miR-892c-5p, hsa-miR-890-5p, hsa-miR-888-5p, hsa-miR-892a-3p, hsa-miR-892b-3p, hsa-miR-891b-5p, and hsa-miR-891a-5p (referred to as miR-892c, miR-890, miR-888, miR-892a, miR-892b, miR-891b, miR-891a throughout this study) was measured by qRT-PCR in paired syngeneic human prostate cell lines that differed in their metastatic status and response to androgen, which included non-malignant epithelial RWPE-1 & its metastatic, androgen-sensitive subclone WPE1-NB26; non-aggressive, androgen-sensitive LNCaP (lymph node metastasis-derived) & its aggressive, hormone-refractory subclone C4-2; non-aggressive, hormone-refractory PC3-N (bone metastasis-derived) & its metastatic, hormone-refractory subclone PC3-ML. We found that the miR-888 cluster was similarly enriched in aggressive PC3-ML and underexpressed in non-aggressive PC3-N relative to non-malignant RWPE-1 prostate cells (Fig. 1B).
Focusing on PC3-N and PC3-ML, we tested if miR-888 cluster levels were differentially expressed in exosomes secreted from these cell lines. Exosomes are membrane-bound microvesicles measuring 50–150 nm in diameter secreted by a large range of cell types, including prostate tumor cells (22). Exosomes selectively concentrate and transport miRNA cargo intercellularly (22) (23). We isolated PC3-N and PC3-ML microvesicles via ultracentrifugation methods that were ~127 nm in diameter according to NanoSight tracking and were visualized by electron microscopy (Suppl. Fig. S1A). Prostate exosome preparations were further verified by western blot analysis to be enriched in the transmembrane exosome marker CD9, the cytosolic membrane-binding exosome markers TSG101 & Alix, and (as expected) were under-represented in the intracellular endoplasmic reticulum marker Calnexin (Suppl. Fig. S1B) (24). Interestingly, metastatic PC3-ML secreted more exosomes per cell (12.17 × 10E3) than non-aggressive PC3-N (9.91 × 10E3) (Suppl. Fig. S1C). PC3-ML exosomes contained higher levels of miR-888 cluster members relative to PC3-N exosomes (Fig. 1C).
We postulated that exosomes isolated from prostatic fluids of cancer patients would be enriched for the miR-888 cluster and their expression would correlate with advanced prostate disease. Our previous work indicated miR-888 as a discriminating marker for high-grade prostate cancer in EPS urine (3). In that study, differential miR-888 expression was detected only in the EPS urine supernatant (fraction collected following 2500 rpm spin) but not in the sedimented EPS urine pellet. Exosomes within the EPS urine supernatant were likely responsible for the differential miRNA profiles. We therefore compared miR-888 cluster expression by qRT-PCR in exosomes isolated from EPS urine supernatant of patients grouped by clinical grade: high-grade prostate cancer (Gleason 8–9, 9 patients), low-grade disease (Gleason 6, 16 patients), and non-cancer (15 patients). Indeed, members of the miR-888 cluster were elevated in EPS urine exosomes obtained from high-grade prostate cancer patients compared to exosomes from low-grade cancer and non-cancer patients (Fig. 1D). These findings indicated the potential for the miR-888 cluster as discriminating exosome-derived biomarkers for advanced prostate cancer.
miR-888 cluster members modulated prostate cell proliferation, migration, invasion and colony formation in vitro
miR-888 promotes prostate cell proliferation, migration and colony formation in vitro (3). Since miR-888 cluster members share similar expression profiles with miR-888 in human prostate cell lines and patient EPS urine-derived exosomes, we hypothesized that additional members of this cluster would also modulate oncogenic activities in the prostate. We first investigated the influence of the miR-888 cluster on prostate cell growth using WST-1 assays. miR-888 cluster members were individually overexpressed via transfection with miRNA mimics in non-aggressive, castration-resistant PC3-N cells and hormone-sensitive LNCaP adenocarcinoma cells and their effects were compared to cells transfected with SCR control mimics (Suppl. Fig. S2A–C). Mimic treatment for miR-888, miR-891b, miR-891a (and to an extent miR-892a) increased PC3-N proliferation rates (Fig. 2A). However, miR-890 and miR-892c overexpression in PC3-N cells decreased cell growth. In LNCaP cells, forced expression of miR-891a, miR-892a as well as miR-892b promoted proliferation, whereas miR-888 and miR-891b showed only moderate effects (Fig. 2B). In contrast to PC3-N cells, miR-890 or miR-892c mimic treatment in LNCaP cells did not significantly decrease growth. Our work indicated that miR-888 cluster members have distinct abilities to promote or suppress prostate cell proliferation and their effects are likely cell type specific.
Figure 2. The miR-888 cluster modulated hormone-sensitive and castration-resistant prostate cancer cell proliferation.
WST-1 assays were performed 24, 48, and 72 h post-transfection in (A) PC3-N and (B) LNCaP prostate cells treated individually with miR-888 cluster miRNA mimics (50 nM) compared to cells treated with negative SCR (scrambled) control mimics (50 nM). miR-888, miR-891b, miR-891a (and to an extent miR-892a) induced PC3-N growth, whereas miR-892c or miR-890 treatment decreased proliferation. In LNCaP cells, miR-891a, miR-892a, or miR-892b overexpression showed significant growth.
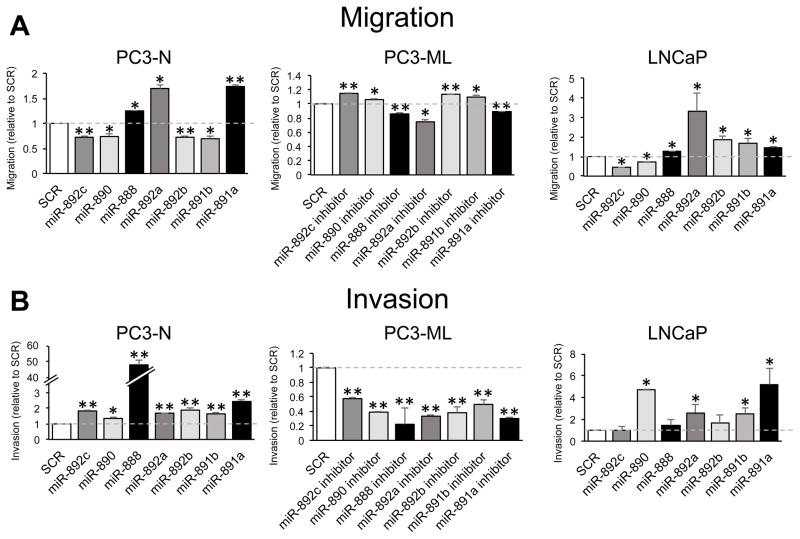
We tested if miR-888 cluster members influenced prostate cell migration and invasion, cellular activities commonly associated with metastasis. In Boyden chamber migration assays, PC3-N cells individually treated with miR-888, miR-892a or miR-891a mimics exhibited enhanced migration compared to cells treated with SCR controls (Fig. 3A, left). In contrast, individual mimic treatment of miR-892c, miR-890, miR-892b or miR-891b significantly suppressed PC3-N cell migration in these assays. Consistent with these results, when we blocked endogenous miR-888 cluster activity in aggressive PC3-ML cells (by transfection of individual antisense miRNA inhibitors), the reciprocal migration phenotypes for these miRNAs were observed (Fig. 3A, center). Testing overexpression of the miR-888 cluster in LNCaP cells, we noted that miR-888, miR-892a, miR-892b, miR-891b and miR-891a individually promoted migration, whereas miR-892c or miR-890 overexpression decreased this behavior (Fig. 3A, right). We also tested prostate cell invasion through extracellular matrix (Matrigel) using Boyden chamber assays. PC3-N cell treatment with each miR-888 cluster mimic enhanced this behavior (Fig. 3B, left). Reciprocally, PC3-ML cells individually transfected with miR-888 cluster inhibitors repressed cell invasion (Fig. 3B, center). When the invasion assays were performed in LNCaP cells, only miR-890, miR-892a, miR-891b or miR-891a mimic treatment enhanced invasion, whereas miR-888 or miR-892b overexpression had little effect (Fig. 3B, right).
Figure 3. miR-888 cluster members regulated prostate cell migration and invasion.
Human prostate cancer cells were individually transfected with miRNA mimics (50 nM, PC3-N & LNCaP) or miRNA inhibitors (25 nM, PC3-ML) to modulate miR-888 cluster member expression. Boyden chamber assays measured the ability of transfected cells to (A) migrate across a 0.8 μM polycarbonate insert or (B) invade through a Matrigel matrix before crossing a polycarbonate insert and towards a chemoattractant (10% FBS) for 24 h. A, Overexpression of miR-888, miR-892a or miR-891a increased migration, and miR-892c, miR-890, miR-892b or miR-891b treatment decreased migration relative to SCR mimic control treatment (left panel). Reciprocal effects were noted in aggressive PC3-ML cells transfected with miRNA inhibitors blocking miR-888 cluster activity (middle panel). Unlike PC3-N cells, miR-892b and miR-891b overexpression increased migration in LNCaP cells (right panel). B, Individual miR-888 cluster member overexpression promoted PC3-N invasion, and conversely inhibiting their activity in metastatic PC3-ML cells repressed this phenotype relative to SCR controls. In LNCaP cells, miR-890, miR-892a, miR-891b or miR-891a overexpression increased invasive behavior.
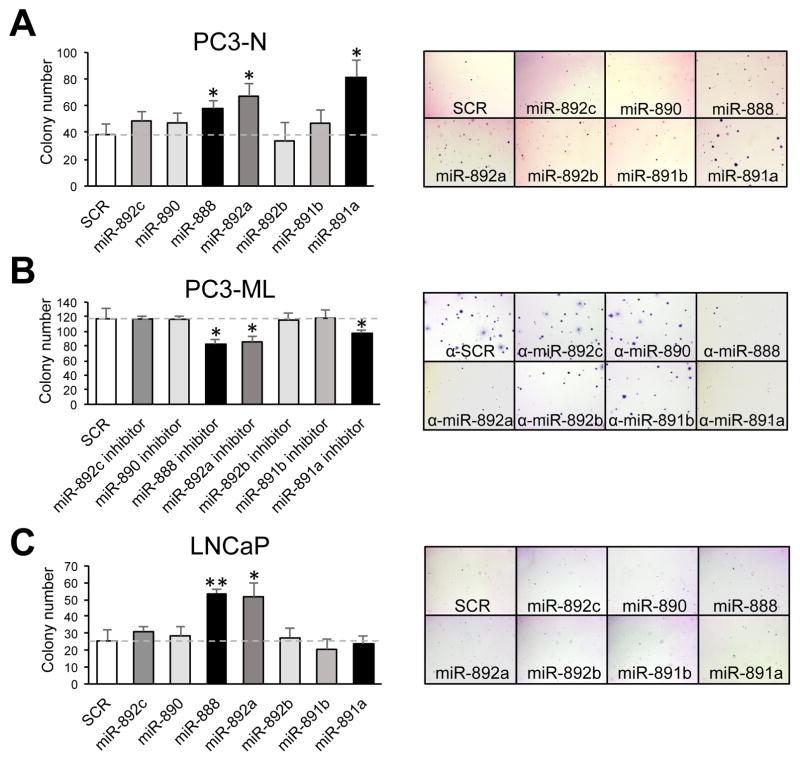
The tumorigenic potential of the miR-888 cluster was tested in vitro using a colony formation assay, which measured anchorage independent growth of prostate cancer cells in soft agar. PC3-N cells transfected with miR-888, miR-892a or miR-891a mimics resulted in higher colony number versus cells transfected with SCR controls (Fig. 4A). Reciprocally, inactivation of endogenous miR-888, miR-892a or miR-891a activity in PC3-ML cells using individual miRNA inhibitors resulted in opposite phenotypes (Fig. 4B). In LNCaP cells, miR-888 and miR-892a (but not miR-891a) mimic treatment significantly increased the cells’ ability to grow in soft agar (Fig. 4C). Taken together, our functional in vitro assays indicated that multiple members of the miR-888 cluster selectively influence human prostate cancer cell proliferation, migration, invasion, as well as colony formation when compared in castration-resistant and hormone-sensitive human prostate cancer cell lines (Suppl. Table 3).
Figure 4. miR-888, miR-892a and miR-891a induced anchorage independent prostate cell growth and colony formation.
Transfected prostate cancer cells were grown in soft agar for 14 days to measure anchorage independent growth and colony formation. A, PC3-N cells individually transfected with miR-888, miR-892a or miR-891a mimics showed increased colony formation relative to cells treated with SCR control mimics. B, Opposite results were observed in PC3-ML cells treated with miRNA inhibitors. C, In LNCaP cells, miR-888 and miR-892a overexpression increased the ability of cells to grow in soft agar.
miR-888 and miR-891a promoted tumor formation in mice
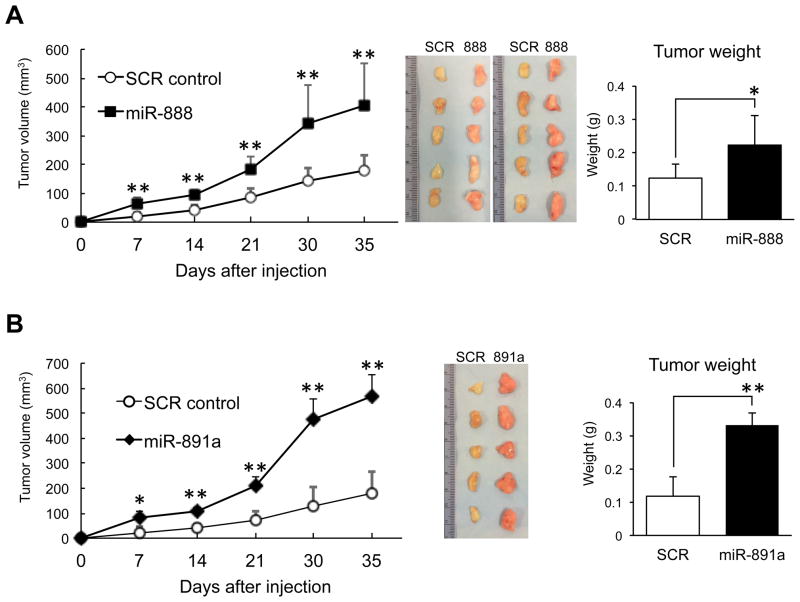
Due to similar pro-oncogenic effects observed for miR-888 and miR-891a in vitro, we tested if they promoted tumor formation in mice. Stable miR-888, miR-891 and SCR mimic overexpressing PC3-N prostate cell lines were generated using shMIMIC lentiviral miRNA vectors (MOI 5). We confirmed these lentivirus infected PC3-N cell lines overexpressing miR-888 or miR-891a exhibited increased proliferation, migration, invasion and colony formation in vitro (Suppl. Fig. S3A–E), reflecting our results using transfected miRNA mimics (Figs. 2–4). These stable miR-888 and miR-891a cell lines (lenti-888, lenti-891a) were subsequently tested in a mouse xenograft tumor model. miR-888 or miR-891a treated PC3-N cells (1 × 106) were mixed 1:1 v/v with Matrigel and injected subcutaneously into the left flank of ten male NOD/SCID mice. As a negative control, 1 × 106 PC3-N cells stably expressing SCR mimics were mixed 1:1 v/v with Matrigel and injected into the right flanks of these same animals. Tumor formation was recorded weekly using digital calipers and mice were sacrificed five weeks post-injection. As predicted, mice injected with PC3-N cells overexpressing miR-888 showed accelerated tumor growth and these tumors were significantly larger and heavier than SCR control tumors (Fig. 5A, Suppl. Fig. S4A). Similar findings were obtained in five male NOD/SCID mice injected with PC3-N cells overexpressing miR-891a (Fig. 5B, Suppl. Fig. S4B). Therefore, miR-888 and miR-891a act as proto-oncogenic factors in prostate cells.
Figure 5. miR-888 and miR-891a promoted tumor growth in mice.
A, PC3-N cell lines stably expressing miR-888 (mixed 1:1 with Matrigel) were implanted subcutaneously into the right flanks of male NOD/SCID mice (n = 10). PC3-N cells stably expressing SCR mimics (mixed 1:1 with Matrigel) were implanted into the left flanks of these same animals. miR-888 tumors grew faster than SCR control tumors (left panel). Mice were sacrificed 5 weeks post-injection. Dissected miR-888 tumors (middle panel) weighed more than SCR tumors (right panel). B, Similar mouse xenograft results were found for miR-891a (n = 5).
miR-888 inhibited RBL1, KLF5, TIMP2 and SMAD4 expression in prostate cells
Focusing on founding cluster member miR-888, we identified potential targets for this miRNA using the miRNA target prediction bioinformatic algorithm TargetScan. Of particular interest were factors i.) possessing miR-888 binding sites in their 3′UTR regions, ii.) underexpressed in prostate cancer patients, and iii.) reported with tumor suppressive and/or anti-metastatic functions. Four factors meeting this criteria warranted further validation; retinoblastoma like 1 (RBL1/p107), a negative cell cycle regulator (25,26); SMAD4, an intracellular TGF-β signaling molecule (27,28); Krupple-like factor 5 (KLF-5), a zinc-finger transcription factor (29,30); and tissue inhibitor of metalloproteinase 2 (TIMP2), an anti-metastatic factor that blocks matrix metalloproteinase activity (31) (Suppl. Fig. S5A). We interrogated these predicted miR-888 targets by cloning the 3′UTR regions of RBL1, KLF5, SMAD4 and TIMP2 (containing miR-888 complementary sequences) downstream of the renilla gene within the dual luciferase psiCHECK2 vector (Promega). The 3′UTR of SMAD4 contained two miR-888 binding sites that were ~4300 base pairs apart, and thus we generated two distinct SMAD4 luciferase constructs (MCS1 & MCS2). In addition to a TargetScan predicted site within the TIMP2 3′UTR (base #2114-2139), we also identified a second potential miR-888 binding site ~50 base pairs away. Therefore, both regions were included in a single TIMP2 luciferase construct. The luciferase constructs were tested in human PC3-N prostate cancer cell lines stably overexpressing miR-888 (via shMIMIC Lentiviral vectors, Suppl. Fig. S3A). Indeed, transfected PC3-N cells overexpressing miR-888 exhibited reduced renilla luciferase expression (relative to firefly luciferase levels) compared to PC3-N cells stably expressing SCR mimics (Fig. 6A). This indicated that RBL1, KLF5, SMAD4 and TIMP2 were negatively regulated by miR-888 in a 3′UTR dependent manner.
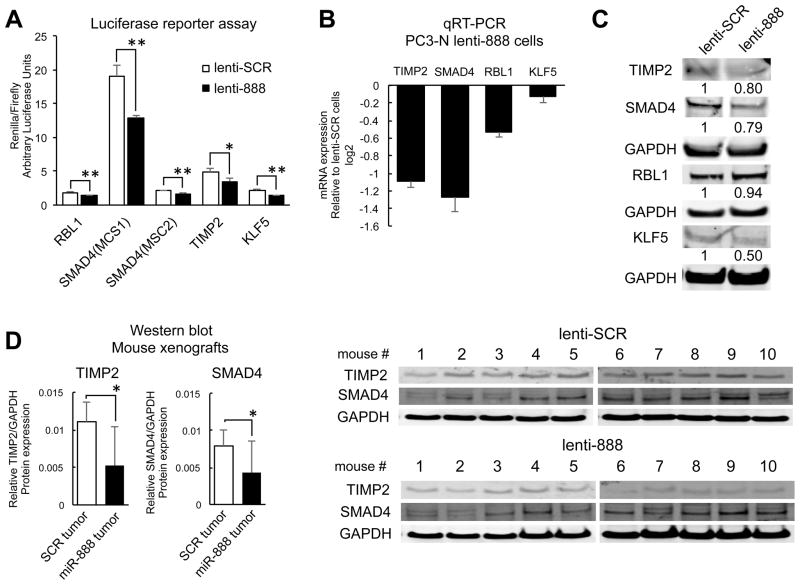
Figure 6. RBL1, KLF5, TIMP2, and SMAD4 are targeted by miR-888 in prostate cells.
A, Luciferase reporter assays measured renilla/firefly levels of prostate cells transfected with the dual luciferase psiCHECK2 vector that contained cloned 3′UTR elements (harboring predicted miR-888 binding sites) for RBL1, SMAD4 (MiRNA Complementary Site 1 or 2), TIMP2, or KLF5 downstream of the renilla cassette. PC3-N cells stably overexpressing miR-888 (lenti-888) significantly repressed renilla expression compared to cells stably overexpressing SCR control mimics (lenti-SCR). Reduced mRNA (B) and protein (C) levels of TIMP2, SMAD4, RBL1 and KLF5 were observed in vitro for PC3-N lenti-888 cells relative to lenti-SCR control cells. D, Western blot analysis of paired PC3-N prostate tumors overexpressing miR-888 or SCR control mimics grown subcutaneously for 5 weeks in the flanks of ten NOD/SCID mice. TIMP2 and SMAD4 protein was reduced in miR-888 treated tumors compared to SCR tumors. Expression was normalized to human 18S rRNA (qRT-PCR) or GAPDH protein (Western blot).
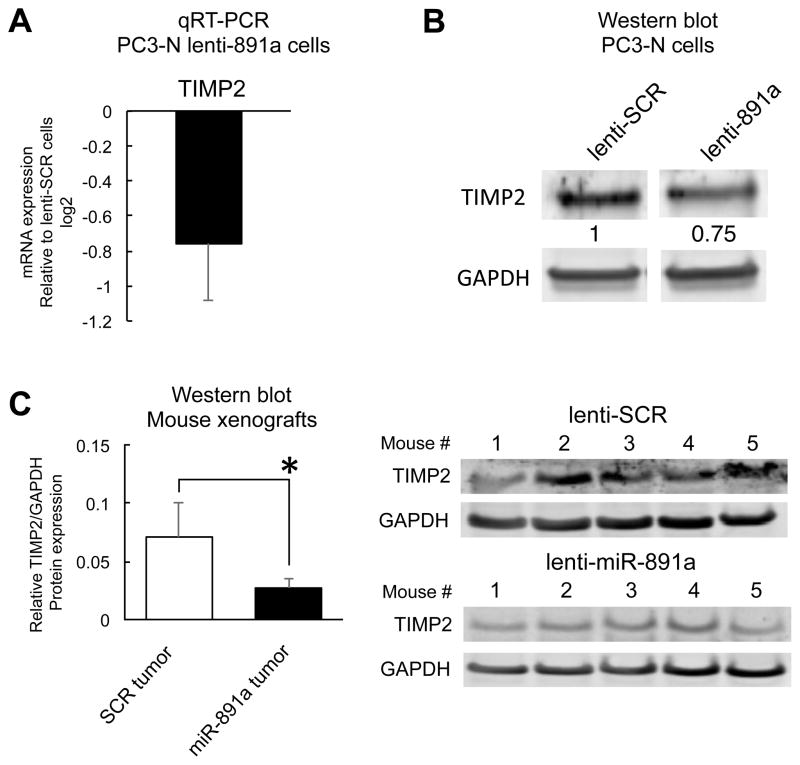
To further confirm that miR-888 repressed RBL1, KLF5, TIMP2 and SMAD4 expression in prostate cells, we isolated total RNA and cell protein lysates from stable lentiviral-infected miR-888 and SCR overexpression PC3-N and LNCaP cell lines (Suppl. Figs. S3A, S6A). Both mRNA and protein levels for RBL1, KLF5, TIMP2 and SMAD4 were reduced in the presence of miR-888 via qRT-PCR and western blot analysis (Fig. 6B–C; Suppl. Fig. S6B). We noted cell type-specific differences between PC3-N and LNCaP. LNCaP lenti-888 cells showed marked RBL-1 protein downregulation (Suppl. Fig. S6B) and did not express appreciable amounts of TIMP2 or KLF5 protein (Suppl. Fig. S6B, previously reported (30),(32)). Thus, additional miR-888 targets, e.g. RBL1 and SMAD4, are likely regulated by miR-888 to influence prostate cell growth, migration/invasion, and colony formation in this cell line. We explored a role for miR-888 in targeting the tumor suppressors RBL1 and TIMP2 in order to promote prostate cell proliferation and invasion. Forced expression of RBL1 or TIMP2 could reverse miR-888’s oncogenic effects in our Matrigel Boyden chamber invasion and WST-1 proliferation assays, and reciprocally RBL1 and TIMP2 RNAi treatment induced prostate growth in a similar manner as miR-888 mimics (Suppl. Fig. S7). Furthermore, TIMP2 and SMAD4 protein were suppressed in vivo by miR-888 in prostate tumors obtained from our mouse xenograft studies (Fig. 5A; Fig. 6D). Intriguingly, TIMP2 also possesses a predicted miR-891a binding site in its 3′UTR (TargetScan, Suppl. Fig. S5B). We thus tested if TIMP2 levels were downregulated in PC3-N cell lines stably overexpressing miR-891a as well as in mouse xenograft tumors treated with this miRNA (Suppl. Fig. S4B; Fig. 5B). TIMP2 levels were suppressed in the presence of miR-891a (Fig. 7A–C). Therefore, the miR-888 cluster may co-regulate common targets associated in prostate cancer progression.
Figure 7. miR-891a modulates TIMP2 mRNA and protein expression in the prostate.
TIMP2 mRNA (A) and protein (B) levels were reduced in PC3-N cells stably overexpressing miR-891a (lenti-891a) relative to those stably overexpressing SCR control mimics (lenti-SCR). C, Mouse xenograft tumors grown subcutaneously in five NOD/SCID mice for 5 weeks showed that PC3-N tumors treated with miR-891a had reduced TIMP2 protein expression compared to PC3-N tumors stably overexpressing SCR control mimics.
Discussion
This is the first comprehensive analysis of the entire miR-888 cluster in any tissue and we focused on their role in the human prostate. Our lab previously identified miR-888 as a novel prostate factor that promotes cell growth, migration, and colony formation and is elevated in patients with advanced prostate cancer (3). We hypothesized miR-888 was part of a larger oncogenic network of noncoding RNAs that influenced prostate tumor progression. Indeed, the miR-888 cluster mapping on human chromosome Xq27.3, is located within the elusive hereditary prostate cancer HPCX1 locus (20,33). We found that cluster member expression correlated in patients with high-grade prostate disease. Using in vitro and in vivo assays, we confirmed that the miR-888 cluster modulated multiple cancer-related activities in both androgen responsive and castration resistant human prostate cancer cell lines. We showed miR-888 and miR-891a were pro-oncogenic factors that similarly accelerated prostate tumor formation and increased tumor size in mice. miR-888 negatively regulated the tumor suppressors RBL1, KLF5, SMAD4 and TIMP2 in prostate cells in a 3′UTR dependent manner and their expression was reduced in prostate cells treated with miR-888 mimics. Our studies implicated that miR-888 mediated-suppression of at least two of these factors (RBL1 and TIMP2) are involved in promoting prostate cell growth and invasion. We also provided evidence that the miR-888 cluster may co-regulate common targets such as TIMP2 in the prostate. These results provide valuable insight into the molecular mechanisms leading to aggressive, lethal prostate disease.
Approximately 50% of human miRNA genes are organized in genomic clusters ranging from 2 to as many as 46 miRNAs (34,35). MiRNA clusters often share high sequence homology and exhibit overlapping expression patterns (36), suggesting that non-coding RNA clusters are co-regulated and control common cellular processes. We found that miR-888 cluster members possess similar expression profiles in a panel of human prostate cell lines differing in metastatic status and response to androgen. Specifically, miR-888 cluster expression correlated with the metastatic status of PC3-derived cell lines and the cluster members were preferentially enriched in aggressive PC3-ML and underexpressed in non-aggressive PC3-N cells. This syngeneic pair is derived from parental PC3, an established cell line originating from the bone metastasis of a prostate cancer patient (21). PC3 cells are commonly studied to represent advanced hormone-refractory prostate disease in vitro and share characteristics with neuroendocrine prostate cancer tumors (37), a late-stage, drug-resistant, and particularly aggressive form of prostate cancer. miR-888 cluster dysregulation in the prostate may therefore contribute to castration-resistance disease.
Our in vitro studies reveal functional complexities for the miR-888 cluster (Suppl. Table 3). For example, when testing all seven miR-888 cluster members using WST-1 assays, miR-888, miR-891a and miR-891b treatment most significantly increased prostate cell proliferation in hormone-refractory PC3-N cells, whereas miR-892a, miR-892b and miR-891a treatment induced the greatest growth effects in hormone-sensitive LNCaP cells. Published reports for miR-892a and miR-892b support an oncogenic role; miR-892a promotes colorectal cancer cell proliferation (38) and miR-892b expression is elevated in non-small-cell lung cancer patients (39). Not all factors within the miR-888 cluster exhibited pro-oncogenic phenotypes however, and miR-890 and miR-892c mimic treatment repressed prostate cell growth and migration in our assays. Hatano et al. also reported a tumor suppressive role for miR-890 (40). All miR-888 cluster members enhanced PC3-N invasion through Matrigel in our Boyden chamber assays, which may reflect a shared pro-oncogenic role for this activity. Taken together, miR-888, miR-891a, and miR-892a most consistently exhibited pro-oncogenic behaviors in our functional assays, but suppressive effects observed for other miR-888 cluster members (miR-890 and miR-892c) underscore the intricacies of this non-coding RNA cluster network in the prostate.
The oncogenic and suppressive abilities of the miR-888 cluster are reminiscent of the miR-17~92 cluster, located on human chromosome 13q31 (mir-17, mir-18a, mir-19a, mir-20a, mir-19b-1, mir-92a). The miR-17~92 cluster is best characterized for its oncogenic activities in leukemia and lung cancer models, and miR-19 is the primary cluster factor responsible for promoting accelerated malignancies in animals (41,42). The miR-17~92 cluster is also reported to repress angiogenesis and may be tumor suppressive in certain cancer contexts, i.e., heterozygous deletions are noted in ovarian cancer, breast cancer, melanomas, and hepatocellular carcinomas (43) (41). Cluster member miR-17 directs anti-proliferative and pro-apoptotic activities in breast cancer cells (44) and miR-17 overexpression in transgenic mice cause stunted growth, small organ size, and reduction of hematopoietic cell linages (41). Reminiscent to these paradoxical roles for the miR-17~92 cluster, our data indicates that specific members of the miR-888 cluster regulate converse effects on cell growth and migration. These antagonistic abilities may be cell type and/or context dependent and could suggest a role for the miR-888 cluster in maintaining prostate homeostasis. Aberrations in miR-888 cluster expression might destabilize prostate function and lead to accelerated growth, tumor formation, and disease progression. Distinct miR-888 cluster activities in our in vitro assays could further reveal genetic distinctions between PC3 cells (castration-resistant, neuroendocrine-like, bone metastasis derived) and LNCaP cells (androgen-responsive, adenocarcinoma, lymph node metastasis derived) as well as unique miRNA target repertoires in these cell lines. Our expression analysis for the miR-888 targets TIMP2, SMAD4, RBL1, and KLF5 in PC3-N and LNCaP cells (Fig. 6B–C, Suppl. Fig. S6A–B) indicated that these tumor suppressors are differentially expressed (and potentially differentially miRNA-regulated) in a cell type specific manner, supporting this notion. Further studies in various organ systems and disease contexts will be required to molecularly dissect the miR-888 cluster.
We subsequently focused on the miR-743 family member, miR-888, and the miR-891 family member, miR-891a, and confirmed that both miRNAs accelerated prostate tumor growth in mice. We initially identified miR-888 and miR-891a in a miRNA-profiling screen, as two of the most differentially expressed miRNAs in aggressive PC3-ML versus non-aggressive PC3-N prostate cell lines (3). Similar to miR-888, elevated miR-891a expression closely correlates with human cancers outside of the prostate. miR-891a is elevated in chromophobe renal cell carcinoma (chRCC) (8), osteosarcoma (45), and non-small-cell lung cancer (46). Functionally, miR-891a (as well as miR-891b) enhances hepatocellular carcinoma (HCC) cell migration (47) and miR-891a is associated with Kaposi’s sarcoma to promote Tat- and K1-induced angiogenesis by targeting IKB alpha (48). We have evidence to suggest that miR-888 and miR-891a act additively/synergistically to promote prostate cell growth (Suppl. Fig S8A). More work is needed to determine how all seven miR-888 cluster members functionally overlap in the prostate and mediate cancer progression.
Furthermore, miR-888 represses the tumor suppressor genes RBL1, SMAD4, KLF5 and TIMP2 in a 3′UTR dependent manner. RBL1 blocks G1-S phase cell cycle progression and expression is decreased in prostate cancer patients in a reciprocal manner to miR-888 (49) (3). KLF5 is one of the most frequent genetic deletions observed in prostate cancer patients (29) and KLF5 loss in PTEN-deficient mice promotes prostate cell proliferation and accelerated tumor formation (29). TargetScan and Microcosm bioinformatic algorithms predict miR-892a and miR-890 binding sites in the KLF5 3′UTR, further implying an interconnected miR-888 cluster network in the prostate. TIMP2 belongs to a family of four metalloproteinases inhibitors (TIMP 1–4), which are often down-regulated in prostate tumors and play critical roles in prostate tumor progression, invasion, metastasis and angiogenesis (50–52). TIMP2 contains binding sites for both miR-888 and miR-891a within its 3′UTR (TargetScan) and may be co-regulated by the miR-888 cluster. Indeed, cell lines and xenograft prostate tumors treated with miR-891a exhibited reduced TIMP2 mRNA and protein levels. Additional miR-888 cluster members have predicted miRNA complementary sites within the TIMP2 3′UTR (miR-891b and miR-892b) as well as in other TIMP family members (miR-891a, miR-892b and miR-890 for TIMP3; miR-892b for TIMP4), which awaits further validation. Lastly, the miR-888 target SMAD4 is closely associated with prostate cancer progression. Decreased SMAD4 expression correlates in prostate cancer patients with aggressive disease, high Gleason score, metastasis, and poor prognosis (27,28). Loss of SMAD4 in PTEN-deficient mice result in accelerated prostate tumor formation as well as 100% penetrance of lethal, metastatic prostate cancer (27). Interestingly, miR-891b is predicted to target PTEN, a gene mutated in 60% of prostate cancer cases (53). Taken together, the miR-888 cluster likely co-regulates multiple signaling pathways associated with prostate cancer progression and metastasis.
Finally, our work showed that miR-888 cluster members were present in exosomes isolated from aggressive human prostate cancer cells and in EPS fluids, particularly from patients with high-grade prostate cancer. Exosomes play important roles in cell-to-cell communication and exosomal miRNAs can be transferred to recipient cells and mediate downstream effects (54). miR-888 cluster members packaged in exosomes isolated from prostate cells and patient fluids may therefore have functional importance in prostate cancer progression. Exosomal miRNA profiles in various human cancers highlight their use as diagnostics (55,56). miR-888 cluster expression from exosomes could be developed as early-detection fluid-based biomarkers that distinguish between indolent and aggressive prostate disease. Ye et al. reported that miR-891a was commonly overexpressed in exosomes isolated from cells and sera of nasopharyngeal carcinoma (NPC) patients versus exosomes isolated from healthy donor sera or normal nasopharyngeal cells (57). Thus, miR-888 cluster members may be useful for a wide range of diagnostic applications.
In conclusion, we have characterized a novel group of oncomiRs (miRNAs associated with cancer) expressed in prostate cells that influence proliferation, migration, invasion, and tumor formation (Suppl. Fig. S8B). Our work indicates that members of the miR-888 cluster act in a complex non-coding RNA network to regulate cancer progression and are promising clinical tools for prostate cancer. In particular, miR-888 and miR-891a are viable candidates for anti-miRNA therapy to block prostate tumorigenesis.
Supplementary Material
Acknowledgments
Financial support: This work was supported by grants to AE-K from the National Cancer Institute (R21CA175894), Department of Defense (PC131691), EVMS Grant Enhancement Fund, Breedan Adams Foundation, Edmondson Fund, Coach Ray Barlow Prostate Cancer Research Fund, EVMS Prostate Cancer Research Funds.
Technical advice was provided by Leonora Balaj (exosome isolation) and Amy Tang & Gregory Nicholson (mouse injections). Phillip Austin Serbin aided in RNA isolation. Raymond Lance, Laurie Wellman, Mary Ann Clements and Brian Main assisted in specimen and clinical data retrieval of EPS Urine from the EVMS Biorepository. PC3-N and PC3-ML were kindly provided by Mark Stearns (Drexel U) and C4-2 cells by Evans Keller (U Michigan). Financial support was provided to AE-K (PI) by the National Institutes of Health (R21CA175894, co-I RD, collaborator OJS), Department of Defense (PC131691), EVMS Grant Enhancement Fund, Breedan Adams Foundation, Edmondson Fund, Coach Ray Barlow Prostate Cancer Research Funds, and EVMS Prostate Cancer Research Funds.
Footnotes
Conflict of interest disclosure statement: We have no financial conflicts to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa T, Lewis H, Esquela-Kerscher A. Chapter 12 – The Role of Noncoding RNAs in Prostate Cancer. In: Laurence J, editor. Translating MicroRNAs to the Clinic. Boston, Massachusetts: Academic Press; 2017. pp. 329–69. [Google Scholar]
- 3.Lewis H, Lance R, Troyer D, Beydoun H, Hadley M, Orians J, et al. miR-888 is an expressed prostatic secretions-derived microRNA that promotes prostate cell growth and migration. Cell Cycle. 2014;13:227–39. doi: 10.4161/cc.26984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol. 2010;42:1316–29. doi: 10.1016/j.biocel.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Xiao M, Li J, Li W, Wang Y, Wu F, Xi Y, et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2016:1–9. doi: 10.1080/15476286.2015.1112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valinezhad Orang A, Safaralizadeh R, Kazemzadeh-Bavili M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int J Genomics. 2014;2014:970607. doi: 10.1155/2014/970607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 8.Youssef YM, White NM, Grigull J, Krizova A, Samy C, Mejia-Guerrero S, et al. Accurate molecular classification of kidney cancer subtypes using microRNA signature. Eur Urol. 2011;59:721–30. doi: 10.1016/j.eururo.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Bobowicz M, Skrzypski M, Czapiewski P, Marczyk M, Maciejewska A, Jankowski M, et al. Prognostic value of 5-microRNA based signature in T2-T3N0 colon cancer. Clin Exp Metastasis. 2016;33:765–73. doi: 10.1007/s10585-016-9810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Cai M, Zheng Y, Zhou L, Wang Q, Chen L. miR-888 in MCF-7 side population sphere cells directly targets E-cadherin. J Genet Genomics. 2014;41:35–42. doi: 10.1016/j.jgg.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Devor EJ, Hovey AM, Goodheart MJ, Ramachandran S, Leslie KK. microRNA expression profiling of endometrial endometrioid adenocarcinomas and serous adenocarcinomas reveals profiles containing shared, unique and differentiating groups of microRNAs. Oncol Rep. 2011;26:995–1002. doi: 10.3892/or.2011.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovey AM, Devor EJ, Breheny PJ, Mott SL, Dai D, Thiel KW, et al. miR-888: A Novel Cancer-Testis Antigen that Targets the Progesterone Receptor in Endometrial Cancer. Transl Oncol. 2015;8:85–96. doi: 10.1016/j.tranon.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S, Chen L. MiR-888 regulates side population properties and cancer metastasis in breast cancer cells. Biochem Biophys Res Commun. 2014;450:1234–40. doi: 10.1016/j.bbrc.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Liu Y, Dong D, Zhang Z. Evolution of an X-linked primate-specific micro RNA cluster. Mol Biol Evol. 2010;27:671–83. doi: 10.1093/molbev/msp284. [DOI] [PubMed] [Google Scholar]
- 16.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–14. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belleannee C, Calvo E, Thimon V, Cyr DG, Legare C, Garneau L, et al. Role of microRNAs in controlling gene expression in different segments of the human epididymis. PLoS One. 2012;7:e34996. doi: 10.1371/journal.pone.0034996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Wang HY, Wan FC, Liu FJ, Liu J, Zhang N, et al. Deep sequencing analysis of small non-coding RNAs reveals the diversity of microRNAs and piRNAs in the human epididymis. Gene. 2012;497:330–5. doi: 10.1016/j.gene.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Meyers D, Freije D, Isaacs S, Wiley K, Nusskern D, et al. Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat Genet. 1998;20:175–9. doi: 10.1038/2477. [DOI] [PubMed] [Google Scholar]
- 20.Farnham JM, Camp NJ, Swensen J, Tavtigian SV, Albright LA. Confirmation of the HPCX prostate cancer predisposition locus in large Utah prostate cancer pedigrees. Hum Genet. 2005;116:179–85. doi: 10.1007/s00439-004-1220-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Stearns ME. Isolation and characterization of PC-3 human prostatic tumor sublines which preferentially metastasize to select organs in S.C.I.D. mice. Differentiation. 1991;48:115–25. doi: 10.1111/j.1432-0436.1991.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, van den Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D, et al. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993;7:1111–25. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]
- 26.Claudio PP, Howard CM, Baldi A, De Luca A, Fu Y, Condorelli G, et al. p130/pRb2 has growth suppressive properties similar to yet distinctive from those of retinoblastoma family members pRb and p107. Cancer Res. 1994;54:5556–60. [PubMed] [Google Scholar]
- 27.Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–73. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang DT, Shi JG, Liu Y, Jiang HM. The prognostic value of Smad4 mRNA in patients with prostate cancer. Tumour Biol. 2014;35:3333–7. doi: 10.1007/s13277-013-1439-y. [DOI] [PubMed] [Google Scholar]
- 29.Xing C, Ci X, Sun X, Fu X, Zhang Z, Dong EN, et al. Klf5 deletion promotes Pten deletion-initiated luminal-type mouse prostate tumors through multiple oncogenic signaling pathways. Neoplasia. 2014;16:883–99. doi: 10.1016/j.neo.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C, Bhalala HV, Vessella RL, Dong JT. KLF5 is frequently deleted and down-regulated but rarely mutated in prostate cancer. Prostate. 2003;55:81–8. doi: 10.1002/pros.10205. [DOI] [PubMed] [Google Scholar]
- 31.Ozden F, Saygin C, Uzunaslan D, Onal B, Durak H, Aki H. Expression of MMP-1, MMP-9 and TIMP-2 in prostate carcinoma and their influence on prognosis and survival. J Cancer Res Clin Oncol. 2013;139:1373–82. doi: 10.1007/s00432-013-1453-x. [DOI] [PubMed] [Google Scholar]
- 32.Pulukuri SM, Patibandla S, Patel J, Estes N, Rao JS. Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors. Oncogene. 2007;26:5229–37. doi: 10.1038/sj.onc.1210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kouprina N, Lee NC, Pavlicek A, Samoshkin A, Kim JH, Lee HS, et al. Exclusion of the 750-kb genetically unstable region at Xq27 as a candidate locus for prostate malignancy in HPCX1-linked families. Genes Chromosomes Cancer. 2012;51:933–48. doi: 10.1002/gcc.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker LE, Lu Z, Chen W, Xiong W, Kong M, Li Y. A systematic screen reveals MicroRNA clusters that significantly regulate four major signaling pathways. PLoS One. 2012;7:e48474. doi: 10.1371/journal.pone.0048474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, et al. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. Rna. 2005;11:241–7. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011;71:1668–79. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang WL, Cao J, Xu B, Yang P, Shen F, Sun Z, et al. miR-892a regulated PPP2R2A expression and promoted cell proliferation of human colorectal cancer cells. Biomed Pharmacother. 2015;72:119–24. doi: 10.1016/j.biopha.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Hu L, Ai J, Long H, Liu W, Wang X, Zuo Y, et al. Integrative microRNA and gene profiling data analysis reveals novel biomarkers and mechanisms for lung cancer. Oncotarget. 2016;7:8441–54. doi: 10.18632/oncotarget.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatano K, Kumar B, Zhang Y, Coulter JB, Hedayati M, Mears B, et al. A functional screen identifies miRNAs that inhibit DNA repair and sensitize prostate cancer cells to ionizing radiation. Nucleic Acids Res. 2015;43:4075–86. doi: 10.1093/nar/gkv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olive V, Li Q, He L. mir-17-92: a polycistronic oncomir with pleiotropic functions. Immunol Rev. 2013;253:158–66. doi: 10.1111/imr.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, et al. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009;23:2839–49. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang J, Wu J. Feud or Friend? The Role of the miR-17-92 Cluster in Tumorigenesis. Curr Genomics. 2010;11:129–35. doi: 10.2174/138920210790886853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006;26:8191–201. doi: 10.1128/MCB.00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Won KY, Kim YW, Kim HS, Lee SK, Jung WW, Park YK. MicroRNA-199b-5p is involved in the Notch signaling pathway in osteosarcoma. Hum Pathol. 2013;44:1648–55. doi: 10.1016/j.humpath.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Lee H-Y, Han S-S, Song S-Y. Serum microRNAs as potential biomarkers for lung cancer. Annals of Oncology. 2016:27. [Google Scholar]
- 47.Zha R, Guo W, Zhang Z, Qiu Z, Wang Q, Ding J, et al. Genome-wide screening identified that miR-134 acts as a metastasis suppressor by targeting integrin beta1 in hepatocellular carcinoma. PLoS One. 2014;9:e87665. doi: 10.1371/journal.pone.0087665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao S, Hu M, Hao T, Li W, Xue X, Xue M, et al. MiRNA-891a-5p mediates HIV-1 Tat and KSHV Orf-K1 synergistic induction of angiogenesis by activating NF-kappaB signaling. Nucleic Acids Res. 2015;43:9362–78. doi: 10.1093/nar/gkv988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claudio PP, Zamparelli A, Garcia FU, Claudio L, Ammirati G, Farina A, et al. Expression of cell-cycle-regulated proteins pRb2/p130, p107, p27(kip1), p53, mdm-2, and Ki-67 (MIB-1) in prostatic gland adenocarcinoma. Clin Cancer Res. 2002;8:1808–15. [PubMed] [Google Scholar]
- 50.Dong Z, Nemeth JA, Cher ML, Palmer KC, Bright RC, Fridman R. Differential regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 expression in co-cultures of prostate cancer and stromal cells. Int J Cancer. 2001;93:507–15. doi: 10.1002/ijc.1358. [DOI] [PubMed] [Google Scholar]
- 51.Brehmer B, Biesterfeld S, Jakse G. Expression of matrix metalloproteinases (MMP-2 and -9) and their inhibitors (TIMP-1 and -2) in prostate cancer tissue. Prostate Cancer Prostatic Dis. 2003;6:217–22. doi: 10.1038/sj.pcan.4500657. [DOI] [PubMed] [Google Scholar]
- 52.Adissu HA, McKerlie C, Di Grappa M, Waterhouse P, Xu Q, Fang H, et al. Timp3 loss accelerates tumour invasion and increases prostate inflammation in a mouse model of prostate cancer. Prostate. 2015;75:1831–43. doi: 10.1002/pros.23056. [DOI] [PubMed] [Google Scholar]
- 53.Phin S, Moore MW, Cotter PD. Genomic Rearrangements of PTEN in Prostate Cancer. Front Oncol. 2013;3:240. doi: 10.3389/fonc.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–21. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11:145–56. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 56.Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. doi: 10.3402/jev.v5.31292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–52. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.