Abstract
Background
The objective of this study was to evaluate creatine as an anti-nociceptive compound in an animal model of thermal and inflammatory pain. Creatine has the structural potential to interact with acid-sensing ion channels (ASIC), which have been involved in pain sensation modulation. The hypothesis evaluated in this study was that creatine will interact with ASICs leading to decreased nociception.
Methods
Male and female C57BL/6J mice were fed with either a control diet or the control diet supplemented with creatine (6.25g/kg diet). After one week on the diet, the mice were tested for thermal hyperalgesia and inflammatory pain response.
Results
The latency to withdraw the tail during the thermal hyperalgesia test was unaffected by sex or diet. During the formalin test, males and females responded differently to the stimulus, and the female mice supplemented with creatine seemed to recover faster than the controls. To determine whether ASICs mediate the action of creatine, GMQ, an ASIC3 agonist, was injected in one paw and pain response was quantified. Females responded more strongly to GMQ injections, and all mice fed creatine had a decreased response to GMQ.
Conclusions
These preliminary data suggest a potential effect of creatine on inflammation-based nociception that may be mediated via ASIC3. While preliminary, this study warrants further research on the potential of creatine as an analgesic and can serve as a stepping stone for the development of ASIC-based therapeutics.
Keywords: Creatine supplementation, Mouse model of pain, Inflammatory pain, Sex differences, Acid sensing ion channels
Introduction
Millions suffer from acute and chronic pain, and the costs have reached over $600 billion each year due to treatments and loss of productivity according to the Institute of Medicine of the National Sciences [1]. While treatments, such as use of opioids, are available, there remains many issues associated with their use, such as addiction [2]. Therefore alternatives are sought after to replace conventional therapies for pain. One such avenue is the study of acid-sensing ion channels (ASICs) [3, 4]: channels that have been associated with pain sensation, mechanosensitivity and synaptic plasticity [5, 6].
These channels are trimeric, proton-gated transmembrane channels that belong to the epithelial sodium channel/degenerin family [6], are sensitive to changes in extracellular pH [7, 8], and exists in 6 known isoforms [9, 10]. In recent studies, nonproton ligands have been determined to modulate the activity of ASICs: amiloride blocks ASICs in a non-selective manner [11]; GMQ (2-guanidine-4-methylquinazoline) can activate ASIC3 in vitro [12] and leads to a stimulating response when binding to the nonproton ligand binding site [5]; and Anthopleura Elegantissima toxin 2 (APETx2), a sea anemone-derived toxin, has ASIC3-specific blocking effects [13].
ASICs are expressed in peripheral nociceptive neurons and in brain areas associated with pain processing [5, 6, 9], especially homomeric ASIC3, which is highly expressed in sensory neurons in the periphery and in the dorsal root ganglion in the spinal cord [9, 14]. Furthermore, conditions causing pain are often associated with tissue acidosis and inflammation [15]. In a human model of acid-induced pain, local amiloride administration reduced the magnitude of pain perception [16]. Inflammation induced by complete Freund’s adjuvant (CFA) leads to increased mRNA levels of ASICs, including ASIC 3 [17], and inflammatory mediators such as nerve growth factor increased sensory neuron excitability and ASIC3 gene transcription [18]. Inflammation-activated signals also activate ASIC3 on nociceptors and initiate pain signaling cascade [5].
As previously mentioned, ASIC3 ligands can alter electrophysiological aspects of ASICs and have been used to demonstrate the implication of ASICs in in vivo nociceptive sensitivity [5, 11, 19, 20]. Local intramuscular and spinal injections of APETx2 prevented mechanical hypersensitivity but did not reduce it once the hyperalgesia was established using an acid-induced pain model [19]. In the same study, inflammatory pain produced by CFA was reduced by APETx2 injection. In a study evaluating the nociceptive effects of the ASIC3 agonist GMQ, wild-type mice injected with GMQ in their hind paw displayed an increase in paw licking time compared to the transgenic mice lacking ASIC3 (asic3−/−) [12]. The increase of the nociceptive paw licking behavior in response to GMQ provided evidence of the involvement in pain and activation of ASIC3 channels in vivo.
Ligands involved in pain modulation of ASIC3, amiloride and GMQ, both have a guanidium group, a common feature for ligands interacting with ASIC3 [12]. A widely available compound, creatine, possesses a modified guanidium group which might confer creatine the ability to interact with ASIC3 and modulate pain sensitivity (along with unpublished electrophysiology results suggesting a possible ASIC modulation by creatine). Creatine is an endogenous organic acid known for its role in cellular metabolism that is used as a buffer to replenish ATP levels [21]. As a dietary supplement, creatine is widely used for enhancing exercise performance [22]. However, its oral consumption has been associated with other reported and proposed health benefits, including neuroprotective effects against ischemic damage and antioxidative protection [21, 23]. Recent human studies demonstrated that creatine supplementation improved quality of life, sleep and reduced pain associated with fibromyalgia following 8 weeks of treatment along with the patient’s regular medications [24, 25]. On the other hand, a 16-week creatine diet treatment produced no changes in pain perception in another group of fibromyalgia patients [26]. Evidence from human studies is not consistent, which may be due to different loading dose, creatine supplementation duration and different types of pain measured. Based on these observations, the current study was aimed to determine whether creatine supplementation can reduce nociceptive sensitivity in a mouse model of thermal and inflammatory pain, and whether the effects of creatine could potentially be mediated via ASIC3.
Materials and methods
Animals
Ninety-five 2–3 months old male and female C57BL/6J mice were obtained from Jackson Laboratories, and acclimated at the UNT Health Science Center vivarium prior to any manipulations. Procedures pertaining to animal handling and maintenance adhered to the NIH guidelines and were approved by the UNT Health Science Center Institutional Animal Care and Use Committee. Each mouse was injected subcutaneously between the shoulder blades with an identification chip (2 × 13 mm biologically inert, Biomark) using a monoject syringe. The mice were separated into two experiments: Squad 1: sixty mice that were used for thermal hyperalgesia and formalin test after one week on treatment; Squad 2: thirty-five mice that were used for GMQ test after 10 days on treatment. All mice were monitored for body weights throughout the study. Food intake was measured daily for five days, starting three days into diet supplementation.
Diets
Mice were randomly assigned to one of the two experimental groups: control group was fed a control diet (Purina LabDiet® cat #: 1813505) or creatine group was fed the control diet supplemented with 6.25 g of creatine/kg diet (Purina TestDiet®; cat #: 1816777-201) (Table 1). Creatine monohydrate ≥98% was purchased from Sigma Aldrich (cat #C3630) and added to the diet by Purina. Animals had ad libitum access to food and water, and were group housed by sex and diet assignment (3–5 animals per cage). Animals were placed under a 12 hour light/dark cycle and all housing and procedures were approved by the UNT Health Science Center Institutional Animal Care and Use Committee. The mice were kept on the diet for one week prior to and throughout testing. Three days after the start of the diet, food intake was measured daily for four days.
Table 1.
Composition of control and creatine diets
| Control | Creatine | |
|---|---|---|
|
| ||
| Creatine, g/kg | N.D.* | 6.25 |
| Protein, g/kg | 183.00 | 174.00 |
| Fat (ether extract), g/kg | 51.00 | 46.00 |
| Fat (acid hydrolysis), g/kg | 60.00 | 55.00 |
| Fiber (max), g/kg | 45.00 | 44.00 |
| Gross energy, kcal/g | 34.30 | 34.00 |
| Ash, g/kg | 62.00 | 63.00 |
| Nitrogen-free extract (by difference), g/kg | 560.00 | 573.00 |
N.D.: not determined, less than 0.05 g/kg diet based on expected recovery.
Vitamins(mg/kg): Choline chloride, 2000-1988; Niacin, 86-83; Pantothenic acid, 31-30; Pyridoxine, 10-9.93; Riboflavin, 9; Thiamine hydrochloride, 26-24; Folic acid, 2; Biotin, 0.3; Carotene, 1.9-1.5; Vitamin K as menadione,22.2-14.9; Vitamin B12, 51-75; Vitamin A, 8; Vitamin D3, 4; Vitamin E, 45.
Minerals (mg/kg): Calcium, 1210-1150; Phosphorus, 9300-9200; Phosphorus (non-phytate), 6680-6800, Sodium,; Chlorine, 4500-4800; Potassium, 6000-6100; Magnesium, 2200; Sulfur, 3300-3000; Iron, 369; Manganese, 154-147; Zinc, 84-90; Copper, 10; Iodine, 2.15-2.12; Cobalt, 0.79-1.09; Selenium, 0.33-0.34; Fluorine, 35.7-7.9; Chromium, 1.94-0.56
Thermal hyperalgesia
The tail immersion test was used to examine thermal hyperalgesia, which models acute pain. Mice were allowed 10 minutes to acclimate to the testing room. Mice were lightly restrained and the distal portion of the tail was immersed in a water bath at 52°C for a maximum latency of 10 seconds. The latency to flex the tail during immersion was recorded. Mice received 3 trials with 1 minute between each. The average latency over the 3 trials was measured and analyzed.
Inflammatory pain
Mice were allowed 10 minutes to acclimate to the testing room and more specifically to the chamber. Each mouse was lightly restrained and injected subcutaneously in their right hind paw with 30 μl of 4% formalin solution, using a 1000 μl U-100 microfine syringe with a 27G needle. Immediately after injection, each mouse was placed in the test chamber (plexiglass box 11 × 8.5 × 15 cm) with mesh flooring elevated 45 cm from the table, supported by PVC tubes and a mirror placed at a 45° angle under the mesh for observation of nociceptive behaviors) and observed for 60 minutes. Behaviors were recorded for 60 minutes. The time spent licking the injected paw was averaged over 10 minute periods and analyzed.
GMQ injections
As with the formalin test, the mice were allowed 10 minutes to acclimate to the testing room. Each mouse was lightly restrained and injected subcutaneously in their left hind paw with 30 μl of 1mM GMQ solution, using a 1000 μl U-100 microfine syringe with a 27G needle. After injection, each mouse was placed in the test chamber (plexiglass box 11 × 8.5 × 15 cm) with mesh flooring elevated 45 cm from the table, supported by PVC tubes and a mirror placed at a 45° angle under the mesh for observation of nociceptive behaviors) and observed for 46 minutes. The time spent licking the injected paw was averaged and analyzed (only the mice with over 10s of licking where used in the results: 5/35 mice did not reach this criterion).
Statistical analysis
The effects of diet and sex on body weights, food intake and nociceptive response to formalin were assessed using three-way analysis of variance (ANOVA) with weeks, days or time-bin as the repeated measure. The effects of diet and sex on thermal hyperalgesia and response to GMQ injection were tested using two-way ANOVA with sex and diet as between-group factors. Planned individual comparisons between different sex groups and diet groups were performed using a single degree-of-freedom F test involving the error term from the overall ANOVA. The alpha level was set at 0.05 for all analyses and Systat 13 statistical package was used to conduct the analyses.
Results
Weekly body weight
Body weights were measured weekly from the time of arrival until the last behavioral test and are presented in Figure 1. Overall, male mice weighed more than females and gained weight during the study while the body weight of females remained stable over the study. There was no difference between the control and creatine-fed female mice. These observations were supported by a repeated measure ANOVA yielding an interaction between Week, Sex and Diet (p = 0.014), a main effect of Sex (p < 0.001) but no main effect of Diet or an interaction between Sex and Diet (all ps > 0.959).
Figure 1. Effects of creatine supplementation on body weight (A) and food intake (B) of male and female C57BL/6J mice.
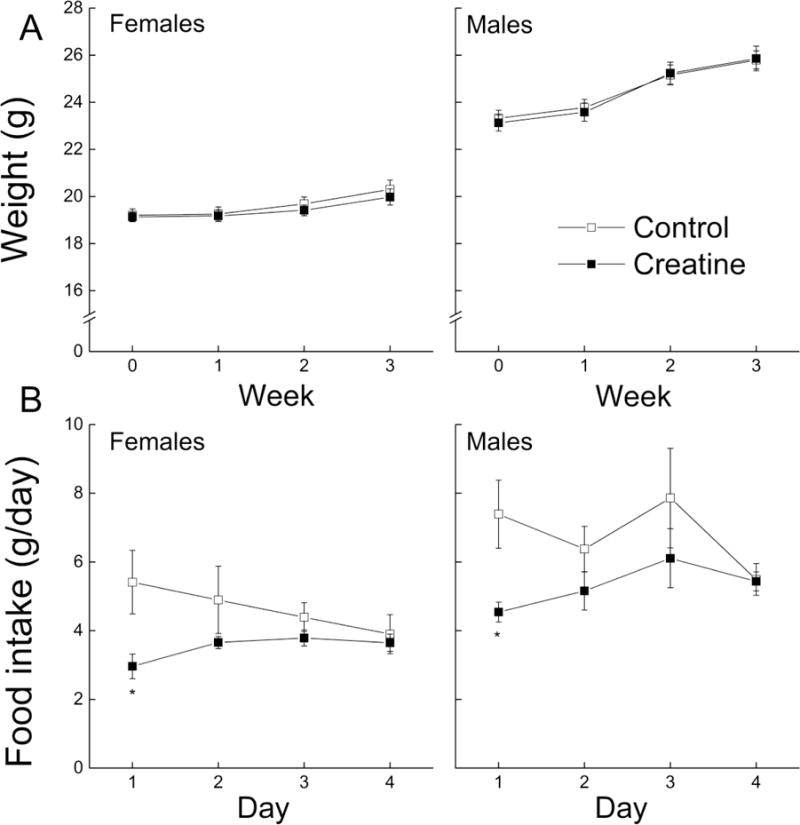
Each value represents the mean ± SEM, n=14–15 for body weight and n=4–5 for food intake. * p < 0.05, compared with sex-matched controls, # p < 0.05 compared to treatment-matched females.
Food Intake
The amount of food consumed by the mice was measured daily for one week and the results are presented in Figure 1B. Overall, male mice consumed more food than females, regardless of which diet they were assigned to. Mice on the creatine diet ate less food than the mice on the control diet at the beginning of food intake measurements, but no differences were found on the last day (one week after starting the diet). A repeated measure ANOVA produced significant main effects of Sex and Diet (all ps < 0.03) and an interaction between Day and Diet (p = 0.018), but did not result in any other interactions (all ps > 0.263).
Tail immersion test
The latency of a mouse to flick its tail out of hot water was averaged across three trials and presented in Figure 2. There was no difference in latency between any of the experimental groups. This observation was supported by an ANOVA yielding no main effect of sex, diet or an interaction of sex and diet (all ps> 0.393).
Figure 2. Effects of short-term creatine supplementation on nociceptive response of young male and female C57BL/6 mice in a tail immersion test.
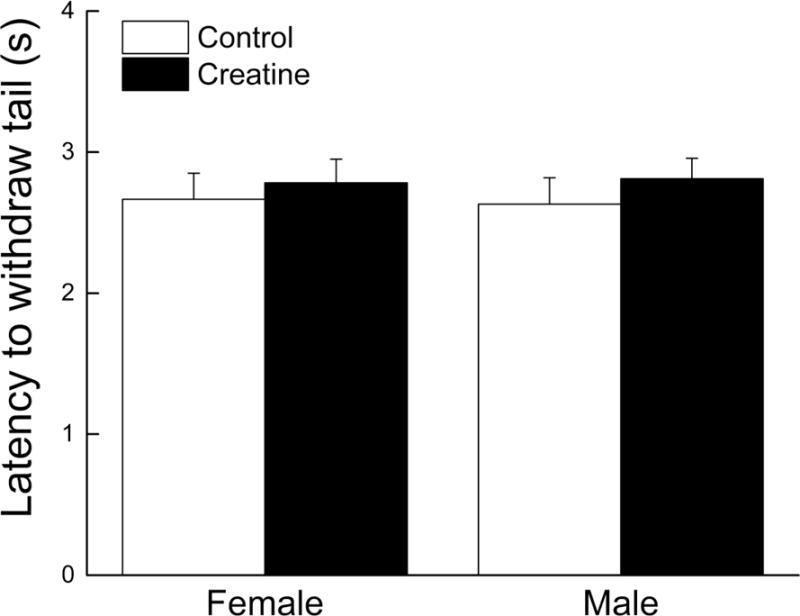
Each value represents the mean ± SEM, n=15.
Formalin test
The duration of paw licking behavior was recorded for 60 minutes and averaged in 10 minute time bins. The results are reported as the percentage of time spent licking and presented in Figure 3. Female mice spent more time licking their paws than male mice, which was most apparent during the first 10min and the last 30 min of observation corresponding to the acute and inflammatory phases, respectively, of the formalin test. The male mice fed creatine had similar response compared to controls, while the female fed creatine had lower paw licking times during the last 20–30 minutes. A three-way ANOVA with time as repeated measure revealed a main effect of Sex (p = 0.013), but did not reveal main effect of Diet (p = 0.078) or an interaction between Sex and Diet (p = 0.55).
Figure 3. Effects of short-term creatine supplementation on nociceptive response of young male and female C57BL/6 mice in a formalin test.
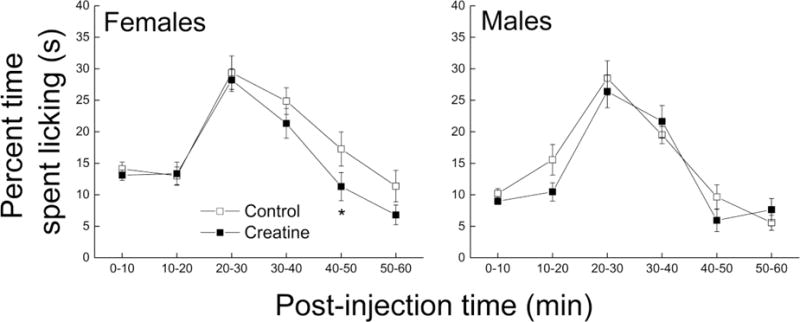
Each value represents the mean ± SEM, n=14–15.
GMQ test
The amount of time spent licking the paw injected with GMQ was recorded and presented in Figure 4. Overall, males licked their GMQ-injected paw ~ 34% less than females regardless of treatment. Creatine-fed mice also licked their GMQ-injected paws ~ 35% less than controls, regardless of sex. These observations were supported by significant main effects of Sex and Diet (all ps < 0.036) and no interaction between Sex and Diet (p = 0.544).
Figure 4. Effects of short-term creatine supplementation on nociceptive response of young male and female C57BL/6 mice in response to GMQ injection.
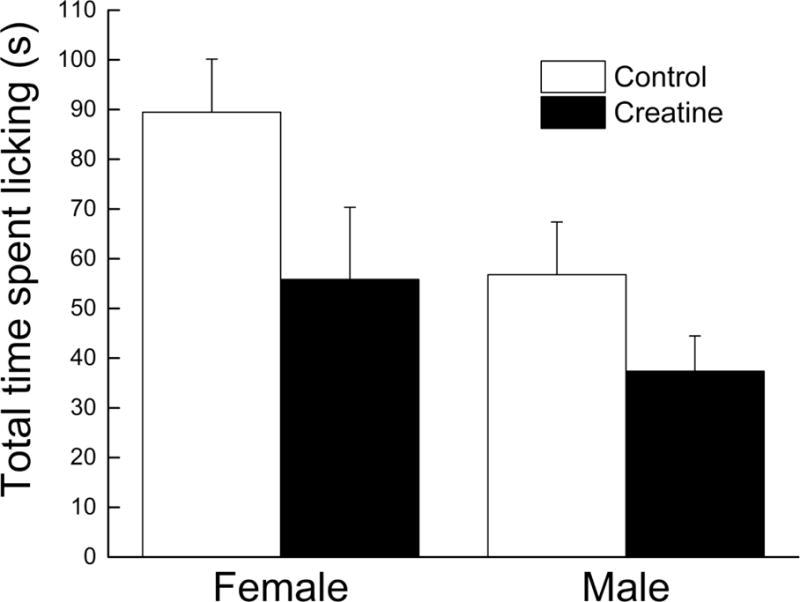
Each value represents the mean ± SEM, n=6–9.
Discussion
The major findings from this study were that short-term creatine supplementation: (1) did not affect the response of male and female C57BL/6J mice to a thermal stimulus, (2) decreased nociceptive licking behavior in response to formalin injection in females only, (3) decreased sensitivity to GMQ injections in both sexes. The data also imply that there are sex differences in response to inflammatory pain and GMQ sensitivity.
Male mice were heavier than the females and gained weight over the duration of the study (3 weeks), while the weight of the female mice remained stable. These differences are consistent with reports by Jackson Laboratories [27]. Supplementation with creatine did not affect the body weight of the mice, which is in contradiction with previous published reports in both humans and mice. Duarte et al. reported that male mice that were given a dose of creatine equivalent to 20 g per day (for humans) for six days, gained 4% more body weight than their control diet counterparts [28]. The dose given was five times the one in our study (equivalent to 5 g per day for humans) and could account for the lack of an effect on body weights in the current study. Allah et al., reported that female albino mice that were on a creatine-supplemented diet (1% and 3% creatine) also gained weight when they were supplemented for 10 weeks [29]. These reports and our data indicate that dose and duration are decisive factors in whether body weight will be affected. Creatine also did not influence food intake in males and females, however a difference in intake between control groups indicates differing growing curves and this observation matches with the weight gain that was observed.
The data for the tail immersion test showed no significant interaction between nociceptive sensitivity and diet or sex. The tail immersion test is designed to provoke a quick reflex reaction [30] which does not involve inflammatory mediators. The temperature of the water at 52°C was considered to be a high intensity pain stimulus [31, 32] directed at stimulating thermal and nociceptive receptors on the mouse tail. While the role of ASIC3 in pain has mostly been linked to inflammatory condition, in which acidification of tissues and inflammatory intermediates modulate the channel [33], some reports have indicated a potential role in thermal sensitivity as well. Chen et al. used male ASIC3 knockout (asic3−/−) and wild-type mice to observe the nociceptive differences using an automated tail-flick apparatus (radiant heat) to test for thermal hyperalgesia on the tail and found that both groups responded similarly to the test [32]. Another study reported that male ASIC3 knockout mice had a reduced tail withdraw latency in the tail immersion test compared to their wild-type counterparts [31]. In our study, thermal sensitivity was not affected by creatine intake implying that perhaps creatine does not affect this pain modality or that the dose used was too low to elicit an effect. Creatine may have the capability to interact with other ASICs besides ASIC3 and influence other types of pain modalities, therefore the mice were also subjected to inflammatory pain stimulation.
In the formalin test, females seem to spend more time licking than males in response to the injection. Interestingly, the effect of sex/gender on pain perception is still being debated both in animals and humans. Many argue that epidemiological research showed that women report pain more often than men in a clinical setting [34, 35], although the magnitude and mechanism underlying this disparity remains unclear. Mogil suggested that women may seek medical attention more than men, or may be more susceptible to chronic pain illnesses, or that they actually have a lower tolerance for pain than men [36]. Several studies in rodents support such sex differences, however confusion persists regarding which sex might be most sensitive especially to acute and thermal nociception [37]. In a study done with 1 week old mice, males showed longer latencies to withdraw in a thermal paw-withdraw test, while the females displayed an increased latency to withdraw on the tail immersion test [38]. In young adult rodents, females have been found to be more sensitive to formalin-induced nociception [39, 40]. The discrepancy in findings regarding which sex might be most sensitive, also depends on the noxious stimulus as well as the strain used in the studies (potential genetic differences might explain the different sex-dependent responses) [37]. Therefore sex/gender might be an important factor to consider when considering the effects of potential anti-nociceptive compounds like creatine. In our study, the female mice supplemented with creatine showed a more rapid recovery in the second phase of the formalin test than the female controls, while there was no difference in the males. Staniland and McMahon showed no difference in the duration of nociceptive behaviors between the wild-type and ASIC3 knockout (asic3−/−) male mice [31]. It is interesting to note that creatine supplementation affected the second phase of the formalin test which corresponds to the inflammatory response phase. Considering that ASIC3 is activated by acidosis and inflammatory factors, it is sensible to assume that creatine could have an effect on this phase. While most studies have focused on one sex, our data indicate that even though minor differences are seen in response to inflammation, the response to potential anti-nociceptive compounds might differ between males and females. Additional studies should consider both sexes/genders for their experiments as analgesic treatment may need to be developed differently based on sex/gender.
Our data have provided some evidence that creatine may have the potential to lower pain sensitivity associated with inflammation, and that it could be achieved via ASIC3 antagonism based on its structural similarity to other ligands. However, one cannot rule out the potential role of other receptors that have been involved in nociception. The transient receptor potential V1 channel (TRPV1) has been known to participate in nociception due to inflammation [31] [41]. A study on rats showed that injecting a TRPV1 antagonist prevented and reversed hyperalgesia and secondary allodynia after formalin injection [41]. ASIC3 is not the only channel that mediates inflammatory pain in the body, so it is possible that other receptors activate and could diminish the observed effect of creatine.
To further determine whether creatine might act via ASIC3, our last experiment used paw-injection of an ASIC3 agonist, GMQ. It has been shown that GMQ injections will increase nociceptive paw licking and that the response is higher in wild-type than the ASIC3 knockout mice [12]. Overall female mice spent more time licking than males, consistent with observations during the formalin test, and the mice fed creatine regardless of sex exhibited lower nociceptive behaviors than the mice on the control diet. These data suggest that ASIC3 could be involved in the mechanisms of creatine anti-nociceptive effects.
While only minor effects were found, creatine intake did reduce pain sensitivity when caused by inflammation or ASIC3 activation by GMQ. As the role of creatine as an antinociception compound begins to be established, a dose and duration study will be necessary to identify a maximal effect of creatine. Furthermore, as pain related issues occur in middle-age and old individuals more frequently, a consequent study should investigate how creatine might affect pain sensitivity in older age groups. Creatine is a safe, relatively inexpensive compound that is readily available and could be used alone or in combination with other treatments to alleviate pain symptoms. Further studies will be needed to determine the mechanism of action of creatine via ASICs, which could lead to the generation of compounds that reduce pain effectively while minimizing the side effects.
Highlights.
Sex differences in inflammatory pain response in young mice.
Preliminary data suggest an antinociception action of creatine.
Creatine supplementation decreased nociceptive behaviors related to inflammation
ASIC3 may be involved in mediating the effects of creatine on pain sensitivity
Acknowledgments
This work was supported by the National Institutes of Health (AG022550 and AG027956) and by intramural grant (RI6144).
List of abbreviations
- APETx2
Anthopleura Elegantissima toxin 2
- ASIC
acid sensing ion channel
- CFA
complete Freund’s adjuvant
- GMQ
2-guanidine-4-methylquinazoline
- TRPV1
transient receptor potential V1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics. J Neurosci. 2015;35(41):13879–88. doi: 10.1523/JNEUROSCI.2711-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron A, Lingueglia E. Pharmacology of acid-sensing ion channels - Physiological and therapeutical perspectives. Neuropharmacology. 2015;94:19–35. doi: 10.1016/j.neuropharm.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Li WG, Xu TL. Acid-sensing ion channels: a novel therapeutic target for pain and anxiety. Curr Pharm Des. 2015;21(7):885–94. doi: 10.2174/1381612820666141027124506. [DOI] [PubMed] [Google Scholar]
- 5.Li WG, Xu TL. ASIC3 channels in multimodal sensory perception. ACS Chem Neurosci. 2011;2(1):26–37. doi: 10.1021/cn100094b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellenberger S, Schild L. International Union of Basic and Clinical Pharmacology. XCI. structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol Rev. 2015;67(1):1–35. doi: 10.1124/pr.114.009225. [DOI] [PubMed] [Google Scholar]
- 7.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118(6):687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Yang ZJ, Ni X, Carter EL, Kibler K, Martin LJ, Koehler RC. Neuroprotective effect of acid-sensing ion channel inhibitor psalmotoxin-1 after hypoxia-ischemia in newborn piglet striatum. Neurobiol Dis. 2011;43(2):446–54. doi: 10.1016/j.nbd.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14(7):461–71. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272(34):20975–8. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 11.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110(8):1185–90. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, et al. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010;68(1):61–72. doi: 10.1016/j.neuron.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, et al. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23(7):1516–25. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linley JE, Rose K, Ooi L, Gamper N. Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflugers Arch. 2010;459(5):657–69. doi: 10.1007/s00424-010-0784-6. [DOI] [PubMed] [Google Scholar]
- 15.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106(3):229–39. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 16.Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24(48):10974–9. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21(20):8026–33. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22(24):10662–70. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, et al. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161(4):950–60. doi: 10.1111/j.1476-5381.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128(3):549–58. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Wyss M, Schulze A. Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience. 2002;112(2):243–60. doi: 10.1016/s0306-4522(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 22.Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gomez AL, et al. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc. 1999;31(8):1147–56. doi: 10.1097/00005768-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Allen PJ. Creatine metabolism and psychiatric disorders: Does creatine supplementation have therapeutic value? Neurosci Biobehav Rev. 2012;36(5):1442–62. doi: 10.1016/j.neubiorev.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amital D, Vishne T, Rubinow A, Levine J. Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. Am J Psychiatry. 2006;163(10):1840–1. doi: 10.1176/ajp.2006.163.10.1840b. [DOI] [PubMed] [Google Scholar]
- 25.Leader A, Amital D, Rubinow A, Amital H. An open-label study adding creatine monohydrate to ongoing medical regimens in patients with the fibromyalgia syndrome. Ann N Y Acad Sci. 2009;1173:829–36. doi: 10.1111/j.1749-6632.2009.04811.x. [DOI] [PubMed] [Google Scholar]
- 26.Alves CR, Santiago BM, Lima FR, Otaduy MC, Calich AL, Tritto AC, et al. Creatine supplementation in fibromyalgia: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken) 2013;65(9):1449–59. doi: 10.1002/acr.22020. [DOI] [PubMed] [Google Scholar]
- 27.Laboratory TJ. Body weight information JAX mice strain C57BL/6J. 2014 [Google Scholar]
- 28.Duarte J, Neuparth M, Soares J, Appell H. Oral creatine supplementation in mice induces hepatic protein overload. Revista Portuguesa de Ciencias do Desporto. 2001;1(3):40–3. [Google Scholar]
- 29.Allah Yar R, Akbar A, Iqbal F. Creatine monohydrate supplementation for 10 weeks mediates neuroprotection and improves learning/memory following neonatal hypoxia ischemia encephalopathy in female albino mice. Brain Res. 2015;1595:92–100. doi: 10.1016/j.brainres.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597–652. [PubMed] [Google Scholar]
- 31.Staniland AA, McMahon SB. Mice lacking acid-sensing ion channels (ASIC) 1 or 2, but not ASIC3, show increased pain behaviour in the formalin test. Eur J Pain. 2009;13(6):554–63. doi: 10.1016/j.ejpain.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99(13):8992–7. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, et al. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27(22):3047–55. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–8. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res. 2010;186:141–57. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- 36.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–66. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 37.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24(3):375–89. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 38.Sternberg WF, Smith L, Scorr L. Nociception and antinociception during the first week of life in mice: sex differences and test dependence. J Pain. 2004;5(8):420–6. doi: 10.1016/j.jpain.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958(1):139–45. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- 40.Kim SJ, Calejesan AA, Li P, Wei F, Zhuo M. Sex differences in late behavioral response to subcutaneous formalin injection in mice. Brain Res. 1999;829(1–2):185–9. doi: 10.1016/s0006-8993(99)01353-0. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Rojas VA, Barragan-Iglesias P, Rocha-Gonzalez HI, Murbartian J, Granados-Soto V. Role of TRPV1 and ASIC3 in formalin-induced secondary allodynia and hyperalgesia. Pharmacol Rep. 2014;66(6):964–71. doi: 10.1016/j.pharep.2014.06.011. [DOI] [PubMed] [Google Scholar]


