Abstract
Sleep apnea is a common sleep disorder characterized by intermittent periods of low blood oxygen levels. The risk for sleep apnea increases with age and is more prevalent in men than women. A common comorbidity of sleep apnea includes male sexual dysfunction, but it is not clear if a causal relationship exists between sleep apnea and sexual dysfunction. Possible mechanisms that link these two disorders include oxidative stress and testosterone. Oxidative stress is elevated in clinical patients with sleep apnea and in rodents exposed to chronic intermittent hypoxia (CIH), an animal model for apnea-induced hypopnea. Further, oxidative stress levels increase with age. Therefore, age may play a role in sleep apnea-induced sexual dysfunction and oxidative stress generation. To investigate this relationship, we exposed gonadally intact 3 (young) and 12 (middle-aged) month old male F344/BN F1 hybrid male rats to 8 days of CIH, and then examined male sexual function. Plasma was used to assess circulating oxidative stress and hormone levels. Middle-aged male rats had lower testosterone levels with increased sexual dysfunction and oxidative stress, independent of CIH. However, CIH decreased testosterone levels and increased sexual dysfunction and oxidative stress only in young gonadally intact male rats, but not in gonadectomized young rats with physiological testosterone replacement. In sum, CIH had a greater impact on younger gonadally intact animals, with respect to sexual behaviors, testosterone, and oxidative stress. Our data indicate CIH mimics the effects of aging on male sexual behavior in young gonadally intact male rats.
Keywords: Testosterone, Oxidative Stress, Sex Dysfunction, Corticosterone, Male Sex Behavior
Introduction
Sleep apnea is a common disorder that consists of interruptions in breathing that cause hypoxia, hypercapnia, and increased thoracic pressure during sleep [1, 2]. One measure of sleep apnea severity is the apnea/hypopnea index (AHI), which is the frequency of apneic/hypoxic events per hour while sleeping [3]. The American Academy of Sleep Medicine classification of AHI as mild, moderate, or severe sleep apnea is considered 5, 15, and >30 hypoxic events per hour, respectively [3]. Notably, age and sex impact sleep apnea. The risk for sleep apnea increases with age and occurs at a higher frequency in men than women [4–6]. Male sexual dysfunction is commonly associated with sleep apnea and can greatly lower quality of life [7, 8].
Although sleep apnea and sexual dysfunction are comorbidities, the exact mechanisms underlying sleep apnea and sexual dysfunction are unknown [9–12]. Possible theories for sleep apnea-induced sexual dysfunction include alterations in testosterone and oxidative stress levels. Clinical studies consistently observe decreased circulating testosterone in middle-aged men (~45 years old) with sleep apnea [12, 13]. Only a few pre-clinical studies on sleep apnea and sexual function have been conducted. Surprisingly, pre-clinical studies are not aligned with clinical findings with respect to testosterone levels. Studies using young mice exposed to chronic intermittent hypoxia (CIH), an animal model of apnea-induced hypopnea, found no effects on testosterone levels, even though CIH decreased sexual activity [14].
Unlike the contrast between clinical and pre-clinical studies on testosterone levels, there is agreement on sleep apnea and CIH-induced oxidative stress [15–20]. Oxidative stress has been linked to both sexual dysfunction [21–25] and sleep apnea in humans [17, 26–29]. A pre-clinical study observed that antioxidants decreased CIH-induced erectile dysfunction in male rats [30], supporting the concept that oxidative stress may play a role in sexual dysfunction in men with sleep apnea.
Aging also contributes to oxidative stress [31], and is a risk factor for sleep apnea. Several studies indicate the involvement of age in the elevated cardiovascular risk for individuals with sleep apnea. Individuals less than 65 years old with sleep apnea have the greatest cardiovascular risk, unlike individuals older than 65 years [32–36]. Similarly in pre-clinical studies, CIH appeared to “age” young (3–4 month) male rats by increasing mean arterial blood pressure to ranges observed in aged (22–24 month) male rats, but had no effect on blood pressure in aged rats [37]. These studies indicate that sleep apnea may have a greater impact on younger individuals compared to older individuals. It is possible that the age-associated increase in oxidative stress may blunt CIH’s effects in older individuals, due to a ceiling effect.
Although there have been some pre-clinical studies on CIH and age, no studies on CIH and age on sexual function have been conducted. This gap is concerning because the number of individuals over the age of 65 increased 213% from 1950 to 2010 in the US [38, 39]. Due to aging of the US population, it is important to have a better understanding of how aging may influence disease modalities, such as sleep apnea and sexual dysfunction. To examine the role of age on sleep apnea and sexual dysfunction, we exposed sexually naïve young and middle-aged male rats to 8 days of CIH, a model of mild sleep apnea. After CIH, we examined sexual function, oxidative stress, and hormone levels.
Methods
Animals
Sexually naïve gonadally intact 3 (young) and 12 (middle-aged) month old male F344/BN F1 hybrid rats were obtained from the National Institute of Aging (through Envigo, Indianapolis, IN). Since two different age groups (young and middle-aged) are examined in this study, we used sexually naïve rats to remove the confound of sexual experience that can increase circulating testosterone and androgen sensitive accessory organ weights [40–43]. Due to the lack of standardized sexual experience across the different age groups, age-wise comparisons would not be appropriate if sexually experienced males were incorporated. Stimulus females were gonadally intact Sprague Dawley (215–225 g) also obtained from Envigo. Animals were doubled housed in a temperature-controlled room (23°C). Food and water were provided ad libitum. Lighting was maintained on a reverse 12:12 light/dark cycle, with lights off at 0900 h. All surgeries were performed under isoflurane (2–3%) anesthesia. Animal care and experimental procedures were performed in accordance with the National Institutes of Health and American Physiological Society’s guidelines for animal care and use. The protocol was approved by the University of North Texas Health Science Center Institutional Animal Care and Use Committee.
Animals were habituated to housing conditions for a week prior to a one-week habituation to the chronic intermittent hypoxia (CIH) apparatus under normoxic conditions. After CIH apparatus habituation, rats were exposed to either normoxia or CIH for 8 days with behavior testing on the last day. Male rats were randomly divided into different experimental groups with the experimental factors of age and hypoxia: Young gonadally intact Normoxia (n=10), Middle-Age gonadally intact Normoxia (n=12), Young gonadally intact CIH (n=13), and Middle-Age gonadally intact CIH (n=10).
To further examine the influence of testosterone, additional young male rats were included: gonadectomized (GDX) Normoxia (n=8), GDX + testosterone replacement treatment (TRT) Normoxia (n=8), GDX-CIH (n=8), and TRT-CIH (n=8). GDX male rats were implanted with either 2 cholesterol-filled or crystalline testosterone-filled (Steraloids, Newport, RI.) continuous release Silastic implants (1.47 mm i.d. × 1.96 mm o.d. × 10 mm length, Dow Corning, Midland, MI). Testosterone-filled capsules resulted in 2–3 ng/ml circulating testosterone (Figure 1B), consistent with the average testosterone levels throughout the diurnal pattern (in the absence of daily hormone peaks occurring at end of the light phase) [44–47]. Male rats were given a 1-week recovery period prior to CIH apparatus habituation. Stimulus females were ovariectomized (OVX) and implanted with 1 Silastic capsule (1.47 mm i.d. × 1.96 mm o.d. × 5 mm length, Dow Corning, Midland, MI) containing 10% crystalline estradiol benzoate (Sigma, St. Louis, MO). Female rats were given at least one-week recovery before behavior testing. Females were made sexually receptive via a subcutaneous injection of 500 μg of progesterone (Sigma, St. Louis, MO) four hours prior to behavioral testing [48, 49].
Figure 1. CIH and age altered steroid hormones.
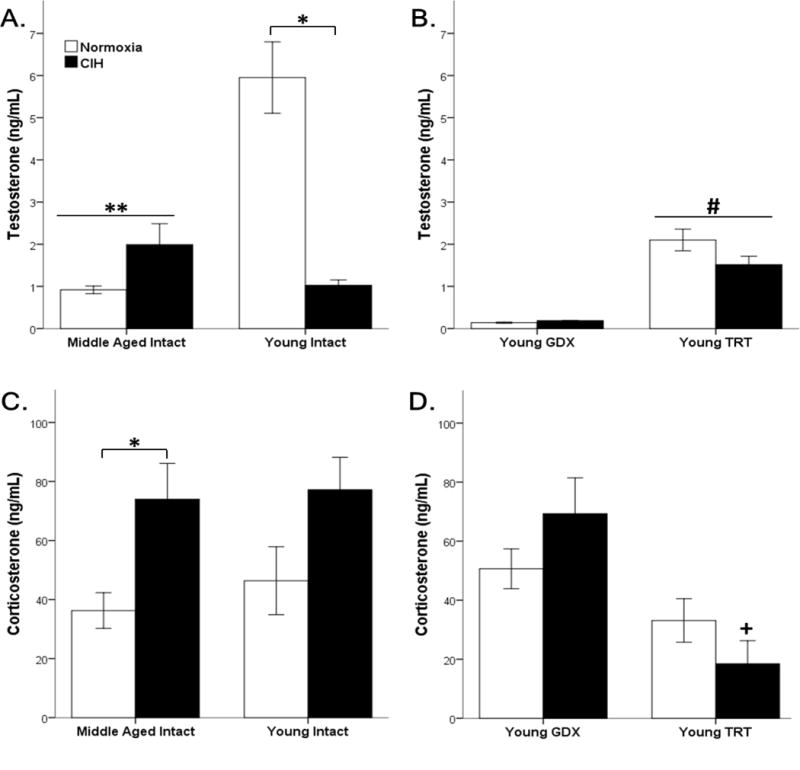
Middle-aged rats have significantly lower circulating testosterone levels than young intact males. 8-day exposure to CIH significantly decreased testosterone in young intact male rats (A). TRT significantly increased testosterone levels compared to GDX males (B). Although age did not impact corticosterone, CIH significantly increased circulating corticosterone levels in middle-aged intact rats (C). Lower corticosterone levels were observed in TRT compared to GDX rats, specifically in the CIH treatment group (D). Fisher’s LSD post hoc analyses: * p ≤ 0.05 normoxia vs. CIH; ** p ≤ 0.05 vs. young normoxia; # p ≤ 0.05 vs. GDX; + p ≤ 0.05 vs. GDX CIH.
Mild Chronic Intermittent Hypoxia (CIH)
One week following arrival to animal facility, rats were placed in Oxycycler chambers (76.2 × 50.8 × 50.8 cm, BioSpherix, Lacona, NY, USA) to acclimate to the CIH equipment under normoxic (21% oxygen) conditions for one week. After habituation, CIH was conducted for 7 days during the light cycle (1700 to 0500 h), as previously described [16, 50, 51]. The mild CIH protocol consists of 8-minute cycles of 5 minutes of hypoxia (10% O2) and 3 minutes of normoxia (21% O2). Hypoxia is initiated by injecting nitrogen into the chamber, resulting in a nadir of 10% oxygen within 4 minutes. Reoxygenation is initiated by oxygen injected over 3 minutes, resulting in peak 21% oxygen within 2.5 minutes. This CIH protocol results in an apnea/hypopnea index (AHI) of 8.
Behavioral Tests
All behavioral tests were carried out between 1200 and 1500 h using dim red lighting and digitally recorded. All tests were conducted in a neutral cage environment (50 × 25 × 30 cm) with clean bedding. No male rat was tested with the same female twice. A rater, blind to the treatment groups, scored male sexual behaviors.
Male Sex Behaviors
Sexually naïve male rats were placed into a neutral testing arena with a sexually receptive female. The appetitive and consummatory sexual behaviors of the male rats were recorded for 10 minutes and quantified: frequencies and latencies for mounts, intromissions, and ejaculations. Appetitive behaviors included latencies to first mount (no penile insertion), a measure of sexual motivation. Consummatory behaviors included intromission (penile insertion) latencies, intromission frequencies, and ejaculatory behavior [49, 52–54]. Hit rate was calculated to determine copulatory efficiency: hit rate = intromission frequency/(intromission frequency + mount frequency) [55, 56].
Biochemical Assays
Plasma Collection
One day after behaviour testing, male rats were anesthetized with isoflurane (2–3%) and sacrificed by decapitation. Trunk blood was collected into chilled 7 mL EDTA tubes. The blood was centrifuged for 10 minutes (2,240 × g) at 4° C. Plasma was promptly separated and placed in microcentrifuge tubes for storage at −80° C until analysis. All samples were collected between 0800–1000 h to examine peak hormone levels occurring at the end of the light phase in gonadally intact rats [44, 45].
A. ELISAs
Samples were assayed by ELISA using a BioTek multi-reader. Plasma testosterone levels were determined by a Competitive Testosterone ELISA, using a polyclonal rabbit anti-testosterone antibody specific to mice and rats (BioVendor, Asheville, NC, USA), according to manufacturer’s instructions. The intra-assay coefficient of variation was 6.50% and the inter-assay coefficient of variation was 11.3%. The sensitivity of this assay is 0.066 ng/ml at the 2 s.d. confidence limit. Specificity of this assay is as follows: testosterone (100%), 5α-DHT (69.6%), androstenedione (≤ 0.1%), androstanediol (≤ 0.1%), progesterone (≤ 0.1%), and androsterone (≤ 0.1%).
Plasma corticosterone levels were determined by a Competitive Corticosterone ELISA, using a polyclonal rabbit anti-corticosterone antibody specific to mice and rats (BioVendor, Asheville, NC, USA), according to manufacturer’s instructions. The intra-assay coefficient of variation was 8.9% and the inter-assay coefficient of variation was 7.2%. The sensitivity of this assay is 6.1 ng/ml at the 2 s.d. confidence limit. Specificity of this assay is as follows: corticosterone (100%), cortisol (2.3%), aldosterone (0.3%), testosterone (≤ 0.1%), progesterone (6.2%), and androsterone (≤ 0.1%).
Plasma oxytocin levels were determined by a Competitive Oxytocin EIA, using rabbit anti-peptide IgG compatible with human, mice, rats, and bovine oxytocin (Phoenix Pharmaceuticals, Burlingame, CA, USA), according to manufacturer’s instructions. The intra-assay coefficient of variation was ≤ 10.0% and the inter-assay coefficient of variation was ≤ 15.0%. The sensitivity of this assay is 0.13 ng/ml at the 2 s.d. confidence limit. Specificity of this assay is as follows: oxytocin (100%), [Lys8]-vasopressin (0.0%), [Arg8]-vasopressin (0.0%), CRF (human, rat) (0.0%), and somatostatin (0.0%).
Basal plasma luteinizing hormone (LH) levels were assayed by Competitive LH ELISA [57–59], using a monoclonal mouse anti-LH antibody that recognizes human and rat LH (Enzo Life Sciences, Farmingdale, NY, USA), according to manufacturer’s instructions. The intra-assay coefficient of variation was 5.3% and the inter-assay coefficient of variation was 15.5%. The sensitivity of this assay is 0.612 ng/ml at the 2 s.d. confidence limit. Specificity of this assay is as follows: LH (100%), FSH (≤0.004%), TSH (0.3%), and hCG (≤ 0.004%).
Plasma follicle stimulating hormone (FSH) levels were measured by Sandwich-ELISA, using a pre-coated microplate with an antibody specific for rat FSH (Elabscience Biotechnology Inc, Wuhan, China), according to manufacturer’s protocol. The intra-assay coefficient of variation was 5.34% and the inter-assay coefficient of variation was 5.84%. The sensitivity of this assay is 1.88 ng/ml with a detection range of 3.13–200 ng/ml. Specificity of this assay recognizes rat FSH with no significant cross-reactivity or interference with analogues.
B. Oxidative Stress
Circulating oxidative stress levels were measured using a colorimetric OxiSelect Advanced Oxidative Protein Products (AOPP) assay (Cell Biolabs, San Diego, CA, USA) [16, 46], that detects proteins damaged by oxidative stress [60]. This assay quantifies oxidized proteins levels (μmol/L) in a sample relative to a known chloramine standard.
Statistical Analysis
All data were analyzed using IBM SPSS (SPSS v.23, IBM, 2015) software and presented as mean ± SEM. Measures were collected according to a 2 × 2 (Young and Middle Age × CIH and Normoxia) and (GDX and TRT × CIH and Normoxia) design and analyzed by ANOVA followed by Fisher Least Significant Difference (LSD) post hoc. P values ≤ 0.05 were designated as significant.
Results
Exposure to CIH alters hormone levels
Peak circulating total testosterone levels were assayed in gonadally intact young (3 month) and middle-aged (12 month) male rats (Figure 1 A). Testosterone levels (5.95 ng/ml ± 2.24 s.d.) in young gonadally intact males were consistent with the reported peak physiological testosterone levels in young male rats that range from 3.5–7 ng/ml [44, 45, 61]. As expected, age significantly affected testosterone levels (F1,25 = 18.036, p ≤ 0.05), wherein middle-aged rats showed decreased total testosterone levels compared to young gonadally intact rats. Furthermore, hypoxia influenced testosterone levels (F1,25 = 16.110, p ≤ 0.05), and there was a significant interaction between Age and Hypoxia (F1,25 = 39.181, p ≤ 0.05). CIH decreased total testosterone in young gonadally intact males but did not alter testosterone levels in middle-aged males (p = 0.053). Further, no differences were observed between middle-aged CIH males and young CIH males (p=0.064). Total testosterone levels were examined in young GDX and TRT male rats (Figure 1 B). As expected, significantly lower testosterone levels were observed in GDX males compared to TRT young male rats (F1,23 = 76.548, p ≤ 0.05). CIH did not affect testosterone levels in either GDX or TRT male rats (F1,23 = 2.062, p = 0.164). The gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which are involved in the hypothalamic-pituitary-gonadal (HPG) axis negative feedback regulation of testosterone, were assayed in young and middle-aged gonadally intact males (Table 1). Gonadotropins in young gonadally intact males were consistent with reported levels in young male rats [62–68]. Neither CIH (F1,18 = 0.493, p = 0.492) nor age (F1,18 = 1.039, p = 0.322) altered basal LH levels in gonadally intact male rats. Similarly, FSH levels were not affected by either CIH (F1,29 = 1.756, p = 0.195) or age (F1,29 = 0.706, p = 0.408).
Table 1.
Circulating gonadotropin levels (ng/mL) in gonadally intact male rats.
| Group | LH | FSH |
|---|---|---|
| Normoxia | ||
| Middle-aged Intact | 52.25 ± 3.79 | 16.14 ± 3.56 |
| Young Intact | 48.96 ± 1.50 | 18.05 ± 4.98 |
| CIH | ||
| Middle-aged Intact | 49.49 ± 4.22 | 19.09 ± 5.41 |
| Young Intact | 49.41 ± 3.58 | 20.79 ± 8.27 |
Unlike testosterone, age did not influence circulating levels of corticosterone (F1,37 = 0.382, p = 0.54; Figure 1 C), but hypoxia significantly increased corticosterone levels (F1,37 = 10.141, p ≤ 0.05). Post hoc analysis showed CIH significantly increased corticosterone in middle-aged gonadally intact male rats, but not in young gonadally intact male rats (p = 0.09). No effects of hypoxia treatment on corticosterone levels were observed in GDX and TRT young male rats (F1,25 = .049, p = 0.83). However, there was a significant effect of hormone treatment on corticosterone levels in GDX and TRT young male rats (F1,25 = 14.045, p ≤ 0.05; Figure 1 D), wherein TRT males had lower corticosterone levels than GDX males.
Only hypoxia, not age, significantly affected oxytocin levels (F1,30 = 4.139, p ≤ 0.05) (Figure 2 A). Specifically, CIH significantly increased oxytocin in middle-aged gonadally intact rats, but did not alter oxytocin levels in young gonadally intact male rats. Since no significant differences were found in young gonadally intact male rats, oxytocin levels were not quantified in GDX and TRT male rats.
Figure 2. CIH and age altered circulating hormones and oxidative stress.
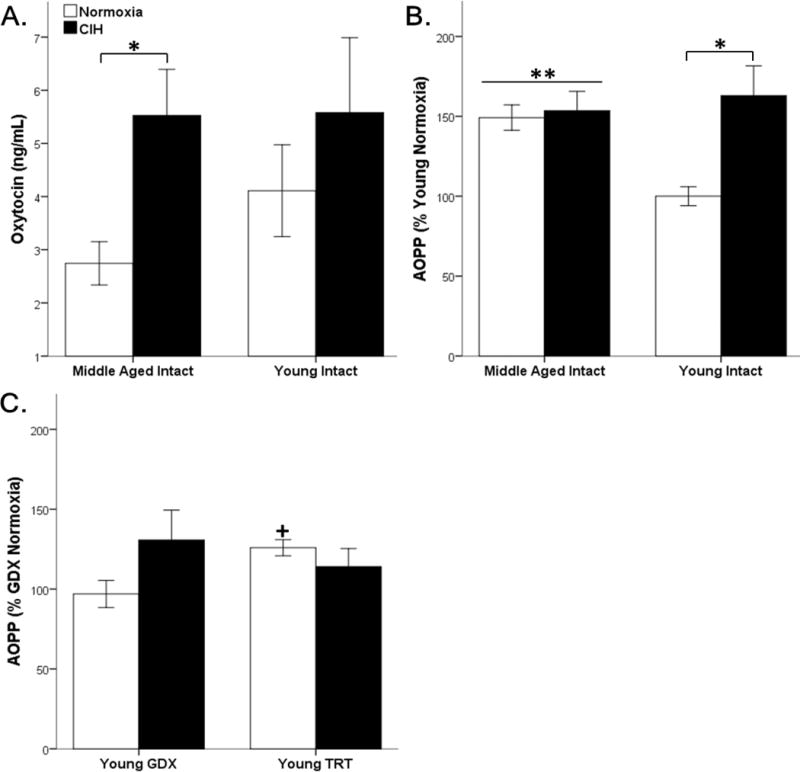
Age did not impact oxytocin, CIH significantly increased oxytocin levels in middle-aged intact rats (A). Middle-aged rats have significantly higher circulating oxidative stress (AOPP) than young intact males. CIH significantly exacerbated oxidative stress in young rats but not middle-aged rats (B). CIH did not impact oxidative stress in GDX and TRT rats. TRT, itself, increased oxidative stress, but only in a normoxic environment (C). Fisher’s LSD post hoc analyses: * p ≤ 0.05 normoxia vs. CIH; ** p ≤ 0.05 vs. young normoxia; + p ≤ 0.05 vs. GDX CIH.
CIH increased circulating oxidative stress in young rats
Oxidative stress was quantified by using AOPP as a marker. There was a main effect for Hypoxia (F1,28 = 7.241, p ≤ 0.05) and an interaction between Age and Hypoxia (F1,28 = 5.511, p ≤ 0.05) on AOPP in gonadally intact young and middle-aged rats. Normoxic middle-aged rats had significantly elevated AOPP compared to normoxic young gonadally intact rats, and young animals exposed to CIH had significantly higher AOPP than normoxic young rats. No significant differences in AOPP were observed between young rats exposed to CIH and middle-aged rats, regardless of CIH exposure (Figure 2 B). Oxidative stress levels were not altered by either hormone (F1,17 = 0.335, p = 0.570) or hypoxia (F1,17 = 1.062, p = 0.317) in young GDX and TRT male rats. However, there was a significant interaction between hormone and hypoxia (F1,17 = 4.614, p ≤ 0.05). Only under normoxic conditions, TRT significantly increased oxidative stress levels compared to GDX (Figure 2 C).
CIH impaired male sex behaviors in young rats
All male rats were sexually naïve. Frequency and latencies (time to first behavior) for male sexual behaviors (mount, intromission, and ejaculation) were quantified in young (3 month), middle-aged (12 month) male rats, young GDX, and young TRT male rats (Figure 3). As expected, Age significantly impacted mount latencies (F1,38 = 23.91, p ≤ 0.05) and mount frequencies (F1,38 = 21.055, p ≤ 0.05), but Hypoxia did not affect mount latencies (F1,38 = 0.821, p = 0.37) and mount frequencies (F1,38 = 0.97, p = 0.33) in young intact and middle-aged gonadally intact male rats. Middle-aged rats showed increased mount latencies and decreased mount frequencies compared to young rats. CIH did not impact mounting behaviors (motivation) (Figure 3 A, C). Similarly, hypoxia did not affect mount latencies (F1,24 = 1.929, p = 0.18) or mount frequencies (F1,25 = 0.202, p = 0.66) in GDX and TRT male rats. As expected, hormone treatment did significantly influence mount latencies (F1,24 = 2203.30, p ≤ 0.05) and mount frequencies (F1,25 = 42.30, p ≤ 0.05) in GDX and TRT male rats. Significantly lower mount latencies were observed in TRT males compared to GDX young male rats. Furthermore, higher mount frequencies were observed in TRT males compared to GDX young male rats (Figure 3 B, D).
Figure 3. Age impaired appetitive mounting behaviors.
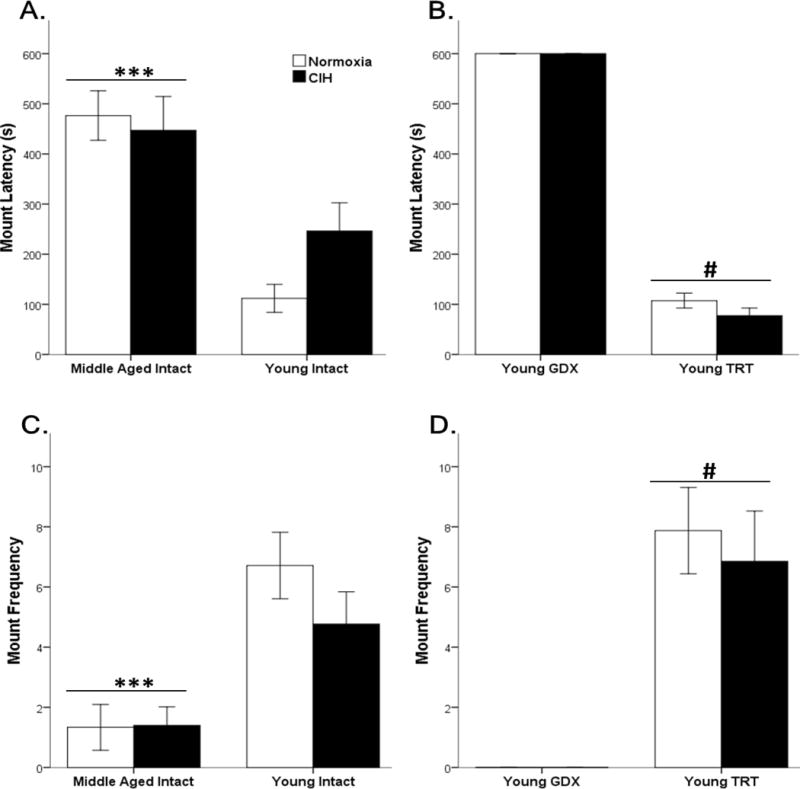
Middle-aged rats had significantly longer mount latency (A) and lower mount frequency (C) than young intact male rats. GDX rats did not exhibit mounting sexual behaviors, whereas testosterone replacement in GDX rats decreased mount latency (B) and increased mount frequency (D). No effects of CIH were observed. Fisher’s LSD post hoc analyses: *** p ≤ 0.05 vs. young intact rats; # p ≤ 0.05 vs. young GDX.
For intromission latency, there was a main effect for Age (F1,38 = 23.111, p ≤ 0.05). Middle-aged rats have increased latency to first intromission compared to young intact rats, and young animals exposed to CIH had significantly higher intromission latency than normoxic young intact rats (Figure 4). No significant differences were found between young gonadally intact rats exposed to CIH and middle-aged rats (Figure 4 A). In TRT and GDX young male rats, hypoxia did not affect intromission latencies (F1,25 = 0.459, p = 0.50), whereas hormone treatment significantly decreased intromission latencies (F1,25 = 131.35, p ≤ 0.05) (Figure 4 B).
Figure 4. CIH and age impaired consummatory intromission behaviors.
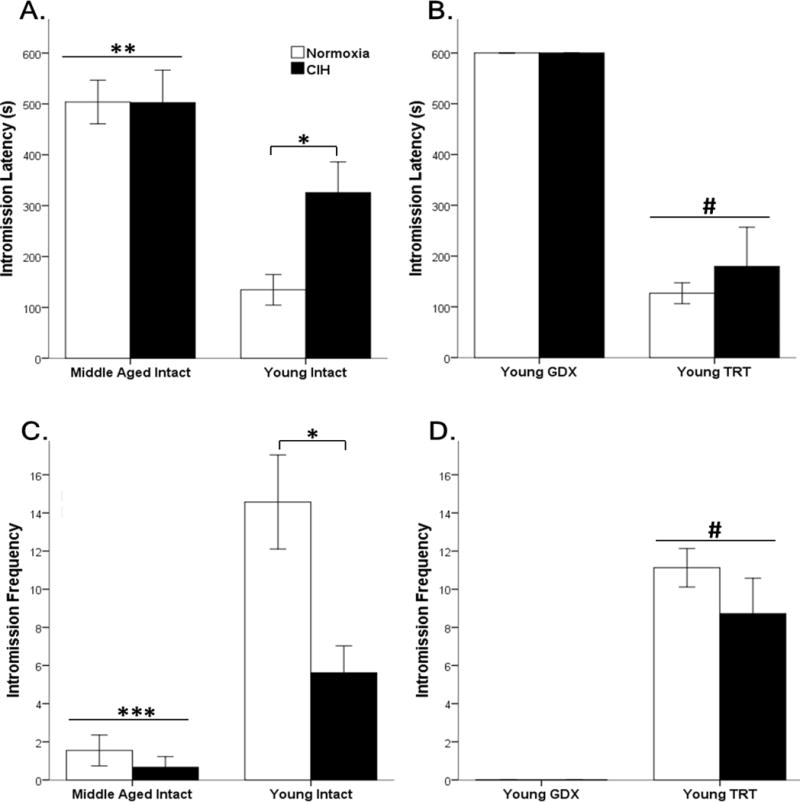
Middle-aged rats had significantly longer intromission latency (A) and fewer intromissions (C) than young intact male rats. CIH did not alter behavior in middle-aged rats. In young intact male rats, CIH increased intromission latencies and decreased the frequencies of intromissions. GDX rats did not exhibit consummatory sexual behaviors, whereas testosterone replacement in GDX rats decreased intromission latency (B) and increased intromission frequency (D). Fisher’s LSD post hoc analyses: * p ≤ 0.05 normoxia vs. CIH; ** p ≤ 0.05 vs. young normoxia; *** p ≤ 0.05 vs. young intact rats; # p ≤ 0.05 vs. young GDX.
However, for intromission frequencies both Age (F1,36 = 41.685, p ≤ 0.05), Hypoxia (F1,36 = 12.479, p ≤ 0.05) and an interaction between these two main effects (F1,36 = 8.42, p ≤ 0.05) were significant in gonadally intact males. Young rats intromitted at a higher frequency than middle-aged rats, and CIH decreased the number of intromissions only in young gonadally intact rats (Figure 4 C). In TRT and GDX young male rats, hypoxia did not affect intromission frequencies (F1,25 = 1.297, p = 0.27), whereas hormone treatment significantly increased intromission frequencies (F1,25 = 87.87, p ≤ 0.05) (Figure 4 D).
To examine copulatory efficiency, the hit rate was quantified. Age significantly impacted the hit rate (F1,36 = 18.37, p ≤ 0.05), but Hypoxia did not significantly influence hit rate (F1,36 = 3.01, p = 0.09) in young and middle-aged gonadally intact male rats. Young male rats had better copulatory efficiency than middle-aged rats (Figure 5 A). Similarly, in TRT and GDX young male rats, hypoxia did not affect the hit rate (F1,25 = 0.27, p = 0.61), whereas hormone treatment significantly increased the hit rate (F1,25 = 92.73, p ≤ 0.05) (Figure 5 B).
Figure 5. Age impaired consummatory efficiency and ejaculations.
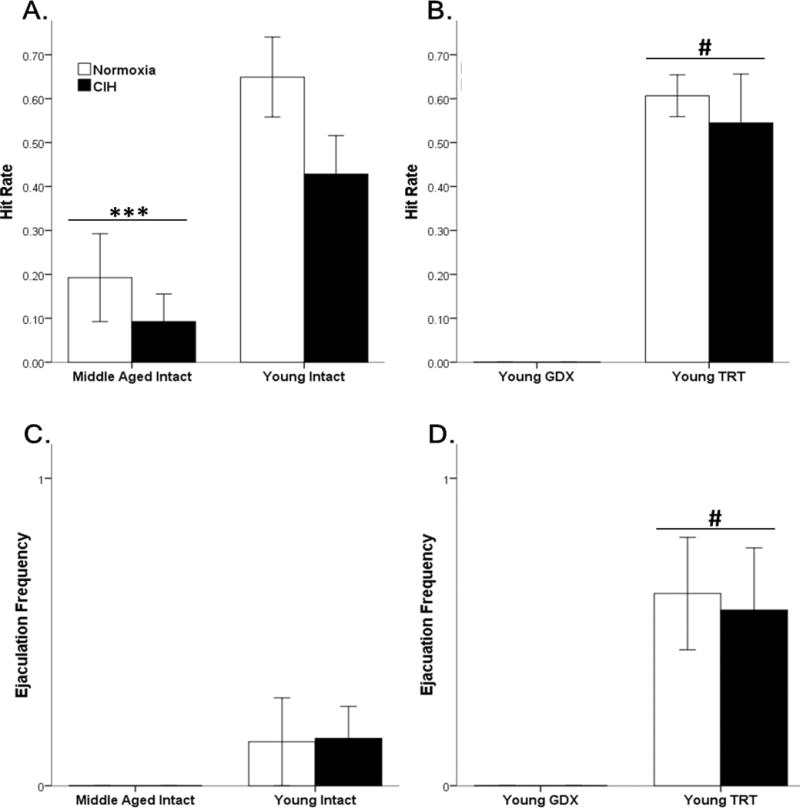
Age decreased hit rate compared to young intact males (A). GDX rats did not exhibit sexual behaviors, whereas testosterone replacement in GDX rats increased the hit rate (B). Middle-aged rats did not ejaculate during the testing period (C). Testosterone replacement in GDX rats increased ejaculation frequency (D). Fisher’s LSD post hoc analyses: *** p ≤ 0.05 vs. young intact males; # p ≤ 0.05 vs. young GDX.
Only ejaculatory frequencies were analyzed, as no middle-aged or GDX rats ejaculated. Neither Age (F1,38 = 3.26, p = 0.08) nor hypoxia (F1,38 = 0.004, p = 0.95) significantly affected ejaculation frequencies in sexually naïve young and middle-aged gonadally intact male rats (Figure 5 C). In TRT and GDX young male rats, hypoxia did not affect ejaculation frequencies (F1,25 = 0.036, p = 0.85), whereas hormone treatment significantly increased ejaculation frequencies (F1,25 = 18.01, p ≤ 0.05) (Figure 5 D).
Discussion
The prevalence of sleep apnea in the US is at least 20% [69, 70]. Strikingly, up to 90% of individuals with sleep apnea are undiagnosed [71–73]. An understudied, but common, co-morbidity of sleep apnea in men and women is sexual dysfunction [74, 75]. Currently, the relationship between sleep apnea and sexual dysfunction is unknown. Furthermore, as aging is associated with both increased prevalence of sleep apnea and sexual dysfunction, it is clinically important to determine if a causal relationship or interaction of age and sleep apnea on sexual function exists. This is the first study to examine the effects of CIH, an animal model of sleep apnea, on sexual function and oxidative stress in young and middle-aged male rats.
In this study we observed increased male sexual dysfunction in middle-aged rats compared to young gonadally male rats. The age-related sexual dysfunction in middle-aged rats was unaffected by CIH. However, CIH did induce sexual dysfunction in young gonadally intact male rats to levels observed in middle-aged male rats. This increase in male sexual behavior dysfunction was accompanied by an increase in circulating oxidative stress and a decrease in circulating testosterone levels. Indeed, CIH exposure in young male rats resulted in behavioral and hormonal profiles consistent with middle age. Therefore, in addition to prior reports of CIH “aging” the cardiovascular system in young (3–4 months) male rats to levels observed in aged (22–24 months) male rats [37], CIH “ages” the reproductive system.
To determine if CIH’s effects on sexual behavior is a direct effect or due to CIH decreasing testosterone levels in young gonadally intact male rats, we included young male rats that were testosterone deficient (GDX) or were given exogenous physiological testosterone replacement (TRT). Interestingly, CIH did not alter any tested parameters, such as corticosterone, AOPP, and male sex behaviors. These findings indicate that CIH induced sexual dysfunction is due to the loss of testosterone. Further, the presence of the exogenous physiological testosterone may be protective with respect to oxidative stress generation, as CIH did not increase oxidative stress in TRT male rats. This is consistent with our previous findings showing testosterone is a mild oxidative stressor, which can protect neurons against subsequent oxidative stress insults via a preconditioning mechanism [76, 77].
Several mechanisms could underlie the decline in testosterone levels in young gonadally intact male rats exposed to CIH. Since CIH increased corticosterone levels, it is possible the hypothalamic-pituitary-adrenal (HPA) axis, via corticosterone, decreased testosterone levels [78–80]. However, it is unlikely CIH-induced corticosterone affected testosterone levels via the hypothalamic-pituitary-gonadal (HPG) axis based on the lack of gonadotropin (LH and FSH) response to CIH. Another possible mechanism could be Leydig cell dysfunction. In men, aging can negatively influence Leydig cell function, resulting in unchanged to elevated luteinizing hormone (LH) with decreased testosterone [81–83]. Similarly, several in vivo studies in rats found decreased testosterone levels with normal LH [62, 63, 84]. Furthermore, it is well established middle-aged Brown Norway male rats exhibit Leydig cell impairments, wherein approximately 50% testosterone loss is observed with unchanged LH levels [64, 85, 86]. Our results showing unchanged LH levels with decreased testosterone levels are consistent with these studies.
Interestingly, prior studies found corticosterone can cause Leydig cell dysfunction and decrease testosterone levels without affecting LH levels via a down-regulation of 17β-hydroxysteroid dehydrogenase (17β -HSD), the final step of testosterone synthesis in Leydig cells [87–92]. Therefore, it is possible CIH-induced corticosterone release is acting directly on Leydig cells, resulting in a decline in testosterone synthesis.
Another mechanism by which CIH could decrease testosterone is oxidative stress. Multiple studies have shown oxidative stress can induce Leydig cell dysfunction, resulting in low testosterone production [93–99]. Interestingly, corticosterone can also increase neuronal oxidative stress generation [100–104]. Both corticosterone and oxidative stress can be decreased by oxytocin [105–114]. CIH increased oxytocin, indicating oxytocin release may be a protective mechanism in response to CIH. Our results show age and CIH are associated with significant reductions in testosterone levels. In young gonadally intact male rats CIH alone can mimic the effect of age on both testosterone and male sexual behaviors. Interestingly, increased oxidative stress, low testosterone, and male sexual dysfunction are shared outcomes between middle-aged rats and CIH exposed young male rats. Therefore, these results suggest CIH-induced oxidative stress is one of the primary factors involved in CIH-induced reproductive aging.
In conclusion, these results indicate oxidative stress generated by CIH could be an important mechanistic link between sleep apnea associated sexual dysfunction. Since young male rats exposed to CIH had similar sexual dysfunction and oxidative stress levels as middle-aged rats, CIH appears to “age” young male rats. Based on these results, a causal relationship exists between sleep apnea and sexual dysfunction.
Highlights.
Middle-aged male rats had increased sexual dysfunction and oxidative stress, independent of CIH.
CIH increased sexual dysfunction and oxidative stress only in young male rats.
CIH “ages” the reproductive system.
Acknowledgments
We would like to thank Jessica Proulx and Drs. Marilyn Y. McGinnis, Augustus Lumia, J. Thomas Cunningham, and Styliani Goulopoulou for their excellent technical assistance and advice. This work was supported by the following NIH grants: NS088514 to R.L.C. and AG049255 to D.A.S. and R.L.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Experimental physiology. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- 3.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 6.Turner AD, Lim AS, Leurgans SE, Bennett DA, Buchman AS, Barnes LL. Self-Reported Sleep in Older African Americans and White Americans. Ethn Dis. 2016;26:521–8. doi: 10.18865/ed.26.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon YJ, Yoon DW, Han DH, Won TB, Kim DY, Shin HW. Low Quality of Life and Depressive Symptoms as an Independent Risk Factor for Erectile Dysfunction in Patients with Obstructive Sleep Apnea. J Sex Med. 2015;12:2168–77. doi: 10.1111/jsm.13021. [DOI] [PubMed] [Google Scholar]
- 8.Kalejaiye O, Abdel Raheem A, Moubasher A, Capece M, McNeillis S, Muneer A, et al. Sleep disorders in patients with Erectile Dysfunction. BJU Int. 2017 doi: 10.1111/bju.13961. [DOI] [PubMed] [Google Scholar]
- 9.Chung SD, Hung SH, Lin HC, Tsai MC, Kao LT. Obstructive sleep apnea and urological comorbidities in males: a population-based study. Sleep Breath. 2016;20:1203–8. doi: 10.1007/s11325-016-1336-x. [DOI] [PubMed] [Google Scholar]
- 10.Bozorgmehri S, Fink HA, Parimi N, Canales B, Ensrud KE, Ancoli-Israel S, et al. Association of Sleep Disordered Breathing with Erectile Dysfunction in Community Dwelling Older Men. J Urol. 2017;197:776–82. doi: 10.1016/j.juro.2016.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen ML, Santos-Silva R, Bittencourt LR, Tufik S. Prevalence of erectile dysfunction complaints associated with sleep disturbances in Sao Paulo, Brazil: a population-based survey. Sleep Med. 2010;11:1019–24. doi: 10.1016/j.sleep.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XB, Lin QC, Zeng HQ, Jiang XT, Chen B, Chen X. Erectile Dysfunction and Sexual Hormone Levels in Men With Obstructive Sleep Apnea: Efficacy of Continuous Positive Airway Pressure. Arch Sex Behav. 2016;45:235–40. doi: 10.1007/s10508-015-0593-2. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Tang T, Wu W, Gu L, Du J, Zhao T, et al. Efficacy of nasal continuous positive airway pressure on patients with OSA with erectile dysfunction and low sex hormone levels. Respir Med. 2016;119:130–4. doi: 10.1016/j.rmed.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Soukhova-O’Hare GK, Shah ZA, Lei Z, Nozdrachev AD, Rao CV, Gozal D. Erectile dysfunction in a murine model of sleep apnea. Am J Respir Crit Care Med. 2008;178:644–50. doi: 10.1164/rccm.200801-190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumaine JE, Ashley NT. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am J Physiol Regul Integr Comp Physiol. 2015;308:R1062–9. doi: 10.1152/ajpregu.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder B, Shell B, Cunningham JT, Cunningham RL. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiological Reports. 2017;5 doi: 10.14814/phy2.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancuso M, Bonanni E, LoGerfo A, Orsucci D, Maestri M, Chico L, et al. Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med. 2012;13:632–6. doi: 10.1016/j.sleep.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Lee T, et al. Endothelial dysfunction and C-reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep. 2007;30:997–1001. doi: 10.1093/sleep/30.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lui MM, Lam JC, Mak HK, Xu A, Ooi C, Lam DC, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–6. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez A, Martinez P, Munoz M, Benedito S, Garcia-Sacristan A, Hernandez M, et al. Endothelin-1 contributes to endothelial dysfunction and enhanced vasoconstriction through augmented superoxide production in penile arteries from insulin-resistant obese rats: role of ET(A) and ET(B) receptors. Br J Pharmacol. 2014;171:5682–95. doi: 10.1111/bph.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musicki B, Burnett AL. Constitutive NOS uncoupling and NADPH oxidase upregulation in the penis of type 2 diabetic men with erectile dysfunction. Andrology. 2017;5:294–8. doi: 10.1111/andr.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Latif MA, Makhlouf AA, Moustafa YM, Gouda TE, Niederberger CS, Elhanbly SM. Diagnostic value of nitric oxide, lipoprotein(a), and malondialdehyde levels in the peripheral venous and cavernous blood of diabetics with erectile dysfunction. Int J Impot Res. 2006;18:544–9. doi: 10.1038/sj.ijir.3901473. [DOI] [PubMed] [Google Scholar]
- 24.Burnett AL, Strong TD, Trock BJ, Jin L, Bivalacqua TJ, Musicki B. Serum biomarker measurements of endothelial function and oxidative stress after daily dosing of sildenafil in type 2 diabetic men with erectile dysfunction. J Urol. 2009;181:245–51. doi: 10.1016/j.juro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Castela A, Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol. 2016;13:266–74. doi: 10.1038/nrurol.2016.23. [DOI] [PubMed] [Google Scholar]
- 26.Serra A, Maiolino L, Cocuzza S, Di Luca M, Campione G, Licciardello L, et al. Assessment of oxidative stress markers and hearing thresholds in patients with obstructive sleep apnea-hypopnoea treated with cysteine and superoxide dismutase therapy. Acta Biomed. 2017;87:253–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 28.Tothova L, Hodosy J, Mucska I, Celec P. Salivary markers of oxidative stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath. 2014;18:563–70. doi: 10.1007/s11325-013-0919-z. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1:61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhu D, Deng Y, Pan Y, Wang Z, Yuan X, Guo X, et al. N-acetylcysteine Ameliorates the Erectile Dysfunction Caused by Chronic Intermittent Hypoxia in Rats: Partly Involvement of Endoplasmic Reticulum Stress. Urology. 2015;86:844e7–e14. doi: 10.1016/j.urology.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, Soler-Cataluna JJ, Almeida-Gonzalez C, De la Cruz Moron I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186:909–16. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 33.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–71. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 35.Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–21. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 36.Goff EA, O’Driscoll DM, Simonds AK, Trinder J, Morrell MJ. The cardiovascular response to arousal from sleep decreases with age in healthy adults. Sleep. 2008;31:1009–17. [PMC free article] [PubMed] [Google Scholar]
- 37.Quintero M, Olea E, Conde SV, Obeso A, Gallego-Martin T, Gonzalez C, et al. Age protects from harmful effects produced by chronic intermittent hypoxia. J Physiol. 2016;594:1773–90. doi: 10.1113/JP270878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United Nations, Department of Economic and Social Affairs. World Population Prospects: 2012 Revision. 2013. [Google Scholar]
- 39.Pew Research Center. Population change in the US and in the world from 1950 to 2050. In: Center PR, editor. Global Attitudes and Trends Pew Research Center. 2014. [Google Scholar]
- 40.Cummings JA, Clinton SM, Perry AN, Akil H, Becker JB. Male rats that differ in novelty exploration demonstrate distinct patterns of sexual behavior. Behav Neurosci. 2013;127:47–58. doi: 10.1037/a0031528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aumuller G, Braun BE, Seitz J, Muller T, Heyns W, Krieg M. Effects of sexual rest or sexual activity on the structure and function of the ventral prostate of the rat. Anat Rec. 1985;212:345–52. doi: 10.1002/ar.1092120404. [DOI] [PubMed] [Google Scholar]
- 42.Drori D, Folman Y. Effects of Cohabitation on the Reproductive System, Kidneys and Body Composition of Male Rats. J Reprod Fertil. 1964;8:351–9. doi: 10.1530/jrf.0.0080351. [DOI] [PubMed] [Google Scholar]
- 43.Thomas TR, Neiman CN. Aspects of copulatory behavior preventing atrophy in male rats’ reproductive system. Endocrinology. 1968;83:633–5. doi: 10.1210/endo-83-3-633. [DOI] [PubMed] [Google Scholar]
- 44.Andersen ML, Bignotto M, Tufik S. Hormone treatment facilitates penile erection in castrated rats after sleep deprivation and cocaine. J Neuroendocrinol. 2004;16:154–9. doi: 10.1111/j.0953-8194.2004.01145.x. [DOI] [PubMed] [Google Scholar]
- 45.Esquifino AI, Chacon F, Jimenez V, Reyes Toso CF, Cardinali DP. d24-hour changes in circulating prolactin, follicle-stimulating hormone, luteinizing hormone and testosterone in male rats subjected to social isolation. J Circadian Rhythms. 2004;2:1. doi: 10.1186/1740-3391-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham RL, Macheda T, Watts LT, Poteet E, Singh M, Roberts JL, et al. Androgens exacerbate motor asymmetry in male rats with unilateral 6-hydroxydopamine lesion. Horm Behav. 2011;60:617–24. doi: 10.1016/j.yhbeh.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pirke KM, Geiss M, Sintermann R. A quantitative study on feedback control of LH by testosterone in young adult and old male rats. Acta Endocrinol (Copenh) 1978;89:789–95. doi: 10.1530/acta.0.0890789. [DOI] [PubMed] [Google Scholar]
- 48.Cunningham RL, McGinnis MY. Physical provocation of pubertal anabolic androgenic steroid exposed male rats elicits aggression towards females. Horm Behav. 2006;50:410–6. doi: 10.1016/j.yhbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Cunningham RL, McGinnis MY. Factors influencing aggression toward females by male rats exposed to anabolic androgenic steroids during puberty. Horm Behav. 2007;51:135–41. doi: 10.1016/j.yhbeh.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol. 2011;589:1463–76. doi: 10.1113/jphysiol.2010.200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faulk KE, Nedungadi TP, Cunningham JT. Angiotensin converting enzyme 1 in the median preoptic nucleus contributes to chronic intermittent hypoxia hypertension. Physiol Rep. 2017;5 doi: 10.14814/phy2.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farrell SF, McGinnis MY. Long-term effects of pubertal anabolic-androgenic steroid exposure on reproductive and aggressive behaviors in male rats. Horm Behav. 2004;46:193–203. doi: 10.1016/j.yhbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Vagell ME, McGinnis MY. The role of gonadal steroid receptor activation in the restoration of sociosexual behavior in adult male rats. Horm Behav. 1998;33:163–79. doi: 10.1006/hbeh.1998.1445. [DOI] [PubMed] [Google Scholar]
- 54.Agmo A. Sexual motivation–an inquiry into events determining the occurrence of sexual behavior. Behav Brain Res. 1999;105:129–50. doi: 10.1016/s0166-4328(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 55.Brotto LA, Gorzalka BB, Hanson LA. Effects of housing conditions and 5-HT2A activation on male rat sexual behavior. Physiol Behav. 1998;63:475–9. doi: 10.1016/s0031-9384(97)00482-4. [DOI] [PubMed] [Google Scholar]
- 56.Shulman LM, Spritzer MD. Changes in the sexual behavior and testosterone levels of male rats in response to daily interactions with estrus females. Physiol Behav. 2014;133:8–13. doi: 10.1016/j.physbeh.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tohei A, Suda S, Taya K, Hashimoto T, Kogo H. Bisphenol A inhibits testicular functions and increases luteinizing hormone secretion in adult male rats. Exp Biol Med (Maywood) 2001;226:216–21. doi: 10.1177/153537020122600309. [DOI] [PubMed] [Google Scholar]
- 58.Siegel RA, Weidenfeld J, Feldman S, Conforti N, Chowers I. Neural pathways mediating basal and stress-induced secretion of luteinizing hormone, follicle-stimulating hormone, and testosterone in the rat. Endocrinology. 1981;108:2302–7. doi: 10.1210/endo-108-6-2302. [DOI] [PubMed] [Google Scholar]
- 59.Roste LS, Tauboll E, Isojarvi JI, Berner A, Berg KA, Pakarinen AJ, et al. Gonadal morphology and sex hormones in male and female Wistar rats after long-term lamotrigine treatment. Seizure. 2003;12:621–7. doi: 10.1016/s1059-1311(03)00056-6. [DOI] [PubMed] [Google Scholar]
- 60.Kalousova M, Zima T, Tesar V, Dusilova-Sulkova S, Skrha J. Advanced glycoxidation end products in chronic diseases-clinical chemistry and genetic background. Mutat Res. 2005;579:37–46. doi: 10.1016/j.mrfmmm.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 61.Heywood LH. Testosterone levels in the male laboratory rat: variation under experimental conditions. Int J Androl. 1980;3:519–29. doi: 10.1111/j.1365-2605.1980.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 62.Orr TE, Taylor MF, Bhattacharyya AK, Collins DC, Mann DR. Acute immobilization stress disrupts testicular steroidogenesis in adult male rats by inhibiting the activities of 17 alpha-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J Androl. 1994;15:302–8. [PubMed] [Google Scholar]
- 63.Balasubramanian K, Aruldhas MM, Govindarajulu P. Effect of corticosterone on rat epididymal lipids. J Androl. 1987;8:69–73. doi: 10.1002/j.1939-4640.1987.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 64.Chen H, Guo J, Ge R, Lian Q, Papadopoulos V, Zirkin BR. Steroidogenic fate of the Leydig cells that repopulate the testes of young and aged Brown Norway rats after elimination of the preexisting Leydig cells. Exp Gerontol. 2015;72:8–15. doi: 10.1016/j.exger.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudenstein RS, Bigdeli H, McDonald MH, Snyder PJ. Administration of gonadal steroids to the castrated male rat prevents a decrease in the release of gonadotropin-releasing hormone from the incubated hypothalamus. J Clin Invest. 1979;63:262–7. doi: 10.1172/JCI109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yilmaz B, Kutlu S, Canpolat S, Sandal S, Ayar A, Mogulkoc R, et al. Effects of paint thinner exposure on serum LH, FSH and testosterone levels and hypothalamic catecholamine contents in the male rat. Biol Pharm Bull. 2001;24:163–6. doi: 10.1248/bpb.24.163. [DOI] [PubMed] [Google Scholar]
- 67.Demura R, Suzuki T, Nakamura S, Komatsu H, Odagiri E, Demura H. Effect of immobilization stress on testosterone and inhibin in male rats. J Androl. 1989;10:210–3. doi: 10.1002/j.1939-4640.1989.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 68.Dahl KD, Jia XC, Hsueh JW. Bioactive follicle-stimulating hormone levels in serum and urine of male and female rats from birth to prepubertal period. Biol Reprod. 1988;39:32–8. doi: 10.1095/biolreprod39.1.32. [DOI] [PubMed] [Google Scholar]
- 69.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 70.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 72.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of Obstructive Sleep Apnea: a Population-based Perspective. Expert Rev Respir Med. 2008;2:349–64. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Finkel KJ, Searleman AC, Tymkew H, Tanaka CY, Saager L, Safer-Zadeh E, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10:753–8. doi: 10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 74.Liu L, Kang R, Zhao S, Zhang T, Zhu W, Li E, et al. Sexual Dysfunction in Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. J Sex Med. 2015;12:1992–2003. doi: 10.1111/jsm.12983. [DOI] [PubMed] [Google Scholar]
- 75.Fanfulla F, Camera A, Fulgoni P, Chiovato L, Nappi RE. Sexual dysfunction in obese women: does obstructive sleep apnea play a role? Sleep Med. 2013;14:252–6. doi: 10.1016/j.sleep.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 76.Holmes S, Abbassi B, Su C, Singh M, Cunningham RL. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology. 2013;154:4281–92. doi: 10.1210/en.2013-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holmes S, Singh M, Su C, Cunningham RL. Effects of Oxidative Stress and Testosterone on Pro-Inflammatory Signaling in a Female Rat Dopaminergic Neuronal Cell Line. Endocrinology. 2016;157:2824–35. doi: 10.1210/en.2015-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor GT, Weiss J, Rupich R. Male rat behavior, endocrinology and reproductive physiology in a mixed-sex, socially stressful colony. Physiol Behav. 1987;39:429–33. doi: 10.1016/0031-9384(87)90368-4. [DOI] [PubMed] [Google Scholar]
- 79.Colborn DR, Thompson DL, Jr, Roth TL, Capehart JS, White KL. Responses of cortisol and prolactin to sexual excitement and stress in stallions and geldings. J Anim Sci. 1991;69:2556–62. doi: 10.2527/1991.6962556x. [DOI] [PubMed] [Google Scholar]
- 80.Collu R, Gibb W, Ducharme JR. Role of catecholamines in the inhibitory effect of immobilization stress on testosterone secretion in rats. Biol Reprod. 1984;30:416–22. doi: 10.1095/biolreprod30.2.416. [DOI] [PubMed] [Google Scholar]
- 81.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 82.Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol. 2012;2012:625434. doi: 10.1155/2012/625434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cunningham RL, Singh M, O’Bryant SE, Hall JR, Barber RC. Oxidative Stress, Testosterone, and Cognition among Caucasian and Mexican-American Men with and without Alzheimer’s Disease. J Alzheimers Dis. 2014;40:563–73. doi: 10.3233/JAD-131994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balasubramanian K, Pereira BM, Govindarajulu P. Epididymal carbohydrate metabolism in experimental hypercorticosteronism: studies on mature male rats. Int J Androl. 1982;5:534–44. doi: 10.1111/j.1365-2605.1982.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 85.Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl. 2000;21:72–84. [PubMed] [Google Scholar]
- 86.Bonavera JJ, Swerdloff RS, Leung A, Lue YH, Baravarian S, Superlano L, et al. In the male brown-Norway (BN) male rat, reproductive aging is associated with decreased LH-pulse amplitude and area. J Androl. 1997;18:359–65. [PubMed] [Google Scholar]
- 87.Sankar BR, Maran RR, Sivakumar R, Govindarajulu P, Balasubramanian K. Chronic administration of corticosterone impairs LH signal transduction and steroidogenesis in rat Leydig cells. J Steroid Biochem Mol Biol. 2000;72:155–62. doi: 10.1016/s0960-0760(00)00019-4. [DOI] [PubMed] [Google Scholar]
- 88.Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, Hardy MP. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology. 2002;143:130–8. doi: 10.1210/endo.143.1.8604. [DOI] [PubMed] [Google Scholar]
- 89.Dong Q, Salva A, Sottas CM, Niu E, Holmes M, Hardy MP. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl. 2004;25:973–81. doi: 10.1002/j.1939-4640.2004.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 90.Kostic TS, Stojkov NJ, Janjic MM, Maric D, Andric SA. The adaptive response of adult rat Leydig cells to repeated immobilization stress: the role of protein kinase A and steroidogenic acute regulatory protein. Stress. 2008;11:370–80. doi: 10.1080/10253890701822378. [DOI] [PubMed] [Google Scholar]
- 91.Maric D, Kostic T, Kovacevic R. Effects of acute and chronic immobilization stress on rat Leydig cell steroidogenesis. J Steroid Biochem Mol Biol. 1996;58:351–5. doi: 10.1016/0960-0760(96)00044-1. [DOI] [PubMed] [Google Scholar]
- 92.Gao HB, Shan LX, Monder C, Hardy MP. Suppression of endogenous corticosterone levels in vivo increases the steroidogenic capacity of purified rat Leydig cells in vitro. Endocrinology. 1996;137:1714–8. doi: 10.1210/endo.137.5.8612506. [DOI] [PubMed] [Google Scholar]
- 93.Quinn PG, Payne AH. Oxygen-mediated damage of microsomal cytochrome P-450 enzymes in cultured leydig cells. Role in steroidogenic desensitization. J Biol Chem. 1984;259:4130–5. [PubMed] [Google Scholar]
- 94.Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38:171–96. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- 95.Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, et al. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36:1361–73. doi: 10.1016/s0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 96.Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143:1637–42. doi: 10.1210/endo.143.5.8802. [DOI] [PubMed] [Google Scholar]
- 97.Chen H, Liu J, Luo L, Baig MU, Kim JM, Zirkin BR. Vitamin E, aging and Leydig cell steroidogenesis. Exp Gerontol. 2005;40:728–36. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Midzak AS, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 2009;299:23–31. doi: 10.1016/j.mce.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 99.Chen H, Jin S, Guo J, Kombairaju P, Biswal S, Zirkin BR. Knockout of the transcription factor Nrf2: Effects on testosterone production by aging mouse Leydig cells. Mol Cell Endocrinol. 2015;409:113–20. doi: 10.1016/j.mce.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A. 2009;106:3543–8. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.You JM, Yun SJ, Nam KN, Kang C, Won R, Lee EH. Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can J Physiol Pharmacol. 2009;87:440–7. doi: 10.1139/y09-027. [DOI] [PubMed] [Google Scholar]
- 102.McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol. 1996;141:201–6. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- 103.Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J Clin Biochem Nutr. 2010;47:224–32. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng Y, Huang J, Tao L, Shen Z, Li H, Mo F, et al. Corticosterone increases intracellular Zn(2+) release in hippocampal HT-22 cells. Neurosci Lett. 2015;588:172–7. doi: 10.1016/j.neulet.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–75. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 106.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–34. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 107.Petersson M, Eklund M, Uvnas-Moberg K. Oxytocin decreases corticosterone and nociception and increases motor activity in OVX rats. Maturitas. 2005;51:426–33. doi: 10.1016/j.maturitas.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Petersson M, Hulting AL, Uvnas-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neurosci Lett. 1999;264:41–4. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- 109.Erbas O, Akman L, Yavasoglu A, Terek MC, Akman T, Taskiran D. Oxytocin improves follicular reserve in a cisplatin-induced gonadotoxicity model in rats. Biomed Res Int. 2014;2014:703691. doi: 10.1155/2014/703691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Erbas O, Taskiran D, Oltulu F, Yavasoglu A, Bora S, Bilge O, et al. Oxytocin provides protection against diabetic polyneuropathy in rats. Neurol Res. 2017;39:45–53. doi: 10.1080/01616412.2016.1249630. [DOI] [PubMed] [Google Scholar]
- 111.Kaneko Y, Pappas C, Tajiri N, Borlongan CV. Oxytocin modulates GABAAR subunits to confer neuroprotection in stroke in vitro. Sci Rep. 2016;6:35659. doi: 10.1038/srep35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bakos J, Strbak V, Ratulovska N, Bacova Z. Effect of oxytocin on neuroblastoma cell viability and growth. Cell Mol Neurobiol. 2012;32:891–6. doi: 10.1007/s10571-012-9799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iseri SO, Gedik IE, Erzik C, Uslu B, Arbak S, Gedik N, et al. Oxytocin ameliorates skin damage and oxidant gastric injury in rats with thermal trauma. Burns. 2008;34:361–9. doi: 10.1016/j.burns.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 114.Moghimian M, Faghihi M, Karimian SM, Imani A, Houshmand F, Azizi Y. Role of central oxytocin in stress-induced cardioprotection in ischemic-reperfused heart model. J Cardiol. 2013;61:79–86. doi: 10.1016/j.jjcc.2012.08.021. [DOI] [PubMed] [Google Scholar]


