Abstract
Background
Little is known about the predictors of sexual intercourse frequency (SIF) among couples trying to conceive despite the well-established link between SIF and fecundity.
Aim
To evaluate the male and female demographic, occupational, and lifestyle predictors of SIF among couples.
Methods
469 couples without a history of infertility participating in the Longitudinal Investigation of Fertility and the Environment Study (2005–2009) were followed for ≤1 year while trying to conceive. At enrollment, both partners were interviewed about demographic, occupational, lifestyle, and psychological characteristics using standardized questionnaires. Multivariable generalized linear mixed models with Poisson distribution was used to estimate the adjusted percent difference in SIF across exposure categories.
Outcomes
SIF was recorded in daily journals and summarized as average SIF per month.
Results
The median (interquartile range) SIF during follow-up was 6 (4–9) acts per month. For every year increase in female and male age, SIF decreased by −0.8% (95% CI −2.5, 1.0%) and −1.7% (95% CI −3.1, −0.3%). Women with high school education or less and those of non-White race had 34.4% and 16.0% higher SIF, respectively. A similar trend was seen for male education and race. Only couples where both partners (but not just one partner) worked rotating shifts had −39.1% (95% CI −61.0, −5.0%) lower SIF compared to couples where neither partner worked rotating shifts. Male (but not female) exercise was associated with 13.2% (95% CI 1.7, 26.0%) higher SIF. Diagnosis of a mood or anxiety disorder in the male (but not female) was associated with a 26.0% (95% CI −42.7, −4.4%) lower SIF. Household income, smoking status, BMI, night work, alcohol intake, psychosocial stress were not associated with SIF.
Clinical Implications
Even among couples trying to conceive, there was substantial variation in SIF. Both partners’ age, education, race, and rotating shift work as well as male exercise and mental health play an important role in determining SIF.
Strengths & Limitations
As this was a secondary analysis of an existing study, we lacked information on many pertinent psychological and relationship quality variables and the hormonal status of participants, which could have affected SIF. The unique population-based couple design, however, captured both partners’ demographics, occupational characteristics, lifestyle behaviors in advance of their daily, prospective reporting of SIF, which was a major strength.
Conclusion
Important predictors of SIF among couples attempting to conceive include male exercise and mental health and both partners’ age, education, race, and rotating shift work.
Keywords: alcohol, body weight, exercise, intercourse, libido, sexual activity, shift work, smoking, stress
Introduction
Sexual intercourse has been positively linked to overall physical and emotional wellbeing as well as increased relationship satisfaction in both men and women [1, 2]. Given the clear associations between sexual activity and quality of life, there has been great interest in identifying the factors that predict sexual intercourse frequency (SIF) but limited actual research. While the research thus far has mostly focused on marriage/cohabitation parameters, age, and race/ethnicity in relation to SIF in older populations, there is increasing interest in the sociodemographic and lifestyle factors that are related to coital frequency in younger age groups.
In reproductive aged couples, intercourse frequency not only plays a significant role in relationship quality and satisfaction [3] but also in determining couple fecundity [4]. While determinants of fecundity are often thought of exclusively as biological factors affecting ovulation, sperm quality, fertilization, and survival of the fertilized oocyte, behavioral factors, such as libido and SIF, could also play a critical role. At present, it is unknown to what extent differences in patterns of intercourse may exist and may explain associations seen between various demographic, occupational, and lifestyle exposures and markers of fecundity such as time to pregnancy, largely because the necessary data are seldom collected. The identification of such factors would have relevance for preconception guidance and general public health guidance.
Thus, using a large prospective cohort of couples without a history of infertility trying to become pregnant, where information on daily frequency of sexual intercourse was collected in journals, we sought to investigate the male and female demographic, occupational, and lifestyle characteristics associated with frequency of sexual intercourse.
Material and Methods
Study Population
The Longitudinal Investigation of Fertility and the Environment (LIFE) Study is a population-based prospective cohort of 501 couples attempting to conceive in two geographic areas (Texas and Michigan) between 2005 and 2009. Couples were eligible to enroll in the study if they were in a committed relationship and the female partner was 18–44 years, had menstrual cycles between 21 and 42 days, and had no hormonal birth control injections during past year; the male partner was ≥18 years; and both partners had the ability to communicate in English or Spanish, and had no sterilization procedures or physician diagnosed infertility. Couples were further excluded if they had been off contraception >2 months. A complete description of the study, including recruitment yield, is presented elsewhere [5]. Briefly, of the 51,715 couples who were screened, 50,527 (98%) were ineligible largely due to age (27%), not being interested in pregnancy (19%), not being in a committed relationship (19%), and planning to move outside the study area (16%). Of the 1188 eligible couples, 501 (42%) enrolled in the study and were followed for up to 12 months or through pregnancy if pregnancy occurred. The protocol was approved by the Institutional Review Boards at each institution and all participants provided written informed consent before enrollment.
Demographic, Lifestyle, and Occupational Characteristics
Research assistants traveled to couples’ homes and completed baseline in-person interviews that were conducted simultaneously but separately with each partner. The baseline interview queried men and women about their demographic and lifestyle characteristics, medical and reproductive history, and occupational activity. Men and women were asked to provide their current age, level of education, ethnicity, race, household income, and their lifetime and current use of cigarettes. Physical activity was assessed by asking participants whether they followed a regular vigorous exercise program in the past 12 months and if so, how many days per week (open response). Due to the low number of men and women reporting exercise 1 day per week and 6+ days per week, the following categories were created: 1–2, 3, 4, and ≥5 days per week. Stress was measured using the 4-item Cohen’s perceived stress scale (PSS-4) [6]. Participants also self-reported a physician diagnosis of anxiety or mood disorders and whether or not they were currently receiving medical treatment for this condition. Men and women were asked if they had consumed ≥12 alcoholic drinks in the past 12 months and if so, how often they consumed alcoholic beverages, how many alcoholic drinks they had on a typical occasion, and whether there was ever a single occasion during which they drank ≥5 alcoholic drinks. For occupational exposures, both partners were asked if they were currently employed and if so whether their current job involved any of the following: night work, rotating shifts, heavy exertion or lifting, or prolonged sitting (men only) or standing (women only). During the in-home interview, all men and women had their weight and height measured using the digital self-calibrating Health-O-Meter scale and a standardized cloth tape, respectively, after removing shoes and excessive clothing. The nurse was instructed to take two measurements and record weight to the nearest pound and height to the nearest 0.5 inch.
Sexual Intercourse Frequency
Men and women recorded daily vaginal-penial intercourse frequency in journals. The women also recorded daily information in the journals on bleeding and Clearblue® Easy home urinary-based fertility monitor results. The monitor date for menses along with daily journal information was used to establish menstrual cycles. As enrollment occurred on various days of women’s menstrual cycles, the length of the first cycle under study was the sum of the prospectively observed portion (median=15, interquartile range=7 to 22 days) and the time since last menstrual period (reported at enrollment). During follow-up, SIF was summarized as the average sexual intercourse frequency per month, which was defined as the total SIF per menstrual cycle divided by the cycle length multiplied by 30. This standardization was done to account for the differing lengths of menstrual cycles during follow-up. On average, there was no significant difference in the reporting of SIF between the man and woman within a couple (average difference=0.8 times/month). Therefore, we used female report of sexual intercourse frequency as the main outcome variable due to the slightly lower amount of missing data.
Statistical Analysis
To evaluate univariate predictors of sexual intercourse frequency, we calculated the median SIF per couple over follow-up and classified couples as either high SIF couples if their median SIF was ≥9 times/month (the 75th percentile) or low-to-average SIF couples if their median SIF was <9 times/month. Male and female demographic, occupational, and lifestyle characteristics were then compared using analysis of variance for continuous variables or chi-square tests (or Fisher’s exact tests when cell counts were <5) for categorical variables.
Multivariable generalized linear mixed models with Poisson distribution were used to analyze the associations between male and female demographic, occupational, and lifestyle characteristics and SIF over follow-up. These models were chosen as they can account for the correlated cycles within couples and an imbalanced number of cycles per couple (when the entire joint distribution is correctly specified).[7] Effect estimates and 95% CIs are presented as the percent difference in SIF for a particular group compared to the reference group for categorical variables and as the percent change in SIF for a one unit increase for continuous variables. These percent difference estimates were calculated using the following formula: [exp(β) − 1] × 100, where β is the effect estimate estimated from the generalized linear mixed models. The ESTIMATE statement was used to predict the average SIF for a specific combination of lifestyle factors to determine the overall magnitude of difference between couples with all of the positive versus all of the negative predictors of SIF. For continuous predictors, the 10th and 90th percentile was used as the high and low exposure. For categorical predictors, the specific level associated with the highest or lowest SIF was chosen.
Confounding was evaluated using prior knowledge and descriptive statistics from our cohort through the use of directed acyclic graphs. Variables retained in the final multivariable models were female age (years), female education level (high school or less, some college, college graduate), female regular exercise (yes, no), the difference between couple’s ages (years), and male employment (yes, no). Interactions between male and female demographic, occupational and lifestyle characteristics within a couple were tested using a cross-product term in the final multivariable model. To avoid any confounding by distress about achieving pregnancy, we conducted a sensitivity analysis restricted to the first 3 cycles of follow-up. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Out of the original 501 couples, 493 (98%) completed at least one cycle of daily journals during follow-up. From there we excluded any cycles with more than half the days missing information on SIF, resulting in a loss of 154 cycles (6% of the total cycles) and 7 couples. We also excluded 16 couples who got pregnant in the first cycle and had less than 14 days of follow-up. Thus, our final sample size consisted of 469 couples contributing 2211 cycles of follow-up.
The median (range) number of cycles contributed by couples was 3 (1–16). During follow-up, the median SIF (interquartile range) was 6 (4–9) acts per month with a range of 0 to 60 times per month in any given cycle. Couples with a median SIF >9 times/month over follow-up tended to have younger male and female partners, were less likely to have female partners with a college education, and were more likely to have male partners that were currently employed compared to couples with a SIF ≤9 times/month (Tables A.1 & A.2).
The significant male and female predictors of sexual intercourse frequency are shown in Figure 1. For every 1 year increase in male and female age, SIF decreased by −2.5 (95% CI - 3.7, −1.2%) and −2.5% (95% CI −3.7, −1.2%), respectively; however when modelled jointly, male age (% change: −1.7%; 95% CI −3.1, −0.3%) was more strongly associated with SIF than female age (% change: −0.8%; 95% CI −2.5, 1.0%) (Table 1). Couples where the female had only a high school education or less had 34.4% (95% CI 7.0, 68.8%) higher SIF than couples where the female had a college education. Couples in which the female partner was a race other than non-Hispanic white also had higher SIF. Similar, but not statistically significant trends were observed for male education and race/ethnicity. Household income was not associated with SIF.
Figure 1.
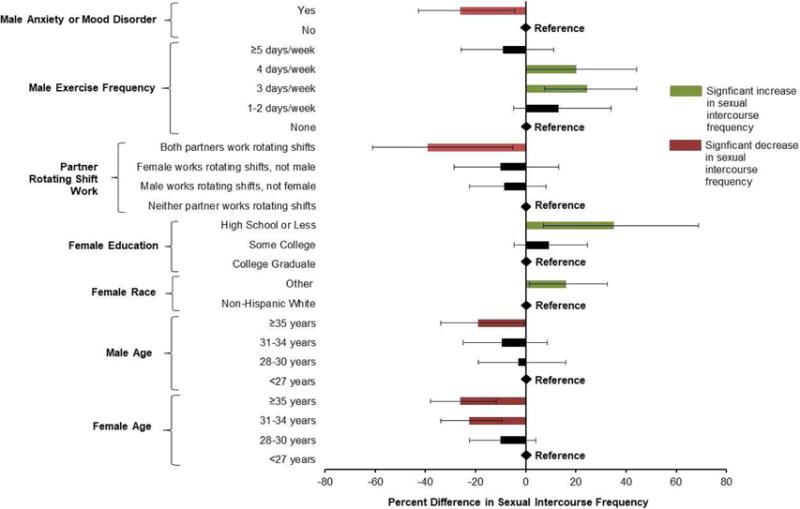
Significant male and female predictors of sexual intercourse frequency among couples trying to conceive in the LIFE Study, 2005–2009 (n=469).
Generalized linear mixed models with Poisson distribution and log link were used to estimate the % difference (95% CI) adjusting for female age (years), education level (high school or less, some college, college graduate), and regular exercise (yes, no), the difference between couple’s ages (years), and male employment (yes, no).
Table 1.
Association of demographic characteristics with frequency of sexual intercourse over follow-up in the LIFE Study, 2005–2009 (n=469 couples).
| Demographic Characteristics | Number of Couples | % Difference in Frequency of Sexual Intercourse (95% CI)1 |
|---|---|---|
| Female Age, per yr | −2.5 (−3.7, −1.2) | |
| <27 years | 93 | REF |
| 28–30 years | 175 | −10.1 (−22.3, 4.0) |
| 31–34 years | 126 | −22.6 (−33.8, −9.4) |
| ≥35 years | 75 | −26.0 (−38.0, −11.7) |
| Male Age, per yr | −2.5 (−3.7, −1.2) | |
| <27 years | 57 | REF |
| 28–30 years | 150 | −3.0 (−18.9, 16.0) |
| 31–34 years | 130 | −9.7 (−25.0, 8.7) |
| ≥35 years | 132 | −19.0 (−33.8, −0.8) |
| Difference in Male and Female Age, per yr | −1.2 (−2.6, 0.2) | |
| Female Age adjusting for Male Age, per yr | −0.8 (−3.1, 1.0) | |
| Male Age adjusting for Female Age, per yr | −1.7 (−3.1, −0.3) | |
| Female Race | ||
| Non-Hispanic white | 385 | REF |
| Other | 84 | 16.0 (1.6, 32.5) |
| Male Race | ||
| Non-Hispanic white | 390 | REF |
| Other | 79 | 15.6 (0.9, 32.3) |
| Female Highest Education | ||
| High School or Less | 26 | 34.4 (7.0, 68.8) |
| Some College | 84 | 9.2 (−4.5, 24.7) |
| College Graduate | 359 | REF |
| Male Highest Education | ||
| High School or Less | 39 | 19.7 (−0.9, 44.7) |
| Some College | 136 | 9.4 (−2.5, 22.7) |
| College Graduate | 292 | REF |
| Couple Household Income | ||
| < $29,999 | 17 | 24.5 (−6.5, 65.8) |
| $30,000–$49,999 | 55 | −9.7 (−23.8, 7.0) |
| $50,000–$69,999 | 81 | 2.3 (−11.5, 18.2) |
| ≥ $70,000 | 314 | REF |
Generalized linear mixed models with Poisson distribution and log link were used to estimate the % difference (95% CI) adjusting for female age (years), education level (high school or less, some college, college graduate), and regular exercise (yes, no), the difference between couple’s ages (years), and male employment (yes, no).
Couples in which the female worked rotating shifts had −23.1% (95% CI −36.4, −6.9%) lower SIF; however, this decrease in SIF was mainly driven by couples in which both partners worked rotating shifts (% difference: −39.1%; 95% CI −61.0, −5.0%) (Table 2). None of the other male or female occupational characteristics including night work, heavy exertion, or prolonged standing or sitting were associated with SIF. Of the lifestyle characteristics, male but not female exercise, was associated with SIF (Table 3). Specifically, couples in which the man engaged in regular exercise had 13.2% (95% CI 1.7, 26.0%) higher SIF than couples in which the man engaged in no regular exercise. The highest SIF was observed among couples where the male partner exercised 3–4 days/week. Neither partner’s BMI, smoking status, or alcohol intakes (in terms of frequency or intensity) were associated with SIF.
Table 2.
Association of occupational characteristics with frequency of sexual intercourse over follow-up in the LIFE Study, 2005–2009 (n=469 couples).
| Occupational Characteristics | Number of Couples | % Difference in Frequency of Sexual Intercourse (95% CI)1 |
|---|---|---|
| Female Paid Employment | ||
| Employed | 372 | REF |
| Not Employed | 97 | −11.5 (−22.1, 0.6) |
| Male Paid Employment | ||
| Employed | 454 | REF |
| Not Employed | 15 | −8.5 (−31.4, 21.9) |
| Female Night Work2 | ||
| No night work | 340 | REF |
| Night work | 32 | −8.5 (−24.5, 11.0) |
| Male Night Work | ||
| No night work | 351 | REF |
| Night work | 103 | −8.2 (−19.0, 4.1) |
| Female Rotating Shifts | ||
| No rotating shifts | 339 | REF |
| Rotating shifts | 33 | −23.1 (−36.4, −6.9) |
| Male Rotating Shifts | ||
| No rotating shifts | 396 | REF |
| Rotating shifts | 83 | −12.2 (−23.9, 1.4) |
| Couple Rotating Shift Work | ||
| Neither partner works rotating shifts | 283 | REF |
| Male works rotating shifts, not female | 46 | −8.5 (−22.5, 8.1) |
| Female works rotating shifts, not male | 22 | −10.0 (−28.5, 13.3) |
| Both partners work rotating shifts | 10 | −39.1 (−61.0, −5.0) |
| Female Heavy Exertion or Lifting | ||
| No heavy exertion or lifting | 327 | REF |
| Heavy exertion or lifting | 45 | −5.6 (−20.1, 11.6) |
| Male Heavy Exertion or Lifting | ||
| No heavy exertion or lifting | 304 | REF |
| Heavy exertion or lifting | 150 | −0.6 (−11.4, 11.4) |
| Female Prolonged Standing | ||
| No prolonged standing | 292 | REF |
| Prolonged standing | 80 | −8.5 (−19.8, 4.4) |
| Male Prolonged Sitting | ||
| No prolonged sitting | 237 | REF |
| Prolonged sitting | 217 | 1.0 (−9.1, 12.3) |
Generalized linear mixed models with Poisson distribution and log link were used to estimate the % difference (95% CI) adjusting for female age (years), education level (high school or less, some college, college graduate), and regular exercise (yes, no), the difference between couple’s ages (years), and male employment (yes, no).
Models for specific occupational characteristics were restricted to employed men, women, or couples.
Table 3.
Association of lifestyle characteristics with frequency of sexual intercourse over followup in the LIFE Study, 2005–2009 (n=469 couples).
| Lifestyle Characteristics | Number of Couples | % Difference in Frequency of Sexual Intercourse (95% CI)1 |
|---|---|---|
| Female BMI, per 1 kg/m2 | 0.1 (−0.7, 0.8) | |
| 18.5–24.9 kg/m2 | 208 | REF |
| 25–29.9 kg/m2 | 129 | 3.4 (−8.6, 17.0) |
| 30–34.9 kg/m2 | 61 | −0.9 (−15.5, 16.1) |
| ≥35 kg/m2 | 60 | 10.6 (−6.2, 30.4) |
| Male BMI, per 1 kg/m2 | 0.0 (−1.0, 1.1) | |
| 18.5–24.9 kg/m2 | 76 | REF |
| 25–29.9 kg/m2 | 194 | 8.9 (−6.2, 26.6) |
| 30–34.9 kg/m2 | 120 | 10.7 (−5.9, 30.2) |
| ≥35 kg/m2 | 63 | 3.5 (−14.4, 25.2) |
| Female Smoking Status | ||
| Never Smoker | 342 | REF |
| Former Smoker | 78 | −6.6 (−19.0, 7.6) |
| Current Smoker | 49 | −4.4 (−19.7, 13.8) |
| Male Smoking Status | ||
| Never Smoker | 298 | REF |
| Former Smoker | 104 | −2.5 (−14.3, 10.8) |
| Current Smoker | 67 | −11.8 (−24.6, 3.2) |
| Female Exercises Regularly | ||
| No | 283 | REF |
| Yes | 186 | 7.01 (−3.7, 18.9) |
| Frequency of Female Exercise | ||
| None | 283 | REF |
| 1–2 days/week | 39 | 2.7 (−14.9, 24.0) |
| 3 days/week | 68 | 3.5 (−11.0, 20.3) |
| 4 days/week | 36 | 8.4 (−10.8, 31.8) |
| ≥5 days/week | 42 | 14.5 (−4.4, 37.1) |
| Male Exercises Regularly | ||
| No | 277 | REF |
| Yes | 192 | 13.2 (1.7, 26.0) |
| Frequency of Male Exercise | ||
| None | 277 | REF |
| 1–2 days/week | 48 | 13.0 (−4.8, 34.1) |
| 3 days/week | 70 | 24.6 (7.5, 44.3) |
| 4 days/week | 40 | 20.1 (0.0, 44.2) |
| ≥5 days/week | 34 | −9.0 (−25.6, 11.2) |
| Frequency of Female Alcohol Intake | ||
| None | 121 | REF |
| ≤ 1 time per month | 117 | −10.8 (−22.7, 3.1) |
| 2–3 times per month | 87 | −6.0 (−19.7, 10/1) |
| 1 time per week | 63 | 1.1 (−15.0, 20.2) |
| ≥2 times per week | 80 | −2.9 (−17.8, 14.6) |
| Intensity of Female Alcohol Intake | ||
| None | 121 | REF |
| 1 drink per occasion | 102 | −12.1 (−24.4, 2.2) |
| 2 drinks per occasion | 157 | −2.2 (−14.6, 12.1) |
| ≥3 drinks per occasion | 88 | −5.4 (−19.1, 10.6) |
| Female had ≥5 Alcoholic Drinks at 1 Occasion | ||
| No | 264 | REF |
| Yes | 205 | −3.1 (−12.6, 7.5) |
| Frequency of Male Alcohol Intake | ||
| None | 70 | REF |
| ≤ 1 time per month | 65 | −1.5 (−18.4, 19.0) |
| 2–3 times per month | 73 | −16.9 (−30.9, 0.0) |
| 1 time per week | 100 | −10.8 (−24.9, 5.9) |
| ≥2 times per week | 161 | −1.9 (−16.3, 15.1) |
| Intensity of Male Alcohol Intake | ||
| None | 70 | REF |
| 1 drink per occasion | 58 | −2.6 (−19.9, 18.5) |
| 2 drinks per occasion | 143 | −6.9 (−20.8, 9.5) |
| 3 drinks per occasion | 94 | −9.5 (−24.0, 7.9) |
| ≥4 drinks per occasion | 104 | −7.5 (−22.1, 9.9) |
| Male had ≥5 Alcoholic Drinks at 1 Occasion | ||
| No | 141 | REF |
| Yes | 328 | −4.8 (−14.8, 6.4) |
Generalized linear mixed models with Poisson distribution and log link were used to estimate the % difference (95% CI) adjusting for female age (years), education level (high school or less, some college, college graduate), and regular exercise (yes, no), the difference between couple’s ages (years), and male employment (yes, no).
Of the psychological factors, only couples in which the male partner had been diagnosed with either an anxiety or mood disorder had lower SIF (% difference: −26%; 95% CI −42.7, −4.4%) (Table 4). Although numbers were small, this inverse association appeared to be driven by men diagnosed but not receiving treatment (n=8) (% difference: −43.5%; 95% CI −61.6, −16.7%) as men who were receiving treatment (n=11) had no difference in SIF (% difference: −9.5%; 95% CI −35.2, 26.4%). Male and female perceived chronic stress, female diagnosis of anxiety or mood disorders, and male and female current treatment for these conditions were also not associated with SIF.
Table 4.
Association of psychological factors with frequency of sexual intercourse over follow-up in the LIFE Study, 2005–2009 (n=469 couples).
| Psychological Factors | Number of Couples | % Difference in Frequency of Sexual Intercourse (95% CI)1 |
|---|---|---|
| Female Stress Level | ||
| C1 (0–1) | 104 | REF |
| C2 (2–3) | 143 | −10.4 (−22.3, 3.3) |
| C3 (4–5) | 131 | −4.1 (−16.9, 10.8) |
| C4 (≥6) | 91 | −6.7 (−20.5, 9.4) |
| Male Stress Level | ||
| C1 (0–1) | 140 | REF |
| C2 (2–3) | 150 | 0.5 (−11.6, 14.4) |
| C3 (4–5) | 102 | −7.3 (−19.7, 7.0) |
| C4 (≥6) | 77 | 3.6 (−11.3, 21.1) |
| Female Anxiety Disorder | ||
| No | 464 | REF |
| Yes | 34 | 7.7 (−11.5, 31.2) |
| Female Mood Disorder | ||
| No | 472 | REF |
| Yes | 28 | −10.1 (−27.6, 11.7) |
| Female Mood or Anxiety Disorder | ||
| No | 416 | REF |
| Yes | 53 | −0.3 (−15.1, 17.0) |
| Female Receiving Treatment for Mood or Anxiety Disorder | ||
| No | 443 | REF |
| Yes | 26 | −10.7 (−28.7, 11.8) |
| Male Anxiety Disorder | ||
| No | 453 | REF |
| Yes | 16 | −17.0 (−37.2, 9.6) |
| Male Mood Disorder | ||
| No | 463 | REF |
| Yes | 6 | −28.5 (−54.5, 12.6) |
| Male Mood or Anxiety Disorder | ||
| No | 450 | REF |
| Yes | 19 | −26.0 (−42.7, −4.4) |
| Male Receiving Treatment for Mood or Anxiety Disorder | ||
| No | 455 | REF |
| Yes | 14 | −18.0 (−39.2, 10.6) |
Generalized linear mixed models with Poisson distribution and log link were used to estimate the % difference (95% CI) adjusting for female age (years), education level (high school or less, some college, college graduate), and regular exercise (yes, no), the difference between couple’s ages (years), and male employment (yes, no).
The estimated SIF for couples with the combination of high SIF predictors (i.e. both partners were 25 years old, the female had a high school education or less and was of non-White race/ethnicity, the male exercised 3 days per week and had not been diagnosed with a mood or anxiety disorder, and neither partner worked rotating shifts) was 14.1 (95% CI 10.8, 18.3) acts per month versus 2.8 (95% CI 2.0, 4.0) acts per month for couples with the combination of low SIF predictors (i.e. both partners were 36 years old, the female was a college graduate and was of non-Hispanic White race/ethnicity, the male did not exercise and had been diagnosed with a mood or anxiety disorder, and both partners worked rotating shifts).
Results were similar with the male partner’s report of sexual intercourse frequency was used as the main outcome variable (instead of female report), when the couples/cycles with missing data were included in the analysis (and missing was assumed to mean no intercourse), and when analyses were restricted to the first 3 cycles of follow-up.
Discussion
In this prospective cohort, the median SIF among couples without a history of infertility trying to conceive was 6 times per month; however there was substantial variation in SIF with reports ranging from 0 to 60 times per month in any given cycle of follow-up. Important positive predictors of SIF included younger male and female age, a lower education level among either partner, having a partner of Hispanic ethnicity or non-White race, having both partners not working rotating shifts, more frequent physical activity among the male partner, and not having a male partner with an anxiety or mood disorder.
Using data from cycle 6 (2002) of the National Survey of Family Growth, Eisenberg and colleagues reported that American men and women between 25 and 45 years have sex on average 5.7 and 6.4 times per month [8]. Yet, not all of these men and women were in committed relationships or trying to get pregnant which challenges direct comparison of the findings. In a prospective cohort of 91 married or cohabitating women (1984–1986), mean intercourse frequency over the 1–3 month follow-up was 1.7 times per week (~6.8 times per month)[9] and among 202 couples in the Dieckmann diethylstilbestrol cohort the median, retrospectively-reported coital frequency was 8 times per month [10]. Results from these latter two studies are closer to our median and mean report of 6 and 7.3 times per month, suggesting two things. First, that couples trying to conceive are having sex slightly more often (but not that much more) than the typical reproductive-aged or married man or woman. Second, that similar to the findings of other validation studies [9, 11–13], retrospective reporting of SIF is most likely an overestimate and could account for the higher median coital frequency reported in the Dieckmann diethylstilbestrol cohort.
Our finding that male rather than female age was a stronger predictor of SIF in couples agrees with several [14–18] but not all [19–21] early studies on this topic. The prevailing hypothesis is that sexual responsiveness reaches a peak around 17 years among males and gradually declines thereafter; however, women supposedly peak somewhere between the late 20s and mid-40s and their responsiveness may not decline until menopause. The stronger inverse association among men could also be due to the direct relationship between male age and erectile dysfunction [22]. The higher SIF observed among men and women with lower educational attainment but not of lower income is also consistent with other studies [8, 23]; however, it is not clear what is driving this association as it persisted after adjustment for work characteristics, race/ethnicity, and age. Increased SIF among men and women who are a race other than non-Hispanic white has also been seen in other studies [8, 23] and has been attributed to cultural differences. Overall, while certain socio-demographic characteristics were strongly related to SIF, these factors are often collected in time to pregnancy studies and thus adjustment for these variables would likely reduce the bias due to confounding by SIF.
Interestingly, we found little differences in SIF across various occupational characteristics such as night shift work and prolonged sitting/standing. Moreover, while rotating shift work was associated with lower SIF among women, this association was driven by couples in which the male and female worked rotating shifts, highlighting the importance of both partner’s work schedules in dictating SIF. This decrement could be attributable to less opportunities for sexual intercourse perhaps due to unpredictable work schedules or there could be a more biologic mechanism related to disruptions of circadian rhythm affecting libido [24]. Unfortunately, given the design of our study, we were unable to address the plausibility of these potential pathways. An important null finding was the lack of relationship between male and female BMI and SIF. It is often assumed that overweight and obese women have less sexual intercourse and that this could be a reasonable explanation for the inverse relationship observed with fecundity [25]. However, the vast majority of studies in reproductive aged women (including ours) provide no evidence to support this assumption [26–29].
The beneficial effects of male exercise on SIF has been reported previously in a randomized controlled trial among men with prostate cancer undergoing androgen suppression therapy [30] and a prospective intervention study of sedentary, healthy men [31]. Exercise has also been linked to decreased risk of erectile dysfunction among younger [32] and older men [33]. The biological mechanisms behind these associations could be due to increases in free and total testosterone levels that occur shortly after physical exertion [34], improvements in markers of endothelial function which could positively affect erectile function [35], or enhanced feelings of masculinity [31], Yet the benefits of male exercise on SIF only persisted with exercise up to 4 days per week with SIF decreasing (albeit non-significantly) for men exercising ≥5 days per week. This non-linear relationship between exercise frequency and SIF could be due to subtle changes in the functioning of the hypothalamic-pituitary-gonadal axis following high levels of intensive exercise training which may result in lower sex steroid and prolactin production [36, 37]. There were no associations between male or female perceived chronic stress at baseline and SIF during follow-up; however, our assessment lacked information on day to day stressors related to work, finances, or family life that occurred during follow-up could have been more important predictors [38].
There was an inverse association between diagnosis with anxiety or depression in the male partner and lower SIF. This was not unexpected, as reduced interest in sexual activity has been shown to be a common symptom of anxiety and depression [25]. Interestingly, while psychotropic medications are among the drug categories most often associated with impairments of the sexual response [24], in this cohort the association between anxiety or depression in the male partner and lower SIF was attenuated among couples in which the male partner was receiving treatment. This suggests that in this specific population, the benefits of treatment seemed to outweigh the potential negative consequences. However, since we had few men and women with these conditions, and no information on the specific types of psychotropic medications future research on this topic is warranted.
Finally, we found no associations between male or female usual alcohol consumption and SIF. As many research papers have confirmed, the relationship between alcohol intake and sexual activity is complex with both pharmacologic and psychological dimensions. While alcohol intake generally disinhibits psychological sexual arousal at low doses, it tends to suppress physiological sexual response at higher doses [39]. However, the point at which this reversal occurs varies from person to person, primarily as a function of tolerance and expectancy based on personal experiences [40]. Therefore, these intra-individual differences could have contributed to our overall null findings across couples.
Several other important limitations of our study are noteworthy. First, many of our occupational and lifestyle characteristics were self-reported on the baseline questionnaire and thus misclassification of exposure is possible. For example, while self-reported usual alcohol intake has been shown to be positively correlated with prospectively collected intake, it is not a perfect assessment of usual consumption [41]. Yet, given the prospective nature of this study, any exposure misclassification would be expected to attenuate associations towards the null. Second, in our study, we lacked information on average hours spent at work and while sleeping per week as well as information on marital and relationship satisfaction which could have been important predictors of SIF and important confounders. We also lacked information on the hormonal status of participants. Since testosterone levels influence sexual drive, lacking data on testosterone and other hormones prevented us from understanding whether the relations of demographic, occupational, and lifestyle factors and SIF were mediated by hormonal factors. Future work could address this issue. Moreover, while the expected the prevalence of hypogonadism, PCOS, and other hormonal disorders to be rare in this population (given the strict inclusion criteria of no history of infertility and regular menstrual cycles) they could still be influencing our findings. Despite these limitations, our study has many strengths including its unique population-based couple design that captured both partners’ demographics, occupational characteristics, lifestyle behaviors in advance of their reporting of SIF. Moreover, the prospective collection of information on SIF in daily journals completed throughout follow-up is considered highly valid. In a previous validation study, a woman’s reports of coitus within the previous 48 hours was strongly correlated with the observation of sperm in urine [42]. By studying a population of men and women in committed relationships who were all trying to conceive, we also inadvertently controlled for many important confounders such as relationship status and pregnancy intentions by design.
Conclusions
Among couples without a history of infertility trying to conceive there was substantial variation in SIF, with a median (range) frequency per month of 6 (0–60) acts. Important predictors of sexual intercourse frequency include both partners’ age, education, race, and rotating shift work as well as male exercise and mental health. Given the well-established relationship between SIF and fecundity, our results highlight the potential importance of collecting information on sexual activity in studies on time to pregnancy particularly for certain demographic, occupational, and lifestyle exposures of interest. Our results also suggest that public health interventions, such as those aimed at increasing male physical activity levels, which may increase frequency of sexual intercourse could be a means to increase fertility among couples trying to conceive.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–74. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field N, Mercer CH, Sonnenberg P, Tanton C, Clifton S, Mitchell KR, et al. Associations between health and sexual lifestyles in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3) Lancet. 2013;382:1830–44. doi: 10.1016/S0140-6736(13)62222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa RM, Brody S. Women’s relationship quality is associated with specifically penile-vaginal intercourse orgasm and frequency. J Sex Marital Ther. 2007;33:319–27. doi: 10.1080/00926230701385548. [DOI] [PubMed] [Google Scholar]
- 4.Stanford JB, Dunson DB. Effects of sexual intercourse patterns in time to pregnancy studies. Am J Epidemiol. 2007;165:1088–95. doi: 10.1093/aje/kwk111. [DOI] [PubMed] [Google Scholar]
- 5.Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development–the LIFE Study. Paediatr Perinat Epidemiol. 2011;25:413–24. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Scamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1998. [Google Scholar]
- 7.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, New Jersey: John Wiley and Sons, Inc; 2004. Chapter 14: Missing Data and Dropout. [Google Scholar]
- 8.Eisenberg ML, Shindel AW, Smith JF, Breyer BN, Lipshultz LI. Socioeconomic, anthropomorphic, and demographic predictors of adult sexual activity in the United States: data from the national survey of family growth. J Sex Med. 2010;7:50–8. doi: 10.1111/j.1743-6109.2009.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornsby PP, Wilcox AJ. Validity of questionnaire information on frequency of coitus. Am J Epidemiol. 1989;130:94–9. doi: 10.1093/oxfordjournals.aje.a115326. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen RH, Wilcox AJ, Baird DD. Can men provide accurate confounder data about their partners for Time-to-Pregnancy studies? Ann Epidemiol. 2007;17:186–90. doi: 10.1016/j.annepidem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Gillmore MR, Leigh BC, Hoppe MJ, Morrison DM. Comparison of daily and retrospective reports of vaginal sex in heterosexual men and women. J Sex Res. 2010;47:279–84. doi: 10.1080/00224490903050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber LR, Lyerly JE, Young AM, Dmochowski J, Vick TM, Scholes D. Comparison of prospective and retrospective measurements of frequency of sexual intercourse. Matern Child Health J. 2014;18:1293–9. doi: 10.1007/s10995-013-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner MJ, Hertz-Picciotto I, Taylor D, Schoenbach V, Wheeless A. Retrospective vs. prospective coital frequency and menstrual cycle length in a contraceptive effectiveness trial. Ann Epidemiol. 2001;11:428–33. doi: 10.1016/s1047-2797(01)00248-4. [DOI] [PubMed] [Google Scholar]
- 14.Kinsey AC, Pomeroy WB, Martin CE. Sexual Behavior in the Human Male. Bloomington, IN: Indiana University Press; 1948. [Google Scholar]
- 15.Kinsey AC, Pomeroy WB, Martin CE, Gebhard PH. Sexual Behavior in the Human Female. Bloomington, IN: Indiana University Press; 1953. [Google Scholar]
- 16.Doddridge R, Schumm WR, Bergen B. Factors Related to Decline in Preferred Frequency of Sexual Intercourse among Young Couples. Psychol Rep. 1987;60:391–95. [Google Scholar]
- 17.Edwards JN, Alan B. Sexual Behavior In and Out of Marriage: An Assessment of Correlates. Journal of Marriage and the Family. 1976;38:73–81. [Google Scholar]
- 18.James WH. Marital coital rates, spouses’ ages, family size and social class. Journal of Sex Research. 1974;10:205–18. doi: 10.1080/00224497409550851. [DOI] [PubMed] [Google Scholar]
- 19.Rao KV, Demaris A. Coital frequency among married and cohabiting couples in the United States. J Biosoc Sci. 1995;27:135–50. doi: 10.1017/s0021932000022653. [DOI] [PubMed] [Google Scholar]
- 20.Udry JR, Deven FR, Coleman SJ. A cross-national comparison of the relative influence of male and female age on the frequency of marital intercourse. J Biosoc Sci. 1982;14:1–6. doi: 10.1017/s0021932000013808. [DOI] [PubMed] [Google Scholar]
- 21.Udry JR, Morris NM. Relative contribution of male and female age to the frequency of marital intercourse. Soc Biol. 1978;25:128–34. doi: 10.1080/19485565.1978.9988330. [DOI] [PubMed] [Google Scholar]
- 22.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–7. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Kivela SL, Nissinen A, Puska P. Dimensions of health behaviour among the 65–74-year-old population in eastern Finland. Funct Neurol. 1988;3:309–25. [PubMed] [Google Scholar]
- 24.Ferguson JM. The effects of antidepressants on sexual functioning in depressed patients: a review. J Clin Psychiatry. 2001;62(Suppl 3):22–34. [PubMed] [Google Scholar]
- 25.McCabe M, Althof SE, Assalian P, Chevret-Measson M, Leiblum SR, Simonelli C, et al. Psychological and interpersonal dimensions of sexual function and dysfunction. J Sex Med. 2010;7:327–36. doi: 10.1111/j.1743-6109.2009.01618.x. [DOI] [PubMed] [Google Scholar]
- 26.Esposito K, Ciotola M, Giugliano F, Bisogni C, Schisano B, Autorino R, et al. Association of body weight with sexual function in women. Int J Impot Res. 2007;19:353–7. doi: 10.1038/sj.ijir.3901548. [DOI] [PubMed] [Google Scholar]
- 27.Yaylali GF, Tekekoglu S, Akin F. Sexual dysfunction in obese and overweight women. Int J Impot Res. 2010;22:220–6. doi: 10.1038/ijir.2010.7. [DOI] [PubMed] [Google Scholar]
- 28.Brunner Huber LR, Stanley WA, Broadhurst L, Dmochowski J, Vick TM, Scholes D. No association between body size and frequency of sexual intercourse among oral contraceptive users. Ann Epidemiol. 2014;24:655–9. doi: 10.1016/j.annepidem.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneshiro B, Jensen JT, Carlson NE, Harvey SM, Nichols MD, Edelman AB. Body mass index and sexual behavior. Obstet Gynecol. 2008;112:586–92. doi: 10.1097/AOG.0b013e31818425ec. [DOI] [PubMed] [Google Scholar]
- 30.Cormie P, Newton RU, Taaffe DR, Spry N, Joseph D, Akhlil Hamid M, et al. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trial. Prostate Cancer Prostatic Dis. 2013;16:170–5. doi: 10.1038/pcan.2012.52. [DOI] [PubMed] [Google Scholar]
- 31.White JR, Case DA, McWhirter D, Mattison AM. Enhanced sexual behavior in exercising men. Arch Sex Behav. 1990;19:193–209. doi: 10.1007/BF01541546. [DOI] [PubMed] [Google Scholar]
- 32.Hsiao W, Shrewsberry AB, Moses KA, Johnson TV, Cai AW, Stuhldreher P, et al. Exercise is associated with better erectile function in men under 40 as evaluated by the International Index of Erectile Function. J Sex Med. 2012;9:524–30. doi: 10.1111/j.1743-6109.2011.02560.x. [DOI] [PubMed] [Google Scholar]
- 33.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–8. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 34.Schwab R, Johnson GO, Housh TJ, Kinder JE, Weir JP. Acute effects of different intensities of weight lifting on serum testosterone. Medicine and science in sports and exercise. 1993;25:1381–5. [PubMed] [Google Scholar]
- 35.Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, et al. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015;45:279–96. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- 36.Hackney AC, Lane AR, Register-Mihalik J, O’Leary CB. Endurance Exercise Training and Male Sexual Libido. Med Sci Sports Exerc. 2017;49:1383–88. doi: 10.1249/MSS.0000000000001235. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler GD, Wall SR, Belcastro AN, Cumming DC. Reduced serum testosterone and prolactin levels in male distance runners. JAMA. 1984;252:514–6. [PubMed] [Google Scholar]
- 38.Bodenmann G, Ledermann T, B TN. Stress, sex, and satisfaction in marriage. Personal Relationships. 2007;14:551–69. [Google Scholar]
- 39.Crowe LC, George WH. Alcohol and human sexuality: review and integration. Psychol Bull. 1989;105:374–86. doi: 10.1037/0033-2909.105.3.374. [DOI] [PubMed] [Google Scholar]
- 40.Southwick L, Steele C, Marlatt A, Lindell M. Alcohol-related expectancies: defined by phase of intoxication and drinking experience. J Consult Clin Psychol. 1981;49:713–21. doi: 10.1037//0022-006x.49.5.713. [DOI] [PubMed] [Google Scholar]
- 41.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–7. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 42.Kunin CM, Ames RE. Methods for determining the frequency of sexual intercourse and activities of daily living in young women. Am J Epidemiol. 1981;113:55–61. doi: 10.1093/oxfordjournals.aje.a113066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


