Abstract
Obesity is associated with an increased incidence of high-grade prostate cancer (PC) and poor prognosis for PC patients. Recently, we showed that obesity-related periprostatic white adipose tissue (WAT) inflammation, characterized by crown-like structures (CLS) consisting of dead or dying adipocytes surrounded by macrophages, was associated with high-grade PC. It’s possible, therefore, that agents that suppress periprostatic WAT inflammation will alter the development or progression of PC. Pioglitazone, a ligand of PPARγ, is used to treat diabetes and possesses anti-inflammatory properties. Here our main objectives were to determine if pioglitazone inhibited obesity-related periprostatic WAT inflammation in mice and then to elucidate the underlying mechanism. Treatment with pioglitazone reduced the density of CLS in periprostatic fat, and suppressed levels of TNF-α,TGF-β and the chemokine monocyte chemoattractant protein-1 (MCP-1). Importantly, the ability of pioglitazone to suppress periprostatic WAT inflammation was abrogated in MCP-1 knock out mice. Pioglitazone caused dose-dependent induction of both adiponectin, an anti-inflammatory adipokine, and its receptor AdipoR2 in cultured 3T3-L1 cells and in periprostatic WAT of obese mice. Pioglitazone blocked TNF-α–mediated induction of MCP-1 in 3T3-L1 cells, an effect that was attenuated when either adiponectin or AdipoR2 were silenced. Taken together, pioglitazone-mediated induction of adiponectin suppressed the elevation in MCP-1 levels thereby attenuating obesity-related periprostatic WAT inflammation. These findings strengthen the rationale for future efforts to determine whether targeting the PPARγ–adiponectin–MCP-1 axis will decrease periprostatic adipose inflammation and thereby reduce the risk of high-grade PC or improve outcomes for men with PC.
Keywords: pioglitazone, prostate cancer, inflammation, adiponectin, adipose
Introduction
Obesity is both a risk factor for the development of high-grade prostate cancer (PC) and a poor prognostic factor for PC survivors (1–3). Obesity-related effects on levels of hormones, adipokines and proinflammatory mediators have been suggested to contribute to the pathogenesis of PC (4–6). Chronic low grade inflammation is commonly found in the visceral and subcutaneous white adipose tissue (WAT) of obese humans and mice (7–9). In both mouse models of obesity and obese humans, macrophages infiltrate WAT and surround dead or dying adipocytes forming crown-like structures (CLS) (10). The prostate is contained within a thin connective tissue capsule and is surrounded by adipose tissue. Recently, we showed that WAT inflammation occurred in the periprostatic fat of approximately fifty percent of men undergoing radical prostatectomy as a treatment for PC (11). Importantly, periprostatic WAT inflammation, as defined by the presence of CLS, was associated with high-grade prostate cancer suggesting a potential contributory role (11). Periprostatic WAT inflammation was also associated with systemic effects that have been linked to the pathogenesis of PC including reduced levels of adiponectin, an anti-inflammatory adipokine, and insulin resistance (11,12). It is possible, therefore, that interventions that attenuate periprostatic WAT inflammation will either reduce the risk of PC or improve the outcomes of PC patients.
PPARγ, a member of the nuclear receptor family of transcription factors, plays a significant role in regulating lipid and glucose metabolism (13). Ligand-activated PPARγ induces adipocyte differentiation, stimulates mitochondrial biogenesis and inhibits the production of proinflammatory mediators (14). Pioglitazone, a PPARγ-agonist that belongs to a class of drugs called thiazolidinediones, is used to treat type 2 diabetes. Pioglitazone improves insulin resistance, and has a range of anti-inflammatory properties, including being able to induce adiponectin levels and reduce the number of macrophages in adipose tissue (15,16). Whether pioglitazone can suppress obesity-related periprostatic WAT inflammation is unknown.
In this study, we had two major objectives. The first was to determine whether pioglitazone could suppress periprostatic WAT inflammation in a mouse model of obesity. Our second goal was to elucidate the mechanism underlying the anti-inflammatory effect of pioglitazone. Here we demonstrate that treatment with pioglitazone attenuated obesity-related periprostatic WAT inflammation. Moreover, we provide evidence that pioglitazone induced adiponectin which acted, in turn, to suppress the induction of monocyte chemoattractant-1 (MCP-1) in adipose tissue leading to decreased periprostatic WAT inflammation. Taken together, we show for the first time that it’s possible to use a pharmacological strategy to suppress periprostatic WAT inflammation. Our data highlight the importance of targeting the PPARγ-adiponectin-MCP-1 axis as a strategy to reduce periprostatic WAT inflammation and potentially reduce the risk of PC or improve outcomes for men with PC.
Materials and Methods
Materials
Pioglitazone was obtained from the NCI Chemical Repository. Mouse recombinant TNF-α, and ELISA kits for MCP-1 and adiponectin were obtained from R&D Systems. RNeasy mini kits, real-time PCR mouse primers for CD68 (Cat # QT00254051), MCP-1 (Cat # QT00167832), TGF-β (Cat # QT00145250), GAPDH (Cat # QT01658692), adiponectin (Cat # QT01048047), AdipoR1 (Cat # QT00154217), AdipoR2 (Cat # QT00165326) and siRNA for adiponectin were purchased from Qiagen. TNF-α primers were obtained from Sigma (17). Non-targeting siRNA, AdipoR2 siRNA and DharmaFECT4 were obtained from Dharmacon. Murine leukemia virus reverse transcriptase, RNase inhibitor, oligo (dT)16, and Fast SYBR green PCR master mix were obtained from Applied Biosystems. Insulin, IBMX and dexamethasone were obtained from Sigma.
Animal models
Male wild-type C57BL/6J and MCP-1 knock out mice (B6.129S4-Ccl2tm1Rol/J (The Jackson Laboratory) were used. Mice received either low fat (LF) diet (12450Bi; 10 kcal% fat), high fat (HF) diet (D12492i; 60 kcal% fat) or HF diet containing 0.006% or 0.06% w/w pioglitazone (Research Diets). Previously, 0.05% w/w pioglitazone was found to activate PPARγ in adipose tissue (18). Following sacrifice, periprostatic fat tissue was snap-frozen in liquid nitrogen and stored at −80°C for molecular analyses or formalin fixed for histologic analyses. The animal protocol was approved by the Institutional Animal Care and Use Committee at Weill Cornell Medicine (New York, NY).
Adipocyte diameter
Adipocyte diameter was quantified as previously described (19).
Cell culture
3T3-L1 preadipocytes (Zenbio) were seeded 5,000 cells/cm2 in cell culture plates and maintained in preadipocyte medium (Zenbio). All experiments were carried out within one year (2015–2016) of purchasing the cell line. Independent authentication of the purchased cell line was not carried out. Once the cells were confluent, cells were incubated an additional 48 hours before initiating differentiation. The cells were then switched to differentiation medium containing 10% fetal bovine serum, 50 U/mL penicillin, 50 µg/mL streptomycin, 500 µmol/L IBMX, 50 µmol/L dexamethasone, and 2.5 µg/mL insulin for 3 days. Three days after the initiation of differentiation, cells were switched to maintenance medium containing 10% fetal bovine serum, 50 U/mL penicillin, 50 µg/ml streptomycin, 50 µmol/L dexamethasone, and 2.5 µg/mL insulin and maintained until 7 days post differentiation. All treatments were performed on cells that were maintained in serum free medium overnight.
RNA interference
siRNAs were transfected into 3T3-L1 adipocytes using DharmaFECT4 (20). Cells transfected with non-targeting siRNA were used as negative controls. Seventy-two hours after transfection, the cells were analyzed.
Quantitative real-time PCR
Total RNA was isolated from periprostatic fat and cells using the RNeasy Mini Kit. RNA was reversed transcribed using murine leukemia virus reverse transcriptase and oligo (dT)16 primer and the resulting cDNA was then used for amplification. GAPDH was used as an endogenous normalization control. Real-time PCR was performed using 2× Fast SYBR green PCR master mix on a 7500 Fast Real-time PCR system (Applied Biosystems). Relative fold-induction was determined using the ddCT (relative quantification) analysis protocol.
ELISA assay
MCP-1 and adiponectin protein levels in cell culture medium were quantified using ELISA kits from R&D Systems.
Statistical analysis
In the mouse experiments, endpoints of interest include mouse weights over time or at the time of sacrifice, mouse caloric consumption over time, and various biomarker measurements obtained from mouse periprostatic WAT, including number of CLS/cm2, average adipocyte diameter and relative mRNA expression level. Differences in the endpoints across multiple groups were examined using ANOVA or the non-parametric Kruskal-Wallis test, where appropriate. Differences in the endpoints between two groups were examined using t-test or the non-parametric Wilcoxon rank sum test, where appropriate. For data measured longitudinally over time, such as mouse weight and caloric consumption, differences in the average value of the endpoint over time were examined using the mixed effects linear regression model which takes into account both the between and within mouse variations. All statistical tests were two-sided and p-value<0.05 is considered statistically significant.
Results
Pioglitazone inhibits periprostatic white adipose tissue inflammation
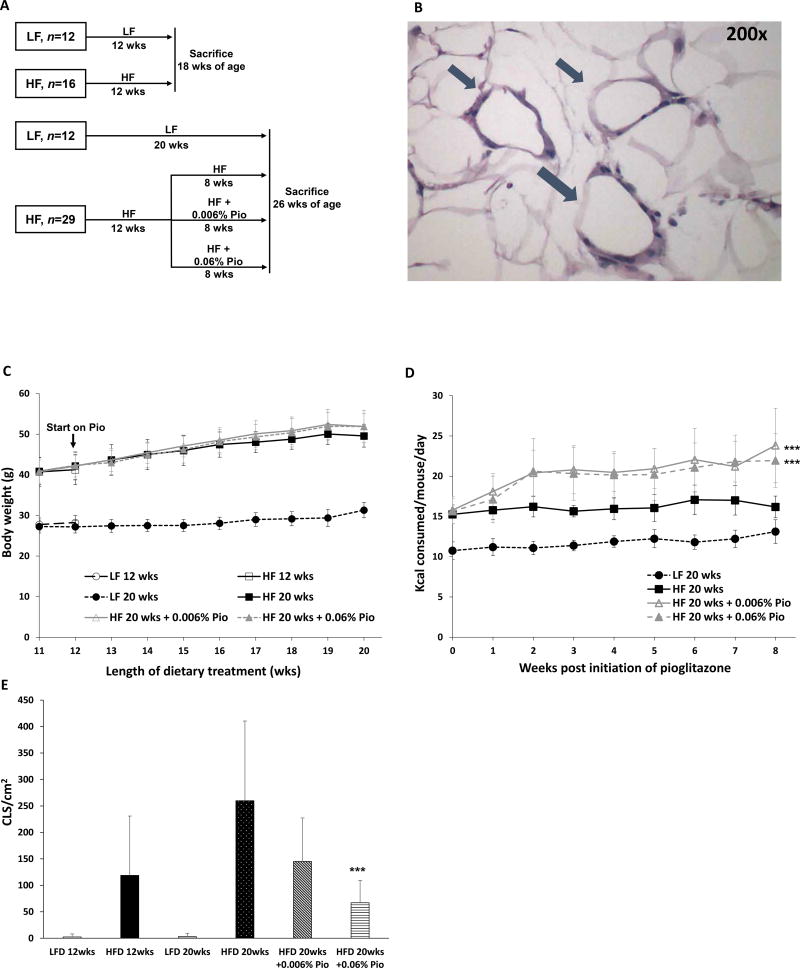
Mice were fed either a HF diet or LF diet to determine if obesity caused periprostatic WAT inflammation as defined by the presence of CLS (Figs. 1A and B). Body weight, calorie consumption and periprostatic WAT inflammation were determined (Figs. 1C–E). After feeding these diets for 12 weeks, the HF diet fed mice weighed approximately 40% more than the mice that received the LF diet. This increase in weight was paralleled by the development of periprostatic WAT inflammation (Figs. 1C and E). To determine if pioglitazone inhibited obesity associated periprostatic WAT inflammation, obese HF diet fed mice were either continued on HF diet alone or treated with HF diet containing two doses (0.006%, 0.06% w/w) of pioglitazone Fig. 1A). Interestingly, treatment with either dose of pioglitazone led to a marked increase in calorie consumption without causing significant weight gain (Figs. 1C and D). Notably, treatment with pioglitazone led to a dose-dependent decrease in periprostatic WAT inflammation (Fig. 1E).
Figure 1. Pioglitazone inhibits high fat diet induced periprostatic white adipose inflammation.
A, study schema. C57BL/6J male mice were treated with low fat (LF) or high fat (HF) diets beginning at 6 weeks of age. After 12 weeks of feeding, two groups of mice fed either LF or HF diets were sacrificed. Two additional groups continued to receive LF diet or HF diet for an additional 8 weeks prior to sacrifice. Other mice received the HF diet for 12 weeks before being switched to HF diet containing either 0.006% or 0.06% w/w pioglitazone for an additional 8 weeks until sacrifice at 26 weeks of age. B, hematoxylin and eosin-stained section showing several CLS (arrows) in periprostatic fat. C, body weights of mice in different treatment groups. D, caloric consumption was monitored weekly. E, periprostatic white adipose inflammation defined as CLS/cm2 was quantified at the time of sacrifice. Mean ± SD (error bars) are shown. D, n=9–10/group. E, n=4–10/group; ***p<0.001 compared with HF diet 20 weeks.
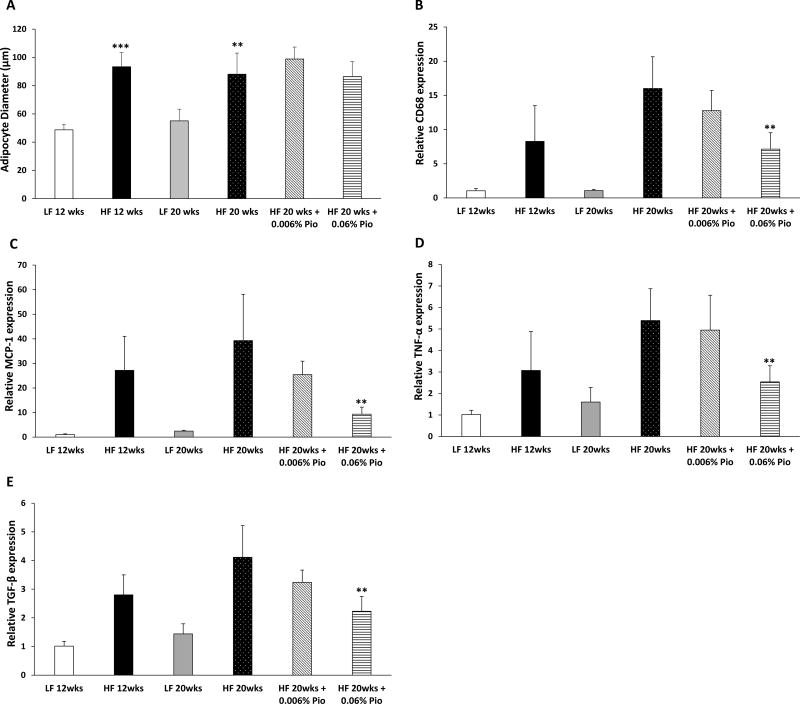
Numerous studies have demonstrated a correlation between adipocyte size and the presence and severity of WAT inflammation (10,19,21). Consistent with the increase in body weight, adipocytes were larger in the periprostatic WAT of HF diet compared with LF diet fed mice (Fig. 2A). Although pioglitazone reduced periprostatic WAT inflammation, it did not affect adipocyte size (Fig. 2A). Next we characterized a series of molecular endpoints in periprostatic WAT. Consistent with the findings for CLS in periprostatic WAT (Fig. 1E), the increase in expression of the macrophage marker CD68, induced by HF diet feeding was attenuated by treatment with 0.06% (w/w) pioglitazone (Fig. 2B). Next, we measured levels of MCP-1, a chemokine that is important for recruitment of blood monocytes into adipose tissue where they undergo differentiation and become macrophages (22,23). As shown in Fig. 2C, HF diet led to an increase in levels of MCP-1, an effect that was suppressed by 0.06% (w/w) pioglitazone. Similarly, periprostatic WAT inflammation was associated with increased levels of TNF-α and TGF-β; these changes were attenuated by treatment with 0.06% (w/w) pioglitazone (Figs. 2D and E).
Figure 2. Pioglitazone suppresses levels of proinflammatory mediators in periprostatic white adipose tissue in mice fed a high fat diet.
At 6 weeks of age, C57BL/6J male mice were fed either low fat (LF) diet or high fat (HF) diet. The LF diet fed mice were randomized to one of two groups. One group was sacrificed at 18 weeks of age after 12 weeks on LF diet while the second group was sacrificed at 26 weeks of age after 20 weeks on LF diet. The other mice were fed HF diet for 12 weeks to induce obesity and then randomized to one of four groups. One group was sacrificed at 18 weeks of age after 12 weeks on HF diet. The second group was continued on HF diet, the third group was switched to HF diet containing 0.006% w/w pioglitazone and the fourth group was switched to HF diet containing 0.06% w/w pioglitazone for an additional 8 weeks until sacrifice at 26 weeks of age. A, average adipocyte diameter. B–E, Real-time PCR was carried out for CD68, MCP-1, TNF-α and TGF-β. Mean ± SD (error bars) are shown. A, n=4–10/group; **p<0.01 compared with LF diet 20 weeks and ***p<0.001 compared with LF diet 12 weeks. B–E, n=3–7/group; **p<0.01 compared with HF diet 20 weeks.
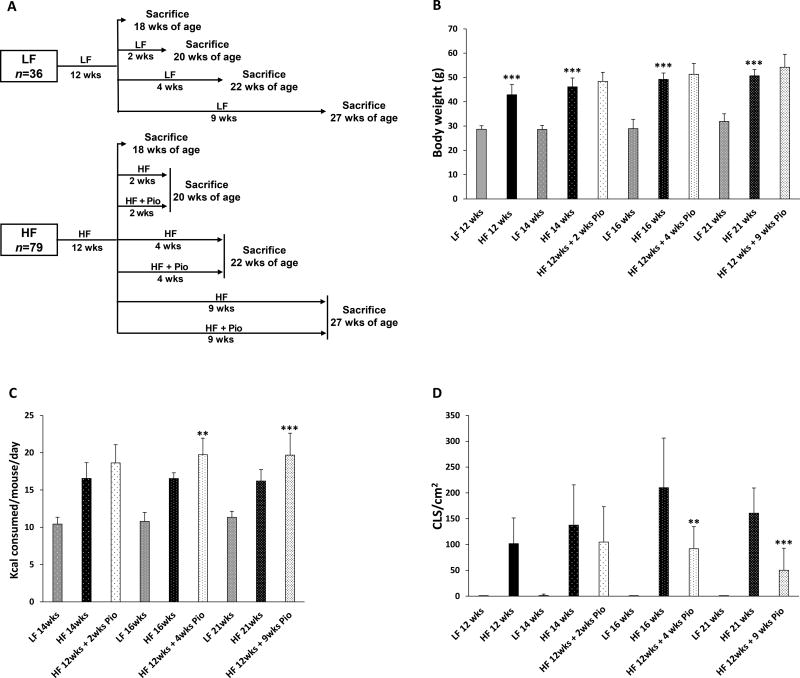
Having established that pioglitazone suppressed HF diet-induced periprostatic WAT inflammation, we next carried out a study to determine the time course of this anti-inflammatory effect. The experimental schema is shown in Fig. 3A. Body weight, calorie consumption and periprostatic WAT inflammation were assessed over time (Figs. 3B–D). Consistent with the findings in Fig. 1, treatment with HF diet for 12 weeks led to weight gain and periprostatic WAT inflammation (Figs. 3B and D). Mice were then either continued on HF or LF diets or treated with HF diet containing 0.06% (w/w) pioglitazone for 2, 4 or 9 weeks. Here, treatment with pioglitazone for 4 or 9 weeks caused hyperphagia (Fig. 3C) in the absence of inducing weight gain (Fig. 3B). Treatment with pioglitazone led to reduced number of CLS/cm2 in WAT after 4 and 9 weeks of treatment (Fig. 3D).
Figure 3. Treatment with pioglitazone led to time-dependent suppression of periprostatic white adipose tissue inflammation.
A, study schema. At 6 weeks of age, C57BL/6J male mice were fed either a low fat (LF) or high fat (HF) diet. A group of LF diet fed mice was sacrificed at 18 weeks of age after 12 weeks on LF diet. A second group of LF diet fed mice was sacrificed at 20 weeks of age after 14 weeks on LF diet. A third group of LF diet fed mice was sacrificed at 22 weeks of age after 16 weeks on LF diet. The fourth group of LF diet fed mice was sacrificed at 27 weeks of age after 21 weeks on LF diet. The HF diet fed mice were randomized to seven groups at 18 weeks of age after 12 weeks on HF diet. One group was sacrificed at 18 weeks of age while three other groups were continued on HF diet for an additional 2, 4 or 9 weeks. The remaining three groups were switched to HF diet containing 0.06% w/w pioglitazone for an additional 2, 4 or 9 weeks. B, body weights of mice in different treatment groups. C, caloric consumption was monitored in mice following initiation of pioglitazone treatment. Average calorie consumption during the treatment period is presented. D, periprostatic white adipose inflammation was quantified as CLS/cm2. B–D, mean ± SD (error bars) are shown. B, n=6–16/group, ***p<0.001 compared with LF diet group. C, n=6–16/group; **p<0.01 compared with HF diet group. D, n=6–12/group; **p<0.01, ***p<0.001 compared with HF diet group.
MCP-1 is important for pioglitazone-mediated suppression of HF diet-induced periprostatic WAT inflammation
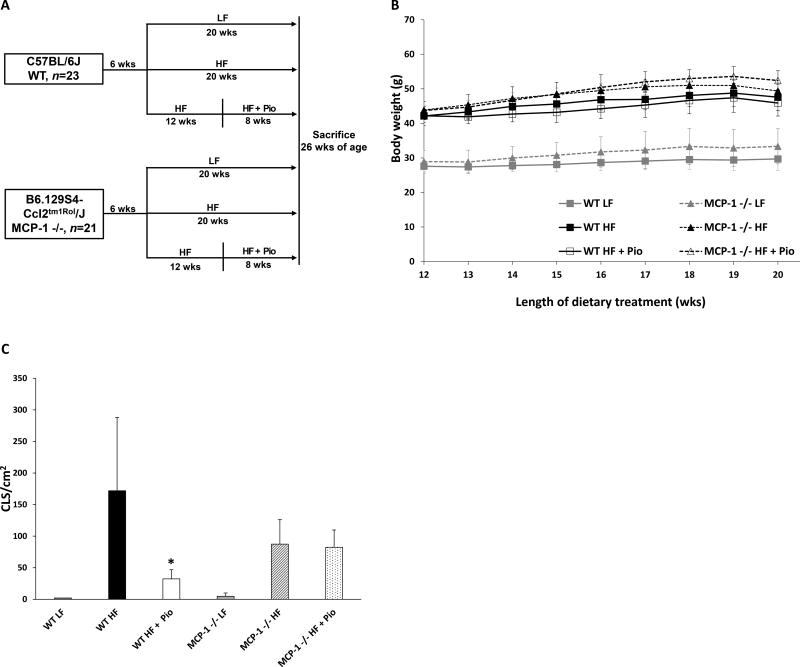
As mentioned above, MCP-1 plays a significant role in regulating the accumulation of macrophages in adipose tissue. The inhibitory effects of pioglitazone on HF diet-mediated induction of periprostatic WAT inflammation (Fig. 1E) were paralleled by changes in MCP-1 expression (Fig. 2C). Hence, we tested whether the observed suppression of MCP-1 expression was required for the anti-inflammatory effects of pioglitazone. To address this question, we compared the anti-inflammatory efficacy of pioglitazone in wild-type vs. MCP-1 −/− mice (Fig. 4A). Body weight and periprostatic WAT inflammation were determined (Figs. 4B and C). Treatment with pioglitazone did not affect the weights of either the wild-type or the MCP-1 −/− mice (Fig. 4B). Consistent with findings in Figs. 1E and 3D, pioglitazone markedly suppressed periprostatic WAT inflammation in wild-type mice (Fig. 4C). Importantly, this anti-inflammatory effect of pioglitazone was abrogated in MCP-1 −/− mice (Fig. 4C). Taken together, these data indicate that the anti-inflammatory effect of pioglitazone is mediated by its ability to suppress HF diet-mediated induction of MCP-1.
Figure 4. The anti-inflammatory effects of pioglitazone are mediated by MCP-1.
A, study schema. Beginning at 6 weeks of age, wild-type and MCP-1 −/− (B6.129S4-Ccl2tm1Rol/J) mice were fed either low fat (LF) or high fat (HF) diets for 20 weeks. Other mice received 12 weeks of HF diet before being switched to HF diet containing 0.06% w/w pioglitazone for 8 weeks. All mice were sacrificed at 26 weeks of age. B, body weight was monitored weekly in different treatment groups. C, periprostatic white adipose inflammation was quantified as CLS/cm2. Mean ± SD (error bars) are shown; n=4–6/group; *p<0.05 compared with HF diet group.
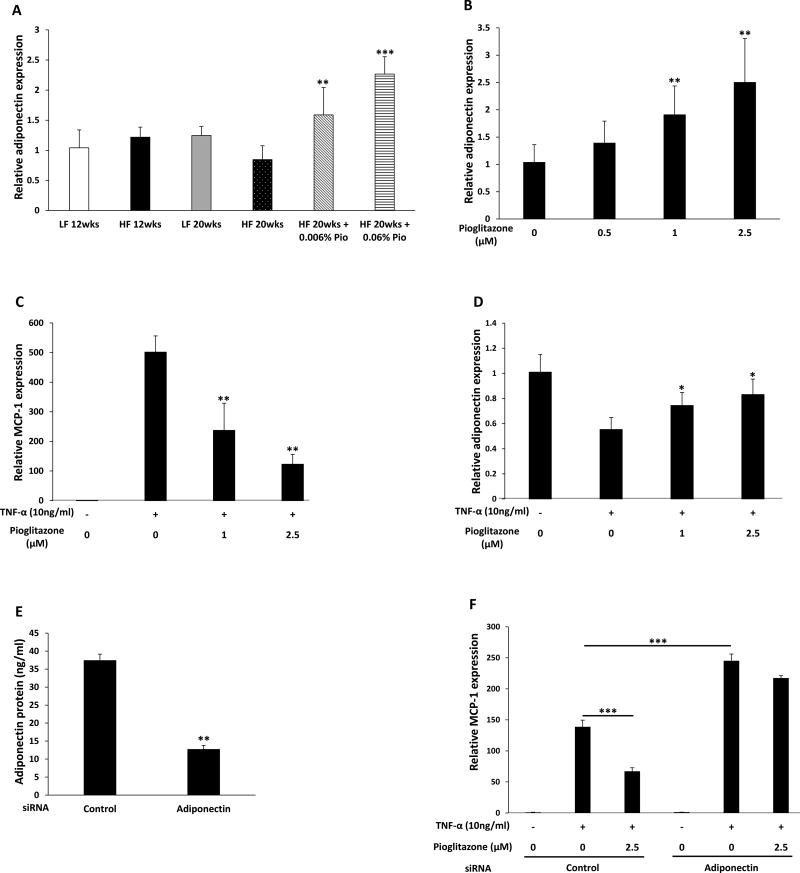
Pioglitazone-mediated suppression of MCP-1 is mediated by adiponectin
Pioglitazone is a known inducer of adiponectin in adipose tissue (24,25). Moreover, adiponectin can inhibit MCP-1 expression (26). Hence, experiments were next carried out to determine if the suppressive effects of pioglitazone on MCP-1 expression were mediated by adiponectin. As shown in Fig. 5A, treatment with pioglitazone led to a dose-dependent increase in adiponectin levels in mice fed a HF diet. To determine if pioglitazone-mediated increases in adiponectin suppressed the induction of MCP-1, a series of in vitro experiments were carried out using 3T3-L1 adipocytes. As shown in Fig. 5B, pioglitazone caused dose-dependent induction of adiponectin. Levels of TNF-α, a known inducer of MCP-1 (27), are elevated in inflamed periprostatic WAT (Fig. 2D). Interestingly, pioglitazone caused dose-dependent suppression of TNF-α-mediated induction of MCP-1 (Fig. 5C). Moreover, treatment of adipocytes with TNF-α inhibited adiponectin mRNA levels, an effect that was attenuated by pioglitazone (Fig. 5D). To confirm that pioglitazone-mediated suppression of MCP-1 induction is mediated by adiponectin, siRNA to adiponectin was used. Adipocytes transfected with siRNA to adiponectin produced approximately 70% less adiponectin protein (Fig. 5E). Importantly, silencing adiponectin both enhanced TNF-α-mediated induction of MCP-1 and attenuated the ability of pioglitazone to suppress the induction of MCP-1 mRNA (Fig. 5F). Collectively, these results indicate that pioglitazone-mediated increases in adiponectin suppress the induction of MCP-1.
Figure 5. Pioglitazone-mediated induction of adiponectin regulates MCP-1 levels.
A, At 6 weeks of age, C57BL/6J male mice were fed either low fat (LF) diet or high fat (HF) diet. The LF diet fed mice were randomized to one of two groups. One group was sacrificed at 18 weeks of age after 12 weeks on LF diet while the second group was sacrificed at 26 weeks of age after 20 weeks on LF diet. The other mice were fed HF diet for 12 weeks to induce obesity and then randomized to one of four groups. One group was sacrificed at 18 weeks of age after 12 weeks on HF diet. The second group was continued on HF diet, the third group was switched to HF diet containing 0.006% w/w pioglitazone and the fourth group was switched to HF diet containing 0.06% w/w pioglitazone for an additional 8 weeks until sacrifice at 26 weeks of age. Adiponectin mRNA levels were measured in periprostatic white adipose tissue. B, 3T3-L1 adipocytes were treated with 0–2.5 µM pioglitazone for 6 hours. Relative adiponectin expression was quantified. C and D, 3T3-L1 adipocytes were pretreated with 0–2.5 µM pioglitazone for 6 hours. Subsequently, cells were treated with TNF-α for an additional 6 hours. MCP-1 (C) and adiponectin (D) mRNA levels were quantified. E, 3T3-L1 adipocytes were transfected with control or adiponectin siRNA. Levels of adiponectin in the medium were quantified by ELISA. F, 3T3-L1 adipocytes were transfected with control siRNA or adiponectin siRNA. Seventy-two hours after transfection, cells were treated with vehicle or pioglitazone for 6 hours. Subsequently, cells received TNF-α (10 ng/mL) for an additional 6 hours. Levels of MCP-1 mRNA were quantified. Mean ± SD (error bars) are shown. A, n=3–7/group; **p<0.01, ***p<0.001 compared to mice fed HF diet for 20 weeks. B–F, n=4–6/treatment group; *p<0.05, **p<0.01, ***p<0.001.
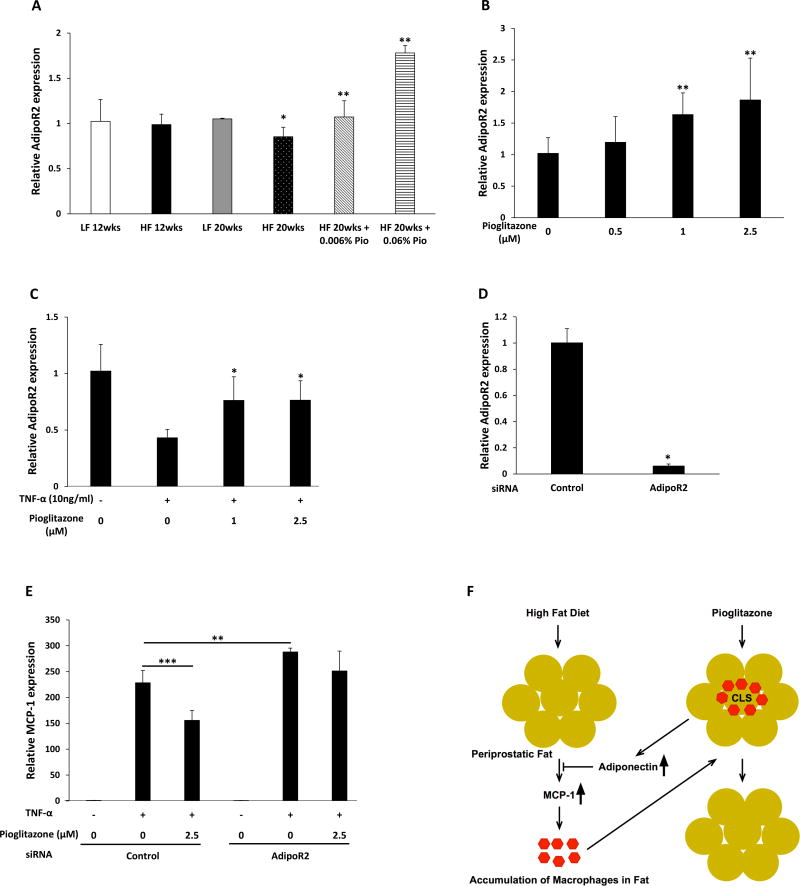
Adiponectin binds to adiponectin receptor R1 (AdipoR1) and R2 (AdipoR2) and regulates gene expression in adipocytes (28). We next investigated whether pioglitazone modulated the expression of the adiponectin receptors. Feeding a HF diet for 20 weeks was associated with decreased expression of AdipoR2 in periprostatic WAT (Fig. 6A). This effect was reversed by treatment with pioglitazone which led to dose-dependent induction of AdipoR2. By contrast, levels of AdipoR1 in periprostatic WAT were unaffected by either the HF diet or pioglitazone. To extend upon these findings, additional experiments were carried out in 3T3-L1 cells. Consistent with the findings in periprostatic WAT (Fig. 6A), treatment of 3T3-L1 cells with pioglitazone caused dose-dependent induction of AdipoR2 levels (Fig. 6B). Similar to the effects on adiponectin, TNF-α–mediated down regulation of AdipoR2 was attenuated by treatment with pioglitazone (Fig. 6C). To confirm that pioglitazone-mediated suppression of MCP-1 induction is mediated by AdipoR2, siRNA was used. Levels of AdipoR2 in 3T3-L1 cells were suppressed more than 80% by the siRNA to AdipoR2 (Fig. 6D). Notably, silencing AdipoR2 both enhanced TNF-α-mediated induction of MCP-1 and attenuated the ability of pioglitazone to suppress the induction of MCP-1 (Fig. 6E). Taken together, our study demonstrates that treatment with pioglitazone suppresses obesity-related periprostatic WAT inflammation by inducing adiponectin which acts, in turn, to block the induction of MCP-1 resulting in reduced macrophage accumulation (Fig. 6F).
Figure 6. Pioglitazone induces AdipoR2.
A, At 6 weeks of age, C57BL/6J male mice were fed either low fat (LF) diet or high fat (HF) diet. The LF diet fed mice were randomized to one of two groups. One group was sacrificed at 18 weeks of age after 12 weeks on LF diet while the second group was sacrificed at 26 weeks of age after 20 weeks on LF diet. The other mice were fed HF diet for 12 weeks to induce obesity and then randomized to one of four groups. One group was sacrificed at 18 weeks of age after 12 weeks on HF diet. The second group was continued on HF diet, the third group was switched to HF diet containing 0.006% w/w pioglitazone and the fourth group was switched to HF diet containing 0.06% w/w pioglitazone for an additional 8 weeks until sacrifice at 26 weeks of age. AdipoR2 mRNA levels were quantified in periprostatic white adipose tissue. B, 3T3-L1 adipocytes were treated with 0–2.5 µM pioglitazone for 6 hours. Relative AdipoR2 expression was quantified. C, 3T3-L1 adipocytes were pretreated with 0–2.5 µM pioglitazone for 6 hours. Subsequently, cells were treated with TNF-α for an additional 6 hours. Relative AdipoR2 expression was quantified. D, 3T3-L1 adipocytes were transfected with control or AdipoR2 siRNA and levels of AdipoR2 were quantified. E, 3T3-L1 adipocytes were transfected with control siRNA or AdipoR2 siRNA. Seventy-two hours after transfection, cells were treated with vehicle or pioglitazone for 6 hours. Subsequently, cells received TNF-α (10 ng/mL) for an additional 6 hours. Levels of MCP-1 mRNA were quantified. For A–E, mean ± SD (error bars) are shown. A, n=3–7/group; *p<0.05, **p<0.01 compared to mice fed with LF diet for 20 weeks. B–E, n=4–6/group; *p<0.05, **p<0.01, ***p<0.001; F, high fat diet feeding causes periprostatic adipocyte hypertrophy and increased production of MCP-1. MCP-1 is a chemokine that is important for recruitment of blood monocytes into periprostatic adipose tissue where they undergo differentiation and become macrophages leading to the formation of crown-like structures (CLS). Pioglitazone induces adiponectin and its receptor (AdipoR2) which act, in turn, to block MCP-1 expression leading to a reduction in CLS.
Discussion
White adipose tissue inflammation is associated with the development, grade or progression of several solid tumors including PC (11,19,29–31). Here we demonstrate that diet induced obesity led to periprostatic WAT inflammation in mice consistent with recent findings in men (11). Importantly, pioglitazone, an anti-diabetic drug, protected against HF diet-induced periprostatic WAT inflammation. Similar anti-inflammatory effects of thiazolidinediones including pioglitazone have been demonstrated in other adipose depots in both mice and humans (16,32–34).
We carried out a series of studies to explore the mechanism underlying the anti-inflammatory effects of pioglitazone. Treatment with pioglitazone led to a dose-dependent decrease in the expression of MCP-1 in the periprostatic fat of obese mice (Fig. 2C). Because MCP-1 plays a key role in the recruitment of blood monocytes into adipose tissue where they differentiate and become macrophages (22,23), it seemed likely that the observed decrease in MCP-1 levels could account for the reduction in periprostatic adipose inflammation. This possibility was supported by our observation that pioglitazone failed to suppress periprostatic adipose inflammation in obese MCP-1 knock out mice (Fig. 4C). The fact that the ability of pioglitazone to suppress periprostatic adipose inflammation was dependent on its effect on MCP-1 is consistent with prior reports that thiazolidinediones can suppress levels of MCP-1 (16,34). In men, periprostatic adipose inflammation is associated with reduced circulating levels of adiponectin, an anti-inflammatory adipokine (11). Pioglitazone is known to induce adiponectin in both humans and mice (24,35–38). Adiponectin has been reported to suppress MCP-1 levels (26). Hence, we next determined if pioglitazone induced adiponectin or its receptor AdipoR2 leading to reduced levels of MCP-1. Pioglitazone caused dose-dependent induction of adiponectin and AdipoR2 in both periprostatic fat and in cultured adipocytes (Figs. 5A and B, Figs. 6A and B). Moreover, pioglitazone blocked TNF-α-mediated induction of MCP-1, an effect that was attenuated when either adiponectin or AdipoR2 were silenced (Figs. 5F and 6E). Collectively, these results indicate that treatment with pioglitazone reduces obesity-related periprostatic WAT inflammation by inducing adiponectin which acted, in turn, to suppress MCP-1 resulting in less macrophage accumulation. In preclinical studies, agonists of the adiponectin receptors can suppress inflammation (39). Whether such agents attenuate obesity-related periprostatic adipose inflammation or inhibit the development or progression of prostate cancer warrants further investigation.
In previous studies, adipocyte hypertrophy has been associated with WAT inflammation (10,19,21). It has been presumed that hypoxia, endoplasmic stress or both stimulate the inflammatory response (40,41). Consistent with findings in human periprostatic fat (11), we found that periprostatic adipocyte hypertrophy was associated with WAT inflammation in obese mice. Behavioral or pharmacological interventions that result in reduced caloric intake lead to decreased adipocyte size and improved WAT inflammation (17,42). In this study, pioglitazone stimulated caloric intake but did not induce weight gain presumably because this agent induces mitochondrial biogenesis and thereby energy expenditure (43). Notably, treatment with pioglitazone reduced periprostatic WAT inflammation in the absence of a reduction in body weight or periprostatic adipocyte size. Recently, pioglitazone was reported to increase capillary density in association with decreasing adipose tissue inflammation which would be anticipated to increase local oxygen tension (44). If similar effects occur in the periprostatic fat, this could explain the observed decrease in periprostatic WAT inflammation in the absence of a reduction in adipocyte size.
Inflamed periprostatic fat is associated with both high-grade PC and insulin resistance in PC patients (11). Elevated insulin levels have been suggested to play a role in PC growth and mortality (12,45,46). Our preclinical study indicates that pioglitazone, an insulin sensitizer, causes a reduction in periprostatic inflammation after relatively short-term treatment. Pioglitazone can also suppress levels of the hormone resistin, which can stimulate the proliferation of prostate cancer cells (47,48). A large-meta-analysis comparing thiazolidinedione use and cancer incidence suggested that pioglitazone may reduce the risk of PC (49). However, another recent study suggested that use of pioglitazone was associated with a small increase in the risk of prostate cancer (50). Hence, it’s unclear from the epidemiological evidence whether pioglitazone will be beneficial to either prostate cancer patients or men at high risk for prostate cancer. These studies failed to select for men with periprostatic adipose inflammation, the group that may be most likely to benefit from this type of agent. Given this uncertainty, a window of opportunity clinical trial should be considered where pioglitazone would be given to men in the preoperative period allowing biological endpoints to be evaluated in both the prostate and periprostatic fat obtained at the time of surgery. Taken together, the current results raise the possibility that targeting the PPARγ–adiponectin–MCP-1 axis will decrease periprostatic adipose inflammation and thereby reduce the risk of high-grade prostate cancer or improve the outcomes of men with prostate cancer.
Acknowledgments
This work was supported by the Prostate Cancer Foundation (Movember-Challenge Award, A.J. Dannenberg), Tokyo Association for Clinical Surgery (M. Miyazawa), Patricia and William Kleh (A.J. Dannenberg), and NIH/NCI P50 CA092629 (A.J. Dannenberg).
Footnotes
Disclosures: None of the authors disclosed potential conflicts of interest.
References
- 1.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(7):1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Zhou G, Sun B, Zhao G, Liu D, Sun J, et al. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol Lett. 2015;9(3):1307–12. doi: 10.3892/ol.2014.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41(18):2887–95. doi: 10.1016/j.ejca.2005.09.003. doi S0959-8049(05)00765-3 [pii] 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2349–56. doi: 10.1158/1055-9965.EPI-14-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010;56(1):34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 9.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 10.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Gucalp A, Iyengar NM, Zhou XK, Giri DD, Falcone DJ, Wang H, et al. Periprostatic adipose inflammation is associated with high-grade prostate cancer. Prostate Cancer Prostatic Dis. 2017 doi: 10.1038/pcan.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsing AW, Gao YT, Chua S, Jr, Deng J, Stanczyk FZ. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95(1):67–71. doi: 10.1093/jnci/95.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557–66. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123(6):993–9. doi: 10.1016/j.cell.2005.11.026. doi S0092-8674(05)01277-8 [pii] 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50(9):2094–9. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 16.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54(8):2305–13. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 17.Bhardwaj P, Du B, Zhou XK, Sue E, Giri D, Harbus MD, et al. Estrogen Protects against Obesity-Induced Mammary Gland Inflammation in Mice. Cancer Prev Res (Phila) 2015;8(8):751–9. doi: 10.1158/1940-6207.CAPR-15-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbaramaiah K, Howe LR, Zhou XK, Yang P, Hudis CA, Kopelovich L, et al. Pioglitazone, a PPARgamma agonist, suppresses CYP19 transcription: evidence for involvement of 15-hydroxyprostaglandin dehydrogenase and BRCA1. Cancer Prev Res (Phila) 2012;5(10):1183–94. doi: 10.1158/1940-6207.CAPR-12-0201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4(7):1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilroy G, Burk DH, Floyd ZE. High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS One. 2009;4(9):e6940. doi: 10.1371/journal.pone.0006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. 10.1172/JCI19246 112/12/1796 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281(36):26602–14. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 24.Bodles AM, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab. 2006;291(5):E1100–5. doi: 10.1152/ajpendo.00187.2006. [DOI] [PubMed] [Google Scholar]
- 25.Hammarstedt A, Sopasakis VR, Gogg S, Jansson PA, Smith U. Improved insulin sensitivity and adipose tissue dysregulation after short-term treatment with pioglitazone in non-diabetic, insulin-resistant subjects. Diabetologia. 2005;48(1):96–104. doi: 10.1007/s00125-004-1612-3. [DOI] [PubMed] [Google Scholar]
- 26.Zoico E, Garbin U, Olioso D, Mazzali G, Fratta Pasini AM, Di Francesco V, et al. The effects of adiponectin on interleukin-6 and MCP-1 secretion in lipopolysaccharide-treated 3T3-L1 adipocytes: role of the NF-kappaB pathway. Int J Mol Med. 2009;24(6):847–51. doi: 10.3892/ijmm_00000302. [DOI] [PubMed] [Google Scholar]
- 27.Murao K, Imachi H, Momoi A, Sayo Y, Hosokawa H, Sato M, et al. Thiazolidinedione inhibits the production of monocyte chemoattractant protein-1 in cytokine-treated human vascular endothelial cells. FEBS Lett. 1999;454(1–2):27–30. doi: 10.1016/s0014-5793(99)00765-6. doi S0014-5793(99)00765-6 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clin Cancer Res. 2016;22(9):2283–9. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Gluck S, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat. 2016;158(1):113–26. doi: 10.1007/s10549-016-3847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyengar NM, Ghossein RA, Morris LG, Zhou XK, Kochhar A, Morris PG, et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer. 2016;122(24):3794–802. doi: 10.1002/cncr.30251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodles AM, Varma V, Yao-Borengasser A, Phanavanh B, Peterson CA, McGehee RE, Jr, et al. Pioglitazone induces apoptosis of macrophages in human adipose tissue. J Lipid Res. 2006;47(9):2080–8. doi: 10.1194/jlr.M600235-JLR200. doi M600235-JLR200 [pii] 10.1194/jlr.M600235-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Mulder P, Morrison MC, Verschuren L, Liang W, van Bockel JH, Kooistra T, et al. Reduction of obesity-associated white adipose tissue inflammation by rosiglitazone is associated with reduced non-alcoholic fatty liver disease in LDLr-deficient mice. Sci Rep. 2016;6:31542. doi: 10.1038/srep31542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koppaka S, Kehlenbrink S, Carey M, Li W, Sanchez E, Lee DE, et al. Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes. 2013;62(6):1843–54. doi: 10.2337/db12-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aso Y, Hara K, Ozeki N, Yatsuka C, Nakano T, Matsumoto S, et al. Low-dose pioglitazone increases serum high molecular weight adiponectin and improves glycemic control in Japanese patients with poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2009;85(2):147–52. doi: 10.1016/j.diabres.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Coletta DK, Sriwijitkamol A, Wajcberg E, Tantiwong P, Li M, Prentki M, et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia. 2009;52(4):723–32. doi: 10.1007/s00125-008-1256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Silva Morais A, Lebrun V, Abarca-Quinones J, Brichard S, Hue L, Guigas B, et al. Prevention of steatohepatitis by pioglitazone: implication of adiponectin-dependent inhibition of SREBP-1c and inflammation. J Hepatol. 2009;50(3):489–500. doi: 10.1016/j.jhep.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Glintborg D, Frystyk J, Hojlund K, Andersen KK, Henriksen JE, Hermann AP, et al. Total and high molecular weight (HMW) adiponectin levels and measures of glucose and lipid metabolism following pioglitazone treatment in a randomized placebo-controlled study in polycystic ovary syndrome. Clin Endocrinol (Oxf) 2008;68(2):165–74. doi: 10.1111/j.1365-2265.2007.03015.x. [DOI] [PubMed] [Google Scholar]
- 39.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503(7477):493–9. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 40.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93(1):1–21. doi: 10.1152/physrev.00017.2012. 10.1152/physrev.00017.2012 93/1/1 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, et al. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila) 2013;6(4):282–9. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54(5):1392–9. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 44.Spencer M, Yang L, Adu A, Finlin BS, Zhu B, Shipp LR, et al. Pioglitazone treatment reduces adipose tissue inflammation through reduction of mast cell and macrophage number and by improving vascularity. PLoS One. 2014;9(7):e102190. doi: 10.1371/journal.pone.0102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9(11):1039–47. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunter JH, Sarkar PL, Lubik AA, Nelson CC. New players for advanced prostate cancer and the rationalisation of insulin-sensitising medication. Int J Cell Biol. 2013;2013:834684. doi: 10.1155/2013/834684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ, Lee YS, Won EH, Chang IH, Kim TH, Park ES, et al. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int. 2011;108(2 Pt 2):E77–83. doi: 10.1111/j.1464-410X.2010.09813.x. [DOI] [PubMed] [Google Scholar]
- 49.Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2012;38(6):475–84. doi: 10.1016/j.diabet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, et al. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA. 2015;314(3):265–77. doi: 10.1001/jama.2015.7996. 10.1001/jama.2015.7996 2397834 [pii] [DOI] [PubMed] [Google Scholar]