Abstract
The pathology of migraine, a common neurological disease, involves sensitization and activation of trigeminal nociceptive neurons to promote hyperalgesia and allodynia during an attack. Migraineurs often exhibit characteristics of a hyperexcitable or hypervigilant nervous system. One of the primary reported risk factors for development of a hyperexcitable trigeminal system is chronic, unmanaged stress and anxiety. While primary traumatic stress is a commonly cited risk factor for many pain conditions, exposure to secondary traumatic stress early in life is also thought to be a contributing risk factor. The goal of this study was to investigate cellular changes within the spinal trigeminal nucleus and trigeminal ganglion mediated by secondary traumatic stress. Male Sprague Dawley rats (sender) were subjected to forced swim testing (primary traumatic stress) and were then housed in close visual, olfactory, and auditory proximity to the breeding male and female rats, pregnant female rats, or female rats and their nursing offspring (all receivers). In response to secondary stress, levels of calcitonin gene-related peptide, active forms of the mitogen activated protein kinases ERK, JNK, and p38, and astrocyte expression of glial fibrillary acidic protein were significantly elevated in the spinal trigeminal nucleus in day 45 offspring when compared to naïve offspring. In addition, increased nuclear expression of ERK and p38 was observed in trigeminal ganglion neurons. Our results demonstrate that secondary traumatic stress promotes cellular events associated with prolonged trigeminal sensitization in the offspring, and provides a mechanism of how early life stress may function as a risk factor for migraine.
Keywords: migraine, secondary traumatic stress, trigeminal ganglion, MAP kinase, sensitization
1. INTRODUCTION
Migraine, prevalent neurological disorder, affects >18% of the general population in the United States, with the prevalence highest in women of childbearing age (Buse et al., 2013a). Migraineurs are thought to have a hyperexcitable nervous system characterized by a heightened sensitivity towards external stimuli that may be responsible for triggering a migraine attack (Burstein et al., 2015; Dodick and Silberstein, 2006). Sensitization and activation of trigeminal nerves, which provide a nociceptive pathway from the peripheral dural tissues to the spinal trigeminal nucleus, is implicated in the underlying pathology of migraine (Pietrobon and Moskowitz, 2013). A lowering of the activation threshold of trigeminal nociceptive neurons that occurs during neuronal sensitization or a primed state is associated with increased neuron-glia interactions and cellular changes in the expression of ion channels and signal transduction receptors (Dodds et al., 2016; Hucho and Levine, 2007). These cellular changes are mediated by increases in neuronal and glial expression of signal transduction proteins in the trigeminal ganglion and spinal trigeminal nucleus. In particular, members of the mitogen-activated protein kinase (MAPK) family mediate intracellular signaling cascades that promote neuron-glia interactions and maintain a hyperexcitable state of nociceptive neurons (Crown, 2012; Ji et al., 2009; Ji et al., 2013). The neuropeptide calcitonin gene-related peptide (CGRP), whose expression is regulated by MAPK and is strongly implicated in migraine pathology, can promote sensitization of primary and secondary trigeminal nociceptive neurons and activation of satellite glial cells in the ganglion and astrocytes and microglia in the spinal cord (Durham, 2016; Iyengar et al., 2017; Seybold, 2009).
The most-cited risk factor for migraine pathology is stress, which if unmanaged can lead to a variety of anxiety disorders including but not limited to major depressive disorders, Post-Traumatic Stress Disorder (PTSD), and panic disorders (Buse et al., 2013b). Symptoms of stress include clenching and grinding of teeth, frequent headaches, and neck pain (Slade et al., 2016). While primary stress is the direct exposure of an organism to a traumatic event outside of normal daily experiences, secondary stress, which is commonly referred to as compassion stress, is caused by exposure to victims of a primary traumatic stress (Klaric et al., 2013). Exposure to traumatic events or situations, especially early in life, can lead to dysmodulation between the responses of the sympathetic and parasympathetic nervous system and the HPA axis leading to increased pain sensitivity (Mooney-Leber and Brummelte, 2017; van Bodegom et al., 2017). Clinically, pain threshold and tolerance is used to define the normal functioning of the nervous system in response to stressors. Frequent or severe stress can mediate cellular changes leading to the development of a hypervigilant nervous system, which becomes dysregulated and maladaptive to future allostatic load (Maleki et al., 2012). Hypervigilance is characterized as a heightened state of sensory nerve excitability and pain sensitivity due to an enhanced response to inflammatory molecules that leads to a lower pain threshold and lower pain tolerance. Migraine-associated allodynia, which is defined as a nociceptive response to normally non-painful stimuli, like brushing one’s hair involves activation of the trigeminal system and development of central sensitization (Burstein et al., 2015). An individual with a maladaptive hypervigilant nervous system, which can arise from unmanaged anxiety caused by early life stress, is at an increased risk for development of persistent central sensitization as reported in migraine pathology (Aurora and Wilkinson, 2007; Borsook et al., 2012).
Although there appears to be a link between childhood maltreatment in the forms of emotional or physical abuse and the risk of developing migraine (Brennenstuhl and Fuller-Thomson, 2015; Tietjen et al., 2012; Tietjen, 2016), the impact of secondary traumatic stress on the developing trigeminal system has not been investigated. Results from our study provide evidence that exposure of parent rats and nursing offspring to secondary traumatic stress is sufficient to cause a sustained increase in the expression of proteins implicated in peripheral and central sensitization of trigeminal nociceptive neurons in young adult rats.
2. RESULTS
2.1 Secondary Stress Increases CGRP and MAPK Expression in the Spinal Trigeminal Nucleus
Initially, brainstem tissue containing the spinal trigeminal nucleus (STN) was isolated and stained with the fluorescent nuclear dye DAPI to visualize the distribution of neuronal and glial cells. The number and pattern of nuclear staining within the STN was not altered by secondary stress when compared to tissues obtained from naïve control animals (data not shown). Similarly, the same tissues were immunostained with the neuronal specific nuclear protein NeuN to visualize the distribution of neurons within the outer laminae. Within the STN, the location and relative number of neurons appeared similar when comparing tissues obtained from naïve control animals and those obtained from animals subjected to prenatal and postnatal secondary stress (n=4 for all conditions; data not shown). As a control for antibody specificity, tissues were incubated with secondary antibodies alone and no specific staining pattern was detected in the absence of primary antibodies (data not shown).
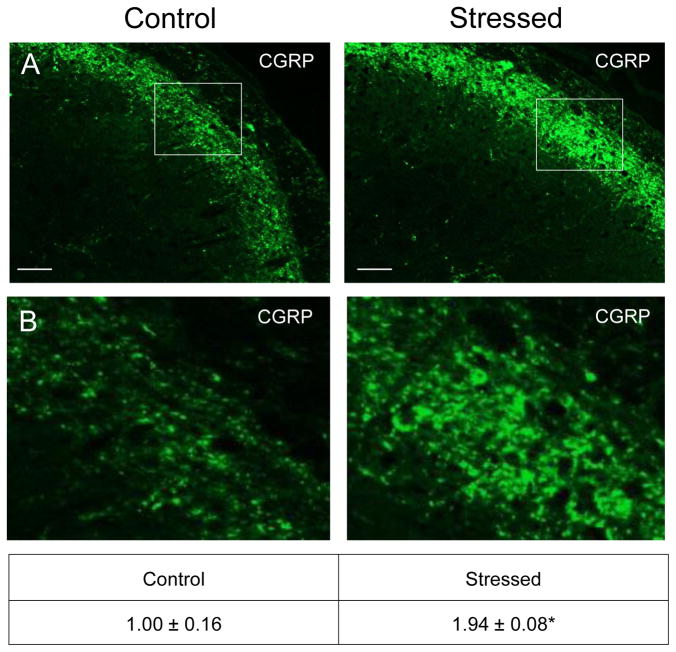
To determine the effects of secondary stress on proteins associated with the development and maintenance of central sensitization, cellular changes in the STN were investigated using immunohistochemistry. An increased level of staining intensity of the neuropeptide CGRP was observed in neurons and glia in the outer lamina region of the STN in animals subjected to secondary stress when compared to naïve control (Fig. 2, nnaive = 4, nstressed = 3). The relative immunostaining intensity was significantly elevated in samples from stressed offspring (1.94 ± 0.08; Cohen’s d = 5.42; t = −5.416; df = 4; CI: −1.424, −0.459; P = 0.006) as compared to levels detected in animals from naïve litters (1.00 ± 0.16).
Figure 2.
Exposure to secondary traumatic stress causes increased expression of CGRP in the STN. Tissues were obtained from unstressed animals (Control), and from animals exposed to pre-natal and post-natal secondary traumatic stress (Stressed). (A) Images of STN stained for CGRP are shown in top panels. (B) An enlarged image of the cells delimited by the box is shown in the bottom panels. The average fold change ± SEM of CGRP staining intensity when compared to the mean of the control that was made equal to one is reported. Control (n = 4) values are reported in comparison to stressed (n = 4) values. *P < 0.05 when compared to control. Scale bars equal 50 μm.
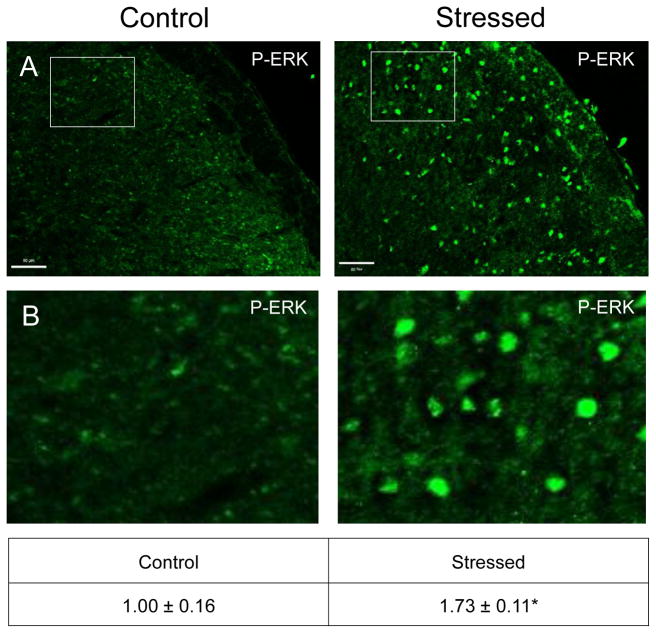
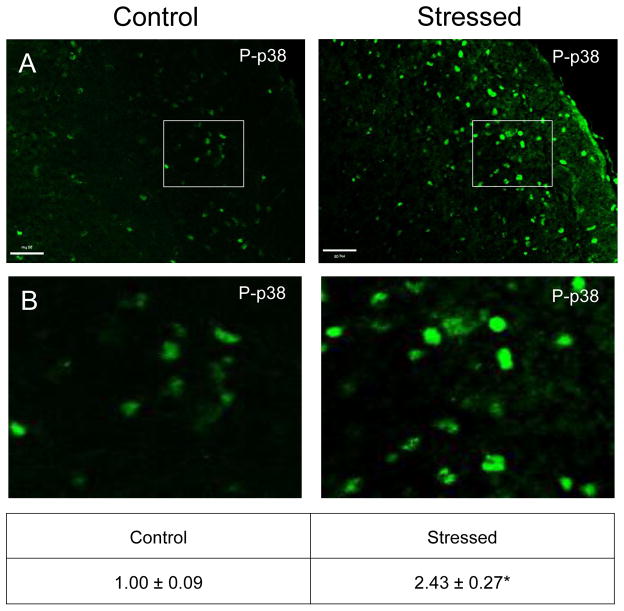
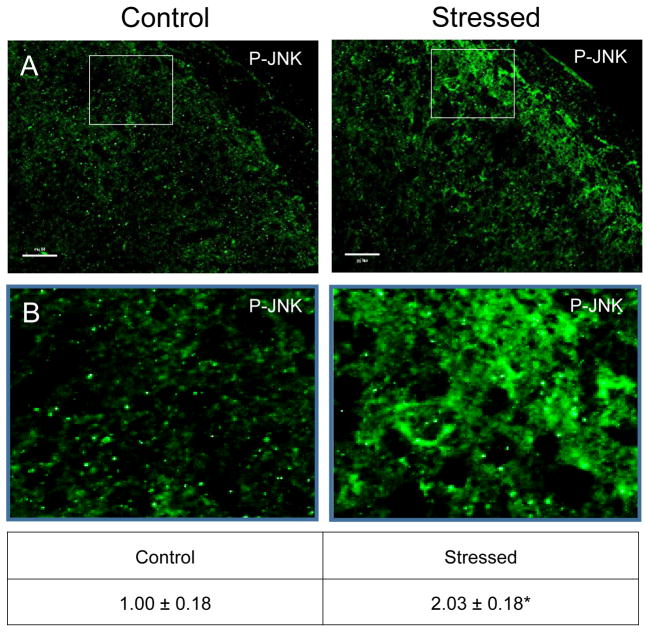
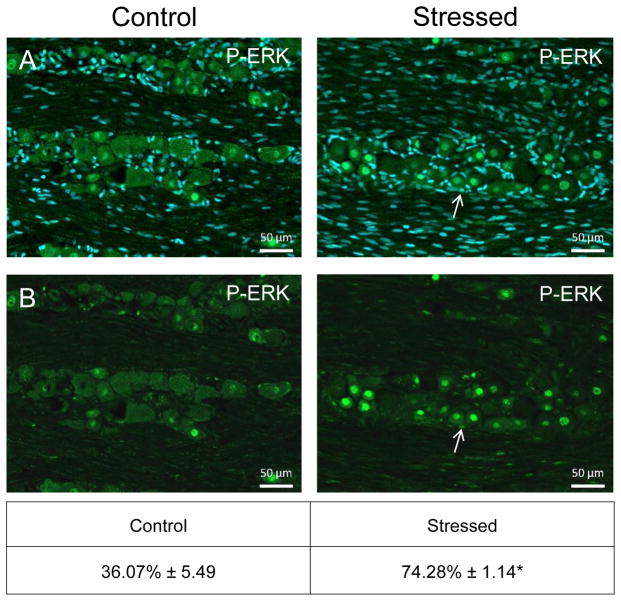
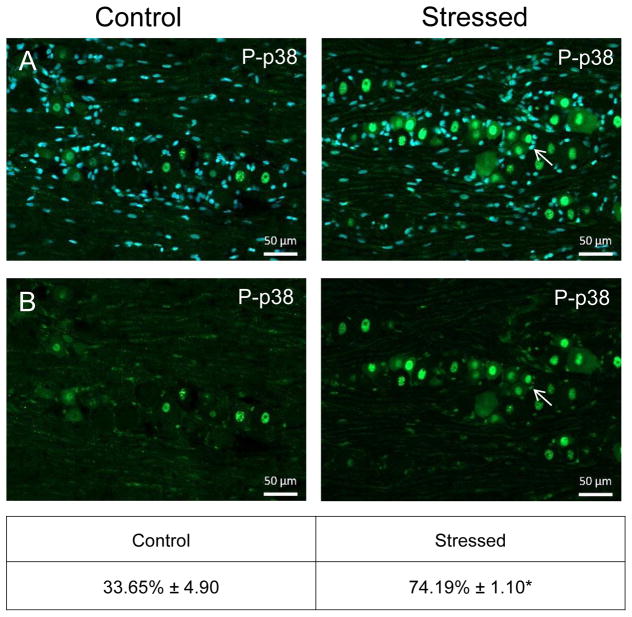
Similar to CGRP, an increase in the intensity of immunostaining for the phosphorylated, active forms of ERK (P-ERK;), p38 (P-p38), and JNK (P-JNK) was detected in laminae I–III of the STN (nNaive = 4, nStressed = 4). Low levels of P-ERK were detected in neurons and glia of control tissues (Fig. 3). However, in stressed offspring, neuronal P-ERK staining was readily observed in a punctate pattern in the outer laminae and the intensity was significantly increased (1.73 ± 0.11; Cohen’s d = 3.6; t = −4.408; df = 6; CI: −1.142, −0.327; P = 0.005) as compared to levels detected in naive animals (1.00 ± 0.18). Similarly, the expression of the active form of p38 was significantly elevated in neurons localized in the medullary region of the STN in offspring exposed to secondary stress (2.43 ± 0.27; Cohen’s d = 4.64; t = −5.685; df = 6; CI: −2.052, −0.817; P = 0.001) when compared to levels in control animals (Fig. 4). Low levels of P-JNK staining were detected in neurons and glia of control tissues (Fig. 5); however, in tissues obtained from stressed animals, a diffuse pattern of P-JNK staining was observed in the outer lamina of the medullary horn. When compared to naïve control animals (1.00 ± 0.18), there was a significant increase in staining intensity after exposure to secondary traumatic stress (2.03 ± 0.18; Cohen’s d = 3.85; t = −4.712; df = 6; CI: −1.563, −0.495; P = 0.003).
Figure 3.
Exposure to secondary traumatic stress causes increased expression of phosphorylated ERK in the STN. Tissues were obtained from unstressed animals (Control), and from animals exposed to pre-natal and post-natal secondary traumatic stress (Stressed). (A) Representative images of STN stained for active P-ERK are shown in the top panels. (B) An enlarged image of the cells delimited by the box is shown in the bottom panels. The average fold change ± SEM of P-ERK staining intensity when compared to the mean of the control that was made equal to one is reported. Control (n = 4) values are reported in comparison to stressed (n = 4) values. *P < 0.05 increases when compared to control. Scale bars equal 50 μm.
Figure 4.
Exposure to secondary traumatic stress causes increased expression of phosphorylated p38 in the STN. Tissues were obtained from unstressed animals (Control), and from animals exposed to pre-natal and post-natal secondary traumatic stress. (A) Representative images of STN stained for active P-p38 are shown in the top panels. (B) An enlarged image of the cells delimited by the box is shown in the bottom panels. The average fold change ± SEM of P-p38 staining intensity when compared to the mean of the control that was made equal to one is reported (n = 4). *P < 0.05 increases when compared to control. Scale bars equal 50 μm.
Figure 5.
Exposure to secondary traumatic stress causes increased expression of active JNK in the STN. Tissues were obtained from unstressed animals (Control), and from animals exposed to pre-natal and post-natal secondary traumatic stress. (A) Representative images of STN stained for active JNK are shown in the top panels. (B) An enlarged image of the cells is delimited by the box is shown in the bottom panels. The average fold change ± SEM of JNK staining intensity when compared to the mean of the control that was made equal to one is reported. Control (n = 3) values are reported in comparison to stressed (n = 4) values. *P < 0.05 increases when compared to control. Scale bars equal 50 μm.
2.2 Effect of Secondary Stress on Astrocytes
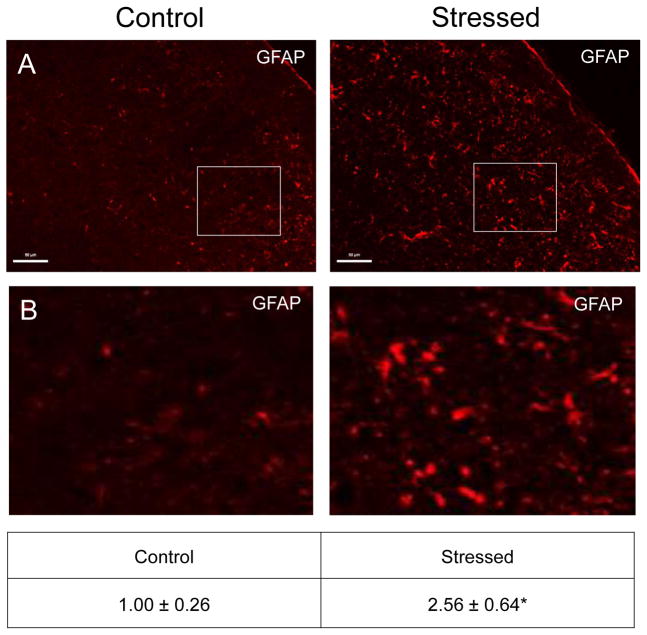
A low level of GFAP expression was detected in laminae I–III in naïve offspring (Fig. 6, nNaive = 4, nStressed = 3). In contrast, the intensity and number of GFAP positive astrocytes was significantly elevated in tissues obtained from offspring exposed to secondary stress (2.56 ± 0.64; Cohen’s d = 2.74; t = −3.058; df = 5; CI: −2.879, −0.249; P = 0.028) as compared to those observed in naïve tissues (1.00 ± 0.26).
Figure 6.
Exposure to secondary traumatic stress causes increased expression of GFAP in the STN. Tissues were obtained from unstressed animals (Control), and from animals exposed to pre-natal and post-natal secondary traumatic stress. (A) Representative images of STN stained for active GFAP are shown in the top panels. (B) An enlarged image of the cells is delimited by the box is shown in the bottom panels. The average fold change ± SEM of GFAP staining intensity when compared to the mean of the control that was made equal to one is reported. Control (n = 4) values are reported in comparison to stressed (n = 3) values. *P < 0.05 increases when compared to control. Scale bars equal 50 μm.
2.3 Increased Expression of P-ERK and P-p38 in Trigeminal Ganglion of Stressed Offspring
In naïve animals, a low level of P-ERK immunostaining was detected in neurons, but not satellite glial cells, in the V1/V2 region of the trigeminal ganglion (Fig. 7, nNaive = 4, nStressed = 8). While the average percentage of neurons exhibiting nuclear localization of P-ERK was 20.79% ± 2.32 in naïve animals, the average number of P-ERK positive neurons was significantly increased in the V1/V2 region (57.61% ± 3.60; Cohen’s d = 4.28; t = −6.77; df = 10; CI: −48.93, −24.70; P < 0.001) in stressed animals. Increased nuclear expression of P-ERK was observed in both large diameter Aδ and small diameter C fiber neurons in ganglion from animals exposed to early life secondary stress. Similar to P-ERK expression, naïve animals exhibited low levels of P-p38 in the nucleus of neuronal cell bodies (33.07% ± 5.49) but significantly increased nuclear levels in Aδ and C fiber neurons in the V1/V2 region (74.28% ± 1.14; η2 = 0.671; U < 0.001; P = 0.004) in stressed animals (Fig. 8). In addition, an increase in the number of satellite glial cells exhibiting P-ERK and P-p38 staining was observed in stressed offspring when compared to naïve animals. Unlike the result in the STN, CGRP levels in the ganglion were not increased in response to early life stress (data not shown).
Figure 7.
Increased expression of P-ERK in the nucleus of trigeminal neurons in response to secondary stress. Images (200x) from a representative V1/V2 region of the ganglia stained for expression of PERK in control animals (left) and stressed animals (right). A merged image of P-ERK and the nuclear dye DAPI is shown in the top panels while only P-ERK staining is shown in the bottom panels.
Figure 8.
Increased expression of P-p38 primarily in trigeminal neurons in response to exposure to secondary stress. Images (200x) from a representative V1/V2 region of the ganglia stained for expression of P-p38 in control animals (left) and stressed animals (right). A merged image of P-p38 and the nuclear dye DAPI is shown in the top panels while only P-ERK staining is shown in the bottom panels.
3. DISCUSSION
A major finding of our study was that exposure of parent rats and nursing pups to secondary early life stress resulted in sustained changes in the levels of proteins associated with central and peripheral sensitization of trigeminal neurons of young adult male rats. The rationale for our study was based on data which supports the notion that early life stress is a risk factor for development of a hypervigilant nervous system implicated in chronic pain disorders including migraine (Tietjen et al., 2012; Tietjen, 2016). Our model involved investigating changes in response to three exposures to secondary traumatic stress mediated by the co-housing of a primary water-stressed male rat during the prenatal (breeding and pregnancy) and postnatal (nursing) periods. Thus, both male and female parents, pregnant mothers, and nursing mothers with their offspring were subjected to environmental stress. While occasional stress elicits an adaptive physiological response involving both central and peripheral nervous systems, more frequent exposure to stressors can lead to recurrent or persistent activation leading to adverse health outcomes (Crofford, 2015; McEwen et al., 2012). For example, early life stressors are reported to cause long-lasting cellular and regulatory changes in the nervous system leading to a maladaptive state characterized by increased anxiety and depression symptoms (van Bodegom et al., 2017). Studies on the mechanisms contributing to chronic pain conditions and development of a hypervigilant nervous system have primarily focused on cellular changes occurring in specific brain regions (Heinricher, 2016; Johnson and Greenwood-Van Meerveld, 2014; Stankiewicz et al., 2013). To our knowledge, this is the first study designed to investigate the effect of secondary traumatic stress on primary and secondary trigeminal neurons, which is the nociceptive pathway central to migraine pathology (Olesen et al., 2009).
The protein most associated with sensitization and activation of primary and secondary trigeminal neurons, and hence, the underlying pathology of migraine, is the neuropeptide CGRP (Bigal et al., 2013; Iyengar et al., 2017). CGRP is proposed to play a fundamental role in migraine since cerebrospinal fluid levels of CGRP are elevated during and between migraine attacks (van Dongen et al., 2017) and CGRP levels correlate with treatment response to anti-migraine drugs (Karsan and Goadsby, 2015). In agreement with the notion that elevated levels of CGRP play an important role in mediating trigeminal sensitization, results from our study provide evidence that CGRP expression is significantly elevated in the STN of young adult rats that have been exposed to secondary stress. CGRP is thought to contribute to migraine pathology by promoting an enhanced state of neuronal excitability or primed state via modulation of the expression of ion channels and receptors on secondary trigeminal neurons (Russo, 2015). In addition, CGRP promotes central sensitization via activation of astrocytes and microglial cells within the STN to mediate the release of cytokines and other pro-inflammatory molecules known to facilitate an inflammatory response and maintain an excitable state (Seybold, 2009). Therefore, it is likely that sustained, elevated CGRP levels in response to early life stress may function to establish a maladaptive positive feedback loop that maintains a hyperexcitable state of the trigeminal system in young adult rats.
A significant finding of our study was the sustained increase in expression of the active forms of the MAP kinases ERK, p38, and JNK in the spinal trigeminal nucleus of young adult animals exposed to early life secondary traumatic stress. MAPK are a family of signal transduction enzymes that mediate cellular activities in response to inflammatory stimuli implicated in the development of central sensitization and maintenance of nociception (Ji et al., 2009). Elevated P-ERK levels are reported in spinal cord glial cells, astrocytes and microglia, in response to noxious stimuli (Ji et al., 2009; Nakagawa and Kaneko, 2010), and are also elevated in spinal neurons involved in nociceptive transmission (Gao and Ji, 2009) that promote and sustain central sensitization (DeLeo et al., 2004; Xie et al., 2007). Results from our study demonstrated an increase in nuclear localization of P-ERK in the second order neurons of stressed offspring, an event associated with an enhanced state of neuronal excitation (Gereau Ji). Similarly, P-JNK levels were upregulated in neurons and glial cells in stressed animals. Increased expression of P-ERK and P-JNK can mediate sensitization of second order nociceptive neurons through increasing neuronal ion channel expression and activity, and causing increased expression of membrane receptors associated with nociception (Ji, 2004; Ji et al., 2009). Also, P-ERK and P-JNK are known to stimulate synthesis and secretion of cytokines from glial cells, which can facilitate a prolonged hyperexcitable state of neurons (Ji, 2004; Ji et al., 2009). In addition, levels of P-p38 were elevated in neurons and glial cells in response to secondary stress. An increase in the expression of p38 is also associated with an inflammatory response and development of more chronic pain states (Thornton & Rincon, 2009). Astrocytes are reported to participate in the upregulation of P-p38 via increased IL-17R signaling, which promotes inflammation (Kang et al., 2010). Taken together, secondary stress resulted in prolonged activation of the MAPKs ERK, JNK, and p38 in neurons and glial cells in the STN that is consistent with the development of a hyperexcitable or hypervigilant trigeminal system characteristic of migraine pathology.
Recently, elevated levels of CGRP in the spinal trigeminal nucleus were shown to mediate increased expression of GFAP, a protein used as a biomarker of activated astrocytes (Cornelison et al., 2016). In agreement, findings from this study provide evidence that early life stress causes an increase in the expression of CGRP, and also GFAP. These results are similar to the effects reported in response to prolonged nicotine exposure, in which levels of CGRP and GFAP were elevated in the spinal trigeminal nucleus and animals exhibited increased sensitivity to mechanical stimulation of trigeminal nerves (Hawkins et al., 2015). Enhanced activity of astrocytes is associated with the initiation and maintenance of central sensitization characterized by a state of prolonged neuronal excitability via the release of pro-inflammatory mediators that include cytokines and chemokines (Abbadie et al., 2009; Miller et al., 2009; Milligan and Watkins, 2009). Interestingly, while CGRP is known to be a MAP kinase-responsive gene such that elevated levels of MAP kinases will promote increased synthesis and secretion of CGRP from trigeminal neurons (Durham and Russo, 2003), CGRP is also known to induce MAP kinase signaling pathways and promote activation of astrocytes (Cady et al., 2011). At this time, we can only speculate about the temporal sequence of cellular events that involves increased neuron-glia interactions associated with a more sensitized state in the spinal trigeminal nucleus indicative of central sensitization. However, the establishment of this type of neuron-glia inflammatory loop has been reported to occur in the trigeminal ganglion to promote peripheral sensitization (Capuano et al., 2009; Freeman et al., 2008; Garrett and Durham, 2009).
Evidence of bidirectional signaling within the trigeminal system may help to explain how elevated levels of CGRP in the spinal trigeminal nucleus, and subsequent activation of astrocytes, may promote sensitization of primary trigeminal neurons by facilitating neuron-glia communication and increased MAP kinase signaling (Cornelison et al., 2016). Similarly, results from this study provide evidence that exposure to prenatal and postnatal secondary traumatic stress can promote a sustained increase in P-ERK and P-p38 levels in primary nociceptive neurons and satellite glial cells within the trigeminal ganglion associated with peripheral sensitization in young adult animals. Increased nuclear expression of MAP kinases is thought to facilitate a sensitized state of primary nociceptive neurons by altering the expression of receptors coupling to pro-inflammatory signal cascades and enhanced activity of selective ion channels (Cheng and Ji, 2008; Ji et al., 2009; Milligan et al., 2003; Takeda et al., 2009; Tsuda et al., 2004). Taken together, our results provide evidence that prolonged exposure to secondary environmental stress leads to sustained changes in the expression of proteins implicated in the development of peripheral and central sensitization of trigeminal neurons, which are indicative of a hypervigilant or hyperexcitable nervous system. Furthermore, our findings may provide a possible explanation of why early life stress can lead to a maladaptive nervous system that is associated with a greater risk of developing migraine or other orofacial pain conditions later in life.
4. EXPERIMENTAL PROCEDURES
4.1 Animals
Animal studies were performed in accord with Missouri State University Institutional Animal Care and Use Committee guidelines and approved protocols, as well as the guidelines set forth by the National Institute of Health, the Animal Welfare Act of 2017, and ARRIVE. A concerted effort was made to reduce the number of animals and minimize suffering. Adult male Sprague-Dawley rats (200–300 grams; Charles River Laboratories Inc., Wilmington, MA) and four adult female Sprague-Dawley rats (175–200g; Charles River) were utilized for breeding purposes to study the effects of secondary traumatic stress on the offspring. All animals were housed in clean plastic cages (VRW, West Chester, PA) on a 12-hour light/dark cycle with unrestricted access to food and water. Prior to use, animals were acclimated to the facility environment for a minimum of one week upon arrival. During pregnancy, female animals were provided with Crink-L’Nest™ (The Andersons Inc., Maamee, OH) and Formulab Diet 5008 (LabDiet, St. Louis, MO). For the immunohistochemical studies, a large effect size (η2 = 0.702, Cohen’s f = 1.53) was anticipated based on previously published data (Cornelison neuroscience ). Utilizing G-Power to conduct power analysis and employing a power of 0.80 at α = 0.05 alpha, the minimum sample size to detect the expected main effect was determined to require a minimum of eight offspring animals. We utilized a total of twelve male offspring (four naïve offspring and eight stressed offspring) to study changes in the levels of signaling proteins in neurons and glial cells in the spinal trigeminal nucleus and trigeminal ganglion.
4.2 Induction of Secondary Traumatic Stress
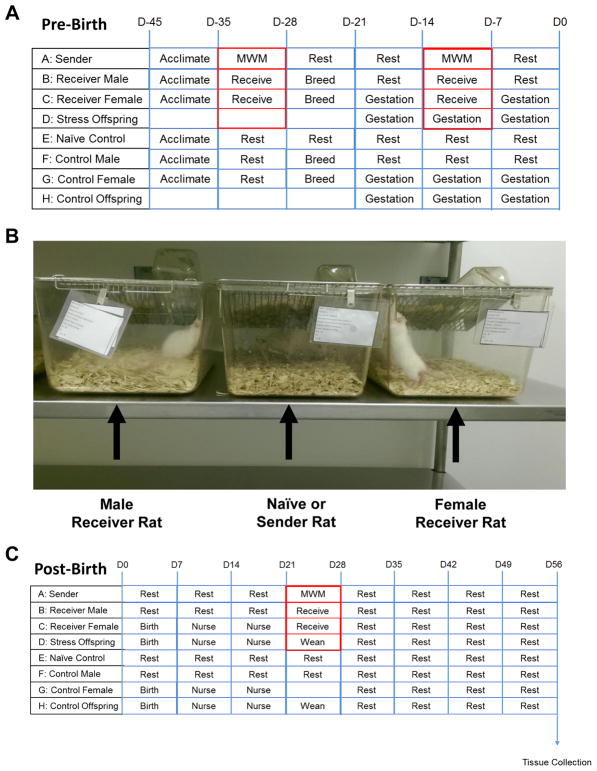
Three identical, but separate rooms were utilized in this study. One room was used to house animals subjected to primary stress, while the other two rooms were used for breeding in a normal environment (naïve offspring) or breeding in a stressful environment (secondary stress offspring). A timeline of our experimental model is shown in Figure 1A. To induce primary stress, an adult male sender rat was subjected to the Morris Water Maze for three minutes or until finding an underwater platform. The animal was then removed and allowed to rest for two minutes before being returned to the water. This method was repeated a total of four times before the rat was placed in its cage to recover overnight. The water maze test was repeated on each animal for seven consecutive days per each episode. During this time, adult male sender rats subjected to the water maze to cause primary traumatic stress were housed in cages in close visual, olfactory, and auditory proximity (Fig. 1B) to male and female breeding rats that would serve as animals receiving secondary traumatic stress (receiver rats). Upon completion of the first round, male and female receiver rats, as well as corresponding naïve controls in the non-stressed environment were co-housed for one week to allow for copulation. After one week, breeding animals were separated and pregnancy was confirmed by observing the vaginal plug.
Figure 1.
Experimental design for inducing secondary traumatic stress in offspring. A. The environmental conditions prior to birth are shown for adult male and female animals. B. Either an unstressed, naïve animal or swim-stressed animal (sender) was placed in a cage in auditory, visual, and olfactory proximity of the male and female breeding animals (receivers). C. The environmental conditions following birth are shown.
During mid-pregnancy, the sender rats were again subjected to the water maze for one week, and then were housed in cages near the pregnant female receiver rats as previously described. The final episode involved placing sender rats in the proximity of lactating females and their nursing offspring prior to weaning (Fig. 1C). At 21 days of age, offspring were weaned and allowed to develop to young adulthood (45 days of age). At this time, the offspring were euthanized via CO2 asphyxiation prior to decapitation and removal of both trigeminal ganglia and tissue from upper spinal cord and brainstem (obex to −6 mm) for immunohistochemical analysis.
4.3 Immunohistochemistry
Immunohistochemistry and image analysis were conducted essentially as described in previous studies (Cady et al., 2011; Koop et al., 2017). Tissues were fixed in a solution of 4% paraformaldehyde overnight at 4°C, and cryoprotected by incubating tissues in 12.5% sucrose in distilled water at 4°C for one hour followed by 25% sucrose overnight at 4°C. Tissues were mounted in Optimal Cutting Temperature compound (OCT; Sakura Finetek, Torrance, CA), and serial 14 μm transverse sections of either the upper spinal cord (4–5 mm caudal to the obex) or the trigeminal ganglion containing all three branches, were obtained using a cryostat (Microtome HM 525, Thermo Scientific, Waltham, MA) set at −25°C. Tissues were mounted on Superfrost Plus microscope slides (Fisher Scientific, Pittsburg, PA, USA).
Slides containing sections from each experimental condition were incubated in 1x Phosphate Buffer Saline (PBS) for 5 minutes to rehydrate tissues. Following rehydration, sections were incubated for 20 minutes at room temperature with a 1x PBS solution containing 5% donkey serum (Jackson ImmunoReasearch Laboratories, West Grove, PA) and 0.1% Triton X-100 (Life Technologies, Grand Island, NY). Sections were then incubated with primary antibodies against calcitonin gene related peptide (CGRP), the phosphorylated, active forms of extracellular regulated protein kinase (P-ERK), p38 (P-p38), and c-Jun N-terminal kinase (P-JNK). Spinal cord sections were also stained for the intermediate filament protein glial fibrillary acidic protein (GFAP), a marker of activated astrocytes, and the neuronal-specific protein NeuN as described in Table 1. The sections were then incubated with Alexafluor secondary antibodies. As a control, some slides were incubated with only Alexafluor secondary antibodies. Sections were mounted in Vectashield medium (H-1200) containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) to co-stain cell nuclei. A Zeiss Axiocam mRm camera (Carl Zeiss, Thornwood, NY) mounted on a Zeiss Imager Z1 fluorescent microscope equipped with an ApoTome was used to collect three, 200x images of the medullary dorsal horn and V1/V2 areas of the trigeminal ganglion.
Table 1.
Summary of Primary and Secondary Antibodies Used for Immunohistochemistry.
| Antibody | Host | Supplier | Dilution | Incubation |
|---|---|---|---|---|
| P-JNK | Rabbit | Cell Signaling | 1:100 | Overnight, 4°C |
| P-ERK | Rabbit | Bioworld | 1:500 | Overnight, 4°C |
| P-p38 | Rabbit | Cell Signaling | 1:200 | Overnight, 4°C |
| CGRP | Rabbit | Sigma | 1:1000 | 3 h, RT |
| NeuN | Mouse | Millipore | 1:1,000 | 3 h, RT |
| GFAP | Goat | Abcam | 1:5,000 | 3 h, RT |
| Alexa 488 | Rabbit | Life Technologies | 1:200 | 1 h, RT |
| Alexa 567 | Goat | Life Technologies | 1:200 | 1 h, RT |
| Alexa 647 | Mouse | Life Technologies | 1:200 | 1 h, RT |
4.4 Statistical Analysis
Statistical analysis of immunostaining data was performed essentially as described previously (Cornelison et al., 2016; Hawkins et al., 2015; Koop et al., 2017). The relative fluorescent staining intensity in spinal cord tissue was determined by measuring the grayscale intensity from four regions, in laminae I–III, of the medullary dorsal horn. Regions of interest were randomly chosen from the DAPI images that allowed for comparison of neuronal cell densities. Average background intensities were also acquired using a similar procedure from acellular regions of the medullary dorsal horn. The average background intensity acquired from each image was then subtracted from each integrated density value from areas of interest. Staining intensity measurements for each animal are reported as the average of a minimum of three images from each tissue. Intensities acquired in stressed offspring were normalized to levels detected in naïve offspring and are reported as the average fold change ± SEM relative to the mean level for control animals that was made equal to one. For analysis of p-p38 and p-ERK expression in the trigeminal ganglia, the percentage of cells demonstrating nuclear localization was calculated from the total number of visible neuronal nuclei in each DAPI image. Percentages were averaged between the three images taken from each tissue to acquire a representative average for each animal. Values acquired for each condition were averaged and represented as the mean percentage of positive neuronal cells ± SEM. For statistical analysis, SPSS Statistics 21 software (IBM, North Castle, NY) was used to detect outlier values using 3 as a multiplier, and this information was used to remove animals from this study. A Shapiro-Wilk test was utilized to determine the normality of the data set, while a Levene’s test was used to determine equal or unequal variance. Statistical differences were determined using either an independent T-test or a Mann-Whitney U test. All statistical analysis was determined utilizing SPSS software (IBM) and results were considered significantly different when P < 0.05.
HIGHLIGHTS.
Early life stress leads to cellular changes associated with trigeminal sensitization.
Secondary traumatic stress promotes elevated levels of CGRP and GFAP in the STN.
Stress induced expression of the active forms of ERK, JNK, and p38 in the STN.
Levels of P-ERK and P-p38 increased in trigeminal ganglia in response to stress.
Acknowledgments
We would like to thank Angela Goerndt for her assistance with the animals. This work was supported by the National Institutes of Health [DE024629].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, et al. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–34. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia. 2007;27:1442–53. doi: 10.1111/j.1468-2982.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache. 2013;53:1230–44. doi: 10.1111/head.12179. [DOI] [PubMed] [Google Scholar]
- Borsook D, et al. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73:219–34. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Brennenstuhl S, Fuller-Thomson E. The Painful Legacy of Childhood Violence: Migraine Headaches Among Adult Survivors of Adverse Childhood Experiences. Headache. 2015;55:973–83. doi: 10.1111/head.12614. [DOI] [PubMed] [Google Scholar]
- Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35:6619–29. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse DC, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013a;53:1278–99. doi: 10.1111/head.12150. [DOI] [PubMed] [Google Scholar]
- Buse DC, et al. Psychiatric comorbidities of episodic and chronic migraine. J Neurol. 2013b;260:1960–9. doi: 10.1007/s00415-012-6725-x. [DOI] [PubMed] [Google Scholar]
- Cady RJ, et al. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano A, et al. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–8. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison LE, Hawkins JL, Durham PL. Elevated levels of calcitonin gene-related peptide in upper spinal cord promotes sensitization of primary trigeminal nociceptive neurons. Neuroscience. 2016;339:491–501. doi: 10.1016/j.neuroscience.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford LJ. Chronic Pain: Where the Body Meets the Brain. Trans Am Clin Climatol Assoc. 2015;126:167–83. [PMC free article] [PubMed] [Google Scholar]
- Crown ED. The role of mitogen activated protein kinase signaling in microglia and neurons in the initiation and maintenance of chronic pain. Exp Neurol. 2012;234:330–9. doi: 10.1016/j.expneurol.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Dodds KN, et al. Glial contributions to visceral pain: implications for disease etiology and the female predominance of persistent pain. Transl Psychiatry. 2016;6:e888. doi: 10.1038/tp.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46(Suppl 4):S182–91. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Durham PL, Russo AF. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci. 2003;23:807–15. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL. Diverse Physiological Roles of Calcitonin Gene-Related Peptide in Migraine Pathology: Modulation of Neuronal-Glial-Immune Cells to Promote Peripheral and Central Sensitization. Curr Pain Headache Rep. 2016;20:48. doi: 10.1007/s11916-016-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SE, Patil VV, Durham PL. Nitric oxide-proton stimulation of trigeminal ganglion neurons increases mitogen-activated protein kinase and phosphatase expression in neurons and satellite glial cells. Neuroscience. 2008;157:542–55. doi: 10.1016/j.neuroscience.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett FG, Durham PL. Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation. Neuron Glia Biol. 2009:1–12. doi: 10.1017/S1740925X09990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JL, et al. Nicotine stimulates expression of proteins implicated in peripheral and central sensitization. Neuroscience. 2015;290:115–25. doi: 10.1016/j.neuroscience.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM. Pain Modulation and the Transition from Acute to Chronic Pain. Adv Exp Med Biol. 2016;904:105–15. doi: 10.1007/978-94-017-7537-3_8. [DOI] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–76. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158:543–559. doi: 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, et al. MAP kinase and pain. Brain Res Rev. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Greenwood-Van Meerveld B. Stress-induced pain: a target for the development of novel therapeutics. J Pharmacol Exp Ther. 2014;351:327–35. doi: 10.1124/jpet.114.218065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsan N, Goadsby PJ. CGRP mechanism antagonists and migraine management. Curr Neurol Neurosci Rep. 2015;15:25. doi: 10.1007/s11910-015-0547-z. [DOI] [PubMed] [Google Scholar]
- Klaric M, et al. Secondary traumatisation and systemic traumatic stress. Psychiatr Danub. 2013;25(Suppl 1):29–36. [PubMed] [Google Scholar]
- Koop LK, et al. Central Role of Protein Kinase A in Promoting Trigeminal Nociception in an In Vivo Model of Temporomandibular Disorders. J Oral Facial Pain Headache. 2017;31:264–274. doi: 10.11607/ofph.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache. 2012;52(Suppl 2):102–6. doi: 10.1111/j.1526-4610.2012.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, et al. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, et al. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009:417–49. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–40. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney-Leber SM, Brummelte S. Neonatal pain and reduced maternal care: Early-life stressors interacting to impact brain and behavioral development. Neuroscience. 2017;342:21–36. doi: 10.1016/j.neuroscience.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Olesen J, et al. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–90. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–91. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–52. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009:451–91. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- Slade GD, et al. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J Dent Res. 2016;95:1084–92. doi: 10.1177/0022034516653743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz AM, Swiergiel AH, Lisowski P. Epigenetics of stress adaptations in the brain. Brain Res Bull. 2013;98:76–92. doi: 10.1016/j.brainresbull.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33:784–92. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, et al. Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache. 2012;52:920–9. doi: 10.1111/j.1526-4610.2012.02165.x. [DOI] [PubMed] [Google Scholar]
- Tietjen GE. Childhood Maltreatment and Headache Disorders. Curr Pain Headache Rep. 2016;20:26. doi: 10.1007/s11916-016-0554-z. [DOI] [PubMed] [Google Scholar]
- Tsuda M, et al. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- van Bodegom M, Homberg JR, Henckens M. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci. 2017;11:87. doi: 10.3389/fncel.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen RM, et al. Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia. 2017;37:49–63. doi: 10.1177/0333102415625614. [DOI] [PubMed] [Google Scholar]