Abstract
Aging is associated with a substantial decline in the expression of social behavior, as well as increased neuroinflammation. Since immune activation and subsequent increased expression of cytokines can suppress social behavior in young rodents, we examined age and sex differences in microglia within brain regions critical to social behavior regulation (PVN, BNST, and MEA) as well as in the hippocampus. Adult (3-month) and aged (18-month) male and female F344 (N = 26, n = 5–8/group) rats were perfused and Iba-1 immunopositive microglia were assessed using unbiased stereology and optical density. For stereology, microglia were classified based on the following criteria: (i) thin ramified processes, (ii) thick long processes, (iii) stout processes, or (iv) round/ameboid shape. Among the structures examined, the highest density of microglia was evident in the BNST and MEA. Aged rats of both sexes displayed increased total number of microglia number exclusively in the MEA. Sex differences also emerged, whereby aged females (but not males) displayed greater total number of microglia in the BNST relative to young adult counterparts. When morphological features of microglia were assessed, aged rats exhibited increased soma size in the BNST, MEA, and CA3. Together, these findings provide a comprehensive characterization of microglia number and morphology under ambient conditions in CNS sites critical for the normal expression of social processes. To the extent that microglia morphology is predictive of reactivity and subsequent cytokine release, these data suggest that the expression of social behavior in late aging may be adversely influenced by heightened inflammation.
Keywords: aging, senescence, microglia, morphology, social behavior, rat, limbic system, Fischer 344
1.0 Introduction
Neuroinflammation is a significant consequence of normal aging (for review, see Barrientos, Kitt, Watkins, & Maier, 2015; Eggen, Raj, Hanisch, & Boddeke, 2013; Jurgens & Johnson, 2012; Norden & Godbout, 2013), and inflammation or illness is associated with a reduction in social behavior as part of a larger repertoire of ‘sickness behavior’. In fact, aged mice exhibit prolonged expression of sickness-related reductions in social interaction (Godbout et al., 2005; Huang et al., 2008), supporting a role for age-related neuroinflammation in the regulation of social behavior. Furthermore, aging is accompanied by a decline in the expression of social behavior, even in the absence of overt illness (Hunt et al., 2011; Perkins et al., 2016; Salchner et al., 2004). The consequences of decreased social behavior may be substantial, since positive social interactions are beneficial for overall health (Carter, 1998; DeVries et al., 2007; Grippo et al., 2007). Thus, identification of mechanisms by which social behavior is reduced in late aging remains critical.
Microglia represent a unique population of immune cells, as they are found exclusively within the central nervous system (Kettenmann et al., 2011; Lawson et al., 1990). Derived from myeloid progenitor cells that infiltrate the brain predominantly before the formation of the blood brain barrier (Ginhoux et al., 2010), microglia are the resident immune cells of the brain, performing numerous functions that include surveillance of the brain microenvironment. Microglia also respond to injury or illness by releasing pro-inflammatory cytokines (such as IL-1β and TNFα) and chemokines (Dantzer, 2004). However, recent advances in the understanding of microglia function have shown that in addition to their role as immune cells, microglia are integral in the formation and pruning of synapses across the lifespan (Kettenmann et al., 2013; Paolicelli et al., 2011; Salter and Beggs, 2014; Tremblay, 2011), help regulate neurogenesis (Sierra et al., 2010), and even contain receptors for neurotransmitters and neuropeptides that allow them to respond to neuronal activity (Kettenmann et al., 2011; Pocock and Kettenmann, 2007). Thus, microglia help maintain homeostasis in the CNS and respond to perturbations in homeostasis (e.g. pathogens) by releasing a cascade of inflammatory factors.
Importantly, natural aging is associated with tell-tale signs of neuroinflammation that can manifest in multiple ways (for review, see Barrientos, Kitt, Watkins, & Maier, 2015; Eggen, Raj, Hanisch, & Boddeke, 2013; Jurgens & Johnson, 2012; Norden & Godbout, 2013). For example, microglia isolated from the aged brain have enhanced expression of MHCII (Griffin et al., 2006; Henry et al., 2009), CD86 (Griffin et al., 2006), CD68 (Wong et al., 2005), CD11b (Hart et al., 2012; Stichel and Luebbert, 2007), IL-1β (Griffin et al., 2006; Sierra et al., 2007; Stichel and Luebbert, 2007), and IL-6 (Sierra et al., 2007). Other studies have demonstrated that the aged brain is ‘primed’ in response to an immune challenge (reviewed in Barrientos et al., 2015; Norden and Godbout, 2013). In this case, ‘primed’ indicates that there are no basal differences in neuroimmune markers, but rather that a secondary challenge is required for age-related alterations in neuroimmune function to become evident. For example, administration of LPS produces a robust increase in central IL-1β and IL-6 mRNA expression that is exacerbated in aged mice (Godbout et al., 2005; Huang et al., 2008). In addition, hippocampal microglia isolated from aged rats exhibit a sensitized cytokine response to peripheral immune challenge with LPS (Frank et al., 2010b) or E. coli (Frank et al., 2010a). However, the mechanisms that drive age-related neuroinflammation are not well understood. One possibility is that aging is associated with increased number or reactivity of microglia, particularly in response to injury or immune challenge. Indeed, microglia isolated from the hippocampus (Abraham and Johnson, 2009; Huang et al., 2008), cortex (Henry et al., 2009), and cerebellum (Huang et al., 2008) of aged mice exhibit a prolonged pro-inflammatory response to endotoxin administration. Aging is also accompanied by a significant decline in the expression of fractalkine (CX3CL1), a negative regulator of microglia activation (Fenn et al., 2013). Interestingly, CX3CR1 knockout mice display altered patterns of social behavior, providing evidence for a functional role of microglia in social behavior regulation (Zhan et al., 2014). In addition, aged rats have high levels of corticosterone and this is related to greater glucocorticoid receptor activation within the hippocampus, both of which contribute to the sensitization of microglia (Barrientos et al., 2015b). Taken together, late aging is clearly associated with significant alterations in the inflammatory environment that may contribute to altered social behavior.
Increased neuroinflammation in late aging may be related to changes in the number and/or morphology of microglia in senescence. For example, using in vivo 2-photon microscopy, Hefendehl et al. (2014) found that cortical microglia in the aged murine brain are abnormal, exhibiting increased soma size and decreased process length (Hefendehl et al., 2014). These characteristics are thought to result in a decrease in the ability of microglia to surveil the brain parenchyma and respond to perturbations of homeostasis. Using unbiased stereology, Mouton et al. (2002) found that microglia number in the dentate gyrus and CA1 increased with age in female C57 mice; females also exhibited greater numbers of microglia regardless of age (Mouton et al., 2002). Other studies have found no late aging- or sex-differences in microglia number in the hippocampus (Khan et al., 2015; Kohman et al., 2013). Despite a rich literature examining microglia number and morphology in late aging, no studies have examined microglia specifically in CNS structures that play an established role in social behavior regulation.
The neural circuitry governing social behavior is well established in younger animals and is referred to as the social behavior neural network (SBNN) or social salience network (SSN) (Freeman and Young, 2016; Johnson et al., 2017; Johnson and Young, 2017). Oxytocin (OT), a neuropeptide heavily involved in social behavior, is produced in the paraventricular nucleus of the hypothalamus (PVN) and supraoptic nucleus (SON). Oxytocin receptor is expressed in the prefrontal cortex, nucleus accumbens, bed nucleus of the stria terminalis (BNST), hippocampus (HPC), and medial amygdala (MEA) in the rodent brain (Freeman and Young, 2016; Knobloch and Grinevich, 2014). The PVN receives substantial brainstem inputs and as such is a key site of integration of ascending signals and the neuroendocrine responses to stress (Myers et al., 2017; Pacak and Palkovits, 2001). The BNST is involved in social recognition (Dumais et al., 2016b) and exhibits sex-specific patterns of immediate early gene (c-Fos) activation following a social experience (Perkins et al., 2017). The amygdala has been implicated in social information processing in humans (Adolphs, 2003) and the MEA specifically exhibits induction of c-Fos in response to social interaction in adult rats that is blunted in aged rats (Salchner et al., 2004). Thus, these three regions (PVN, BNST, and MEA) were chosen to assess potential age and sex differences in microglia morphology that may relate to social behavior. The HPC was assessed since age-related changes in neuroimmune function have been extensively characterized in this structure, including examination of microglia morphology (Khan et al., 2015; Mouton et al., 2002) and age-related microglia priming (Frank et al., 2010b; Huang et al., 2008; Wynne et al., 2010).
Given the strong ability for illness and inflammation-related events to suppress social behavior, we reasoned that late aging associated reductions in social behavior might be a result of increased number and/or altered activational states of resident microglia within these socially-relevant nuclei. Thus, to examine age-related changes in microglia, we used immunohistochemistry and unbiased stereology to estimate total microglia number, average area (µm2), and average volume (µm3) in three socially-relevant brain structures: BNST, PVN, and MEA in male and female F344 rats of different ages. Microglia were also assessed within the HPC as a referent site to help contextualize measures of morphology within CNS sites more critically involved in social behavior regulation, and because the extant literature includes numerous assessments of microglia in this structure.
2.0 Materials & Methods
2.1 Subjects
Subjects were adult (3 month) and aged (18 month) male and female F344 rats (N = 26; n = 5–8 per group). Rats were obtained from the National Institute of Aging (NIA) colony maintained by Charles River Laboratories and were given at least 2 weeks to acclimate to colony conditions (22 ± 1°C; 12:12 light:dark cycle with lights on at 0700) prior to experimentation. All rats were pair-housed and provided ad libitum access to both food and water throughout the experiment. All rats were handled for 3 min on each of two days prior to tissue collection. Rats were maintained and treated in accordance with the guidelines set forth by the Institute of Laboratory Animal Resources, (1996) and in accordance with the protocol approved by the IACUC at Binghamton University.
2.2 Experimental Design
To assess the number of microglia, we used a 2 [Age] × 2 [Sex] design. Animals were perfused directly from their home cages, since we were interested in assessing microglia under basal conditions. Animals were injected (i.p.) with sodium pentobarbital and transcardially perfused with ice-cold (4° C) 0.1 M phosphate buffered saline [PBS; 0.01 M phosphate buffer, 120 mM NaCl; 2.7 mM KCl, Sigma-Aldrich Chemical, St. Louis, MO; cat. no. P3813] followed by ice-cold (4° C) 4% paraformaldehyde (Sigma-Aldrich Chemical, St. Louis, MO; cat. no. 158127). Brains were removed and post-fixed in 4% paraformaldehyde overnight, after which they were transferred to 30% sucrose in 0.01 M PBS for 3–4 days. Brains were then rapidly frozen using 2-methylbutane [EMD Millipore, Darmstadt, Germany; cat. no. MX0760-1] on dry ice, and stored at −20° C until sectioning. Brains were sectioned coronally (40 µm) on a cryostat from the prefrontal cortex through the hippocampus. Sections were placed sequentially into 96-well plates containing a cryoprotectant solution [30% ethylene glycol, 30% glycerol in 0.05 M phosphate buffer] and stored at −20° C until immunohistochemistry was performed.
2.3 Tissue processing: Immunohistochemistry
All tissue was processed in cohorts counterbalanced by experimental group such that all groups were represented in each cohort to control for any potential differences in immunohistochemistry procedures. Systematic random sampling was used and every fourth section was selected for each brain region. Sections were chosen based on the atlas of Paxinos & Watson (1986; Figure 1A). Immunohistochemistry was performed with Vector Labs Elite Rabbit IgG ABC Kits [Vector Laboratories, Burlingame, CA; cat. no. PK-6101]. Briefly, free-floating sections were washed in 0.1 M PBS, followed by a 30-minute incubation in 0.3% hydrogen peroxide [VWR; cat. no. BDH7690-1] made with 0.1 M PBS. After rinsing in PBS, sections were placed into a blocking solution [1.5% Normal Goat Serum in PBS + 0.5% Trition X-100; Vector Laboratories, Burlingame, CA; cat. no. PK-6101] for 2 hr. before incubation in primary antibody overnight at 4° C [1:4000 dilution rabbit polyclonal anti-Iba1; Wako Chemicals, Richmond, VA; cat. no. 019-19741]. The next day, sections were washed in PBS, followed by a 2 hr. incubation in secondary antibody [1:5000 in 1.5% Normal Goat Serum in PBS + 0.5% Trition X-100; Vector Laboratories, Burlingame, CA; cat. no. PK-6101]. After a brief wash in PBS, sections were transferred to an avidin/biotin complex solution for 2 hr. Finally, the reaction was visualized with a diaminobenzidine (DAB) solution with nickel-cobalt, prepared according to the manufacturer’s instructions [Vector Laboratories, Burlingame, CA; cat. no. SK-4100]. Tissue was mounted onto gelatin-coated slides, allowed to dry overnight, and coverslipped with Permount® [Fisher Scientific, Waltham, MA].
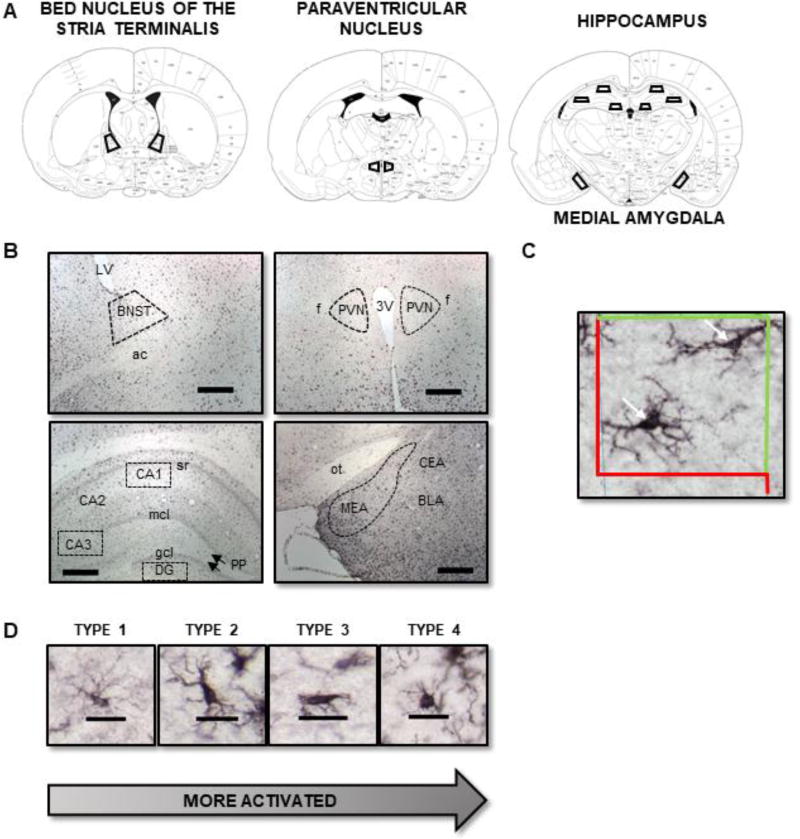
Figure 1.
(A) Regions of interest for stereological analysis of microglia (adapted from Paxinos & Watson, 1986). (B) Representative photomicrographs (scale bar = 500 µm) showing areas for stereological analysis of microglia (BNST, PVN, CA1, CA3, DG, and MEA). (C) Optical fractionator in Stereo Investigator. Microglia that were within the counting frame (or touching the green line) were included, whereas those that touched the red line were excluded. This prevents duplicate counting of microglia. Arrows point to example Iba-1 positive microglia. (D) Representative photomicrographs (scale bar = 25 µm) of Type 1 (thin ramified processes), Type 2 (thick long processes), Type 3 (stout processes), and Type 4 (round/ameboid shape).
2.4 Unbiased Stereological Quantification of Iba1+ Microglia
Slides were coded such that the experimenter performing cell counts was blind to experimental group. A Zeiss microscope (Axioscope 2-Plus, Thornwood, NY, USA) attached to a digital camera (DVC-1310; DVC Company, Austin, TX) and a three-axis motorized stage was used. The camera was attached to a computer with StereoInvestigator software (MicroBrightField Bioscience, Williston, VT). The regions of interest (BNST, PVN, MEA, CA1, CA3, and DG) were outlined using a 5× objective (Zeiss Plan-NEOFLUAR) according to the atlas of Paxinos & Watson (1986; Figure 1A–B); cells were counted using a 40× dry-objective lens (Zeiss Plan-NEOFLUAR). Cells were excluded if they were located on the edge of the tissue (Schwarz et al., 2012). The total number of microglia in the BNST, PVN, MEA, CA1, CA3, and DG were estimated using the optical fractionator method. The sampling grid was applied randomly to the sections, such that the counting frames were placed randomly onto the region of interest (ROI). For each region, a counting frame size of (75 µm × 75 µm) and a grid size of (100 µm × 100 µm) were used, with a guard zone of 2 µm and a disector height of 20 µm (Figure 1C).
Concurrent with cell counting, microglia were categorized as one of four types: Type 1: thin ramified processes, Type 2: thick long processes, Type 3: stout processes, or Type 4: round/ameboid shape (Bolton et al., 2017; Schwarz et al., 2012) (Figure 1D). For ease of presentation, Types 1 and 2 were summed and are shown at Type 1/2. The same thing was done for Types 3 and 4. Thus, Type 1/2 should be considered ‘ramified/quiescent’, whereas Type 3/4 should be considered ‘transitioning to activated/activated’. For each animal, six-eight sections were quantified for each brain region. For each microglia counted, estimated soma areas (µm2) and volumes (µm3) were obtained using the nucleator software function with the number of rays set to 8. Stereological parameters were chosen based on pilot studies that assured that the Gundersen-Jensen estimator for the error coefficient (smoothness factor = 1) was below 0.10 for all animals. All stereological parameters can be found in Table 1.
Table 1.
Stereological parameters used to estimate the total population of Iba1+ microglia.
| Stereological Variable | BNST | PVN | MEA | CA1 | CA3 | DG |
|---|---|---|---|---|---|---|
| Section sampling faction (ssf) | 1/4 | |||||
| Fraction of area of section samples (asf) | (75 µm×75 µm)/(100 µm×100 µm) | |||||
| Thickness sampling fraction (h/t;tsf) | Average = 0.56 | Average = 0.57 | Average = 0.56 | Average = 0.49 | Average = 0.49 | Average = 0.48 |
| Disector volume (h×Aframe) - µm3 | 112,500 µm3 | 146,250 µm3 | 112,500 µm3 | 146,250 µm3 | 146,250 µm3 | 146,250 µm3 |
| Average thickness of mounted section (t) | ~ 36 µm | ~ 35 µm | ~ 36 µm | ~ 41 µm | ~ 41 µm | ~ 42 µm |
| Height of disector (h) | 20 µm | |||||
| Guard zone | 2 µm | |||||
| Observed coefficient of variation of estimator (OCE) | 0.074 ± 0.003 | 0.097 ± 0.007 | 0.067 ± 0.002 | 0.087 ± 0.003 | 0.087 ± 0.003 | 0.093 ± 0.004 |
| Smoothness factor (m) | 1.0 | |||||
| Number of sections used (i) | 6 (bilateral) | 6 (unilateral) | 6–8 (unilateral) | 6 (unilateral) | 6 (unilateral) | 6 (unilateral) |
2.5 Optical Density
To assess microglia density, photomicrographs were taken with a 10× dry-objective lens (Nikon LU PLANFLUOR) in each ROI. For MEA, BNST, CA1, CA3, and DG, 2–4 unilateral images were obtained per animal. For the PVN, images were obtained bilaterally in 2–4 sections per animal. Analysis of optical density was completed with NIH ImageJ (Abràmoff et al., 2004). Briefly, images were converted to 16-bit images. The “moments” threshold was applied with the same value used for each section and ROI, to allow for comparison between ROIs. This thresholding procedure best captured microglia soma and processes. Data for each animal were averaged to create a single data point.
2.6 Data Analysis
All data were analyzed with Statistica (Dell Statistica, Tulsa, OK). The total number of microglia, average estimated area of the soma (µm2), average estimated volume of the soma (µm3), and microglia density were assessed separately for each brain region with 2 (Age) × 2 (Sex) ANOVAs. Microglia density was also analyzed with a repeated-measures ANOVA with ROI as the within-subjects factor and Age and Sex as between-subjects factors. Since sex differences in the number of microglia have been observed consistently in the literature, we examined age differences in microglia morphology (Type 1/2 vs Type 3/4) separately in males and females. To analyze these data by microglia type, mixed model ANOVAs were used with Type as a within-subjects factor and Age as a between-subject’s factors. Fisher’s Least Significant Difference (LSD) post-hoc tests were used for all analyses. There were unequal sample sizes and some dependent measures violated homogeneity of variance assumptions, so all data were transformed using a natural log transformation. However, for ease of presentation, all data in graphs represent untransformed data. Given the large number of dependent measures, we opted to display key findings graphically in Figures, whereas Table 2 displays more comprehensively all data collected.
Table 2.
Average (± SEM) number, density, area, and volume of microglia.
| Males | Females | ||||
|---|---|---|---|---|---|
|
| |||||
| Region | Measure | Adult | Aged | Adult | Aged |
| BNST | Number | 2617.3 ± 349.1 | 2871.0 ± 269.6 | 1996.3 ± 266.8 | 2765.0 ± 178.9 |
| Density (OD) | 21.23 ± 3.79a | 17.47 ± 2.46 a,b | 9.81 ± 1.55 b | 20.56 ± 1.01a | |
| Area (µm2) | 52.6 ± 0.9 | 57.5 ± 1.3* | 51.8 ± 1.2 | 57.3 ± 1.6* | |
| Volume (µm3) | 336.4 ± 9.4 | 381.3 ± 14.3* | 328.7 ± 15.7 | 377.9 ± 17.3* | |
|
| |||||
| PVN | Number | 1818.3 ± 385.8 | 2047.9 ± 489.7 | 1115.6 ± 444.8 | 1289.7 ± 375.5 |
| Density (OD) | 17.53 ± 5.05 | 15.07 ± 3.26 | 8.23 ± 4.01 | 12.00 ± 2.62 | |
| Area (µm2) | 50.9 ± 1.1a | 51.3 ± 0.7*a | 44.7 ± 0.5b | 52.4 ± 1.7*a | |
| Volume (µm3) | 328.5 ± 11.1a | 312.9 ± 11.0*a | 259.3 ± 4.4b | 337.9 ± 18.8*a | |
|
| |||||
| MEA | Number | 3383.2 ± 313.3 | 3966.7 ± 452.5* | 2395.00 ± 380.6 | 3360.1 ± 514.3* |
| Density (OD) | 30.46 ± 5.50a | 26.76 ± 3.71a | 10.01 ± 0.91b | 23.11 ± 4.22a | |
| Area (µm2) | 51.8 ± 1.5 | 55.4 ± 0.6* | 48.7 ± 2.2 | 55.3 ± 0.7* | |
| Volume (µm3) | 329.2 ± 14.2 | 364.1 ± 7.6* | 310.6 ± 29.0 | 361.9 ± 6.6* | |
|
| |||||
| CA1 | Number | 2199.0± 215.7 | 2095.5 ± 229.3 | 1743.2 ± 170.3 | 2104.8 ± 206.9 |
| Density (OD) | 13.85 ± 2.33 | 10.53 ± 0.78 | 7.41 ± 1.59# | 8.54 ± 1.62# | |
| Area (µm2) | 59.2 ± 2.5 | 65.4 ± 2.8 | 59.2 ± 3.2 | 63.5 ± 3.2 | |
| Volume (µm3) | 421.4 ± 29.2 | 495.6 ± 31.2* | 401.1 ± 23.5 | 469.6 ± 29.9* | |
|
| |||||
| CA3 | Number | 1651.6 ± 167.5 | 1733.9 ± 193.7 | 1231.0 ± 200.5 | 1724.3 ± 161.5 |
| Density (OD) | 13.07 ± 2.41a | 9.29 ± 1.55a,b | 7.11 ± 1.85b | 10.15 ± 1.50a,b | |
| Area (µm2) | 64.6 ± 2.2 | 67.4 ± 1.9* | 60.0 ± 2.2 | 67.7 ± 3.2* | |
| Volume (µm3) | 501.7 ± 33.2 | 514.5 ± 24.3 | 428.7 ± 25.9 | 520.1 ± 39.7 | |
|
| |||||
| DG | Number | 1676.7 ± 199.6 | 1885.8 ± 304.1 | 940.9 ± 132.6 | 1639.0 ± 172.3 |
| Density (OD) | 9.89 ± 2.03 | 7.83 ± 0.72 | 5.28 ± 2.22# | 7.94 ± 1.01# | |
| Area (µm2) | 68.9 ± 3.3 | 72.1 ± 2.4 | 66.6 ± 1.4 | 70.5 ± 3.4 | |
| Volume (µm3) | 511.1 ± 35.6 | 583.1 ± 38.3 | 487.4 ± 17.6 | 532.8 ± 44.4 | |
Note:
indicates a main effect of Age;
indicates a main effect of Sex;
significant interactions are indicated by bold text: different letters denote groups that are significantly different from one another.
3.0 Results
3.1 General results
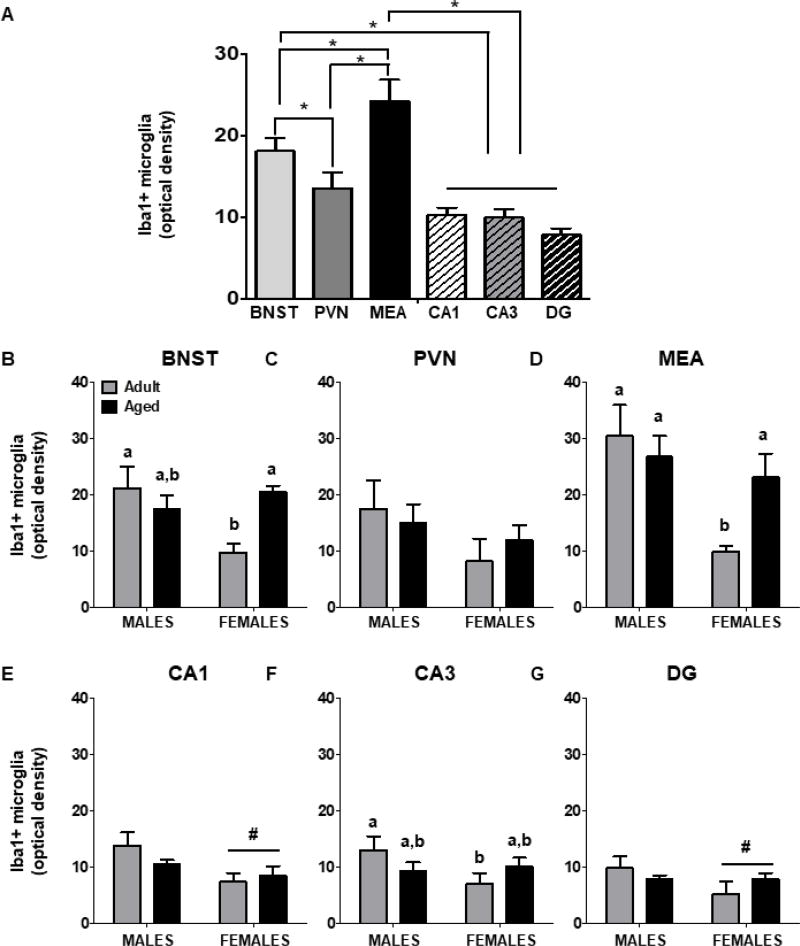
Microglia density varied as a function of ROI [F(5,65) = 21.90, p < 0.00001]. Post-hoc tests revealed that the density of microglia was highest in the MEA relative to all other ROIs. Density was also higher in BNST relative to PVN and HPC subregions. However, PVN and HPC did not differ from each other (Figure 2). Microglia density was lowest in adult females, relative to adult males and aged rats of both sexes [Age × Sex interaction: F(1,65) = 5.82, p = 0.03], although there was no interaction with ROI. Not surprisingly, soma area and volume were significantly correlated in all regions examined: BNST (r2 = 0.91), PVN (r2 = 0.78), MEA, (r2 = 0.95), CA1, (r2 = 0.90), CA3 (r2 = 0.90), and DG (r2 = 0.91).
Figure 2.
(A) Regional differences in microglia density. Significant differences between ROIs indicated with (*). Density of Iba1+ microglia in BNST (B), PVN (C), MEA (D), CA1 (E), CA3 (F), and DG (G). Significant main effects of Sex are indicated by (#). For significant interactions, different letters indicate groups that differ significantly from one another.
3.2 Bed nucleus of the stria terminalis
3.2a Estimated Total Number
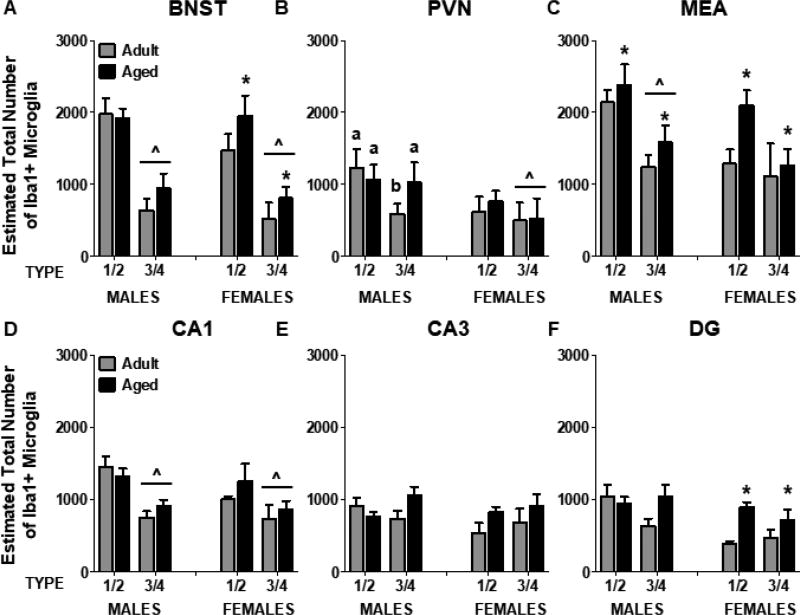
The total number of microglia in BNST was not significantly different between males and females (p = 0.33), nor did it differ as a function of Age (p = 0.066). To assess the number of microglia by Type, males and females were examined separately. In males, there were significantly fewer Type 3/4 microglia relative to Type 1/2 microglia [F(1,14) = 42.98, p < 0.0001] (Figure 3A). Aged females had more microglia, relative to adult females, and this did not vary as a function of microglia type [F(1,9) = 5.94, p = 0.04]. Thus, age differences in the number of microglia in BNST were observed exclusively in females. Similar to what was observed in males, there were more Type 1/2 microglia than Type 3/4 microglia in females [F(1,9) = 16.41, p = 0.003]. Microglia density was lower in adult females, relative to adult males and aged females [Age × Sex interaction: F(1,20) = 5.50, p = 0.03] (Figure 2B).
Figure 3.
Estimated total number of Type 1/2 and Type 3/4 Iba1+ microglia in BNST (A), PVN (B), MEA (C), CA1 (D), CA3 (E), and DG (F). Aged rats had significantly more microglia in the MEA, regardless of microglia type, whereas aged females (but not males) had more microglia in the BNST and DG. Significant differences in microglia type are indicated by (^) and significant differences between adult and aged rats are indicated by (*). For significant interactions, different letters indicate groups that differ significantly from one another.
3.2b Estimated Area & Volume
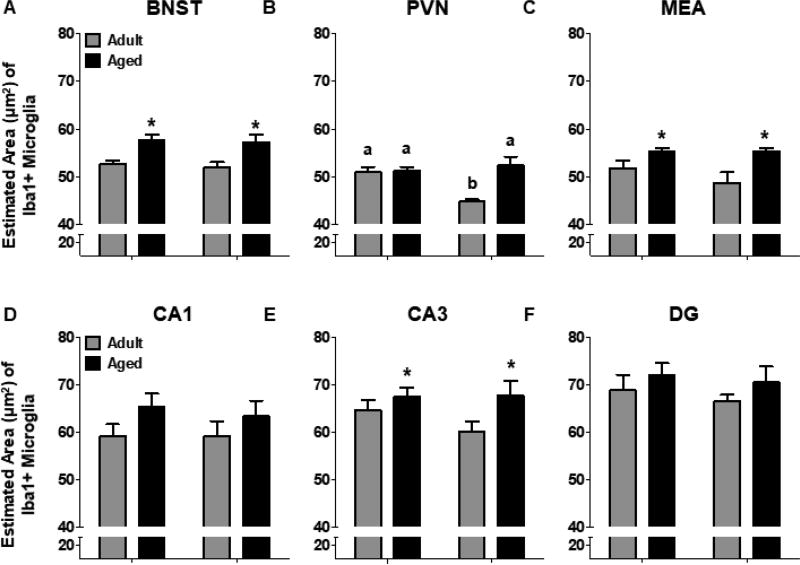
The estimated soma area of microglia in the BNST was larger in aged rats relative to adult rats [F(1,23) = 16.70, p = 0.0005] and this was observed in both males and females (Figure 4A). Type 3/4 microglia were larger than Type 1/2 microglia in males [F(1,14) = 22.88, p < 0.0001] and females [F(1,9) = 11.94, p = 0.007]. The estimated volume of microglia in BNST was also significantly greater in aged rats, regardless of sex [F(1,23) = 11.33, p = 0.003]. The volume of Type 3/4 microglia was larger than that of Type 1/2 microglia in males [F(1,14) = 37.50, p < 0.0001] and females [F(1,9) = 14.22, p = 0.004].
Figure 4.
Average estimated soma area (µm2) of microglia in the (A) BNST, (B) PVN, (C) MEA, (D) CA1, (E) CA3, and (F) DG. Microglia soma were significantly larger in 18-month-old rats in BNST, MEA, and CA3. In the PVN, 3-month-old females had significantly lower soma areas, relative to all other experimental groups. significant differences between adult and aged rats are indicated by (*). For significant interactions, different letters indicate groups that differ significantly from one another.
3.3 Paraventricular nucleus of the hypothalamus
3.3a Estimated Total Number
The estimated total number of microglia did not differ as a function of Age (p = 0.54) or Sex (p = 0.12), nor were there significant differences in microglia density within the PVN (all p’s > 0.13) (Figure 2C). In aged males, there were no differences between the numbers of Type 1/2 and Type 3/4 microglia, whereas in adult males, there were fewer Type 3/4 than Type 1/2 microglia [Type × Age interaction: F(1,14) = 9.08, p = 0.009]. In females, the number of Type 1/2 microglia was greater than the number of Type 3/4 microglia; this did not vary as a function of Age [F(1,8) = 7.30, p = 0.027] (Figure 3B).
3.3b Estimated Area & Volume
Microglia soma in the PVN of adult females were significantly smaller than those in adult males or in aged rats of either sex [Age × Sex interaction: F(1,22) = 12.78, p = 0.002] (Figure 4B). Not surprisingly, the estimated area of Type 3/4 microglia was larger in adult males, relative to the area of Type 1/2 microglia. However, in aged males, there were no significant differences between the estimated area of Type 1/2 or Type 3/4 microglia [Type × Age interaction: F(1,14) = 5.77, p = 0.03]. In females, there was a significant main effect of Age, wherein microglia somas were larger overall in aged females, relative to adult females [F(1,8) = 22.33, p = 0.001]. In addition, Type 3/4 microglia were larger than Type 1/2 microglia [main effect of Type: F(1,8) = 15.81, p = 0.004]. Similar effects were observed with the estimated volume of microglia.
3.4 Medial amygdala
3.4a Estimated Total Number
There were significantly more microglia in the MEA of aged rats, relative to adult rats [F(1,21) = 4.71, p = 0.04), although males and females were not different from one another (p = 0.053). In males, there were significantly more Type 1/2 microglia than Type 3/4 microglia [F(1,13) = 32.67, p < 0.0001]. In contrast, females had similar levels of each type of microglia (F(1,8) = 3.17, p = 0.11]. Aged females had more microglia than adult females [F(1,8) = 6.76, p = 0.03], an effect that was not observed in males (Figure 3C). Microglia density was lower in adult females, compared to all other groups [Age × Sex interaction: F(1,18) = 7.02, p = 0.02] (Figure 2D).
3.4b Estimated Area & Volume
Overall, the estimated area of microglia in MEA was larger in aged rats [F(1,20) = 11.67, p = 0.003] (Figure 4C). Similarly, microglia volume was higher in aged rats [F(1,21) = 8.70, p = 0.008]. Consistent with other brain regions, the somas of Type 3/4 microglia were significantly larger than those of Type 1/2 microglia in the MEA in males [F(1,13) = 52.24, p < 0.0001] and females [F(1,7) = 62.56, p < 0.0001]. Similarly, Type 1/2 microglia volumes were significantly smaller in males [F(1,13) = 56.63, p < 0.0001] and females [F(1,8) = 30.38, p = 0.0006].
3.5 Hippocampus
3.5a Estimated Total Number
Microglia number did not differ between males and females in CA1, CA3, or DG (p’s = 0.40, 0.28, and 0.08, respectively), nor were there differences between adult and aged rats (p’s = 0.59, 0.16, and 0.09, respectively). Both males and females had more Type 1/2 microglia than Type 3/4 microglia in CA1 [Males: F(1,13) = 52.16, p < 0.0001; Females: F(1,8) = 5.66, p = 0.04] (Figure 3D). In CA3, although there was a significant Type × Age interaction in males [F(1,13) = 5.98, p = 0.03], post hoc tests revealed no significant group differences. Similarly, in females, the number of Type 1/2 and Type 3/4 microglia in CA3 did not differ (Figure 3E). In males, the number of microglia did not vary as a function of Age or Type (p = 0.07) in DG. However, aged females had significantly more microglia than adult females, regardless of microglia type [F(1,8) = 8.44, p = 0.02] (Figure 3F). In CA1 and DG, males had a higher density of microglia relative to females [F(1,23) = 9.35, p = 0.006; F(1,23) = 4.37, p = 0.048, respectively] (Figure 2E,G). In CA3, adult females had a lower density of microglia relative to adult males, although they were not different from aged rats of either sex [Age × Sex interaction: F(1,23) = 4.38, p = 0.048] (Figure 2F).
3.5b Estimated Area & Volume
The overall estimated area of microglia soma was not affected by Age or Sex in CA1 (p’s = 0.09 & 0.73, respectively) or DG (p’s 0.19 & 0.49, respectively) (Figure 4D,F). However, the estimated soma area was larger in aged rats in CA3 [F(1,23) = 4.87, p = 0.04] (Figure 4E). In males, the estimated area of microglia was slightly lower in Type 3/4 microglia relative to Type 1/2 microglia in CA1 [F(1,13) = 5.76, p = 0.03], although this was not observed in females (p = 0.67). Similarly, there were no age differences in soma size in DG. Soma size of Type 1/2 microglia was slightly larger in aged females, relative to adult females, whereas no age differences were observed in the estimated area of Type 3/4 microglia in DG [Type × Age interaction: F(1,8) = 7.54, p = 0.03]. Soma volume was larger in aged rats in CA1 [F(1,23) = 5.68, p = 0.03], but no significant age differences were observed in CA3 (p = 0.10) or DG (p = 0.12). Analysis of microglia phenotype separately in males and females revealed that the increase in CA1 microglia volume in aged rats was driven by females [F(1,8) = 6.02, p = 0.04], since no significant age differences were observed in males (p = 0.12). Similar to what was found with microglia area, microglia volume in DG was not different between adult and aged males [F(1,13) = 1.03, p = 0.33], whereas aged females had a larger volume of Type 1/2, but not Type 3/4 microglia [Type × Age interaction: F(1,8) = 5.36, p = 0.049].
4.0 Discussion
The current study investigated whether the number and/or morphology of microglia varied as a function of age and sex in the hippocampus and in areas implicated in the regulation of social behavior. There were several key findings: (1) microglia density was highest in the MEA and BNST, pointing to the social behavior circuit as a region of interest for late aging alterations in neuroinflammation; (2) in the MEA, aged rats of both sexes had greater numbers of microglia relative to their adult counterparts whereas within the BNST, aged females had significantly more microglia than adult females, an effect that was not observed in aged males; and (3) across several structures that were examined, microglial soma were significantly larger in aged rats. Overall, it appeared as if the most sensitive measure of activation state was not in categorical classification of morphology, but rather in measures of area and volume. Thus, the present examination of microglia in socially-relevant brain structures fills an important void in our understanding of aging-related manifestations of neuroinflammation.
Regional differences in microglia density have been previously observed in mice (Lawson et al., 1990) and rats (Schwarz et al., 2012). For example, in postnatal day (P) 30 Sprague-Dawley rats, high numbers of microglia were observed in cortex, substantia nigra, and amygdala, whereas substantially fewer were observed in the HPC or PVN (Schwarz et al., 2012). Consistent with these data, we observed the highest density of microglia within the MEA, followed by the BNST, whereas fewer microglia were evident in PVN, CA1, CA3, and DG. Although examination of optical density did not allow us to assess the density of microglia by activation state, an interesting pattern emerged wherein density of Iba1+ microglia was lower adult females relative to adult males, whereas no sex differences were evident in aged rats. This pattern was observed in the BNST, MEA, and CA3, whereas in CA1 and DG, microglia density was lower in females, regardless of age. Interestingly, previous data from our lab demonstrated no increase in mRNA expression of cytokines (IL-6, TNF-α) and chemokines (MCP-1) in late aging (18–19 months) in the HPC or PVN (Gano et al., 2017), an effect that is not surprising given the relative lack of age differences in microglia number we observed within these structures.
Aging is associated with an increase in neuroinflammation. Studies have demonstrated increased expression of cytokines following immune challenge (Godbout et al., 2005; Huang et al., 2008), increased number and/or density of microglia in several brain regions (Mouton et al., 2002; Ogura et al., 1994; Tremblay et al., 2012), an increase in the soma size of cortical microglia (Hefendehl et al., 2014), and altered microglia morphology (e.g. dystrophy) in the aged brain (Streit et al., 2004). However, it has also been reported that there are no age differences in the number of microglia (Khan et al., 2015; Kohman et al., 2013; Perry et al., 1993; Stark et al., 2007). Importantly, the preponderance of studies examining microglia in the aged brain have focused on hippocampal and cortical structures. We observed no significant age- or sex-differences in microglia number in CA1 or CA3, although there were more microglia in the DG of aged females. Microglia in CA3 were significantly larger in aged rats, relative to young adults. To our knowledge, no study to date has examined whether late aging is associated with altered cytokine expression or differences in the number/morphology of microglia within brain regions known to be involved in the regulation of social behavior, specifically the PVN, BNST, and MEA.
Since aging is accompanied by reduced expression of social behavior (Hunt et al., 2011; Perkins et al., 2016; Salchner et al., 2004) that may be related to increased neuroinflammation in aging, we hypothesized that aged rats would exhibit an increase in the number and size of microglia within these regions. Indeed, there was a ~25% increase in the total number of microglia in the MEA of aged males and females and a significant increase (~ 25%) in the number of microglia in the BNST of aged females (but not males) On average, microglia somas were larger in aged rats, relative to young adults. Here, we used microglia morphology as an indirect measure of activity. To the extent that increased soma size is indicative of a shift to a more activated state (Hanisch and Kettenmann, 2007), these data indicate that microglia in in the aged brain may be more reactive. When combined with an increase in microglia number in MEA and BNST (in females), these data suggest that the MEA and BSNT are sites of significant alterations in microglia function in late aging. The MEA and BNST are key components of the social behavior neural network and oxytocin (OT) and vasopressin (AVP) binding within these regions are associated with behaviors such as social motivation (Dumais et al., 2013) and social recognition (Dumais et al., 2016a; Ferguson et al., 2001). Few studies have examined the relationship between inflammation and neuropeptide release directly. For example, i.c.v. administration of IL-1β increases the release of AVP and OT within the SON, but not PVN (Landgraf et al., 1995). It remains to be determined whether pro-inflammatory cytokines can alter the release of AVP and OT within other socially-relevant, such as the BNST and MEA.
Previous studies have demonstrated sex differences in the number and morphology of microglia across development. During neonatal development, males have significantly more microglia than females (Lenz and McCarthy, 2015; Schwarz et al., 2012). For example, male Sprague Dawley rats had more microglia in parietal cortex, hippocampus, and amygdala at P 4, while at P30, females had a greater population of microglia with stout processes, consistent with an activated phenotype (Schwarz et al., 2012). This pattern was maintained at P60 in these rats, with increased numbers of stout/ameboid microglia in females, relative to males, in the parietal cortex, hippocampus, and amygdala (Schwarz et al., 2012). In contrast, we observed fewer total number of microglia in the MEA of females, but no sex differences in BNST, PVN, CA1, CA3, or DG. One possible explanation for this is the use of a different rat strain. F344 rats are hyper-adrenergic and stress reactive (Sternberg et al., 1992; Tonelli et al., 2001) relative to Sprague Dawley rats and other commonly used strains. In addition, there are strain/sex differences in the expression of social behavior. Specifically, in Sprague Dawley rats, adult males exhibit greater levels of social behavior relative to females (Stack et al., 2010; Varlinskaya et al., 2014). In contrast, adult female F344 rats exhibit greater levels of social investigation relative to adult male F344 rats (Perkins et al., 2017). This is consistent with the hypothesis that increased number and/or activation of microglia, such as what was observed in the MEA here might adversely influence the expression of social behavior. Importantly, we also observed an increase in the number of microglia in the MEA of aged animals, which may be related to suppression of social behavior observed in senescence.
On average, microglia somas were larger in aged rats, relative to adult rats, consistent with the few studies in which microglia soma size has been examined. For example, Hefendehl et al. (2014) reported increased microglia soma volume in cortex of 26–27-month-old mice. Similar late-aging increases in soma area of dentate gyrus microglia have been reported in 23-month-old male Wistar rats (Viana et al., 2013). To our knowledge, this is the first study to assess soma size in PVN, BNST, and MEA. Within the PVN, a brain region related to HPA axis function, microglia somas were larger in aged females, whereas no age difference was observed in males. This suggests that aged females could be more susceptible to the neuroimmune/neuroendocrine consequences of stress. Within the BNST and MEA, aging was associated with an increase in soma size that was similar in magnitude between males and females. Increased soma size is thought to indicate a shift toward an activated phenotype, which would result in increased release of pro-inflammatory cytokines. Behaviorally, an increase in microglia activation in late aging in BNST and MEA could relate not only to social behavior, but also to anxiety-related behavior. Indeed, there is already evidence that increased expression of pro-inflammatory cytokines (e.g. IL-1β, TNF-α) in the amygdala can promote anxiety-like behavior (Klaus et al., 2016). In addition, exposure to repeated social defeat can promote recruitment of peripheral macrophages to the brain, which was related to the expression of anxiety-like behavior (Wohleb et al., 2013). Within the hippocampus, increased soma size was observed in CA3, but not CA1 or DG. Although many studies have demonstrated age-related increases in cytokine expression within the HPC, few have analyzed specific subregions of the HPC. The CA3 subregion of the hippocampus plays a major role in memory encoding (Rebola et al., 2017) and displays significant hyperexcitability in late aging that is likely mediated by a reduction in GABAergic inhibition (Villanueva-Castillo et al., 2017). Aging is also accompanied by a significant loss of synapses in CA3 (Adams et al., 2010, 2008; Yu et al., 2011), which could be related to increased microglia activation in this region. Further research would be needed to determine a causal link between microglia soma size and synaptic dysfunction in late aging.
Although these data support the hypothesis that increased inflammation in senescence may be one mechanism driving age-related suppression of social behavior, this observation is at present correlational. However, we do know that increased immune activation leads to a pattern of behavior termed ‘sickness behavior’, which includes a decrease in social behavior. Whether this is the mechanism involved in age-related reductions in social behavior will have to be confirmed, perhaps with pharmacological studies. In addition, we chose to examine microglia under basal, non-stimulated conditions. Although some could view this as a limitation, we see it as a strength of the current experiment, adding to the already growing literature regarding the neurobiology of healthy aging. Furthermore, we expanded our analyses to brain regions that have not been investigated previously, owing to their potential role in the regulation of social behavior. Future studies should assess expression of cytokines and chemokines within these important brain regions.
Taken together, we demonstrated for the first time an age-related increase in the number of microglia in the MEA, a region heavily implicated in the regulation of social behavior. In addition, a similar pattern was evident in the BNST (~25% increase), but only in females. Finally, we showed that microglia somas overall, were larger in aged rats. This was consistently observed across all ROIs and suggests a presumptive increase in reactive state of microglia more broadly across the CNS. Thus, these data contribute to the mounting evidence demonstrating increased inflammation in senescence, and point to the neuroimmune system as a potential modulator of age-related suppression of social behavior.
Highlights.
The MEA and BNST displayed greater microglial density than other sites examined.
Late aging was associated with greater numbers of microglia in MEA in males and females.
Aged females (but not males) displayed a greater number of microglia in the BNST.
Microglia in aged rats were larger in BNST, MEA, and CA3, regardless of sex.
Acknowledgments
Supported by NIH grant number R01AG043467 to T.D. and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain. Behav. Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with imageJ. Biophotonics Int. 2004;11:36–41. doi: 10.1117/1.3589100. [DOI] [Google Scholar]
- Adams MM, Donohue HS, Linville MC, Iversen EA, Newton IG, Brunso-Bechtold JK. Age-related synapse loss in hippocampal CA3 is not reversed by caloric restriction. Neuroscience. 2010;171:373–382. doi: 10.1016/j.neuroscience.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp. Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience: Cognitive neuroscience of human social behaviour. Nat. Rev. Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, Maier SF. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015a;309:84–99. doi: 10.1016/j.neuroscience.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Thompson VM, Kitt MM, Amat J, Hale MW, Frank MG, Crysdale NY, Stamper CE, Hennessey PA, Watkins LR, Spencer RL, Lowry CA, Maier SF. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol. Aging. 2015b;36:1483–1495. doi: 10.1016/j.neurobiolaging.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD. Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front. Synaptic Neurosci. 2017;9:1–16. doi: 10.3389/fnsyn.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/S0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- DeVries aC, Craft TKS, Glasper ER, Neigh GN, Alexander JK, DeVries CA, Craft TKS, Glasper ER, Neigh GN, Alexander JK. 2006 Curt P. Richter award winner. Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Bredewold R, Veenema AH. Role of the oxytocin system in amygdala subregions in the regulation of social interest in male and female rats. Neuroscience. 2016a;330:138–149. doi: 10.1016/j.neuroscience.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH. Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology. 2016b;64:79–88. doi: 10.1016/j.psyneuen.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: Correlations with social interest in brain region- and sex- specific ways. Horm. Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Eggen BJL, Raj D, Hanisch UK, Boddeke HWGM. Microglial phenotype and adaptation. J. Neuroimmune Pharmacol. 2013;8:807–823. doi: 10.1007/s11481-013-9490-4. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Smith KM, Lovett-Racke AE, Guerau-de-Arellano M, Whitacre CC, Godbout JP. Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiol. Aging. 2013;34:2748–2758. doi: 10.1016/j.neurobiolaging.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. doi:21/20/8278 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain. Behav. Immun. 2010a;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J. Neuroimmunol. 2010b;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative Perspectives on Oxytocin and Vasopressin Receptor Research in Rodents and Primates: Translational Implications. J. Neuroendocrinol. 2016:28. doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Doremus-fitzwater TL, Deak T. A cross-sectional comparison of ethanol-related cytokine expression in the hippocampus of young and aged Fischer 344 rats. Neurobiol. Aging. 2017;54:40–53. doi: 10.1016/j.neurobiolaging.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Igor M, Merad M, Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway S, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science (80-. ) 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine aF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB. J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J. Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U-KK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hart AD, Wyttenbach A, Hugh Perry V, Teeling JL. Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain. Behav. Immun. 2012;26:754–765. doi: 10.1016/j.bbi.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefendehl JK, Neher JJ, Sühs RB, Kohsaka S, Skodras A, Jucker M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell. 2014;13:60–69. doi: 10.1111/acel.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain. Behav. Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol. Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. doi:S0197-4580(07)00176-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GE, Van Nieuwenhuijzen PS, Chan-Ling T, McGregor IS. “When an old rat smells a cat”: A decline in defense-related, but not accessory olfactory, Fos expression in aged rats. Neurobiol. Aging. 2011;32:737–749. doi: 10.1016/j.neurobiolaging.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Xiao Y, Riefkohl PC, Young LJ. Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm. Behav. 2017;87:16–24. doi: 10.1016/j.yhbeh.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 2017;76:87–98. doi: 10.1016/j.neubiorev.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal–microglial cross-talk during aging, stress and inflammation. Exp. Neurol. 2012;233:40–48. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A. Physiology of microglia. Physiol.Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: New Roles for the Synaptic Stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Khan AM, Babcock Aa, Saeed H, Myhre CL, Kassem M, Finsen B. Telomere dysfunction reduces microglial numbers without fully inducing an aging phenotype. Neurobiol. Aging. 2015;36:1–12. doi: 10.1016/j.neurobiolaging.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Klaus F, Paterna J-C, Marzorati E, Sigrist H, Götze L, Schwendener S, Bergamini G, Jehli E, Azzinnari D, Fuertig R, Fontana A, Seifritz E, Pryce CR. Differential effects of peripheral and brain tumor necrosis factor on inflammation, sickness, emotional behavior and memory in mice. Brain. Behav. Immun. 2016;58:310–326. doi: 10.1016/j.bbi.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Kilby C, Bucko P, Rhodes JS. Effects of Minocycline on Spatial Learning, Hippocampal Neurogenesis and Microglia in Aged and Adult Mice. Behav. Brain Res. 2013;242:17–24. doi: 10.1111/j.1600-6143.2008.02497.x.Plasma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Holsboer F, Pittman Q. Interleukin-1beta stimulates both central and peripheral release of vasopressin and oxytocin in the rat. Eur. J. Neurosci. 1995;7:592–598. doi: 10.1111/j.1460-9568.1995.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-W. [DOI] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neurosci. 2015;21:306–321. doi: 10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–35. doi: 10.1016/S0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci. Biobehav. Rev. 2017;74:366–375. doi: 10.1016/j.neubiorev.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x.Microglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor Specificity of Central Neuroendocrine Responses : Implications for Stress-Related Disorders. Endocr. Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Perkins AE, Doremus-Fitzwater TL, Spencer RL, Varlinskaya EI, Conti MM, Bishop C, Deak T. A working model for the assessment of disruptions in social behavior among aged rats: The role of sex differences, social recognition, and sensorimotor processes. Exp. Gerontol. 2016;76:46–57. doi: 10.1016/j.exger.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AE, Woodruff ER, Chun LE, Spencer RL, Varlinskaya E, Deak T. Analysis of c-Fos induction in response to social interaction in male and female Fisher 344 rats. Brain Res. 2017;1672:113–121. doi: 10.1016/j.brainres.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Rebola N, Carta M, Mulle C. Operation and plasticity of hippocampal CA3 circuits: Implications for memory encoding. Nat. Rev. Neurosci. 2017;18:209–221. doi: 10.1038/nrn.2017.10. [DOI] [PubMed] [Google Scholar]
- Salchner P, Lubec G, Singewald N. Decreased social interaction in aged rats may not reflect changes in anxiety-related behaviour. Behav. Brain Res. 2004;151:1–8. doi: 10.1016/j.bbr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Salter MW, Beggs S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012;120:948–963. doi: 10.1016/j.surg.2006.10.010.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-couple phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014.Microglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit and altered inflammatory profiled. Glia. 2007;55:412–424. doi: 10.1002/glia. [DOI] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex Differences in Social Interaction in Rats: Role of the Immediate-Early Gene zif268. Neuropsychopharmacology. 2010;35:570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AK, Petersen AO, Gardi J, Gundersen HJG, Pakkenberg B. Spatial distribution of human neocortical neurons and glial cells according to sex and age measured by the saucer method. J. Neurosci. Methods. 2007;164:19–26. doi: 10.1016/j.jneumeth.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Sternberg EM, Glowa JR, Smith MA, Cologero AE, Listwak SJ, Aksentijevich S, Chrousos GP, Wilder RL, Gold PW. Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Res. 1992;570:54–60. doi: 10.1016/0006-8993(92)90563-O. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: Participation of dendritic cells and T-cells. Neurobiol. Aging. 2007;28:1507–1521. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic Microglia in the Aging Human Brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Tonelli L, Webster JI, Rapp KL, Sternberg E. Neuroendocrine responses regulating susceptibility and resistance to autoimmune/inflammatory disease in inbred rat strains. Immunol. Rev. 2001;184:203–211. doi: 10.1034/j.1600-065x.2001.1840118.x. [DOI] [PubMed] [Google Scholar]
- Tremblay M-È. The role of microglia at synapses in the healthy CNS: novel insights from recent imaging studies. Neuron Glia Biol. 2011;7:67–76. doi: 10.1017/S1740925X12000038. [DOI] [PubMed] [Google Scholar]
- Tremblay M-È, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012;60:541–558. doi: 10.1002/glia.22287.Effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, Spear LP. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol. 2014;45:433–444. doi: 10.1016/j.pestbp.2011.02.012.Investigations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LC, Lima CM, Oliveira Ma, Borges RP, Cardoso TT, Almeida INF, Diniz DG, Bento-Torres J, Pereira a, Batista-de-Oliveira M, Lopes aaC, Silva RFM, Abadie-Guedes R, Amâncio dos Santos a, Lima DSC, Vasconcelos PFC, Cunningham C, Guedes RCa, Picanço-Diniz CW. Litter size, age-related memory impairments, and microglial changes in rat dentate gyrus: Stereological analysis and three dimensional morphometry. Neuroscience. 2013;238:280–296. doi: 10.1016/j.neuroscience.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Villanueva-Castillo C, Tecuatl C, Herrera-López G, Galván EJ. Aging-related impairments of hippocampal mossy fibers synapses on CA3 pyramidal cells. Neurobiol. Aging. 2017;49:119–137. doi: 10.1016/j.neurobiolaging.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-Induced Recruitment of Bone Marrow-Derived Monocytes to the Brain Promotes Anxiety-Like Behavior. J. Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, De Beer MC, De Villiers WJS, Finch CE. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neurosci. Lett. 2005;390:76–80. doi: 10.1016/j.neulet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav. Immun. 2010;24:1190–1201. doi: 10.1016/j.surg.2006.10.010.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YF, Zhai F, Dai CF, Hu JJ. The relationship between age-related hearing loss and synaptic changes in the hippocampus of C57BL/6J mice. Exp. Gerontol. 2011;46:716–722. doi: 10.1016/j.exger.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]