Abstract
Despite production having stopped in the 1970s, polychlorinated biphenyls (PCBs) represent persistent organic pollutants that continue to pose a serious human health risk. Exposure to PCBs has been linked to chronic inflammatory diseases, such as cardiovascular disease, type 2 diabetes, obesity, as well as hepatic disorders, endocrine dysfunction, neurological deficits and many others. This is further complicated by PCB’s strong hydrophobicity, resulting in their ability to accumulate up the food chain and to be stored in fat deposits. This means that completely avoiding exposure is not possible, thus requiring the need to develop intervention strategies that can mitigate disease risks associated with exposure to PCBs. Currently, there is excitement in the use of nutritional compounds as a way of inhibiting the inflammation associated with PCBs, yet the suboptimal delivery and pharmacology of these compounds may not be sufficient in more acute exposures. In this review, we discuss the current state of knowledge of PCB toxicity and some of the antioxidant and anti-inflammatory nanocarrier systems that may be useful as an enhanced treatment modality for reducing PCB toxicity.
Keywords: Antioxidant, nanocarriers, PCBs, Toxicity
1. Polychlorinated biphenyls – history and health implications
Persistent organic environmental pollutants, such as polychlorinated biphenyls (PCBs), are a global health concern due to their negative impact on human health and the ecosystem. This negative impact largely hinges upon pathologies that include oxidative stress and inflammation at their root, and are linked to numerous non-communicable diseases. Therefore, this presents an interesting paradigm in which PCB-induced toxicity may be decreased through specific therapies that target oxidative stress and inflammatory pathways.
PCBs are a toxic class of chlorinated aromatic compounds that have been and continue to be an issue in environmental health since their introduction in the 1920s [1, 2]. At least 1.3 million tons of PCBs, comprising about 130 identified individual congeners, were manufactured worldwide prior to their banning [3]. PCB production was global with PCB mixtures being manufactured in the United States and overseas under different brand names including Aroclors (USA), Clophens (Germany), Phenoclors and Pyralenes (France), Fenclors (Italy), Fenochlors (Spain), Kanechlors (Japan) and Sovol (former USSR) [4]. Although PCBs were first synthesized in Germany during the late nineteenth century, commercial PCB production only started in 1929 with large scale production initiating in 1945, and were primarily used for industrial purposes, including as an insulator for capacitors and transformers due to their chemical and thermal stability [5].
PCB production ended in the United States (as part of the Toxic Substances Control Act in 1979) and western Europe during the 1970s but continued in eastern Europe until the 1990s and worldwide production was stopped after the Stockholm Convention in 2001. Currently, there is no known PCB production; however, PCBs may still be formed inadvertently during industrial processes such as pigment production [6]. Moreover, PCB pollution and contamination is worldwide with incidences of PCB exposure and detection in humans and the environment reported in numerous countries across the globe such as Belgium, Italy, Spain, Slovakia, Russia, Kenya, Egypt and Japan to name a few [7–11]. However, their resistance to chemical and thermal degradation translates to bioaccumulation in humans and marine life. Specifically, after sequestering in riverbeds and other hydrophobic environments, PCBs are consumed by bottom-dwelling organisms and are often not degraded, leading to biomagnification along trophic levels of the food chain and increased PCB concentrations and toxicity in animals and humans.
2. Polychlorinated biphenyl congeners and chemistry
PCBs are lipophilic compounds that still persist in the environment to this day, despite efforts of remediation and excavation. Due to their lipophilicity, PCBs are found in hydrophobic environments such as soil and riverbeds, and eventually bioaccumulate in adipose tissue of living organisms. However, the ability to sequester within humans and exert toxicity also depends on the physical structure and geometry of PCBs, which is largely dictated by their degree of chlorination.
Depending on the number and position of chlorine atom substitutions, there are 209 possible PCB congeners, all of which were manufactured commercially. This degree of chlorination has a significant impact on PCB exposure, metabolism, and toxicity. Specifically, PCBs are metabolized via hydroxylation by cytochrome P450 enzymes. However, lower chlorine-containing congeners (mono and di substituted) have a higher propensity to be metabolized and excreted, while higher chlorine-containing congeners are less extensively metabolized and thus are more likely to be sequestered and display toxicity [12].
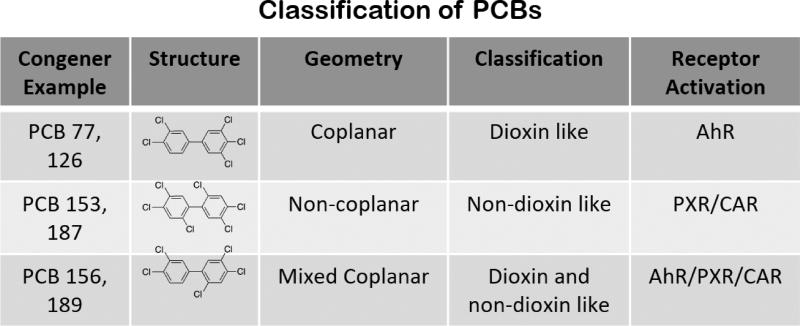
Structurally, PCBs are grouped into two major classes: non-coplanar PCBs and coplanar PCBs (Fig. 1). Non-coplanar PCB chlorine substitution is either ortho- or di-ortho- substituted, leading to steric pressure and a distorted geometry, and are described as “non-dioxin like PCBs,” while coplanar PCB chlorine substitution lacks ortho- substitution, leading to a planar structure and a “dioxin-like” classification [13]. Some mono-ortho PCBs appear to possess both “non-dioxin like” as well as “dioxin-like” properties and are referred to as mixed congeners [14]. Notably, some PCBs (mixed congeners), with characteristics of both coplanar and non-coplanar congeners, have affinity to the pregnane-xenobiotic receptor (PXR) and constitutive androstane receptor (CAR), as well as the AhR, giving rise to pathologies such as non-alcoholic steatohepatitis (NASH), obesity, and diabetes [15]. While mechanisms of action of both non-coplanar and coplanar PCBs can result in inflammation, oxidative stress, and toxicity, non-coplanar PCBs tend to exert their toxic properties via endocrine disruption, neurotoxicity, and an immunotoxicity-related mechanism, whereas coplanar PCBs display toxicity via an aryl hydrocarbon receptor (AhR)-dependent mechanism.
Figure 1.
In addition, PCBs were commercially manufactured as mixtures of congeners and not as a single entity. In North America, Monsanto Corporation produced and marketed these mixtures under the brand name “Aroclor”. Older generation Aroclors such as Aroclor 1260 contained approximately 60% chlorine content by weight and were found to be toxic to occupational workers. They were later replaced by newer generation Aroclors such as 1016 that possessed lower chlorine content [16]. As such, the exact composition of contaminated sites can vary greatly, resulting in a varied health risk potential that these sites pose.
3. Exposure routes and disease risks
Historically, PCB exposure in humans were either occupational or accidental, however the most relevant route of PCB exposure today is through ingestion of PCB-contaminated food and water. In fact, studies have shown that PCBs are present in fish globally, which results in human exposure via the oral route [12, 17–19]. This chronic, low concentration exposure results in inflammation and toxicity, as well as the development and progression of chronic inflammatory diseases, such as obesity, cardiovascular disease, various cancers such as liver, stomach, intestinal, and thyroid cancers, as well as non-Hodgkin lymphoma, and diabetes. In contrast, acute toxicity has also been the subject of investigation, due to symptoms of skin lesions and immunocompromised individuals, resulting from immunosuppression [20–26].
Overall, PCB toxicity is a critical and central issue in environmental and human health, and it is an issue without a specific therapy. Interestingly, healthful nutrition, such as those enriched in polyphenols and antioxidants has been found to decrease the oxidative stress and inflammation induced via PCB exposure [24]. However, therapy through the consumption of diet-derived bioactive compounds with antioxidants and anti-inflammatory properties has limitations that include a lack of bioavailability and stability [27]. These limitations may be overcome through employment of nanotherapy options that specifically treat PCB-induced oxidative stress and inflammation through targeted, controlled, and effective delivery of antioxidants and other protective bioactive compounds.
3.1 PCB exposure: Acute and chronic disorders
Health disorders associated with PCB exposure can be acute or chronic depending on the dose, duration of exposure, type of congener and degree of chlorination. Acute disorders occur as a result of accidental or occupational exposures that are associated with high doses and take place over a short period of time. Acute disorders were reported in accidental PCB poisonings such as the Yusho disease in 1968 that affected about 14,000 people in Japan and the Yu-cheng disease in 1979 that affected approximately 2,000 people in Taiwan [28–31]. During those incidences, mass PCB poisoning occurred in people consuming rice bran oil that was previously contaminated with PCBs and polychlorinated dibenzofurans (PCDFs) during production. Common symptoms included dermal and ocular effects such as chloracne, skin rashes and ocular lesions, irregular menstrual cycle and carcinogenesis [32–34]. High dose PCB exposure in occupational populations that worked at PCB manufacturing plants or dealt with PCB-containing equipment reportedly resulted in elevated liver enzymes levels consequently leading to hepatic effects, dermal effects such as chloracne characterized by acne-like eruptions on the face frequently on the cheeks and behind the ears, respiratory problems and cancer [35–38]. Although these may be considered acute effects, due to their severity and sudden onset, they persist over time considering that most PCB congeners are resistant to bio-degradation and can bioaccumulate. In fact, follow up studies on persons that had high dose PCB exposure demonstrated that these cohorts suffered from a range of health disorders including malignant neoplasms of the liver and stomach, as well as neoplasia in lymphatic and hematopoietic tissues affecting the immune response. Onset of diseases due to PCB exposure are also found in multiple organ systems such as the cardiovascular and circulatory system, gastrointestinal and digestive system, musculoskeletal and connective tissue systems and include neurological, cognitive development and reproductive defects [32, 39, 40].
Chronic or long-term PCB exposure in humans can lead to health effects including disorders of the hepatic system, cardiovascular complications, endocrine dysfunction, reproductive and developmental abnormalities, neurological defects and effects on the immune system. These chronic effects are becoming increasingly relevant to human health research because they reflect the effects of exposure levels in the general population and are not restricted to electrical capacitor workers or victims of PCB poisonings. Furthermore, identifying a particular disease pathology and elucidating the mechanistic evidence that link PCB exposure to such disorders can prove challenging, given the span of chemicals pertaining to the human exposome. Nonetheless, evidence from epidemiologic studies suggest strong correlations of various disease risks with PCB exposure, and numerous experimental studies on animal models have validated such reported findings as discussed below.
PCB effects on the liver include liver cancer, non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) that are correlated with elevation of liver enzymes including gamma-glutamyltransferase (GGT), alanine transaminase (ALT) and aspartate transaminase (AST). Studies on human populations have shown positive associations between serum PCB concentrations and elevated liver enzymes, indicative of liver injury [41–43]. Non-coplanar PCBs such as PCB 153 and Aroclor mixtures such as Aroclor 1260 that are heavily non-coplanar in composition have been shown to worsen NAFLD and promote hepatic inflammation in the presence of diet-induced obesity [16, 44]. Coplanar PCBs such as PCB 126 have also been shown to promote fatty liver or steatosis similar to other dioxin-like chemicals [45]. Additionally, studies by the National Toxicology Program (NTP) and other groups have shown that both PCB 153 and PCB 126 can induce liver cancer in rats [46, 47]. The liver functions as a site for both xenobiotic metabolism and maintenance of energy homeostasis through regulation of multiple key metabolic pathways. Since PCBs are xenobiotics, the liver becomes the first target organ of PCB toxicity provided that PCB exposure occurs through the oral route. Given the liver’s role in metabolic regulation, hepatic dysfunction caused by PCB exposure can escalate into associated metabolic disorders such as insulin resistance and diabetes, obesity, dyslipidemia and the metabolic syndrome [48–50].
PCB effects on the cardiovascular system include associations of PCBs with incidences of hypertension in residents of Anniston, Alabama where the Monsanto Corporation plant was located, as well as in a PCB-exposed Spanish cohort and in the NHANES population [51–53]. However, PCB effects on the cardiovascular system encompasses other complications such as myocardial infarction, stroke and vascular injury [54, 55]. Rodent studies have also reported PCB-induced vascular inflammation and injury, consequently leading to undesirable cardiovascular aftermaths such as inflamed vascular pathology indicative of endothelial cell dysfunction and atherosclerosis [56]. Interestingly, PCBs indirectly act as a risk factor for cardiovascular diseases through hepatic disrupting effects such as increased synthesis of cholesterol and triglycerides and dyslipidemia [57]. Indeed, NAFLD/NASH has been proposed as a risk factor for cardiovascular disease, further adding complexity to PCB-induced multi-organ toxicity.
PCB impact on the endocrine system is majorly perturbed thyroid function due to reduced circulating thyroid hormone levels with PCB exposure, eventually affecting thyroid hormone-associated neurodevelopment and neurological defects [58–60]. PCBs are also viewed as endocrine disruptors with another relevant endocrine effect being association with type 2 diabetes mellitus due to PCB toxicity on pancreatic beta cells and compromised insulin secretion [61, 62]. Other PCB effects include alterations in immunological responses particularly deficient immune function in humans [63] and demonstrated immunosuppression in rodent studies [64] and adverse effects in the reproductive system [65, 66].
4. Mechanisms of toxicity: Metabolic pathways and oxidative stress
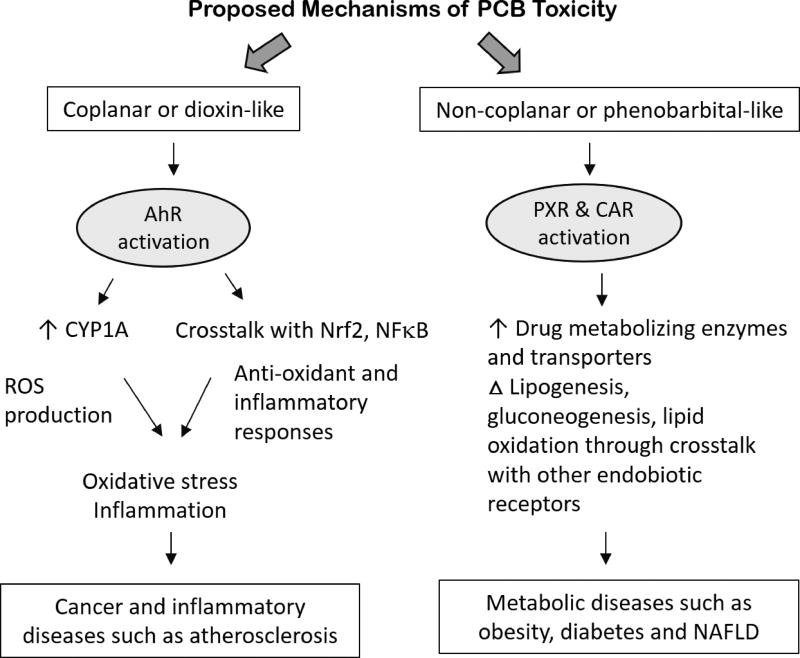
The well-studied mechanisms of PCB toxicity depending on their structural classification is demonstrated in Fig. 2. For most of the observable effects, major PCB-mechanisms of action have been attributed to AhR-activation and upregulation of AhR target genes that precedes unwanted effects such as inflammatory response and oxidative stress. The AhR is a ligand-activated transcription factor primarily expressed in the liver but also found in extra-hepatic tissues. PCB binding to this hepatic receptor causes its nuclear translocation and induction of xenobiotic genes such as cytochrome P450 enzymes such as CYP1A1 and CYP1A2 [67]. However, AhR activation is not restricted to xenobiotic metabolism, but it also plays an important role in many developmental pathways, such as hematopoiesis and differentiation. Hence, its activation is also linked to cell cycle pathways, cell proliferation and cancer [68]. There are also cross-talks between AhR activation and inflammation-related pathways such as the nuclear factor kappa B (NFκB) and nuclear factor (erythroid-derived 2) -like 2 (Nrf2) activation pathways; hence PCB activation of the AhR can result in upregulation of inflammatory cytokines, including interleukins (IL-6, IL-2) and tumor necrosis factor alpha (TNFα), formation of reactive oxygen species (ROS), and induction of antioxidant enzymes [69–71]. The imbalance that ensues due to the amount of ROS produced and the body’s inability to eliminate such reactive intermediates results in oxidative stress and tissue damage. Additional mechanistic studies have shown that PCBs can induce inflammation and oxidative stress through epigenetic modification [72, 73].
Figure 2.
Coplanar PCBs often referred to as “dioxin-like” PCBs elicit their toxicity through AhR induction; however, multiple non-coplanar PCBs are “phenobarbital-like” and activate other hepatic, xenobiotic receptors namely the pregnane-xenobiotic receptor (PXR) and the constitutive androstane receptor (CAR) [13, 15]. Outcomes of CAR and PXR activation are associated with metabolic effects such as NAFLD/NASH, dysregulated energy metabolism and obesity. Nonetheless, and because PCBs occur as mixtures of both congener classes, outcomes of PCB exposure therefore can result in pro-inflammatory and cardio-metabolic disorders. Notably, other mechanisms of PCB-induced toxicity include microRNA alterations, interference with epidermal growth factor receptor (EGFR) signaling and direct effects on vascular endothelium that can promote oxidative stress responses [24, 74, 75].
5. Antioxidant therapy as a possible pathway to combat PCB derived toxicity
Given the critical role that chronic oxidative stress plays in PCB toxicity, it is reasonable to assume that treatments, which can directly counter this pathology, could be a potential antidote to PCB exposure. Since oxidative stress is a state of excess production of oxidants/ROS/free radicals, increasing the antioxidant concentration via external supplementation could counter excess ROS production, and thereby alleviate the oxidative stress condition. Indeed, to maintain red/ox balance, dietary antioxidants, either as a part of fruits and vegetable consumption or vitamin supplementation, have been considered a part of a healthy life style. For example, long term and moderate intake of red wine, a source of resveratrol, has been linked to decreased cardiovascular and cerebrovascular ischemic occurrence [76]. Yet, despite these intended goals, to date, clinical studies evaluating the use of dietary antioxidants for chronic disorders, including diabetes, cardiovascular diseases and neurological as well, have failed to demonstrate significant benefits. For example, antioxidants, such as curcumin, have failed to show significant protective effects against Alzheimer’s disease in a clinical trial with oral intake of the antioxidant over 48 weeks, although pre-clinical trials showed protection in neurodegenerative disorders in mice or rat model [77–80]. Nevertheless, longitudinal and observational human studies support the health promoting benefits of diet-derived bioactive compounds that have antioxidant and/or anti-inflammatory properties [81–83]. As such, along with proper nutrition, there remains a need for treatments that can provide interventional therapy for environmental toxic exposure. To understand the source of the inconsistency between observational studies and clinical trials and identify potential solutions, a brief overview of cellular oxidative stress and a summary of strategies attempted to date are presented.
5.1 Antioxidant action pathway in the vascular endothelium
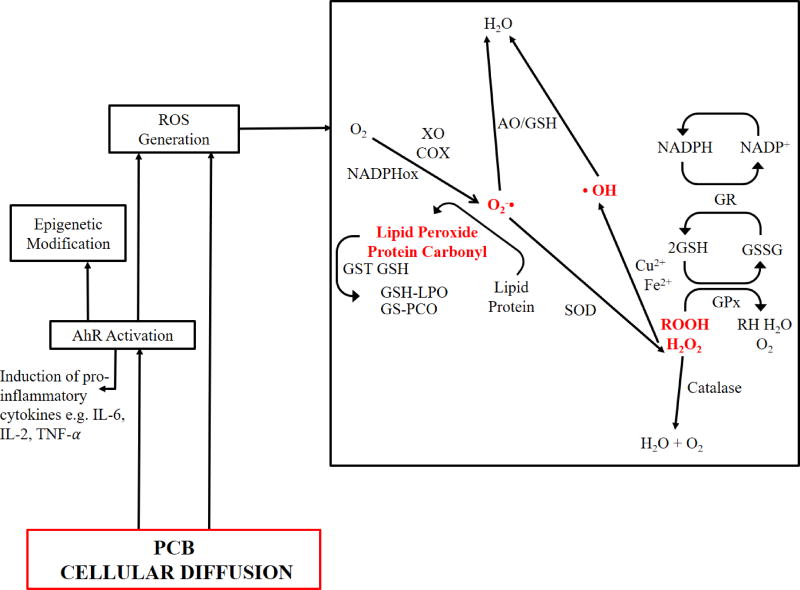
During cellular oxidative stress, especially vascular oxidative stress, a host of cellular pathways are activated that directly result in the enzymatic formation of ROS and reactive nitrogen species (RNS), (Fig. 3). The terms ROS/RNS are general terms used for the reactive species derived from oxygen or nitrogen atoms. As such, it is difficult to imagine a scenario where all of the species formed result in a unified physiological outcome. However, given their short half-lives, it is still challenging to decouple the specific action of individual ROS/RNS molecules in each pathway. In the case of PCB induced toxicity, uncoupling cytochrome P450 1A1 (CYP1A1) and activation of AhR is thought to play a major role of ROS generation, which in turn results in the excess production of free radicals, leading to chronic inflammation and disease [84, 85]. Another mechanism common to oxidative stress induction that has been identified in PCB toxicity is the formation of superoxide anions via the NADPH oxidase enzyme that results in the oxidation of NADPH to NADP+ [86]. Within the cytosol, this superoxide anion is subsequently converted to hydrogen peroxide in the presence of superoxide dismutase (SOD). In turn, hydrogen peroxide in the presence of transition metal ions (Cu, Fe) can form the highly reactive, hydroxyl radicals [87].
Figure 3.
Yet, despite these radical generating mechanisms, cells also possess endogenous antioxidant defense systems to counteract ROS production. For instance, hydrogen peroxide can be “inactivated” by enzymes, including catalase, which converts hydrogen peroxide into water and oxygen [88, 89], and the peroxiredoxins that can reduce peroxide molecules [90]. In reducing peroxides, the peroxiredoxins oxidize glutathione (GSH) into the oxidized disulfide, GSSG [91]. Glutathione is a highly abundant cytosolic molecule that serves to maintain a reducing environment in the cytosol. The level of glutathione is maintained by the conversion of GSSG back to GSH, catalyzed by glutathione reductase. This natural cellular redox cycle continues until disrupted by external factors, leading to accumulation of ROS and oxidized lipids, proteins and DNA, which can stimulate inflammation, apoptosis and cell death.
Based upon these pathways, antioxidant therapy could potentially serve as an intervention for PCB induced oxidative stress related toxicity. It has been theorized and published multiple times that external supplementation of antioxidants (i.e., enzymes, synthetic antioxidant or natural nutritional antioxidants) can counter the excess radical production, restoring natural cellular function. These externally supplemented antioxidants can act in multiple ways, 1) through the downregulation of ROS generating enzymes and upregulating the natural antioxidant defense system, 2) by metal ion chelation, reducing the production of hydroxyl radicals and/or 3) direct scavenging of radical electrons, thereby inactivating ROS [92, 93]. The following section summarizes some of the attempts at controlling oxidative stress through antioxidants and the observations or limitations noted.
5.2 Current antioxidant therapies
5.2.1 Direct Antioxidant Treatment
Antioxidants can be broadly divided into two main categories: antioxidant enzymes and small molecule antioxidants. Antioxidant enzymes have the advantage of high specificity of action. Some of the most common antioxidant enzymes for oxidative stress defense are superoxide dismutase (SOD), catalase, glutathione peroxidase, glutathione reductase, and peroxiredoxins. SOD is capable of converting superoxide radical to hydrogen peroxide (H2O2). H2O2 can further be reduced to water and oxygen via catalase action. Therefore, combined therapy of SOD/ catalase might be suitable for suppression of ROS derived oxidative stress. Glutathione peroxidase helps in detoxification of H2O2, hydroperoxides, lipid peroxides, by utilizing the glutathione redox cycle. Through this high specificity, tailored approaches to treatment can be obtained. In addition, these enzymes function through a catalytic pathway, allowing for multiple copies of ROS (often millions of copies) to be scavenged for each enzyme molecule introduced, leading to a highly potent function. However, antioxidant enzymes are not without their drawbacks, including limited stability in vivo, the challenge to formulate delivery strategies, and the requirement of parenteral routes of administration.
Alternatively, small molecule antioxidants typically have a broad mechanism of action, as they are able to scavenge a variety of ROS/RNS, and potentially provide a robust protection strategy. There are numerous small molecule antioxidants, typically derived from dietary sources, that have been studied for suppression of cellular oxidative stress or used for clinical and pre-clinical testing of vascular oxidative stress, including natural dietary antioxidants like vitamin C and E. Curcumin, quercetin, epigallocatechin gallate (EGCG), from the flavonoid class of molecules, are characterized by the presence of at least one phenolic group in their molecular structure, and also have been found to mitigate cellular oxidative stress [94].
For instance, curcumin, a yellow pigment from curcumin longa, with two phenoxy groups, has been shown to have anti-inflammatory properties and possess an antioxidant potential of about 2.5–2.8 times more potent than Trolox, a common reference standard [95]. Because of its free radical scavenging properties, it has been shown to suppress induced oxidative stress in various in vitro models [96]. It is also known to show its anti-inflammatory effect through inhibition of clooxygenase-2 (COX-2), lipoxygenase (LOX), and inducible nitric oxide synthase (iNOS). The upregulation of these enzymes has been associated with the pathogenesis of tumor progression and other inflammatory disorders [97]. Curcumin in in vitro models has shown to possess a protective effect against diabetes. For instance, curcumin treatment slows the formation of advanced glycation end products and to inhibit activation of hepatic stellate cells, which is linked to hyperglycemia in diabetic cases [98]. In the case of tumor cell growth, Perry et al. showed that administration of curcumin to athymic mice, xenografted with glioma U-87 cells resulted in slower tumor growth subcutaneously and in the intracerebral regions, inhibiting the glioma induced angiogenesis [99]. In another study conducted by Niu et al., 2µM curcumin was able to achieve 80% cell death of melanoma A375 cells, when coupled with light photosensitization, as opposed to only 20% with curcumin alone [100].
Another polyphenolic flavonoid, quercetin, has been shown to be highly effective at suppressing cellular oxidative stress. In a study conducted with retinal pigment epithelial cells (RPE), quercetin demonstrated a dose dependent protective effect against hydrogen peroxide generated cellular oxidative stress, showing its potential towards treatment of retinal degeneration [101, 102]. Damage of RPE cells is associated with age related macular degeneration resulting in loss of vision and oxidative stress, which is considered to be one of the major underlying cause for its pathogenesis. In the case of PCB induced toxicity, quercetin has also been used in vitro and in vivo to suppress toxicity, suggesting a promising approach to therapeutic use. For instance, during in vitro studies with vascular endothelial cells, quercetin inhibited PCB 77 induced CYP1A1 expression and suppressed with AhR activation, which is responsible for excess ROS production [85]. Quercetin was also shown to inhibit PCB induced phosphorylation of caveolin-1, which plays a significant role in pathogenesis of atherosclerosis [103],[104]. In another study conducted by Selvakumar et. al., quercetin was shown to have a neuroprotective effect against PCB induced oxidative stress and hippocampal apoptosis in rats, where decreased levels hydrogen peroxide, lipid peroxidation and protein carbonyl content were experienced 30 days after the simultaneous treatment of quercetin along with Aroclor 1254 as compared to only Aroclor exposure [105]. Although, quercetin is a direct scavenger of ROS/RNS and in vitro studies have shown this action at treatment concentrations of 5–50 µM [106], it is found to be only in nanomolar concentrations in the brain upon administration. This concentration is not likely sufficient to show neuroprotective effect by direct antioxidant action, but is rather acting as a pro-oxidant creating a mild oxidative stress state to stimulate cellular antioxidant defense mechanisms [107–109]. One of the proposed pathways of such stimulation self-defense is activation Nrf2-ARE (nuclear factor (erythroid-derived 2)-like 2- antioxidant response elements) pathway, which controls the various proteins including heme-oxygenase-1, GPX, SOD etc. [110, 111]. Induction of PON2, (a gene from paroxonase family, known to exert antioxidant effect) is another suggested mechanism of self-antioxidant stimulation upon quercetin exposure to neuronal cells at low concentrations [112–114].
Apart from flavonoids, carotenoids have also been explored showing protective effect on RPE cells, acting as blue light filters and ROS intermediate quenchers, help suppressing oxidative stress [115]. The alpha-tocopherol form of Vitamin E has also shown superior antioxidant properties, demonstrating the benefits of vitamin E supplementation towards cardiovascular diseases. For example, dietary supplementation of Vitamin E (specifically α-tocopherol form) significantly reduced aortic lesions in mice over 4–10 weeks, reduced restenosis after angioplasty in rabbits [116]. Table 1 lists some antioxidants and possible mechanism of action pathways and functions.
Table 1.
Known antioxidants/ antioxidant enzymes and their mechanism of action towards suppression of oxidative stress
| Antioxidants | Mechanism of action/ functions |
|---|---|
| Enzymes | |
| Superoxide dismutase (CuZn-SOD, Mn-SOD found in mitochondria | |
| Catalase |
|
| Glutathione peroxidase | |
| Thioredoxin reductase (bronchial, alveolar epithelium) |
|
| Glutathione peroxidase (GPX) |
|
| Glutathione s-transferase | |
| Non-Enzymatic Systemic Antioxidants | |
| Vitamin E (α-tocopherol) (cell membrane-bound antioxidant) |
|
| Vitamin C (ascorbic acid) (water soluble) | |
| Glutathione (abundant in cell compartments) |
|
| Retinoic acid | |
| Melatonin (N-acetyl-5-methoxytryptamine) (hormone in animals, algae) | |
| Dietary Antioxidants | |
| Carotenoids (β-carotene) (pigment found in plants) | |
| Epigallocatechin gallate (EGCG) | |
| Quercetin |
|
| Curcumin | |
| Resveratrol |
|
| Synthetic antioxidant molecules | |
| N-acetyl cysteine (glutathione precursor) | |
| Trolox |
|
| MitoQ |
|
5.2.2 Known limitations of antioxidant therapy and oxidative stress management
Despite much of the promise of antioxidant therapy, there has been limited clinical evidence to suggest that oral antioxidant delivery can be an effective treatment for oxidative stress related diseases. Indeed, recent reports have continued to emerge as highly critical of this approach. At the core of all these findings, the data suggests that poor pharmacology prevents these compounds from functioning as hypothesized. For instance, many antioxidant compounds are plagued by short half-lives due to fast renal clearance, inactivation of activity before reaching the site of treatment as a result of first pass metabolism, poor solubility, lack of natural accumulation at sites of interest and hepatic uptake. The bioavailability, site of absorption (gastro-intestinal tract or intestines), chemical metabolite formation (glycosides, esters etc) and antioxidant capacity of polyphenols are different from each other [145, 146]. During metabolism, polyphenols serve as substrates for several enzymes in the small intestine and colon, resulting in their biotransformation through esterase, glucosidase, decarboxylation, and demethylation [147, 148]. Hence, the therapeutic activity of the antioxidant polyphenols is also dictated by their metabolic state and not only the plasma concentration. Resveratrol, with anti-microbial and anti-inflammatory properties, has reduced bioavailability upon oral administration due to its metabolization into glucuronides and sulfates [149]. Curcumin bioavailability is reduced by several factors including its instability at neutral and alkaline pH, inability to permeate through the intestinal lumen to the blood stream, and its rapid conversion into metabolites within the body. Some of these curcumin metabolite forms such as tetra-, and hexahydrocurcumin show significant antioxidant activity, while other forms of sulfo- or octahydrocurcumin have shown minimal to no anti-inflammatory properties. Hence, it becomes important which form of metabolite is formed upon administration [95, 150–152].
PCB induced toxicity and vascular oxidative stress often go hand-in-hand and there is in vitro evidence showing the protective effect of antioxidants like quercetin and EGCG against PCB 77 induced inflammation. In addition, vitamin C and E have been reported to be protective against oxidative stress caused by PCB mixtures[153]. These examples of antioxidant protection are usually successful in in vitro models but in vivo administration of antioxidants often fail to be effective due to their fragile structural properties under systemic environment. As such, in order to determine if these antioxidant compounds can provide an effective treatment against PCB toxicity, it becomes important to control the method of delivery to ensure intact compounds reach their intended site of action for the required duration of action.
5.3 Antioxidant Therapy through Payloads for Controlled and Effective Delivery
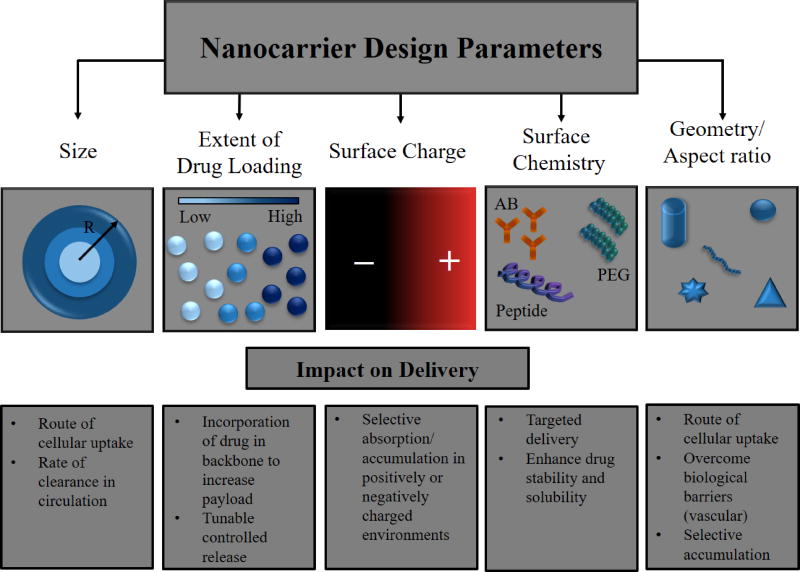
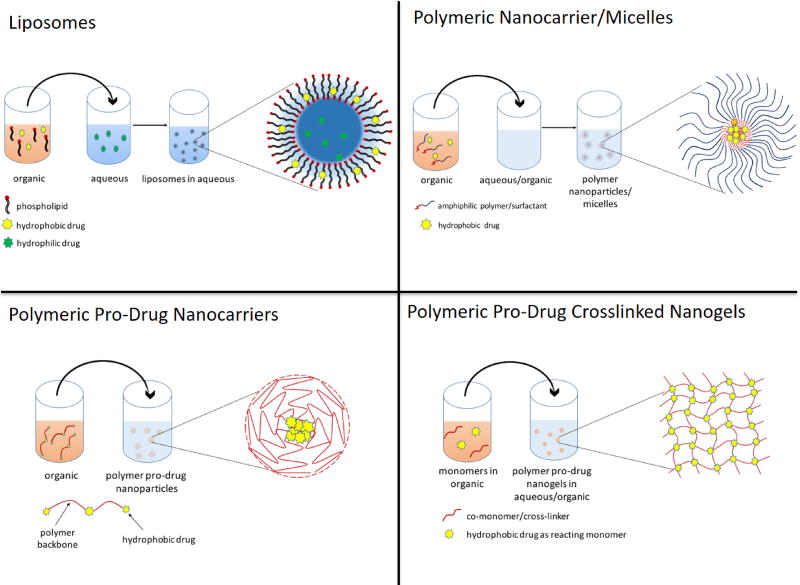
As antioxidants possess promising effective properties, but also possess significant pharmacological limitations, they represent ideal candidates for nanocarrier drug delivery systems. Figure 4 illustrates some of the design factors in nanocarrier design for drug delivery systems and their synthesis processes. Nanoparticles have been considered for many years as a way of modifying the inherent pharmacokinetics of the drug, by stabilizing the compound, enhancing accumulation at sites of action, controlling release and thereby widening the duration of efficacy [154]. The advantages of these systems are their physiochemical properties, including surface chemistry, charge, size and shape, dictate the ultimate fate of the loaded drug (Fig. 4, Fig. 5). For instance, the size of nanocarriers determines the ultimate transport in the circulatory and transcellular systems, with 30–100 nm diameter particles being effective for transcellular and pericellular transport; 50–500 nm particle systems are ideal for long bloodstream circulation [155]; 200–800 nm are best for targeting of sinusoidal cells in liver and spleen [156], while greater than 5 µm being used for organ delivery via mechanical retention [157]. However, these trends are also related to the surface chemistry of materials as well. While nanocarriers in the 50–500nm can provide long circulation, this is only possible when the surface of the particles are of neutral charge and highly hydrophilic, as is the case of poly(ethylene glycol) PEG coated particles [158–160]. When hydrophobic particles in this size range are i.v. injected, they are rapidly cleared by the hepatic system, which provides a unique passively targeted system for fast hepatic uptake, serving as an effective treatment for acute liver disorders [161, 162]. Alternatively, long circulating particles of sizes that can pass through leaky vasculature can result in local particle accumulation, a process that known as the enhanced permeation and retention (EPR) effect [163]. In addition to these considerations, one must also be mindful of the drug loading and associated release rate. To date, there have been many systems pursued to enhance the delivery and efficacy of antioxidant systems, including, liposomal encapsulation systems, polymeric nanoparticles (encapsulated or polymeric pro-drug forms), magnetic nanocarriers, polyplex complexes, and exosomes. In this section, a review of these approaches is provided along with discussion of the potential impact these systems could have on PCB related toxicity.
Figure 4.
Figure 5.
5.3.1 Liposomes
Liposomes are spherical vesicles comprising of one or more phospholipid bilayers, containing an internal aqueous core that is isolated from the external aqueous space. They are artificially synthesized analogs of natural cellular vesicles, including endosomes and exosomes. They are well known for their biocompatibility, low toxicity, and bioabsorbable nature [164]. The major advantage of such systems lies in their ability to load both hydrophilic and lipophilic drugs, due to presence of aqueous core and lipid bilayer in their structure [165]. With its versatile properties, many liposomal systems have been designed with the aim to encapsulate unstable, fragile drugs to get long circulation times and increased bioavailability. They can be internalized by cells via lipid bilayer fusion and are able to facilitate delivery by diffusion or temperature dependent unfolding of the vesicles. Hence, liposomes have been explored extensively for delivery of small molecule antioxidants and antioxidant enzymes to the organs and tissues with oxidative insults.
N-acetylcysteine (NAC), curcumin, quercetin, resveratrol, alpha-tocopherol (Vitamin E), coenzyme Q, enzymes such as GSH, SOD, and catalase are several antioxidants and antioxidant enzymes delivered through liposomes [166]. Liposomal NAC has shown protection against liver injury in a rat sepsis model and better potency against acute respiratory-distress in a rat model [167–169]. While combination of NAC with tocopherol showed protection against mustard gas induced lung damage in guinea pigs [170]. In another study by Alipour et. al., liposomal NAC was able to inhibit acetaminophen induced hepatotoxicity in rats [171].
In the flavonoids category, the use of liposomal curcumin has shown to increase curcumin plasma concentration upon oral administration [172], which demonstrated better inhibition of pancreatic carcinoma cell growth and anticancer properties both in vitro and in vivo [164, 173–176]. In a similar manner, in a pre-clinical study, liposomal quercetin formulations have also shown protective effects against carbon tetrachloride induced hepatotoxicity [177]. Quercetin loaded liposome delivery also helped reduce the peroxynitrite induced myocardial injury [178], while PEGylated quercetin liposomes enhanced anti-tumor activity towards lung and colon cancer in mice [179]. These PEGylated quercetin liposomes not only increased the half-life of quercetin from a few minutes to 2 hours, they also demonstrated higher accumulation and retention in organs.
As a therapy against environmental toxicity, quercetin has also been explored in fighting oxidative stress due to environmental toxicants such as arsenic, which is normally found in contaminated water. In a study conducted by Ghosh et al., quercetin encapsulated liposomes were to inhibit arsenic toxicity by preventing hepatic oxidative stress, decreased arsenic accumulation in liver while free quercetin administration failed to show any significant effect upon sodium arsenite exposure [180]. Resveratrol is another flavonoid that has been loaded into liposomal systems, and has shown the ability to inhibit vascular intimal thickening in rats upon intraperitoneal injection [181], while combination of curcumin-resveratrol encapsulated liposomal delivery showed highest antioxidant serum concentration, longest retention time and demonstrated reduced prostate cancer incidence in mice [182]. These studies strongly suggest the impact antioxidant nanocarriers could have as an intervention to PCB exposure.
In the case of antioxidant enzymes, SOD and catalase encapsulated liposomes resulted in 90% of the survival of newborn rat pups upon exposure to lethal 95% oxygen exposure, while placebo treated newborns resulted in 60% mortality [183]. CuZn-SOD loaded liposomes also showed their protective effect against cerebral ischemia-reperfusion injury in rats. Longer circulation times and ability to cross the blood brain barrier aided longer retention and higher accumulation of CuZn-SOD (upto 2 hours) via liposomal delivery when compared with CuZn-SOD delivered in its free form (30 minutes of retention time). [184, 185]. Another observation made was that enzyme activity was always higher in the injured brain than in the non-injured control at any given time-point, suggesting the activation of natural antioxidant enzyme defense mechanisms upon sufficient antioxidant delivery. In the case of cardiac disorders, one of the pre-clinical trials conducted by Laursen et al. demonstrated that the delivery of SOD resulted in the reduction of blood pressure by 50 mmHg in angiotensin II induced hypertension and enhanced hypotensive response to acetylcholine [186]. Liposome-encapsulated SOD has also demonstrated enhanced efficacy in topical application in a pre-clinical study, where a reduction in post-burn edema and wound size was observed due to enhanced antioxidant effect of SOD against neutrophil mediated injury [187]. In a clinical trial, Cu/Zn SOD-liposome systems showed regression in radiation induced fibrosis by the third week of treatment [188]. They have also shown complete regression with the prophylactic action, in the cases where fibrosis development was certain [189] .
5.3.2 Polymeric nanocarriers
Liposomes were one of the first nanocarrier systems to have been developed, with initial research dating back to the 1960s. Since then, other nanocarrier systems have been explored, with polymer nanoparticles representing one of the most versatile and promising systems in terms of controlled release, tunable shape, and the shear variety of chemistries available, all relying on the mechanism of bulk degradation and/or diffusion for drug release, tuned most commonly by pH and temperature. Most of these systems have focused on the formulation and design of biodegradable systems that provide sustainable and controlled release of fragile small molecule antioxidants or enzymes.
Poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) nanospheres for antioxidant encapsulation have been studied extensively for controlled delivery. PLA/PLGA is a biodegradable polymer that breaks down into lactic acid and glycolic acid and has been FDA approved for various implantable and injectable drug delivery applications. Antioxidant enzyme PLGA encapsulated microspheres have been formulated as a means of overcoming the short-lived stability issues associated with enzyme delivery, especially where delivered enzymes ultimately are shuttled to the lysosomes, as is the case with delivery of SOD or catalase [190]. Quercetin loaded nanocarriers composed of PLA backbone have been shown to reduce arsenic induced oxidative stress and associated gene expression in liver. In fact, the PLA carriers were found to be more effective at reducing oxidative stress and inhibiting fibrosis compared to liposomal quercetin administration [180]. Curcumin loaded into PLGA nanoparticles was also able to reduce arsenic toxicity in a rat model through the inhibition of oxidative damage in rat kidney and brain. A significant reduction in the levels of lipid peroxides and a revival of the natural antioxidant enzyme defense system were some of the important observations made with curcumin nanoparticles [191]. Co-enzyme Q10 (CoQ10) loaded PLGA nanoparticles administered orally have also demonstrated enhanced ejection fraction over the span of three months in rats induced with myocardial ischemia [192]. In another pre-clinical study with renal hypertensive rat model, CoQ10 encapsulated PLGA nanoparticles showed improved blood pressure (30 and 15 mmHg decrease in systolic and diastolic pressure respectively) at 60% lower dosage frequency than the rats treated with just CoQ10 suspension (23 and 10mmHg pressure reduction) [193] Yet, one limitation of PLA/PLGA is the potential local accumulation of acid byproducts that can lead to a decrease in pH, resulting in acidic protein inactivation and local inflammation [194]. As an alternative, polyketal polymers have also been studied. The advantage of polyketal polymers is that their degradation products are neutral, forming diols and acetone. Polyketal SOD microspheres were shown to alleviate muscular ischemic reperfusion injury and bleomycin induced pulmonary injury upon intratracheal administration. These microparticle systems, in a rat model, also showed sustained scavenging of excess superoxide oxide production after ischemia-reperfusion injury up to at least 3 days after infarction [195, 196].
While these microsphere systems were able to show some activity locally, in order to promote systemic delivery, catalase was loaded into PEG-PLA nanoparticles in the size range of 200–300 nm. These carrier systems demonstrated the ability to possess prolonged activity for over 24 hours, even when residing in proteolytic environments, such as lysosomes [197]. Further exploring the PEG chemistry in nanoparticle formulation, chung et al. formulated micellar nanocomplexes comprising PEG-EGCG-herceptin shell and core system. The combination of antioxidant EGCG and anticancer protein Herceptin in these micellar systems demonstrated better tumor selectivity, growth reduction and longer half-life of Herceptin than the direct treatment in mice [198].
Most of these nanoparticle systems are synthesized through a simple single or double emulsion technique, where a solution of polymer and antioxidant are placed into an organic solvent, which is then added to an aqueous solution, allowing the organic solvent to either evaporate or extract out, leaving behind nanoparticles. This physically loaded system typically results in low drug loading efficiency, and a high burst release once purified and placed back into an aqueous environment [199–202]. An alternative method that has been developed is to conjugate the drug to the polymer or to actually polymerize the antioxidant into a degradable polymeric form. The idea is derived from the growing market of pro-drugs of pharmaceutically active yet structurally fragile drugs, where any selected functional group of the drug molecule is modified to protect from deactivation and upon systemic administration, are converted back to the original compound [203]. Carboxylic, hydroxyl, amino, carbonyl groups present in active drug molecules are a few groups explored for transformation into esters, carbonates, carbamates, amides, phosphates and oximes [204]. Ester forms are one of the most common used form of pro-drugs due to its ability to be hydrolyzed systemically into an active molecule [205, 206].
Based on this concept, pro-drug polymeric nanocarriers have also been synthesized and explored for antioxidant delivery. Since most of the small molecule antioxidant possess one or more hydroxy or thiol groups, they can be functionalized and formulated into polyester, polyanhydride or poly(β-amino ester). For example, Wattamwar et al. were able to polymerize trolox (synthetic analogue of Vitamin E) into poly(trolox ester) via Steglich esterification[207]. Nanoparticles composed of poly(trolox ester) were formed via a single emulsion nanoprecipitation method, resulting in particles of 200 nm. Importantly, these particles were able protect pulmonary microvascular endothelial cells against nanocobalt toxicity, suppressing ROS formation [207, 208]. In addition to these findings, the particles possessed a unique ability to inhibit protein oxidation, an effect that was not observed with the native antioxidant, emphasizing the importance of the mode of delivery and how it can augment the observed outcome.
Poly(beta-amino ester) (PBAE) chemistry was further employed by our group for the conjugation of quercetin and curcumin into crosslinked polymers films, microparticles, nanoparticles or nanogel systems [209]. The single-phase reaction-precipitation method was versatile enough to render nanogels in the range of 50 to 800 nm, with uniform release over 24–36 hours by bulk hydrolytic degradation. Both curcumin and quercetin conjugated systems demonstrated protection against induced oxidative stress in endothelial cells [210, 211]. Curcumin PBAE nanogels were specifically explored for their action on mitochondrial oxidative stress, since mitochondria are considered to be a major source of ROS production once triggered. It was shown that free curcumin at low dosage did not show any potential towards OS while at higher concentration resulted in pro-oxidant damage. Importantly, curcumin PBAE nanogels demonstrated an overall higher TC50 value of 100 µg/ml as compared to free curcumin (TC50 5 µg/ml) and was able to effectively suppressed mitochondrial oxidative stress over 24 hours. This widening of the therapeutic window for curcumin represents a critical feature and benefit of nanocarrier delivery.
5.3.3 Targeted Antioxidant Nanocarriers
As mentioned, PCB exposure can lead to acute liver, skin, ocular inflammation and induce/ aid carcinogenesis, while its bioaccumulation in multiple organs results in chronic effects leading to cardiovascular disease and the development of carcinogenic malignancies. While antioxidant nanocarriers have shown benefit in liver organ injury, as nanoparticles do not naturally accumulate in the vascular endothelium, a targeting method must be employed in the nanocarrier design. By coating nanoparticles in affinity molecules directed against cell surface epitopes of the vascular endothelium, it becomes possible to turn antioxidant nanoparticles into vascular specific treatments. One such example is coupling the stealth properties of catalase encapsulated into biotinylated PEG-PLGA polymer nanocarrier with streptavidin modified PECAM-1 antibody. These immunotargeted nanoparticles resulted in 12% of the injected dose binding to the pulmonary vasculature, as compared to only 2% with the non-targeted (IgG coated) nanoparticles. Additionally, by loading the enzyme within the nanocarrier, it was able to elicit its function for at least 20 hours against oxidative stress in endothelial cells, where unloaded enzyme resulted in only a 3 hour function duration due to lysosomal trafficking and protein degradation [212]. In another study, nanoparticles synthesized via interaction of tocopherol phosphate and manganese porphyrin SOD-mimetic, with controlled release profile was conjugated with anti-PECAM, which demonstrated endothelial targeting and reduced expression of pro-inflammatory VCAM, E-selectin, IL-8 [213]. Excitingly, this group demonstrated SOD/catalase loaded anti-PECAM coated nanoparticles were able to accumulate up to 30% of the injected dose in the mouse pulmonary endothelium. Interestingly catalase targeted particles reduced pulmonary edema and leukocyte infiltration after exposure to endotoxin induced lung injury, while SOD loaded particles alleviated the associated lung inflammation [214]. In a preclinical study with mice, it was demonstrated that SOD-loaded, NR-1 PEGylated liposomes, polybutylcyanoacrylate (PBCA), as well as PLGA nanoparticles, showed protection against cerebral ischemia-reperfusion injury, due to localized accumulation in the hippocampus and significantly reduced the observed infarct volume [215]. Surface modifying poly(trolox ester) nanoparticles with anti-PECAM-1 was also able to target endothelial cells. These targeted particles were able to suppress iron oxide induced oxidative stress better than non-targeted, IgG-coated nanoparticles [216]. In case of curcumin, about 100 nm sized nanoparticles conjugated with poly(butylcyanocylate), poly(lactide-co-glycolide), chitosan, albumin or acrylamide polymers have also shown higher peak serum levels and hence enhanced bioavailability [217–219].
5.3.4 Routes of administration and features of acute vs chronic care
A final consideration to be made in the use of antioxidant therapies is that of the route of administration. Given their size and use, most studies have focused upon i.v. injection of nanocarrier systems. Yet, such intervention strategies are likely to be limited to acute and sub-acute exposures, as prolonged i.v. administration is highly undesirable. As such, exploration of alternative delivery methods, including inhalation, intratracheal, intraperitoneal, and topical administration are needed. All of these methods have been tried for the delivery of antioxidant in free form. Clinical trials employing oral delivery of antioxidants such as curcumin in free form have been conducted several times, with variable dosage towards suppression of oxidative stress induced inflammation. Curcumin as such has shown to reduce the inflammation in chronic diseases upon long term daily dosage such as in case of diabetes, by inhibiting the progression of type 2 diabetes. But, oral administration comes with very low bio-distribution of the antioxidant, because of which even doses as high as 4–8 g per day resulted in maximum of 3.6 µM after 1 hour [220]. Intraperitoneal administration on the other hand increased the curcumin plasma levels 10 fold as compared to oral route [221], while curcumin liposomes administered intraperitoneally to obese mice showed improved insulin resistance [222].
Antioxidant encapsulated nanoparticles, comprising resveratrol, resveratrol-curcumin co-delivery, quercetin, and CoQ10 have been explored for skin disorders, with the goal of treating UV radiation induced oxidative stress and skin cancer. Indeed, resveratrol loaded solid lipid nanoparticles showed rapid diffusion and retention in cell membranes [223] There has also been some evidence showing effectiveness of antioxidant NPs towards acute renal failure, where CoQ10 encapsulated NPs when administered intravenously helped in reducing the blood pressure of mice induced with renovascular hypertension [224]. In another example, intratracheally administered SOD/catalase loaded liposomes resulted in protection from acute lung injury by increasing the antioxidant activity of alveolar type II cells [225, 226]. Aerosolization of liposomal encapsulated Cu/Zn SOD has also demonstrated long systemic circulation of antioxidant enzymes [226]. Yet, despite these studies, there still remains a significant need for the evaluation of the impact alternative routes of administration have nanocarrier therapeutic potential.
6. Conclusions
Chronic exposure of environmental pollutants remains a significant health concern. Even now, PCBs pose a continuous threat to the health and safety of our population. As a result, we need a wide array of tools and strategies to counteract these potential risks. While effective and healthful nutrition is likely to be a major player in our strategies to minimize health hazards, as seen by clinical trials of antioxidant interventions, it is unlikely that nutrition alone is enough to treat or prevent all PCB exposure-induced disorders. As such, strategies that can reduce body burden, enhance antioxidant delivery to target cells, and capture PCBs before entering the body can potentially be used to provide defense against PCB toxicity. Furthermore, we know from other treatments, such as NAC for acetaminophen toxicity, where antioxidant therapy can be an effective antidote. In order to enhance antioxidant therapy, strategies for effectively delivering antioxidants, such as nanocarriers, are likely required. Further studies for ideal candidates will be needed to best assess which compounds will be most effective at countering the toxicity of co-planar and non-coplanar PCBs. Finally, while studies with injectable nanocarriers provide some promising results, such routes of administration are not likely acceptable for chronic delivery systems. Thus, research into inhalation, intranasal, buccal or oral drug delivery systems are likely to be a fruitful area of research to determine if they can provide the needed exposure to treat PCB toxicity.
Table 2.
Antioxidant nanocarriers and drug delivery applications
| Antioxidant | Payload/ drug delivery system | Application |
|---|---|---|
|
| ||
| Resveratrol | Targeted solid lipid nanoparticles with Apolipoprotein E, 150–200 nm | In vitro: controlled release, higher permeability across blood brain barrier, hCMEC/D3 cells [227] |
|
| ||
| Curcumin | Curcusomes (curcumin liposomal nanoparticles) | In vivo: improved peripheral insulin resistance in genetically obese and high fat diet obese mice [228] |
|
| ||
| Hepatocyte-targeted galactosylated chitosan-polycaprolactone curcumin nanoparticles | In vitro: 6-fold increase in apoptosis and necrosis of cancerous HepG2 cells as compared to curcumin [229] | |
|
| ||
| Curcumin loaded nanoparticle hydrogels, 140 nm | In vivo: Aflatoxin B1-induced liver damage in rats [230] | |
|
| ||
| Poly(beta-amino ester) nanogels (50–400 nm) | In vitro: inhibition of hydrogen peroxide induced mitochondrial oxidative stress in endothelial cells [211] | |
|
| ||
| Curcumin Liposomes | Phase I clinical trial: Intravenous delivery safe up to 120mg/m2 in healthy subjects [231] | |
| Phase 1 clinical trial: intravenous infusion in patients with locally advanced or metastatic cancer to analyze the dose safety, on-going [232] | ||
|
| ||
| (−)-Epigallocatechin-3-O-gallate (EGCG) | EGCG-folic acid0PEG nanoparticles, 140 – 180 nm | In vitro: inhibition of MCF-7 cell proliferation [233] |
|
| ||
| Bioactive peptide/chitosan-EGCG encapsulated nanoparticle for oral delivery, 150nm | In vitro: increased permeability in Caco-2 cells for Nano chemoprevention via oral route [234] | |
|
| ||
| Quercetin | Hyaluronic acid-quercetin complexed micelles | In vitro: increased cytotoxicity inMCF-7 cell in vivo: inhibition of tumor growth in H22 tumor-bearing mice [235] |
|
| ||
| PEGylated Poly (beta amino ester) nanogels (250–600 nm) | In vitro: decreased hydrogen peroxide induced toxicity in HUVECs cells [210] | |
|
| ||
| N-acetyl cysteine | Pro-drug polymeric nanoparticles conjugated through disulfide bond | Inhibition of activation of microglia stimulated by lipopolysaccharide [236] |
Footnotes
Conflict of Interest Disclosure
All authors declare they have no conflicts of interest.
References
- 1.Grimm FA, et al. Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol. 2015;45(3):245–72. doi: 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond ML, et al. Estimation of PCB stocks, emissions, and urban fate: will our policies reduce concentrations and exposure? Environ Sci Technol. 2010;44(8):2777–83. doi: 10.1021/es9012036. [DOI] [PubMed] [Google Scholar]
- 3.Breivik K, et al. Towards a global historical emission inventory for selected PCB congeners--a mass balance approach. 2. Emissions. Sci Total Environ. 2002;290(1–3):199–224. doi: 10.1016/s0048-9697(01)01076-2. [DOI] [PubMed] [Google Scholar]
- 4.Giesy JP, Kannan K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Crit Rev Toxicol. 1998;28(6):511–69. doi: 10.1080/10408449891344263. [DOI] [PubMed] [Google Scholar]
- 5.Salhotra AM. Human health risk assessment for contaminated properties. Prog Mol Biol Transl Sci. 2012;112:285–306. doi: 10.1016/B978-0-12-415813-9.00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Vorkamp K. An overlooked environmental issue? A review of the inadvertent formation of PCB-11 and other PCB congeners and their occurrence in consumer products and in the environment. Sci Total Environ. 2016;541:1463–1476. doi: 10.1016/j.scitotenv.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Barakat AO, Khairy M, Aukaily I. Persistent organochlorine pesticide and PCB residues in surface sediments of Lake Qarun, a protected area of Egypt. Chemosphere. 2013;90(9):2467–76. doi: 10.1016/j.chemosphere.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Covaci A, et al. The Belgian PCB/dioxin crisis-8 years later An overview. Environ Toxicol Pharmacol. 2008;25(2):164–70. doi: 10.1016/j.etap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Jepson PD, et al. PCB pollution continues to impact populations of orcas and other dolphins in European waters. Sci Rep. 2016;6:18573. doi: 10.1038/srep18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wimmerova S, et al. The spatial distribution of human exposure to PCBs around a former production site in Slovakia. Environ Sci Pollut Res Int. 2015;22(19):14405–15. doi: 10.1007/s11356-015-5047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zani C, et al. Polychlorinated biphenyls and cancer: an epidemiological assessment. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2013;31(2):99–144. doi: 10.1080/10590501.2013.782174. [DOI] [PubMed] [Google Scholar]
- 12.Uekusa Y, et al. Determination of polychlorinated biphenyls in marine fish obtained from tsunami-stricken areas of Japan. PLoS One. 2017;12(4):e0174961. doi: 10.1371/journal.pone.0174961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wahlang B, et al. Human receptor activation by aroclor 1260, a polychlorinated biphenyl mixture. Toxicol Sci. 2014;140(2):283–97. doi: 10.1093/toxsci/kfu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environmental Health Perspectives. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahlang B, et al. Polychlorinated Biphenyl-Xenobiotic Nuclear Receptor Interactions Regulate Energy Metabolism, Behavior, and Inflammation in Non-alcoholic-Steatohepatitis. Toxicol Sci. 2016;149(2):396–410. doi: 10.1093/toxsci/kfv250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahlang B, et al. Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol. 2014;279(3):380–90. doi: 10.1016/j.taap.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batang ZB, et al. Congener-specific levels and patterns of polychlorinated biphenyls in edible fish tissue from the central Red Sea coast of Saudi Arabia. Sci Total Environ. 2016;572:915–925. doi: 10.1016/j.scitotenv.2016.07.207. [DOI] [PubMed] [Google Scholar]
- 18.Byrne S, et al. Persistent Organochlorine Pesticide Exposure Related to a Formerly Used Defense Site on St. Lawrence Island, Alaska: Data from Sentinel Fish and Human Sera. J Toxicol Environ Health A. 2015;78(15):976–92. doi: 10.1080/15287394.2015.1037412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorber M, et al. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ Int. 2015;77:55–62. doi: 10.1016/j.envint.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Codru N, et al. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ Health Perspect. 2007;115(10):1442–7. doi: 10.1289/ehp.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goncharov A, et al. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res. 2008;106(2):226–39. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik S, et al. Effect of proximity to hazardous waste sites on the development of congenital heart disease. Arch Environ Health. 2004;59(4):177–81. doi: 10.3200/AEOH.59.4.177-181. [DOI] [PubMed] [Google Scholar]
- 23.Pellequer JL, et al. Structural basis for preferential binding of non-ortho-substituted polychlorinated biphenyls by the monoclonal antibody S2B1. J Mol Recognit. 2005;18(4):282–94. doi: 10.1002/jmr.740. [DOI] [PubMed] [Google Scholar]
- 24.Petriello MC, et al. PCB 126 toxicity is modulated by cross-talk between caveolae and Nrf2 signaling. Toxicol Appl Pharmacol. 2014;277(2):192–9. doi: 10.1016/j.taap.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sergeev AV, Carpenter DO. Hospitalization rates for coronary heart disease in relation to residence near areas contaminated with persistent organic pollutants and other pollutants. Environ Health Perspect. 2005;113(6):756–61. doi: 10.1289/ehp.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverstone AE, et al. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect. 2012;120(5):727–32. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, et al. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25(4):363–76. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuratsune M, et al. Yusho, a poisoning caused by rice oil contaminated with polychlorinated biphenyls. HSMHA Health Rep. 1971;86(12):1083–91. [PMC free article] [PubMed] [Google Scholar]
- 29.Kuratsune M, et al. Epidemiologic study on Yusho, a Poisoning Caused by Ingestion of Rice Oil Contaminated with a Commercial Brand of Polychlorinated Biphenyls. Environ Health Perspect. 1972;1:119–28. doi: 10.1289/ehp.7201119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda Y, et al. PCB and PCDF congeners in the blood and tissues of yusho and yu-cheng patients. Environ Health Perspect. 1985;59:53–8. doi: 10.1289/ehp.59-1568081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seki Y, Kawanishi S, Sano S. Mechanism of PCB-induced porphyria and yusho disease. Ann N Y Acad Sci. 1987;514:222–34. doi: 10.1111/j.1749-6632.1987.tb48777.x. [DOI] [PubMed] [Google Scholar]
- 32.Masuda Y. [Toxic effects of PCB/PCDF to human observed in Yusho and other poisonings] Fukuoka Igaku Zasshi. 2009;100(5):141–55. [PubMed] [Google Scholar]
- 33.Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters--what we have learned from Yusho disease. Environ Res. 2001;86(1):2–11. doi: 10.1006/enrs.2001.4244. [DOI] [PubMed] [Google Scholar]
- 34.Hsu ST, et al. Discovery and epidemiology of PCB poisoning in Taiwan: a four-year followup. Environ Health Perspect. 1985;59:5–10. doi: 10.1289/ehp.59-1568088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loomis D, et al. Cancer mortality among electric utility workers exposed to polychlorinated biphenyls. Occup Environ Med. 1997;54(10):720–8. doi: 10.1136/oem.54.10.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreiss K. Studies on populations exposed to polychlorinated biphenyls. Environ Health Perspect. 1985;60:193–9. doi: 10.1289/ehp.8560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimbrough RD, et al. Mortality among capacitor workers exposed to polychlorinated biphenyls (PCBs), a long-term update. Int Arch Occup Environ Health. 2015;88(1):85–101. doi: 10.1007/s00420-014-0940-y. [DOI] [PubMed] [Google Scholar]
- 38.Maroni M, et al. Occupational exposure to polychlorinated biphenyls in electrical workers. II. Health effects. Br J Ind Med. 1981;38(1):55–60. doi: 10.1136/oem.38.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li MC, et al. Mortality after exposure to polychlorinated biphenyls and dibenzofurans: 30 years after the “Yucheng accident”. Environ Res. 2013;120:71–5. doi: 10.1016/j.envres.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh SF, et al. A cohort study on mortality and exposure to polychlorinated biphenyls. Arch Environ Health. 1996;51(6):417–24. doi: 10.1080/00039896.1996.9936040. [DOI] [PubMed] [Google Scholar]
- 41.Cave M, et al. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect. 2010;118(12):1735–42. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sala M, et al. Association between serum concentrations of hexachlorobenzene and polychlorobiphenyls with thyroid hormone and liver enzymes in a sample of the general population. Occup Environ Med. 2001;58(3):172–7. doi: 10.1136/oem.58.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wahlang B, et al. Toxicant-associated steatohepatitis. Toxicol Pathol. 2013;41(2):343–60. doi: 10.1177/0192623312468517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahlang B, et al. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem. 2013;24(9):1587–95. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai IK, et al. N-acetylcysteine (NAC) diminishes the severity of PCB 126-induced fatty liver in male rodents. Toxicology. 2012;302(1):25–33. doi: 10.1016/j.tox.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rignall B, et al. Biological and tumor-promoting effects of dioxin-like and non-dioxin-like polychlorinated biphenyls in mouse liver after single or combined treatment. Toxicol Sci. 2013;133(1):29–41. doi: 10.1093/toxsci/kft034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Toxicology, P. Toxicology and carcinogenesis studies of a binary mixture of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) (Cas No. 57465-28-8) and 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (gavage studies) Natl Toxicol Program Tech Rep Ser. 2006;(530):1–258. [PubMed] [Google Scholar]
- 48.Zhang S, et al. Chronic Exposure to Aroclor 1254 Disrupts Glucose Homeostasis in Male Mice via Inhibition of the Insulin Receptor Signal Pathway. Environ Sci Technol. 2015;49(16):10084–92. doi: 10.1021/acs.est.5b01597. [DOI] [PubMed] [Google Scholar]
- 49.Chapados NA, Boucher MP. Liver metabolic disruption induced after a single exposure to PCB126 in rats. Environ Sci Pollut Res Int. 2017;24(2):1854–1861. doi: 10.1007/s11356-016-7939-8. [DOI] [PubMed] [Google Scholar]
- 50.Gadupudi GS, et al. PCB126-Induced Disruption in Gluconeogenesis and Fatty Acid Oxidation Precedes Fatty Liver in Male Rats. Toxicol Sci. 2016;149(1):98–110. doi: 10.1093/toxsci/kfv215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goncharov A, et al. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J Hypertens. 2010;28(10):2053–60. doi: 10.1097/HJH.0b013e32833c5f3e. [DOI] [PubMed] [Google Scholar]
- 52.Everett CJ, et al. Association of polychlorinated biphenyls with hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res. 2008;108(1):94–7. doi: 10.1016/j.envres.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Donat-Vargas C, et al. Association between dietary intake of polychlorinated biphenyls and the incidence of hypertension in a Spanish cohort: the Seguimiento Universidad de Navarra project. Hypertension. 2015;65(4):714–21. doi: 10.1161/HYPERTENSIONAHA.114.04435. [DOI] [PubMed] [Google Scholar]
- 54.Bergkvist C, et al. Dietary exposure to polychlorinated biphenyls and risk of myocardial infarction in men - A population-based prospective cohort study. Environ Int. 2016;88:9–14. doi: 10.1016/j.envint.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 55.Bergkvist C, et al. Dietary exposure to polychlorinated biphenyls is associated with increased risk of stroke in women. J Intern Med. 2014;276(3):248–59. doi: 10.1111/joim.12194. [DOI] [PubMed] [Google Scholar]
- 56.Perkins JT, et al. Polychlorinated biphenyls and links to cardiovascular disease. Environ Sci Pollut Res Int. 2016;23(3):2160–72. doi: 10.1007/s11356-015-4479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goncharov A, et al. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res. 2008;106(2):226–39. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osius N, et al. Exposure to polychlorinated biphenyls and levels of thyroid hormones in children. Environ Health Perspect. 1999;107(10):843–9. doi: 10.1289/ehp.99107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koopman-Esseboom C, et al. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics. 1996;97(5):700–6. [PubMed] [Google Scholar]
- 60.Parham F, et al. Adverse effects in risk assessment: modeling polychlorinated biphenyls and thyroid hormone disruption outcomes in animals and humans. Environ Res. 2012;116:74–84. doi: 10.1016/j.envres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rylander L, Rignell-Hydbom A, Hagmar L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ Health. 2005;4:28. doi: 10.1186/1476-069X-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crinnion WJ. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Altern Med Rev. 2011;16(1):5–13. [PubMed] [Google Scholar]
- 63.Ziegler S, et al. Accelerated telomere shortening in peripheral blood lymphocytes after occupational polychlorinated biphenyls exposure. Arch Toxicol. 2017;91(1):289–300. doi: 10.1007/s00204-016-1725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis D, Safe S. Immunosuppressive activities of polychlorinated biphenyls in C57BL/6N mice: structure-activity relationships as Ah receptor agonists and partial antagonists. Toxicology. 1990;63(1):97–111. doi: 10.1016/0300-483x(90)90072-o. [DOI] [PubMed] [Google Scholar]
- 65.Meeker JD, Hauser R. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst Biol Reprod Med. 2010;56(2):122–31. doi: 10.3109/19396360903443658. [DOI] [PubMed] [Google Scholar]
- 66.Tsuji M, et al. Polychlorinated biphenyls (PCBs) decrease the placental syncytiotrophoblast volume and increase Placental Growth Factor (PlGF) in the placenta of normal pregnancy. Placenta. 2013;34(7):619–23. doi: 10.1016/j.placenta.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Beischlag TV, et al. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–50. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindsey S, Papoutsakis ET. The evolving role of the aryl hydrocarbon receptor (AHR) in the normophysiology of hematopoiesis. Stem Cell Rev. 2012;8(4):1223–35. doi: 10.1007/s12015-012-9384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawal AO. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol Lett. 2017;270:88–95. doi: 10.1016/j.toxlet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Lara L, et al. Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J Neurosci Res. 2015;93(9):1423–33. doi: 10.1002/jnr.23595. [DOI] [PubMed] [Google Scholar]
- 71.Vogel CF, et al. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J Biol Chem. 2014;289(3):1866–75. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu D, Perkins JT, Hennig B. EGCG prevents PCB-126-induced endothelial cell inflammation via epigenetic modifications of NF-kappaB target genes in human endothelial cells. J Nutr Biochem. 2016;28:164–70. doi: 10.1016/j.jnutbio.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu D, et al. Exposure to coplanar PCBs induces endothelial cell inflammation through epigenetic regulation of NF-kappaB subunit p65. Toxicol Appl Pharmacol. 2015;289(3):457–65. doi: 10.1016/j.taap.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wahlang B, et al. Polychlorinated biphenyl exposure alters the expression profile of microRNAs associated with vascular diseases. Toxicol In Vitro. 2016;35:180–7. doi: 10.1016/j.tiv.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hardesty JE, et al. Polychlorinated Biphenyls Disrupt Hepatic Epidermal Growth Factor Receptor Signaling. Xenobiotica. 2016:1–40. doi: 10.1080/00498254.2016.1217572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saleem TSM, Basha SD. Red wine: A drink to your heart. Journal of Cardiovascular Disease Research. 2010;1(4):171–176. doi: 10.4103/0975-3583.74259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sood PK, Nahar U, Nehru B. Curcumin attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain. Neurotox Res. 2011;20(4):351–61. doi: 10.1007/s12640-011-9249-8. [DOI] [PubMed] [Google Scholar]
- 78.Tapia E, et al. Curcumin prevents maleate-induced nephrotoxicity: Relation to hemodynamic alterations, oxidative stress, mitochondrial oxygen consumption and activity of respiratory complex I. Free Radical Research. 2014;48(11):1342–1354. doi: 10.3109/10715762.2014.954109. [DOI] [PubMed] [Google Scholar]
- 79.Kuo JJ, et al. Positive effect of curcumin on inflammation and mitochondrial dysfunction in obese mice with liver steatosis. Int J Mol Med. 2012;30(3):673–9. doi: 10.3892/ijmm.2012.1049. [DOI] [PubMed] [Google Scholar]
- 80.Ringman J, et al. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Research & Therapy. 2012;4(5):43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leermakers ET, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):481–94. doi: 10.3945/ajcn.115.120931. [DOI] [PubMed] [Google Scholar]
- 82.Ng TP, et al. Dietary and supplemental antioxidant and anti-inflammatory nutrient intakes and pulmonary function. Public Health Nutr. 2014;17(9):2081–6. doi: 10.1017/S1368980013002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wood AD, et al. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr. 2014;112(8):1341–52. doi: 10.1017/S0007114514001962. [DOI] [PubMed] [Google Scholar]
- 84.De S, et al. PCB Congener Specific Oxidative Stress Response by Microarray Analysis using Human Liver Cell Line. Environment international. 2010;36(8):907–917. doi: 10.1016/j.envint.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramadass P, et al. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76(1):212–9. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- 86.Bandiera SM. Cytochrome P450 Enzymes as Biomarkers of PCB Exposure and Modulators of Toxicity. In: Larry LGH, Robertson W, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. The University Press of Kentucky; Lexington, KY: 2001. [Google Scholar]
- 87.Gutowski M, Kowalczyk S. A study of free radical chemistry: their role and pathophysiological significance. Acta Biochim Pol. 2013;60(1):1–16. [PubMed] [Google Scholar]
- 88.Scibior D, Czeczot H. Postepy Hig Med Dosw (Online) [Catalase: structure, properties, functions] 2006;60:170–80. [PubMed] [Google Scholar]
- 89.Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta. 1991;196(2–3):143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 90.Rhee SG, et al. Peroxiredoxin Functions as a Peroxidase and a Regulator and Sensor of Local Peroxides. Journal of Biological Chemistry. 2012;287(7):4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta P, Lakes A, Dziubla T. Oxidative Stress and Biomaterials. Academic Press; 2016. Chapter One - A Free Radical Primer; pp. 1–33. [Google Scholar]
- 92.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 93.Zhang YMC. Protective Effect of Quercetin on Aroclor 1254-Induced Oxidative Damage in Cultured Chicken Spermatogonial Cells. Toxicological Sciences. 2005;88(2):545–550. doi: 10.1093/toxsci/kfi333. [DOI] [PubMed] [Google Scholar]
- 94.Kris-Etherton PM, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. The American Journal of Medicine. 2002;113(9, Supplement 2):71–88. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 95.Somparn P, et al. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull. 2007;30(1):74–8. doi: 10.1248/bpb.30.74. [DOI] [PubMed] [Google Scholar]
- 96.Nakamura Y, et al. Inhibitory Effects of Curcumin and Tetrahydrocurcuminoids on the Tumor Promoter-induced Reactive Oxygen Species Generation in Leukocytes in vitro and in vivo. Japanese Journal of Cancer Research. 1998;89(4):361–370. doi: 10.1111/j.1349-7006.1998.tb00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–25. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 98.Lin J, et al. Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARγ activity and attenuating oxidative stress. British Journal of Pharmacology. 2012;166(8):2212–2227. doi: 10.1111/j.1476-5381.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perry MC, et al. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol Nutr Food Res. 2010;54(8):1192–201. doi: 10.1002/mnfr.200900277. [DOI] [PubMed] [Google Scholar]
- 100.Niu T, et al. Inhibition of Autophagy Enhances Curcumin United light irradiation-induced Oxidative Stress and Tumor Growth Suppression in Human Melanoma Cells. Scientific Reports. 2016;6:31383. doi: 10.1038/srep31383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beatty S, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 102.Kook D, et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008;49(4):1712–20. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- 103.Choi YJ, et al. Quercetin blocks caveolae-dependent proinflammatory responses induced by coplanar PCBs. Environment international. 2010;36(8):931–934. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.D’Alessio A, et al. Caveolae Participate in Tumor Necrosis Factor Receptor 1 Signaling and Internalization in a Human Endothelial Cell Line. The American Journal of Pathology. 2005;166(4):1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Selvakumar K, et al. Protective Role of Quercetin on PCBs-Induced Oxidative Stress and Apoptosis in Hippocampus of Adult Rats. Neurochemical Research. 2012;37(4):708–721. doi: 10.1007/s11064-011-0661-5. [DOI] [PubMed] [Google Scholar]
- 106.Saw CLL, et al. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food and Chemical Toxicology. 2014;72:303–311. doi: 10.1016/j.fct.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 107.Fraga CG, et al. Basic biochemical mechanisms behind the health benefits of polyphenols. Molecular Aspects of Medicine. 2010;31(6):435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 108.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Archives of Biochemistry and Biophysics. 2008;476(2):107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 109.Costa LG, et al. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Medicine and Cellular Longevity. 2016;2016:10. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shih AY, et al. Induction of the Nrf2-driven Antioxidant Response Confers Neuroprotection during Mitochondrial Stress in Vivo. Journal of Biological Chemistry. 2005;280(24):22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- 111.Gan L, Johnson JA. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014;1842(8):1208–1218. doi: 10.1016/j.bbadis.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 112.Giordano G, et al. Paraoxonase 2 (PON2) in the mouse central nervous system: A neuroprotective role? Toxicology and Applied Pharmacology. 2011;256(3):369–378. doi: 10.1016/j.taap.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Costa LG, et al. Modulation of Paraoxonase 2 (PON2) in Mouse Brain by the Polyphenol Quercetin: A Mechanism of Neuroprotection? Neurochemical Research. 2013;38(9):1809–1818. doi: 10.1007/s11064-013-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang Y-F, et al. Quercetin Induces Oxidative Stress and Potentiates the Apoptotic Action of 2-Methoxyestradiol in Human Hepatoma Cells. Nutrition and Cancer. 2009;61(5):735–745. doi: 10.1080/01635580902825571. [DOI] [PubMed] [Google Scholar]
- 115.Tokarz P, Kaarniranta K, Blasiak J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD) Biogerontology. 2013;14(5):461–482. doi: 10.1007/s10522-013-9463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–74. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 117.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167(12):1600–19. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 118.Kinnula VL. Production and degradation of oxygen metabolites during inflammatory states in the human lung. Curr Drug Targets Inflamm Allergy. 2005;4(4):465–70. doi: 10.2174/1568010054526368. [DOI] [PubMed] [Google Scholar]
- 119.Kirkman HN, et al. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J Biol Chem. 1999;274(20):13908–14. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]
- 120.Flohe L. Glutathione peroxidase. Basic Life Sci. 1988;49:663–8. doi: 10.1007/978-1-4684-5568-7_104. [DOI] [PubMed] [Google Scholar]
- 121.Comhair SA, et al. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. Faseb j. 2001;15(1):70–78. doi: 10.1096/fj.00-0085com. [DOI] [PubMed] [Google Scholar]
- 122.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radical Biology and Medicine. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 123.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57(13–14):1825–35. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ladner JE, et al. Parallel evolutionary pathways for glutathione transferases: structure and mechanism of the mitochondrial class kappa enzyme rGSTK1-1. Biochemistry. 2004;43(2):352–61. doi: 10.1021/bi035832z. [DOI] [PubMed] [Google Scholar]
- 125.Robinson A, et al. Modelling and bioinformatics studies of the human Kappa-class glutathione transferase predict a novel third glutathione transferase family with similarity to prokaryotic 2-hydroxychromene-2-carboxylate isomerases. Biochemical Journal. 2004;379(Pt 3):541–552. doi: 10.1042/BJ20031656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.White E, Shannon JS, Patterson RE. Relationship between vitamin and calcium supplement use and colon cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(10):769–74. [PubMed] [Google Scholar]
- 127.Bunker VW. Free radicals, antioxidants and ageing. Med Lab Sci. 1992;49(4):299–312. [PubMed] [Google Scholar]
- 128.Mezzetti A, et al. Systemic oxidative stress and its relationship with age and illness. Associazione Medica “Sabin”. J Am Geriatr Soc. 1996;44(7):823–7. doi: 10.1111/j.1532-5415.1996.tb03741.x. [DOI] [PubMed] [Google Scholar]
- 129.Masella R, et al. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16(10):577–86. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 130.Niles RM. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat Res. 2004;555(1–2):81–96. doi: 10.1016/j.mrfmmm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 131.Donato LJ, Noy N. Suppression of mammary carcinoma growth by retinoic acid: proapoptotic genes are targets for retinoic acid receptor and cellular retinoic acid-binding protein II signaling. Cancer Res. 2005;65(18):8193–9. doi: 10.1158/0008-5472.CAN-05-1177. [DOI] [PubMed] [Google Scholar]
- 132.Lobo V, et al. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reiter RJ, et al. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50(4):1129–46. [PubMed] [Google Scholar]
- 134.El-Agamey A, et al. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430(1):37–48. doi: 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 135.Rice-Evans CA, et al. Why do we expect carotenoids to be antioxidants in vivo? Free Radic Res. 1997;26(4):381–98. doi: 10.3109/10715769709097818. [DOI] [PubMed] [Google Scholar]
- 136.Fang MZ, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63(22):7563–70. [PubMed] [Google Scholar]
- 137.Fang JY, et al. Transdermal delivery of tea catechins and theophylline enhanced by terpenes: a mechanistic study. Biol Pharm Bull. 2007;30(2):343–9. doi: 10.1248/bpb.30.343. [DOI] [PubMed] [Google Scholar]
- 138.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2–3):325–37. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 139.Thangapazham RL, Sharma A, Maheshwari RK. Beneficial role of curcumin in skin diseases. Adv Exp Med Biol. 2007;595:343–57. doi: 10.1007/978-0-387-46401-5_15. [DOI] [PubMed] [Google Scholar]
- 140.Madhyastha R, et al. Curcumin facilitates fibrinolysis and cellular migration during wound healing by modulating urokinase plasminogen activator expression. Pathophysiol Haemost Thromb. 2010;37(2–4):59–66. doi: 10.1159/000321375. [DOI] [PubMed] [Google Scholar]
- 141.Stojanović S, Sprinz H, Brede O. Efficiency and Mechanism of the Antioxidant Action of trans-Resveratrol and Its Analogues in the Radical Liposome Oxidation. Archives of Biochemistry and Biophysics. 2001;391(1):79–89. doi: 10.1006/abbi.2001.2388. [DOI] [PubMed] [Google Scholar]
- 142.Aruoma OI, et al. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biology and Medicine. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 143.Dodd S, et al. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opinion on Biological Therapy. 2008;8(12):1955–1962. doi: 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- 144.Smith RAJ, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Annals of the New York Academy of Sciences. 2010;1201(1):96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 145.D’Archivio M, et al. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43(4):348–61. [PubMed] [Google Scholar]
- 146.Setchell KD, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C–labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77(2):411–9. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 147.Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med. 2004;36(7):829–37. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 148.Rechner AR, et al. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med. 2004;36(2):212–25. doi: 10.1016/j.freeradbiomed.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 149.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49(5):472–81. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 150.Okada K, et al. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr. 2001;131(8):2090–5. doi: 10.1093/jn/131.8.2090. [DOI] [PubMed] [Google Scholar]
- 151.Lai CS, et al. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol Nutr Food Res. 2011;55(12):1819–28. doi: 10.1002/mnfr.201100290. [DOI] [PubMed] [Google Scholar]
- 152.Ireson C, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–64. [PubMed] [Google Scholar]
- 153.Studies on the protective role of vitamin C and E against polychlorinated biphenyl (Aroclor 1254)—induced oxidative damage in Leydig cells. Free Radical Research. 2005;39(11):1259–1272. doi: 10.1080/10715760500308154. [DOI] [PubMed] [Google Scholar]
- 154.Dziubla TD, Muzykantov VR. Synthetic carriers for vascular delivery of protein therapeutics. Biotechnol Genet Eng Rev. 2006;22:267–98. doi: 10.1080/02648725.2006.10648074. [DOI] [PubMed] [Google Scholar]
- 155.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Progress in lipid research. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 156.Ulrich AS. Biophysical aspects of using liposomes as delivery vehicles. Biosci Rep. 2002;22(2):129–50. doi: 10.1023/a:1020178304031. [DOI] [PubMed] [Google Scholar]
- 157.Yoon W. Embolic agents used for bronchial artery embolisation in massive haemoptysis. Expert Opin Pharmacother. 2004;5(2):361–7. doi: 10.1517/14656566.5.2.361. [DOI] [PubMed] [Google Scholar]
- 158.Photos PJ, et al. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90(3):323–34. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 159.Abuchowski A, et al. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252(11):3582–6. [PubMed] [Google Scholar]
- 160.Dziubla T, et al. Oxidative Stress, Disease and Cancer. World Scientific; 2006. Nanoscale antioxidant therapeutics; pp. 1023–1043. [Google Scholar]
- 161.Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34(1):1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- 162.Feher J, Lengyel G, Blazovics A. Oxidative stress in the liver and biliary tract diseases. Scand J Gastroenterol Suppl. 1998;228:38–46. doi: 10.1080/003655298750026543. [DOI] [PubMed] [Google Scholar]
- 163.Stylianopoulos T. EPR-effect: utilizing size-dependent nanoparticle delivery to solid tumors. Ther Deliv. 2013;4(4):421–3. doi: 10.4155/tde.13.8. [DOI] [PubMed] [Google Scholar]
- 164.Akbarzadeh A, et al. Liposome: classification, preparation, and applications. Nanoscale Research Letters. 2013;8(1):102–102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hood ED, Shuvaev VV, Muzykantov VR. Chapter Twelve - Targeted Antioxidant Interventions for Vascular Pathologies A2 - Dziubla, Thomas. In: Butterfield DA, editor. Oxidative Stress and Biomaterials. Academic Press; 2016. pp. 323–349. [Google Scholar]
- 166.Suntres ZE. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. Journal of Toxicology. 2011;2011:16. doi: 10.1155/2011/152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mitsopoulos P, et al. Effectiveness of liposomal-N-acetylcysteine against LPS-induced lung injuries in rodents. Int J Pharm. 2008;363(1–2):106–11. doi: 10.1016/j.ijpharm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 168.Alipour M, et al. Prophylactic effect of liposomal N-acetylcysteine against LPS-induced liver injuries. J Endotoxin Res. 2007;13(5):297–304. doi: 10.1177/0968051907085062. [DOI] [PubMed] [Google Scholar]
- 169.Fan J, et al. Liposomal antioxidants provide prolonged protection against acute respiratory distress syndrome. Surgery. 2000;128(2):332–8. doi: 10.1067/msy.2000.108060. [DOI] [PubMed] [Google Scholar]
- 170.Mukhopadhyay S, et al. Role of MAPK/AP-1 signaling pathway in the protection of CEES-induced lung injury by antioxidant liposome. Toxicology. 2009;261(3):143–51. doi: 10.1016/j.tox.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 171.Alipour M, et al. Therapeutic effect of liposomal-N-acetylcysteine against acetaminophen-induced hepatotoxicity. J Drug Target. 2013;21(5):466–73. doi: 10.3109/1061186X.2013.765443. [DOI] [PubMed] [Google Scholar]
- 172.Takahashi M, et al. Evaluation of an Oral Carrier System in Rats: Bioavailability and Antioxidant Properties of Liposome-Encapsulated Curcumin. Journal of Agricultural and Food Chemistry. 2009;57(19):9141–9146. doi: 10.1021/jf9013923. [DOI] [PubMed] [Google Scholar]
- 173.Thangapazham RL, et al. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32(5):1119–23. [PMC free article] [PubMed] [Google Scholar]
- 174.Anand P, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochemical Pharmacology. 2008;76(11):1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 175.Kumar A, et al. Conundrum and Therapeutic Potential of Curcumin in Drug Delivery. 2010;27(4):279–312. doi: 10.1615/critrevtherdrugcarriersyst.v27.i4.10. [DOI] [PubMed] [Google Scholar]
- 176.Padhye S, et al. Perspectives on Chemopreventive and Therapeutic Potential of Curcumin Analogs in Medicinal Chemistry. Mini Reviews in Medicinal Chemistry. 2010;10(5):372–387. doi: 10.2174/138955710791330891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mandal AK, et al. Hepatoprotective Activity of Liposomal Flavonoid against Arsenite-Induced Liver Fibrosis. Journal of Pharmacology and Experimental Therapeutics. 2007;320(3):994–1001. doi: 10.1124/jpet.106.114215. [DOI] [PubMed] [Google Scholar]
- 178.Soloviev A, et al. Arrhythmogenic peroxynitrite-induced alterations in mammalian heart contractility and its prevention with quercetin-filled liposomes. Cardiovascular Toxicology. 2002;2(2):129–139. doi: 10.1385/ct:2:2:129. [DOI] [PubMed] [Google Scholar]
- 179.Yuan Z-p, et al. Liposomal Quercetin Efficiently Suppresses Growth of Solid Tumors in Murine Models. Clinical Cancer Research. 2006;12(10):3193–3199. doi: 10.1158/1078-0432.CCR-05-2365. [DOI] [PubMed] [Google Scholar]
- 180.Ghosh D, et al. Quercetin in vesicular delivery systems: evaluation in combating arsenic-induced acute liver toxicity associated gene expression in rat model. Chem Biol Interact. 2010;186(1):61–71. doi: 10.1016/j.cbi.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 181.Hung CF, et al. Development and evaluation of emulsion-liposome blends for resveratrol delivery. J Nanosci Nanotechnol. 2006;6(9–10):2950–8. doi: 10.1166/jnn.2006.420. [DOI] [PubMed] [Google Scholar]
- 182.Narayanan NK, et al. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125(1):1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 183.Tanswell AK, Freeman BA. Liposome-entrapped antioxidant enzymes prevent lethal O2 toxicity in the newborn rat. J Appl Physiol (1985) 1987;63(1):347–52. doi: 10.1152/jappl.1987.63.1.347. [DOI] [PubMed] [Google Scholar]
- 184.Chan PH, Longar S, Fishman RA. Protective effects of liposome-entrapped superoxide dismutase on posttraumatic brain edema. Ann Neurol. 1987;21(6):540–7. doi: 10.1002/ana.410210604. [DOI] [PubMed] [Google Scholar]
- 185.Imaizumi S, et al. Liposome-entrapped superoxide dismutase ameliorates infarct volume in focal cerebral ischaemia. Acta Neurochir Suppl (Wien) 1990;51:236–8. doi: 10.1007/978-3-7091-9115-6_79. [DOI] [PubMed] [Google Scholar]
- 186.Laursen JB, et al. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95(3):588–93. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 187.Vorauer-Uhl K, et al. Topically applied liposome encapsulated superoxide dismutase reduces postburn wound size and edema formation. Eur J Pharm Sci. 2001;14(1):63–7. doi: 10.1016/s0928-0987(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 188.Delanian S, et al. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol. 1994;32(1):12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 189.Baillet F, et al. Treatment of radiofibrosis with liposomal superoxide dismutase. Preliminary results of 50 cases. Free Radic Res Commun. 1986;1(6):387–94. doi: 10.3109/10715768609051643. [DOI] [PubMed] [Google Scholar]
- 190.Giovagnoli S, et al. Long-term delivery of superoxide dismutase and catalase entrapped in poly(lactide-co-glycolide) microspheres: in vitro effects on isolated neonatal porcine pancreatic cell clusters. J Control Release. 2005;107(1):65–77. doi: 10.1016/j.jconrel.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 191.Sankar P, et al. Oral nanoparticulate curcumin combating arsenic-induced oxidative damage in kidney and brain of rats. Toxicology and Industrial Health. 2016;32(3):410–421. doi: 10.1177/0748233713498455. [DOI] [PubMed] [Google Scholar]
- 192.Simón-Yarza T, et al. Functional benefits of PLGA particulates carrying VEGF and CoQ10 in an animal of myocardial ischemia. International Journal of Pharmaceutics. 2013;454(2):784–790. doi: 10.1016/j.ijpharm.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 193.Ankola DD, et al. Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: Can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy? European Journal of Pharmaceutics and Biopharmaceutics. 2007;67(2):361–369. doi: 10.1016/j.ejpb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 194.Taylor MS, et al. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater. 1994;5(2):151–7. doi: 10.1002/jab.770050208. [DOI] [PubMed] [Google Scholar]
- 195.Seshadri G, et al. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials. 2010;31(6):1372–9. doi: 10.1016/j.biomaterials.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fiore VF, et al. Polyketal microparticles for therapeutic delivery to the lung. Biomaterials. 2010;31(5):810–7. doi: 10.1016/j.biomaterials.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release. 2005;102(2):427–39. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 198.Chung JE, et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nature nanotechnology. 2014;9(11):907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jain AK, Thanki K, Jain S. Co-encapsulation of Tamoxifen and Quercetin in Polymeric Nanoparticles: Implications on Oral Bioavailability, Antitumor Efficacy, and Drug-Induced Toxicity. Molecular Pharmaceutics. 2013;10(9):3459–3474. doi: 10.1021/mp400311j. [DOI] [PubMed] [Google Scholar]
- 200.Kumar V, Prud’homme RK. Thermodynamic limits on drug loading in nanoparticle cores. J Pharm Sci. 2008;97(11):4904–14. doi: 10.1002/jps.21342. [DOI] [PubMed] [Google Scholar]
- 201.Gref R, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–3. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 202.Wilczewska AZ, et al. Nanoparticles as drug delivery systems. Pharmacological Reports. 2012;64(5):1020–1037. doi: 10.1016/s1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 203.Ettmayer P, et al. Lessons learned from marketed and investigational prodrugs. J Med Chem. 2004;47(10):2393–404. doi: 10.1021/jm0303812. [DOI] [PubMed] [Google Scholar]
- 204.Rautio J, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7(3):255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 205.Beaumont K, et al. Design of ester prodrugs to enhance oral absorption of poorly permeable compounds: challenges to the discovery scientist. Curr Drug Metab. 2003;4(6):461–85. doi: 10.2174/1389200033489253. [DOI] [PubMed] [Google Scholar]
- 206.Taylor MD. Improved passive oral drug delivery via prodrugs. Advanced Drug Delivery Reviews. 1996;19(2):131–148. [Google Scholar]
- 207.Wattamwar PP, et al. Antioxidant Activity of Degradable Polymer Poly(trolox ester) to Suppress Oxidative Stress Injury in the Cells. Advanced Functional Materials. 2010;20(1):147–154. [Google Scholar]
- 208.Wattamwar PP, et al. Tuning of the pro-oxidant and antioxidant activity of trolox through the controlled release from biodegradable poly(trolox ester) polymers. J Biomed Mater Res A. 2011;99(2):184–91. doi: 10.1002/jbm.a.33174. [DOI] [PubMed] [Google Scholar]
- 209.Wattamwar PP, et al. Synthesis and characterization of poly(antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomaterialia. 2012;8(7):2529–2537. doi: 10.1016/j.actbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 210.Gupta P, et al. Quercetin conjugated poly(beta-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015;27:194–204. doi: 10.1016/j.actbio.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gupta P, et al. Controlled curcumin release via conjugation into PBAE nanogels enhances mitochondrial protection against oxidative stress. Int J Pharm. 2016;511(2):1012–21. doi: 10.1016/j.ijpharm.2016.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dziubla TD, et al. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29(2):215–27. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Howard MD, et al. Targeting to endothelial cells augments the protective effect of novel dual bioactive antioxidant/anti-inflammatory nanoparticles. Mol Pharm. 2014;11(7):2262–70. doi: 10.1021/mp400677y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hood ED, et al. Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials. 2014;35(11):3708–15. doi: 10.1016/j.biomaterials.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yun X, et al. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. Journal of Cerebral Blood Flow & Metabolism. 2013;33(4):583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cochran DB, et al. Suppressing iron oxide nanoparticle toxicity by vascular targeted antioxidant polymer nanoparticles. Biomaterials. 2013;34(37):9615–9622. doi: 10.1016/j.biomaterials.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 217.Maradana MR, Thomas R, O’Sullivan BJ. Targeted delivery of cur cumin for treating type 2 diabetes. Mol Nutr Food Res. 2013;57(9):1550–6. doi: 10.1002/mnfr.201200791. [DOI] [PubMed] [Google Scholar]
- 218.Sun M, et al. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine (Lond) 2012;7(7):1085–100. doi: 10.2217/nnm.12.80. [DOI] [PubMed] [Google Scholar]
- 219.Mohanty C, Das M, Sahoo SK. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin Drug Deliv. 2012;9(11):1347–64. doi: 10.1517/17425247.2012.724676. [DOI] [PubMed] [Google Scholar]
- 220.Lao CD, et al. Dose escalation of a curcuminoid formulation. BMC Complementary and Alternative Medicine. 2006;6(1):10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Anand P, et al. Bioavailability of Curcumin: Problems and Promises. Molecular Pharmaceutics. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 222.Appendino G, et al. Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med. 2011;53(3 Suppl 1):43–9. [PubMed] [Google Scholar]
- 223.Teskač K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. International Journal of Pharmaceutics. 2010;390(1):61–69. doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 224.Ankola DD, et al. Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy? Eur J Pharm Biopharm. 2007;67(2):361–9. doi: 10.1016/j.ejpb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 225.Barnard ML, Baker RR, Matalon S. Mitigation of oxidant injury to lung microvasculature by intratracheal instillation of antioxidant enzymes. Am J Physiol. 1993;265(4 Pt 1):L340–5. doi: 10.1152/ajplung.1993.265.4.L340. [DOI] [PubMed] [Google Scholar]
- 226.Walther FJ, David-Cu R, Lopez SL. Antioxidant-surfactant liposomes mitigate hyperoxic lung injury in premature rabbits. Am J Physiol. 1995;269(5 Pt 1):L613–7. doi: 10.1152/ajplung.1995.269.5.L613. [DOI] [PubMed] [Google Scholar]
- 227.Neves AR, Queiroz JF, Reis S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. Journal of Nanobiotechnology. 2016;14(1):27. doi: 10.1186/s12951-016-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yekollu SK, Thomas R, O’Sullivan B. Targeting Curcusomes to Inflammatory Dendritic Cells Inhibits NF-κB and Improves Insulin Resistance in Obese Mice. Diabetes. 2011;60(11):2928–2938. doi: 10.2337/db11-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhou N, et al. Galactosylated chitosan-polycaprolactone nanoparticles for hepatocyte-targeted delivery of curcumin. Carbohydr Polym. 2013;94(1):420–9. doi: 10.1016/j.carbpol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 230.Abdel-Wahhab MA, et al. Curcumin nanoparticles loaded hydrogels protects against aflatoxin B1-induced genotoxicity in rat liver. Food and Chemical Toxicology. 2016;94:159–171. doi: 10.1016/j.fct.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 231.Storka A, et al. Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int J Clin Pharmacol Ther. 2015;53(1):54–65. doi: 10.5414/CP202076. [DOI] [PubMed] [Google Scholar]
- 232.clinicaltrials.gov A Phase IB Dose Escalation Study of Lipocurc in Patients With Cancer. National Library of Medicine; Bethesda (MD): 2017. [Google Scholar]
- 233.Zeng L, et al. Preparation and characterization of (−)-Epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on Human breast cancer MCF-7 cells. Scientific Reports. 2017;7:45521. doi: 10.1038/srep45521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hu B, et al. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem Commun (Camb) 2012;48(18):2421–3. doi: 10.1039/c2cc17295j. [DOI] [PubMed] [Google Scholar]
- 235.Pang X, et al. Hyaluronic acid-quercetin conjugate micelles: synthesis, characterization, in vitro and in vivo evaluation. Colloids Surf B Biointerfaces. 2014;123:778–86. doi: 10.1016/j.colsurfb.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 236.Suk JS, et al. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine (London, England) 2011;6(2):365–375. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]