Abstract
Background
Pre-operative detection of corticotropin (ACTH) secreting microadenomas causing Cushing's disease (CD) improves surgical outcomes. Current best magnetic resonance imaging fails to detect up to 40% of these microadenomas. 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) is specific, but not sensitive in detecting corticotropinomas. Theoretically, secretagogue stimulation with corticotropin releasing hormone (CRH) could improve detection of adenomas with 18F-FDG PET. Previous attempts with simultaneous CRH stimulation have failed to demonstrate increased 18F-FDG uptake in corticotropinomas. We hypothesized that CRH stimulation leads to a delayed elevation in glucose uptake in corticotropinomas.
Methods
Clinical data was analyzed for efficacy of CRH in improving 18FDG-PET detection of corticotropinomas in CD. Glucose transporter 1 (GLUT1) immunoreactivity was performed on surgical specimens. Ex-vivo, viable cells from these tumors were tested for secretagogue effects (colorimetric glucose uptake), and for fate of intracellular glucose (glycolysis stress analysis). Validation of ex-vivo findings was performed with AtT-20 cells.
Results
CRH increased glucose uptake in human-derived corticotroph tumor cells and AtT-20, but not in normal murine or human corticotrophs (p<0.0001). Continuous and intermittent (1h) CRH exposure increased glucose uptake in AtT-20 with maximal effect at 4h (p≤0.001). Similarly, CRH and 8-Br-cAMP led to robust GLUT1 upregulation and increased membrane translocation at 2h, while fasentin suppressed baseline (p<0.0001) and CRH-mediated glucose uptake. Expectedly, intra-operatively collected corticotropinomas demonstrated GLUT1 overexpression. Lastly, human derived corticotroph tumor cells demonstrated increased glycolysis and low glucose oxidation.
Conclusion
Increased and delayed CRH-mediated glucose uptake differentially occurs in adenomatous corticotrophs. Delayed secretagogue-stimulated 18F-FDG PET could improve microadenoma detection.
Keywords: Cushing's disease, corticotropinoma, PET, FDG, glucose uptake, secretagogue, CRH, metabolic reprogramming
1. Introduction
Improved remission rates and decreased adverse events following transsphenoidal surgery in Cushing's disease (CD) rely on accurate pre-operative localization of microadenomas.(1,2) Magnetic resonance imaging (MRI) remains the gold standard for detection of pituitary adenomas, although modern MRI modalities such as dynamic or volumetric sequences fail to detect an adenoma in 40% of CD cases.(3–5) Incidental sellar uptake of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) is highly specific but not sensitive for pituitary adenomas.(6–8)
Corticotropin releasing hormone (CRH) has secretagogue effects on adrenocorticotropic hormone or corticotropin (ACTH) secreting pituitary adenomas(9), but not on suppressed adjacent normal gland. This effect could theoretically improve the imaging detection of adenomas with 18F-FDG PET imaging following secretagogue stimulation. However, our group (unpublished data) and others(10), have failed to demonstrate increased 18F-FDG uptake in corticotropinomas by simultaneous CRH administration. To date, it remains unclear whether corticotropinomas are resistant to CRH-mediated glucose uptake, or if the effects are delayed.
We hypothesized that CRH stimulation leads to a delayed elevation in glucose uptake in corticotropinomas. In this study, we investigated the kinetics of CRH-modulated glucose uptake. A time-course in-vitro study revealed that maximum glucose uptake occurs approximately 4 hours post CRH administration. Moreover, we demonstrate for the first time that CRH stimulation results in a differential glucose uptake in adenomatous, but not in normal corticotrophs. Mechanistically, this was associated with a robust increase in glucose transporter 1 (GLUT1) expression. Taken together, these novel findings support the potential use of delayed 18F-FDG PET imaging following CRH stimulation to improve microadenoma detection in CD.
2. Materials and Methods
2.1. Tissue sample collection
A total of 10 patients with CD were enrolled in a clinical trial (NIH 12-N-0067, NCT01459237) conducted at the National Institute of Neurological Diseases and Stroke (NINDS) to evaluate the utility of CRH-stimulated 18F-FDG PET. Study was approved by the Combined Neuroscience Institutional Review Board (IRB). Pituitary adenoma tissues were obtained from these patients at the time of transsphenoidal adenomectomy at the National Institutes of Health Clinical Center (NIHCC) under a clinical trial (NIH 03-N-0164, NCT00060541) for subsequent immunohistochemical and metabolic analyses. Written informed consent was obtained from each patient for research study participation. The study was conducted in accordance to the standards and guidelines established by the IRB.
2.2. CRH-stimulated 18F-FDG PET Study
PET imaging was performed using a high-resolution research tomography scanner (Siemens AG). Each subject underwent two randomly ordered 18F-FDG high resolution PET (hrPET) studies with and without CRH stimulation separated by at least 24h. For CRH stimulated studies, ovine CRH (oCRH or Acthrel®) 1 mcg/kg (up to a maximum dose of 100 mcg) was administered immediately preceding 18F-FDG administration (10 mCi). Pituitary metabolic activity was quantified using the standardized uptake values (SUV). Maximum SUV (SUV-Max) and averaged SUV (SUV-Avg) were calculated as previously described.(11)
2.3. Cell culture
AtT-20/D16:16 (a generous gift from Dr. Steven L. Sabol at the National Heart Lung and Blood Institute) murine corticotroph tumor cell lines were cultured in DMEM (Thermo Fisher Scientific, USA), 10% FCS (Thermo Fisher Scientific) following comparison with commercially available AtT-20 cells (Supplementary figure 1). Cells were incubated for 2 h prior to all experiments in serum-free DMEM supplemented with 0.25% BSA (hereafter DMEM-BSA) and 90 mg/dL glucose, to simulate physiologic glucose levels. Pooled corticotroph cells were harvested (< 30 min post-euthanasia) from female, BALB/c mice (aged 6-8 weeks; Taconic Biosciences, USA), digested and homogenized in 1mg/mL collagenase (Sigma-Aldrich, USA) for 30 min, and cultured as above. All animals were euthanized in accordance with the standards and guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health (NIH). Western blot analysis was performed with anti glucocortidoid receptor antibody (Santa Cruz Biotechnology), anti Serine 211 phophorylated glucocorticoid receptor antibody (antibodies-online.com), and anti CRH receptor 1 antibody (Thermo Fisher Scientific)
2.4. Fluorescence activating sorting of murine corticotrophs
After dissociation of murine hypophyseal cells, corticotrophs were isolated by flow cytometry. Briefly, pooled cells were incubated with anti-CRH receptor 1 antibody (CRH-R1) (Thermo Fisher Scientific) for 1 h, followed by Alexa Fluor-555 conjugated antibody (Invitrogen, USA) and finally stained with DAPI (Thermo Fisher Scientific) for 30 min. Cell sorting analysis was carried out using MoFlo Astrios Cell Sorter and Summit Acquistion and Analysis software (Beckman Coulter, USA).
2.5. ACTH release studies
Following several washes in PBS, cells were incubated in serum-free DMEM-BSA and stimulated with 50nM CRH (Sigma-Aldrich, USA), 100nM arginine vasopressin (Sigma-Aldrich), 5mM 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP; Sigma-Aldrich), and/or 10nM dexamethasone (DEX; Sigma-Aldrich) for the time points indicated. Media was then diluted and assessed via enzyme linked immunosorbant assay (MD Bioproducts, USA) according to manufacturer instructions.
2.6. Glucose kinetics studies
Glucose uptake experiments were conducted with Screen Quest Colorimetric Glucose Uptake Assay Kit (AAT Bioquest, USA) per manufacturer instructions. In brief, cells were cultured in DMEM-BSA on 96-well plates, washed with Krebs-Ringer-Phosphate-HEPES (KRPH) buffer and incubated in 90 μl/well of Glucose Uptake Buffer (AAT Bioquest) for 1h. Subsequently, cells were exposed to 10 μl/well of Deoxy-D-glucose (2DOG) for 40min; cells in the CRH stimulation arm were exposed to 50nM of CRH for 1h. Following treatment, cells were washed with KRPH buffer and lysed. Lastly, 50μl of the Uptake Assay Mixure (AAT Bioquest) was added to each sample and absorbance ratio was read with 570 nm wavelenght.
2.7. RNA extraction and quantitative real-time PCR
Total RNA was extracted from cells using RNeasy Mini Kit (Qiagen, Germany) and reverse transcribed to complementary DNA (cDNA) with Superscript III qRT-PCR Supermix (Life Technologies, USA). Quantitative RT-PCR was performed using target-specific primers (Sigma-Aldrich; Supplementary Table 1) and SYBR Select Master Mix (Life Technologies) on an Illumina Eco Real-Time PCR System (Illumina, USA). Each reaction was performed in triplicate (technical and biological), and relative gene expression values were calculated using the ΔΔCt method with ACTB as an internal control.
2.8. Immunofluorescence and immunohistochemistry
After CRH stimulation for various time periods, cells were fixed using 4% paraformaldehyde (Thermo Fisher Scientific) and blocked using 2% donkey serum in 2% PBSA. Tris-buffered saline with Tween was used as wash buffer. Cells were then incubated with primary antibody against GLUT1 (Abcam, UK) for 1 h and secondary donkey anti-rabbit FITC antibody (Jackson ImmunoResearch, USA) along with DAPI (Thermo Fisher Scientific) was applied to the cells for 30 min. Rabbit IgG was used as negative control. Plasma membrane translocation of GLUT1 was calculated as previously described.(12) Briefly, ImageJ software (ImageJ 1.50i, NIH, Bethesda) was used to measure the relative fluorescence intensity of GLUT1 in the plasma membrane adjacent region and the cytosolic region. The ratio of these measurements was calculated in at least 8 cells per condition tested. Formalin fixed human derived surgical adenoma specimens were stained with GLUT1 antibody (Abcam, UK) using Leica BondMax (Leica Biosystems, Buffalo Grove, IL) and reviewed by blinded neuropathologist (ARC).
2.9. Cell metabolism assessment
To understand the intracellular fate of glucose, glycolytic pathways were investigated using Seahorse XF Glycolysis Stress Test Kit (Agilent Technologies, USA) according to the manufacturer's instructions. To determine the contribution of glucose oxidation to mitochondrial respiration, a Seahorse XF Mito Fuel Flex Test Kit (Agilent) was used. For glucose utilization assessment, cells were seeded at a density of 15,000 cells/well and cultured overnight. Medium was changed to Basal Minimal DMEM (Agilent) with or without glutamine (1mM), glucose (5mM), etomoxir (ETO; 4μM) and bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES; 3μM), and cells were subjected to non-CO2 incubation for 1 h prior to detection. For each sample, three groups were designed. The first group was sequentially treated with glutamine (1mM), ETO (4μM)/BPTES (3μM), glucose (5mM), glucose (10 mM). The second group was sequentially treated with glucose (5mM), glucose (10mM), oxamate (50mM), carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP; 2μM), and antimycin/rotenone (0.5μM). The third group was sequentially treated with glucose (5mM), glucose (10mM), UK5099 (2μM), oligomycin (2μM), and 2DOG (50mM). All chemicals used were from Agilent except oxamate (Sigma-Aldrich). For glucose oxidation analysis, oxygen consumption rate (OCR) was presented for groups 1 and 2. For glycolysis analysis, extracellular acidification rate (ECAR) was presented for groups 1 and 3. Dependency of glucose use was calculated by the following formulas: glycolysis dependency = (ECARglucose –ECARoxamate) / (ECARoligomycin – ECAR2DOG), glucose oxidation dependency = (OCR glucose –OCRUK5099)/(OCRFCCP – OCRAA/Rot). The overlapped stages were averaged and time was extended to mimic continuous treatment for enhanced presentation. However, raw data of each group were used for calculation of glycolysis dependency.
2.10. Statistical analysis
Statistical analyses were determined by one-factor ANOVA using GraphPad Prism version 6.0 software program (GraphPad, USA) for in-vitro and in-vivo results. Grouped analyses were evaluated using two-way ANOVA. Alpha level threshold of 0.05 was used to assess statistical significance.
3. Results
3.1. CRH modulates glucose uptake in adenoma but not normal corticotroph cells
AtT20/D16-16 strain (hereafter referred to as AtT20) was selected based on consistent ACTH release response to secretagogue stimulation (Supplementary Figure 1). This strain of AtT20 expressed glucorticoid receptor (GR), that was phosphorylated (Serine 211)(13) with dexamethasone exposure (Supplementary Figure 2). CRH receptor type 1 (CRHR1) was also detected with western blot and quantitative RT-PCR. Expectedly(14), CRHR1 expression was depressed with exposure to dexamethasone (10 μM) for 3-hours (Supplementary Figure 2). Murine pituitary cells also expressed GR, phosphorylated GR similar to AtT20 cells. However, CRHR1 expression was lower in unsorted normal murine pituitary cells, with non-significant change upon dexamethasone exposure (Supplementary Figure 3). As expected, levels of measured ACTH were substantially lower in isolated normal murine corticotrophs (following fluorescence activating sorting), when compared to AtT-20 cells (Figure 1A). When stimulated with CRH, a significant increase in ACTH secretion was observed in AtT-20 cells after 4 h (mean difference 84.7%, [95% CI 64.5-104.9], ANOVA p<0.0001) (Figure 1A). AtT-20 cells exposed to CRH displayed a significant increase in 2DOG uptake, while the same effect was not observed in normal murine corticotrophs (Figure 1B). We then tested the effect of continuous CRH stimulation (at 1 and 4 h) on human-derived pituitary corticotroph tumor cells. At 1 and 4 h following initiation of CRH stimulation, a consistent increase in 2DOG uptake (mean difference 216 ± 38% and 222 ± 59%, respectively) was observed (Figure 1C). We then confirmed the selectivity of secretagogue effect on glucose uptake in normal pituitary and corticotropinoma tissues ex-vivo from one patient. Expectedly, CRH stimulation led to a significant increase in ACTH secretion from human derived corticotroph tumor cells but not normal corticotrophs cells in primary culture (mean difference 225.6% [95% CI 21.1-430.2], ANOVA p=0.04) (Figure 1D). Furthermore, 2DOG uptake was differentially increased in human derived corticotroph tumor cells but not in normal corticotrophs when exposed to CRH (mean difference 76.3%, [95% CI 50.5-102.2], ANOVA p<0.0001) (Figure 1E). These results suggest that adenomatous cells but not normal corticotrophs have increased glucose uptake that may be modulated with secretagogues stimulation.
Figure 1. CRH stimulation results in selective augmented glucose uptake in adenomatous but not normal corticotrophs.
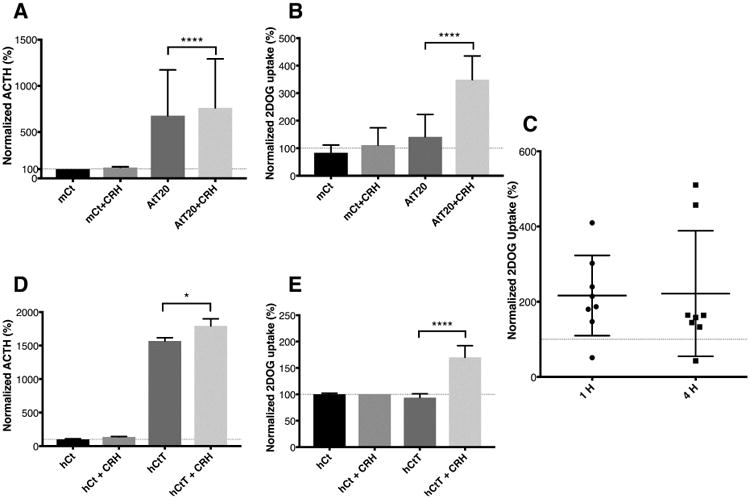
Normal murine corticotrophs cells were separated from harvested pituitary glands using CRH-R1 antibody and flow cytometry. As expected, murine derived AtT-20/D16:16 cells demonstrated a robust CRH-stimulated ACTH secretion response not seen in normal murine corticotrophs (A). At 4 h, a significant increase in normalized 2DOG uptake was noted in AtT-20 cells exposed to CRH but not in normal murine corticotrophs (B). At 1 and 4 h, CRH stimulation resulted in a consistent increase in 2DOG uptake in human derived corticotroph tumor cells (C). Horizontal line at 100 represents 2DOG uptake by adenomatous cells at baseline. The individual data points represent 2DOG uptake normalized to adenoma without stimulation (y axis = 100) at 1 and 4 hours. When human derived corticotroph tumor cells cells were exposed to CRH for 4 hours, a substantial increase in both ACTH release and glucose uptake was seen (D and E). Normal corticotrophs did not respond to CRH stimulation for both ACTH secretion or 2DOG uptake demonstrating the selective CRH-mediated effect on adenomatous cells only. * p ≤ 0.05, **** p ≤ 0.0001 compared with corresponding control values. Horizontal bars represent mean ± standard deviation. Abbreviations: ACTH - adrenocorticotropic hormone, CRH - corticotropin-releasing hormone, hCt – normal corticotrophs, hCtT – human derived corticotroph cells, mCt – normal murine corticotrophs.
3.2. CRH-mediated glucose uptake is delayed
To investigate the kinetics of glucose uptake following secretagogue stimulation, we exposed AtT-20/D16:16 cells to CRH continuously or transiently (1 h) in the presence of 2DOG. Cellular 2DOG uptake was assessed at 1, 2, 4, 8 and 24 h of continuous CRH stimulation (Figure 2A). We found no 2DOG uptake difference at 1 and 2 h between control and CRH-exposed cells (mean difference 35.2%, [95% CI -191.8-262.4] and 216.8%, [95% CI -10.33-443.9], ANOVA, respectively). CRH-stimulated 2DOG uptake was maximally elevated at 4 h (mean difference 341.2%, [95% CI 114.1-568.3], ANOVA p≤0.001) while uptake at 8 and 24 h was not statistically significant (mean differences 127.2%, [95% CI -99.95-354.3] and 134.6%, [95% CI -92.5-361.7], ANOVA, respectively). Since persistent conditions of elevated CRH levels are not clinically relevant, and because the half-life of CRH is approximately 45 min(15), we designed experiments to simulate a short-term exposure to CRH. On transiently exposing AtT-20/D16:16 cells to CRH for 1 h followed by a washout and exposure to 2DOG, we detected a robust and significant increase in 2DOG uptake at 2 and 4 h (mean difference 94%, [95% CI 27.3-160.7], ANOVA p ≤ 0.01, and 68.9%, [95% CI 2.3-135.6], ANOVA p ≤ 0.05 respectively) (Figure 2B).
Figure 2. Continuous and short-term exposure to CRH or 8-Br-cAMP leads to increased glucose uptake in AtT-20/D16:16 cells.
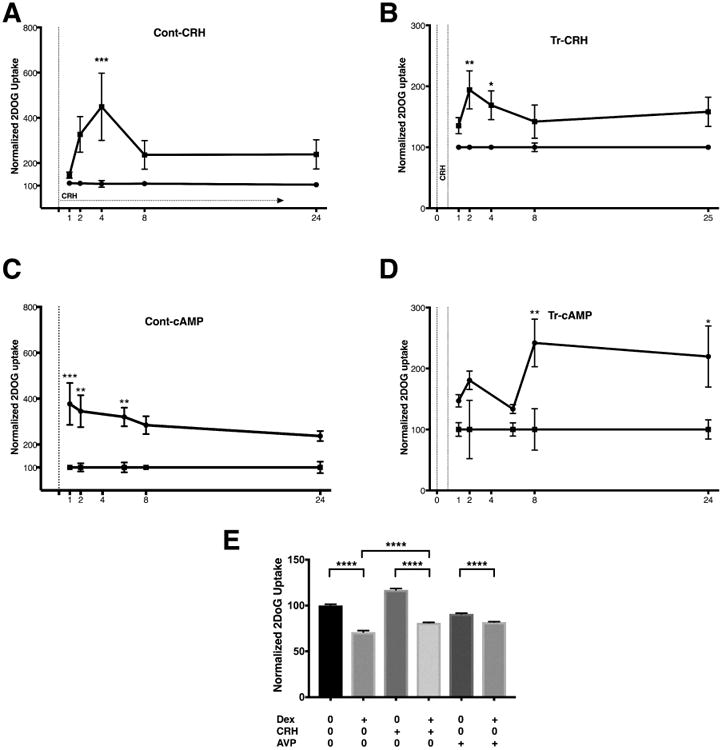
Panels A and B represent normalized 2DOG uptake by AtT-20 cells. When exposed to CRH continuously (A), no increase in 2DOG was seen at early time points (1 and 2 h). 2DOG uptake was significantly elevated at 4 h with further delay up to 24 h. With a short-term 1 h (Tr-CRH) exposure to CRH (B), increased 2DOG was seen at 2 h followed by later time points. Exposure to 8-Br-cAMP resulted in delayed increases in 2DOG uptake with both continuous (Cont-cAMP) and transient (Tr-cAMP) exposure (C and D). The increase in CRH-mediated 2DOG uptake in AtT-20 cells was reduced in the presence of DEX (E). Despite a 3 hour exposure to DEX, CRH stimulation still resulted in a significant increase in 2DOG uptake when compared to the control group (E). In AtT20 cells, AVP failed to induce significant increase in 2DOG uptake, with or without DEX (E). The data presented is representative of experiments carried out with biological duplicates and technical quadruplicates. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001 compared with corresponding control values. The horizontal line at 100 represents 2DOG uptake under control conditions. Horizontal bars represent mean ± standard deviation. Abbreviations: 2DOG -2deoxyglucose, 8-Br-cAMP - 8Br-cyclic adenosine monophosphate, CRH - corticotropin-releasing hormone, DEX – dexamethasone, Cont-CRH – continuous corticotropin-releasing hormone, Cont cAMP – continuous cyclic adenosine monophosphate Tr-CRH – transient corticotropin-releasing hormone, Tr-cAMP – transient cyclic adenosine monophosphate.
Intracellular effects of CRH stimulation are largely mediated by the ubiquitous second messenger cyclic adenosine monophosphate (cAMP)(16), therefore, we hypothesized that glucose uptake would increase in response to cAMP. Continuous exposure to 8-Br-cAMP revealed a slightly different pattern of delayed increase in 2DOG uptake as significant glucose uptake was seen at the 1, 2, 4 and 8-hour mark (mean differences 277%, [95% CI 114.7-439.3], ANOVA p=0.0002, 245.1%, [95% CI 82.8-407.4], ANOVA p=0.0009, 220.1%, [95% CI 57.8-382.4], ANOVA p=0.003, and 184.4%, [95% CI 22.1-346.7], ANOVA p=0.02, respectively) (Figure 2C). Interestingly, transient 8-Br-cAMP exposure resulted in a different, delayed pattern of increased 2DOG uptake compared to CRH exposure as statistical significance was reached in later time points at 8 and 24 h (mean differences 141.9%, [95% CI 34.5-249.3], ANOVA p=0.004, and 119.7%, [95% CI 12.3-227.1], ANOVA p=0.02, respectively) (Figure 2D). Taken together, these results suggest that secretagogues stimulation may lead to a delayed (2 – 4 hour) increase in glucose uptake in adenomatous corticotrophs.
3.3. Dexamethasone reduces glucose uptake response to CRH and AVP in AtT-20 cells
To further investigate the CRH-induced glucose uptake response, the hypercortisolemic state of CD was simulated in-vitro with a potent synthetic glucocorticoid dexamethasone (DEX). Basal levels of glucose uptake from AtT-20 cells were abrogated in presence of DEX (mean differences 29%, [95% CI 27.2-30.63], ANOVA p<0.0001) (Figure 2E). The increase in CRH-mediated 2DOG uptake in AtT-20 cells (mean differences 17%, [95% CI 15.4-18.8], ANOVA p<0.0001) was similarly reduced by the addition of DEX (mean differences 18.8%, [95% CI 17.1-20.5], ANOVA p<0.0001). Importantly, despite the presence of DEX, CRH stimulation led to considerable increase in 2DOG uptake (mean differences 10.1%, [95% CI 8.5-11.9], ANOVA p<0.0001 (Figure 2E). These findings suggest that corticotropinomas may increase glucose uptake in response to CRH stimulation in the setting of hypercortisolemia.
Since arginine vasopressin (AVP) can simulate secretagogue activity in CD(17,18), we next sought to evaluate the effect of AVP on 2DOG uptake in AtT-20/D16:16 cells. Unexpectedly, in AtT-20/D16:16 cells, AVP exposure led to a reduction in 2DOG uptake (91 ± 0.8%, ANOVA p<0.0001). DEX further diminished glucose uptake in AVP-treated cells (82 ± 0.4%, ANOVA p<0.0001) (Figure 2E). The clinical relevance of AVP effect in our in-vitro model is unclear. These findings suggest that at least in AtT20 D16:16 strain, AVP does not induce glucose uptake.
3.4. GLUT1 transcription increases in response to CRH stimulation
To characterize the specific glucose transporters (GLUT) involved in the increased glucose uptake response, AtT-20/D16:16 cells were incubated with both CRH and 8-Br-cAMP and mRNA levels of GLUT 1, 2, 3, 4 and 8 were quantified at 1 to 24 h post-exposure. Cells treated with CRH demonstrated a robust increase in GLUT1 mRNA levels at 2h (205.3 ± 30.8%, p=0.0004), 4h (175.2 ± 8.6%, p<0.0001), 8h (196.4 ± 16.7%, p<0.0001) and 24h (156.3 ± 5.8%, p=0.0002) (Figure 3A). The response to CRH was modest at early time points for GLUT2 (2h, 4h) and GLUT 3 (2h) whilst GLUT3 seemed to have a delayed response at 24h (Figure 3). No significant increase was noted for GLUT4. GLUT8 reflected a similar pattern of expression when compared to GLUT1 with a less robust increase.
Figure 3. CRH stimulation results in transcriptional upregulation of GLUT1.
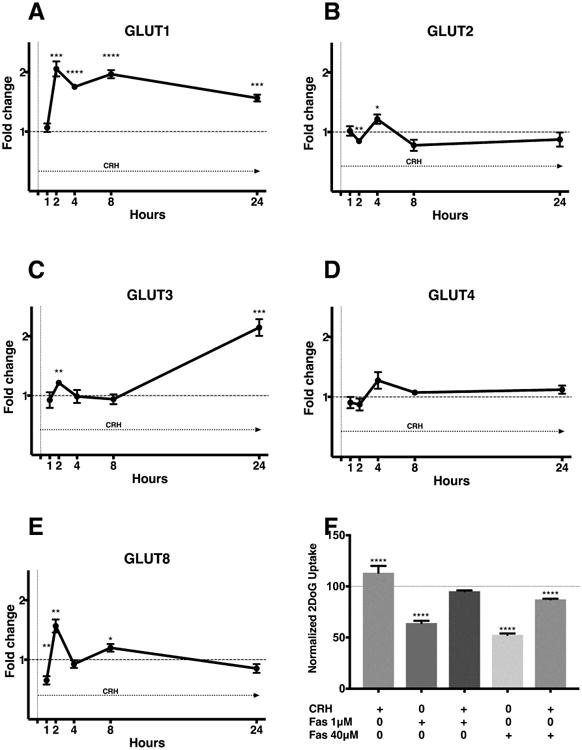
Expression of glucose transporters was calculated by ΔΔCt method following RT-qPCR. GLUT1 is overexpressed throughout the period of continuous exposure to CRH in AtT20 D16:16 cells starting at 2 h (A). Other glucose transporters are variably expressed during CRH exposure. The response to CRH was modest at early time points for GLUT2 (B) and GLUT 3 (C) whilst GLUT3 seemed to have a delayed response at 24h (C). No significant increase was noted for GLUT4 (D). GLUT8 reflected a similar pattern of expression when compared to GLUT1 with a less robust increase (E). The duration of exposure is denoted within each panel with an arrow underneath ‘CRH’. CRH-mediated glucose uptake in AtT-20 cells was blunted by GLUT1 inhibitor Fasentin following a 3-hour exposure (F). However, CRH exposure reversed the decreased glucose uptake caused by a a lower dose of Fasentin (1μM). A higher dose of fasentin (40μM) dose prevented CRH mediated normalization of glucose uptake. This reduction below baseline likely due to the large effect of high dose Fasentin on of glucose uptake at baseline (F). The data shown is representative of experiments carried out in both biological and technical triplicates. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001 compared with corresponding control values. Horizontal bars represent mean ± standard errors of mean (SEM). Abbreviations: CRH - corticotropin-releasing hormone, GLUT – glucose transporter, PCR -polymerase chain reaction
Next, we wanted to understand the contribution of cAMP mediated effects of CRH receptor activation on glucose uptake. When AtT-20 cells were stimulated with continuous 8-Br-cAMP, GLUT1 exhibited significant transcriptional upregulation at 1h, 4h, 8h and 24h (Supplementary figure 2). Expression of the remaining glucose transporters decreased when exposed to 8-Br-cAMP (Supplementary figure 2). These findings also suggest transcriptional regulation of GLUT1 with secretagogue stimulation via cAMP production.
3.5. CRH-mediated glucose uptake from AtT-20 cells is blunted by GLUT1 inhibitor
We hypothesized that fasentin, a selective GLUT1 inhibitor(19) could inhibit CRH-mediated 2DOG uptake. As previously demonstrated, CRH led to increase glucose uptake from AtT-20 cells (mean difference 13.4%, [95% CI 7.3-19.46], ANOVA p<0.0001) (Figure 3F). Low dose fasentin (1 μM) decreased baseline glucose uptake. However, addition of CRH reversed this decrease in glucose uptake. Higher dose of fasentin (40 μM) significantly decreased both baseline (mean difference 47.3% [95% CI 41.2-53.4], ANOVA p<0.0001)and CRH stimulated glucose uptake compared to controls (12.7%, [95% CI 6.591-18.7], ANOVA p<0.0001) (Figure 3F). The findings suggest that selective inhibition of GLUT1 can abrogate baseline and CRH mediated glucose uptake in corticotropinomas.
3.6. CRH augments GLUT1 expression and translocation to the membrane
Next, we confirmed the effect of secretagogue stimulation on GLUT1 expression and translocation in AtT-20/D16:16 cells. We exposed cells to both CRH and 8-Br-cAMP continuously for 2h, 8h and 24h. Immunofluorescence revealed upregulation of GLUT1 within cells in response to stimulation (Figure 4). This effect was seen as early as 2h when compared to control (Figure 4A - a, b and c). Relative fluorescence ratio of GLUT1 signal between plasma membrane adjacent region versus cytosolic region was increased from 0.9 to 1.16 within 2 hours (p < 0.05). At 24 hours of continuous CRH exposure, the ratio increased from 0.68 to 1.21 (p < 0.0001) (Figure 4B). GLUT1 plasma membrane translocation was not increased in AtT20 cells exposed to 8-Br-cAMP (Figures 4A - c, f and I, 4B), suggesting cAMP independent effect of CRH receptor activation.
Figure 4. GLUT1 is overexpressed in pituitary adenomas and its translocation increases with CRH administration.
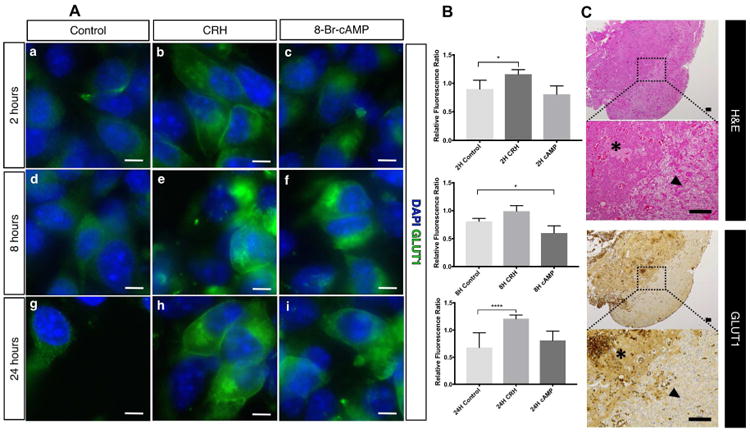
Fluorescence immunocytochemistry revealed increase in total GLUT1 content within AtT20/D16:16 cells with CRH and 8-Br-cAMP stimulation (A: b to i). This effect was seen as early as 2 h (A: b and c). Continuous CRH stimulation resulted in a robust and sustained GLUT1 membrane translocation (A: e and h). Representative immunofluorescence images are shown. GLUT1 membrane tranlocation was quantified as relative fluorescence ratio between averaged plasma membrane region versus cytosolic region using eight representative cells for each condition (B). The middle bars demonstrate a significant increase in GLUT1 localization to plama membrane region with 2-hour (p < 0.05) and 24-hour (p < 0.0001) exposure to CRH. Pituitary adenomas (asterix) harvested from patients who underwent transsphenoidal adenomectomy had higher GLUT1 expression than the surrounding pituitary gland (black arrowhead) (C). Adjacent histopathological sections of surgical specimens were examined with hematoxylin and eosin staining (top) and with GLUT1 immunohistochemical staining (bottom). Corticotropinoma demonstrated increased GLUT1 expression not associated with normal anterior pituitary gland (bottom, black arrowhead). Scale bars for fluorescence immunocytochemistry = 10 μm. Scale bars for immunohistochemistry = 100 μm. Abbreviations: DAPI – 4′,6-diamidino-2-phenylindole, GLUT1 – glucose transporter 1.
3.7. Corticotropinomas have increased GLUT1 expression compared to suppressed normal gland
Clinically, concurrent CRH stimulation and 18F-FDG PET failed to improve standardized uptake value of 18F-FDG or adenoma detection in 10 patients (Table 1). A histopathological analysis was performed blindly on intra-operatively obtained human tissue by an experienced neuropathologist (A.R.) with both H&E stained, and GLUT1 immunostained sections available (Figure 4C). Corticotroph adenomas in 9 out of 10 patients demonstrated immunoreactive cyptosolic GLUT1 expression (Table 1). In 3 patients where adjacent normal gland was available, the adjacent normal gland was negative for immunoreactive GLUT1 (Table 1). Increased immunoreactive cytosolic GLUT1 expression in corticotropinoma was not correlated with increased glucose uptake on FDG-PET scanning (Table 1), suggesting that basal cytosolic GLUT1 expression was not related to increased glucose uptake per se.
Table 1. Corticotropinomas have increased GLUT1 expression compared to the surrounding normal gland.
A blinded analysis of histopathology revealed that 9 out of 10 pituitary adenomas had increased GLUT1 expression. Adjacent normal pituitary tissue was available for 3 patients which were all negative for GLUT1 expression. Despite GLUT1 overexpression, no immediate SUV changes were appreciated in patients that underwent simultaneous CRH-stimulation and 18F-FDG PET, presumably due to delayed effect on glucose uptake.
| GLUT1 Expression | Normal Adjacent Tissue | Non-CRH / CRH-Stimulated PET | Non-CRH SUV Max | CRH-stimulated SUV Max | |
|---|---|---|---|---|---|
| Case 1 | +++ | - | Positive | 12.8 | 12.8 |
| Case 2 | +++ | NA | Negative | 4.3 | 4.3 |
| Case 3 | + | NA | Positive | 11.6 | 11.6 |
| Case 4 | ++ | NA | Negative | 4.3 | 5.3 |
| Case 5 | ++ | NA | Negative | 3.7 | 3.6 |
| Case 6 | +++ | NA | Positive | 21.1 | 11.1 |
| Case 7 | ++ | NA | Negative | 4.7 | 4.3 |
| Case 8 | + | - | Positive | 6 | 6 |
| Case 9 | - | NA | Positive | 7.5 | 7.3 |
| Case 10 | +++ | - | Negative | 5 | 4.3 |
weak immunoreactivity,
moderate immunoreactivity,
strong immunoreactivity,
no immunoreactivity.
Abbreviations: 18F-FDG PET - 18F-fluorodeoxyglucose (18F-FDG) PET, GLUT1 – glucose transporter 1, NA – not available.
3.8. Human-derived corticotroph tumor cells cells are highly dependent on glycolysis
Lastly, we investigated the fate of glucose within human-derived corticotroph tumor cells cells. Normally, the pituitary gland depends on free fatty acids as an energy source(20,21). Expectedly(22), we found that corticotropinoma cells demonstrated higher glycolytic activity compared to normal gland (50.47% vs. 38.99%, Figure 5A). However, the absolute level of glucose oxidation in corticotropinoma cells was quite low (Figure 5B). In order to establish the dependency of corticotroph tumors cells on mitochondrial oxidative phosphorylation and/or anaerobic glycolysis, ETO, BPTES, and UK5099 were employed to block oxidation of fatty acids, glutamine, and glucose, respectively. Similarly, FCCP, an electron transport chain uncoupling agent was subsequently utilized to block oxidative phosphorylation pathways. Human-derived corticotroph tumor cells cells utilized up to 70-80% of their glycolytic capacity but less than 15% of their glucose oxidation capacity (Figure 5) suggesting that these cells were highly dependent on anaerobic glycolysis.
Figure 5. Pituitary adenoma cells demonstrated high glycolytic activity and low glucose oxidation.
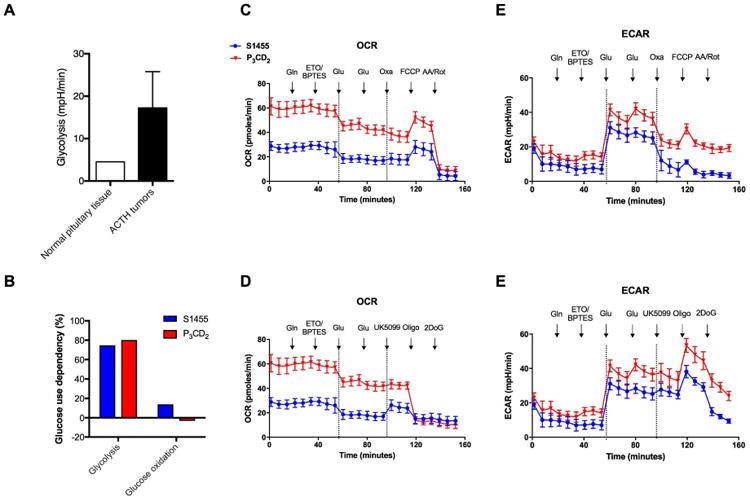
Pituitary adenoma cells had higher glycolytic activity than normal pituitary cells (A). Human primary pituitary adenomatous cells (Tumor 1 and 2) utilized the majority of their glycolytic capacity but barely used glucose for oxidation (B). OCR and ECAR were detected using Seahorse in human primary cells Tumor 1 and Tumor 2. The first stages of C and D and the first stages of E and F were generated using the same data. X-axis was extended to mimic continuous treatments. Data between two dash lines in each part were average of the first and third stages. Note that UK5099 caused minimal changes of OCR while oxamate led to significant alterations of ECAR in both samples. Abbreviations: ECAR – extracellular acidification rate, OCR – oxygen consumption rate.
4. Discussion
18F-FDG PET reveals increased glucose uptake in both non-functioning and hormone secreting pituitary adenomas (23,24), whilst minimal 18F-FDG uptake is usually seen in the normal gland.(6–8) Sellar 18F-FDG uptake is highly specific(6,8,25), but a relatively insensitive (40%) finding for pituitary adenomas (11,26). Low sensitivity of 18F-FDG-PET suggests the need for modulation of glucose uptake in adenomas to improve detection. In hormone secreting cells, secretagogues can further stimulate glucose uptake(27,28), offering an ability to modulate glucose uptake in these cells. Consistent with other reports(10), our study failed to demonstrate an immediate change in standardized uptake value (SUV) with concomitant administration of CRH and 18F-FDG in 10 human subjects (Table 1). These findings led us to investigate whether corticotropinomas are resistant to CRH-mediated glucose uptake, or if the effects are delayed. We hypothesized that CRH stimulation leads to delayed increased glucose uptake in corticotropinomas. Glucose kinetic studies demonstrated an increase in glucose uptake in ACTH-secreting AtT-20/D16:16 cells but not in normal normal murine corticotrophs cells in response to CRH stimulation. Further, selective modulation of glucose uptake with CRH was confirmed in human-derived corticotropinoma when compared to adjacent normal pituitary tissue. These findings are consistent with evidence suggesting that 18F-FDG uptake is specific to residual/recurrent pituitary adenoma following adenomectomy.(29)
A time-course study revealed that both continuous and transient administration of CRH leads to delayed increase glucose uptake in AtT-20 cells. CRH-mediated glucose uptake was maximal at 4h for continuous CRH exposure, and 2 - 4h for transient (1h) CRH exposure. As intracellular effects of CRH stimulation are largely mediated by cAMP(16), we deemed important to assess whether it was the main downstream mediator of CRH effects on glucose uptake. Continuous 8-Br-cAMP administration led to increased uptake at early time points from 1 to 4h whereas transient exposure maximal effect was seen at 8h. The difference in time-dependent stimulation effect between CRH and 8-Br-cAMP might be due to the broad actions of CRH on AtT-20 cells not solely relying on the well-characterized cAMP transduction pathway. Interestingly, previous literature showed that little net oxidation of glucose by either the Krebs' cycle or the pentose phosphate pathway occurs in the pituitary gland in-vivo and that non-esterified fatty acids are one of the main energy substrates.(20,21) Our results suggest that pituitary adenomas undergo metabolic reprogramming to rely on glycolysis as the primary energy source, similar to the Warburg effect in malignant tumors.(30)
CD is characterized by a state of hypercortisolemia that could inhibit glucose uptake in adenomatous (or AtT-20) cells.(31) Our results suggest that even in the presence of DEX, CRH stimulation may lead to significant delayed increase in glucose uptake in corticotropinomas. A number of glucose transporters are involved in glucose uptake by cells of the central nervous system.(32) We identified GLUT1 overexpression in 10 human corticotropinomas and identified GLUT1 as the predominant glucose transporter modulated by CRH stimulation. Both CRH and 8-Br-cAMP led to a robust increase in GLUT1 transcription maintained up to 24h. This is consistent with previous studies in which gonadotrophin releasing hormone (GnRH) increased GLUT1 expression and stimulated glucose uptake in the gonadotrophs.(28) Intriguingly, CRH stimulation resulted in a more robust and sustained membrane translocation of GLUT1 when compared to 8-Br-cAMP. We found a discordance between human corticotropinoma cytosolic GLUT1 expression and 18F-FDG uptake (Table 1) presumably due to delayed glucose uptake effects. We thus suspect that in clinical use, exposure to oCRH may lead to delayed increased membrane translocation of GLUT1 within corticotropinomas, and consequently increase 18F-FDG uptake. Additionally, we found that fasentin, a selective GLUT1 inhibitor, abolished the CRH-mediated increased glucose uptake, confirming the potential role of GLUT1 in glucose uptake in corticotropinomas. Given the dependence of adenomatous cells on glucose as a primary energy source, these results may lead to selective anti-tumor therapies by targeting glucose metabolism.
5. Conclusion
In this study, we demonstrate that CRH stimulation results in differentially increased glucose uptake from adenomatous but not in normal corticortrophs. A clinically relevant transient CRH exposure time-course study revealed that maximum uptake is 4h post exposure. Corticotrophs do not normally utilize glucose at baseline as an energy source, however, glucose kinetic analyses suggest that a potential metabolic switch to aerobic glycolysis occurs in adenomatous cells. Based on these findings, we suggest a pathophysiologic basis for delayed 18F-FDG PET imaging following CRH stimulation for detection of corticotropinomas. We are now confirming these findings with a clinical trial to detect corticotropinomas with 18F-FDG PET imaging 2 - 6 hours following oCRH stimulation.
Supplementary Material
Highlights.
Metabolic reprogramming of corticotropinomas is explored.
Corticotropinomas, but not normal pituitary cells, have significant glucose uptake.
Glucose uptake in corticotropinomas may be modulated by secretagogues via transcriptional regulation of glucose transporter 1.
Acknowledgments
We thank Steven Sabol, at the National Heart Lung and Blood Institute for generously donating the AtT-20 D16/16 cells. This research was supported by the Intramural Research Program of the National Institute of Neurological Diseases and Stroke, Bethesda, MD. This research was also supported by the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program
Financial Support: This research was supported by the Intramural Research Program of the National Institute of Neurological Diseases and Stroke, Bethesda, MD (NIH ZIA NS003150-01).
Abbreviations in the manuscript
- 18F-FDG
18F-fluorodeoxyglucose
- ACTH
adrenocorticotropic hormone or corticotropin
- AtT-20
murine derived ACTH secreting tumor cells
- BPTES
bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide
- CD
Cushing's disease
- DEX
dexamethasone
- CRH
corticotropin releasing hormone
- ECAR
extracellular acidification rate
- ETO
etomoxir
- FCCP
carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- GLUT1
glucose transporter 1
- GLUT2
glucose transporter 2
- GLUT3
glucose transporter 3
- GLUT4
glucose transporter 4
- GLUT8
glucose transporter 8
- MRI
magnetic resonance imaging
- OCR
oxygen consumption rate
- PET
positron emission tomography
- RT-qPCR
real-time quantitative polymerase chain reaction
- SUV
standardized uptake value
Footnotes
Disclosure statement: The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bochicchio D, Losa M, Buchfelder M. Factors influencing the immediate and late outcome of Cushing's disease treated by transsphenoidal surgery: a retrospective study by the European Cushing's Disease Survey Group. J Clin Endocrinol Metab. 1995;80:3114–20. doi: 10.1210/jcem.80.11.7593411. [DOI] [PubMed] [Google Scholar]
- 2.Moshang T. Editorial: Cushing's disease, 70 years later … and the beat goes on. J Clin Endocrinol Metab. 2003;88:31–3. doi: 10.1210/jc.2002-021753. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury IN, Sinaii N, Oldfield EH, Patronas N, Nieman LK. A change in pituitary magnetic resonance imaging protocol detects ACTH-secreting tumours in patients with previously negative results. Clin Endocrinol (Oxf) 2010;72:502–6. doi: 10.1111/j.1365-2265.2009.03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasaliwal R, Sankhe SS, Lila AR, Budyal SR, Jagtap VS, Sarathi V, et al. Volume interpolated 3D-spoiled gradient echo sequence is better than dynamic contrast spin echo sequence for MRI detection of corticotropin secreting pituitary microadenomas. Clin Endocrinol (Oxf) 2013;78:825–30. doi: 10.1111/cen.12069. [DOI] [PubMed] [Google Scholar]
- 5.Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH. Outcome of surgical treatment of 200 children with Cushing's disease. J Clin Endocrinol Metab. 2013;98:892–901. doi: 10.1210/jc.2012-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou JuH, Pan J, LV Y, Zhang JY. Evaluation of pituitary uptake incidentally identified on 18F-FDG PET/CT scan. Oncotarget. 2017 doi: 10.18632/oncotarget.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyun SH, Choi JY, Lee KH, Choe YS, Kim BT. Incidental focal 18F-FDG uptake in the pituitary gland: clinical significance and differential diagnostic criteria. J Nucl Med. 2011;52:547–50. doi: 10.2967/jnumed.110.083733. [DOI] [PubMed] [Google Scholar]
- 8.Jeong SY, Lee SW, Lee HJ, Kang S, Seo JH, Chun KA, et al. Incidental pituitary uptake on whole-body 18F-FDG PET/CT: a multicentre study. Eur J Nucl Med Mol Imaging. 2010;37:2334–43. doi: 10.1007/s00259-010-1571-5. [DOI] [PubMed] [Google Scholar]
- 9.Takano K, Yasufuku-Takano J, Teramoto a, Fujita T. Corticotropin-releasing hormone excites adrenocorticotropin-secreting human pituitary adenoma cells by activating a nonselective cation current. J Clin Invest. 1996;98:2033–41. doi: 10.1172/JCI119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patt HP, Lele V, Lila A, Bandgar T, Shah N. J Med Imaging Radiat Oncol. Vol. 58. Australia: 2014. Utility of hCRH-stimulated (18) F-FDG PET-CT scan in localisation of pituitary microadenoma in Cushing's disease; p. 213. [DOI] [PubMed] [Google Scholar]
- 11.Chittiboina P, Montgomery BK, Millo C, Herscovitch P, Lonser RR. High-resolution 18 F-fluorodeoxyglucose positron emission tomography and magnetic resonance imaging for pituitary adenoma detection in Cushing disease. J Neurosurg United States. 2014;122:1–7. doi: 10.3171/2014.10.JNS14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol Nature Publishing Group. 2013;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, et al. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–83. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- 14.Iredale PA, Duman RS. Glucocorticoid regulation of corticotropin-releasing factor1 receptor expression in pituitary-derived AtT-20 cells. Mol Pharmacol. 1997;51:794–9. doi: 10.1124/mol.51.5.794. [DOI] [PubMed] [Google Scholar]
- 15.Saphier PW, Faria M, Grossman a, Coy DH, Besser GM, Hodson B, et al. A comparison of the clearance of ovine and human corticotrophin-releasing hormone (CRH) in man and sheep: a possible role for CRH-binding protein. J Endocrinol. 1992;133:487–95. doi: 10.1677/joe.0.1330487. [DOI] [PubMed] [Google Scholar]
- 16.Giguère V, Labrie F, Côté J, Coy DH, Sueiras-Diaz J, Schally a V. Stimulation of cyclic AMP accumulation and corticotropin release by synthetic ovine corticotropin-releasing factor in rat anterior pituitary cells: site of glucocorticoid action. Proc Natl Acad Sci U S A. 1982;79:3466–9. doi: 10.1073/pnas.79.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moro M, Putignano P, Losa M, Invitti C, Maraschini C, Cavagnini F. The desmopressin test in the differential diagnosis between Cushing's disease and pseudo-Cushing states. J Clin Endocrinol Metab. 2000;85:3569–74. doi: 10.1210/jcem.85.10.6862. [DOI] [PubMed] [Google Scholar]
- 18.Colombo P, Passini E, Re T, Faglia G, Ambrosi B. Effect of desmopressin on ACTH and cortisol secretion in states of ACTH excess. Clin Endocrinol (Oxf) 1997;46:661–8. doi: 10.1046/j.1365-2265.1997.1330954.x. [DOI] [PubMed] [Google Scholar]
- 19.Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, et al. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther. 2008;7:3546–55. doi: 10.1158/1535-7163.MCT-08-0569. [DOI] [PubMed] [Google Scholar]
- 20.Vannucci S, Hawkins R. Substrates of energy metabolism of the pituitary and pineal glands. J Neurochem. 1983;41:1718–25. doi: 10.1111/j.1471-4159.1983.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 21.Viña JR, Page RB, Davis DW, Hawkins RA. Aerobic glycolysis by the pituitary gland in vivo. J Neurochem. 1984;42:1479–82. doi: 10.1111/j.1471-4159.1984.tb02814.x. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield EH, Merrill MJ. Apoplexy of pituitary adenomas: the perfect storm. J Neurosurg. 2015;122:1444–9. doi: 10.3171/2014.10.JNS141720. [DOI] [PubMed] [Google Scholar]
- 23.De Souza B, Brunetti A, Fulham MJ, Brooks RA, DeMichele D, Cook P, et al. Pituitary microadenomas: a PET study. Radiology. 1990;177:39–44. doi: 10.1148/radiology.177.1.2399336. [DOI] [PubMed] [Google Scholar]
- 24.Bergström M, Muhr C, Ericson K, Lundqvist H, Lilja A, Eriksson L, et al. The normal pituitary examined with positron emission tomography and (methyl-11C)-L-methionine and (methyl-11C)-D-methionine. Neuroradiology. 1987;29:221–5. doi: 10.1007/BF00451757. [DOI] [PubMed] [Google Scholar]
- 25.Koo CW, Bhargava P, Rajagopalan V, Ghesani M, Sims-Childs H, Kagetsu NJ. Incidental detection of clinically occult pituitary adenoma on whole-body FDG PET imaging. Clin Nucl Med. 2006;31:42–3. doi: 10.1097/01.rlu.0000191779.75532.05. [DOI] [PubMed] [Google Scholar]
- 26.Alzahrani AS, Farhat R, Al-Arifi A, Al-Kahtani N, Kanaan I, Abouzied M. The diagnostic value of fused positron emission tomography/computed tomography in the localization of adrenocorticotropin-secreting pituitary adenoma in Cushing's disease. Pituitary. 2009;12:309–14. doi: 10.1007/s11102-009-0180-4. [DOI] [PubMed] [Google Scholar]
- 27.Filetti S, Damante G, Foti D. Thyrotropin stimulates glucose transport in cultured rat thyroid cells. Endocrinology. 1987;120:2576–81. doi: 10.1210/endo-120-6-2576. [DOI] [PubMed] [Google Scholar]
- 28.Harris VM, Bendre SV, Gonzalez De Los Santos F, Fite A, El-Yaman El-Dandachli A, Kurenbekova L, et al. GnRH increases glucose transporter-1 expression and stimulates glucose uptake in the gonadotroph. J Endocrinol. 2012;212:139–47. doi: 10.1530/JOE-11-0359. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Xiao J, Xing B, Wang R, Zhu Z, Li F. Comparison of (68)Ga DOTATATE to 18F-FDG uptake is useful in the differentiation of residual or recurrent pituitary adenoma from the remaining pituitary tissue after transsphenoidal adenomectomy. Clin Nucl Med. 2014;39:605–8. doi: 10.1097/RLU.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 30.Heiden MG Vander, Cantley LC, Thompson CB, Mammalian P, Exhibit C, Metabolism A. Understanding the Warburg Effect : Cell Proliferation. Science (80-) 2009;324:1029–34. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Booth C, Tian L, Shipston MJ. Dissociation of early glucocorticoid inhibition of ACTH secretion and glucose uptake in mouse AtT20 D16:16 corticotrophs. J Neuroendocrinol. 1998;10:447–52. doi: 10.1046/j.1365-2826.1998.00226.x. [DOI] [PubMed] [Google Scholar]
- 32.Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J. 1994;8:1003–11. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


