Abstract
The goal of this study was to investigate 9-month-old infants’ ability to individuate and categorize other-species faces at the subordinate level. We were also interested in examining the effects of initial exposure conditions on infant categorization and individuation processes. Infants were either familiarized with a single monkey face in an individuation procedure or familiarized with multiple exemplars of monkey faces from the same species in a categorization procedure. Event-related potentials were recorded while the infants were presented: familiar faces, novel faces from the familiar species, or novel faces from a novel species. The categorization group categorized monkey faces by species at the subordinate level, whereas the individuation group did not discriminate monkey faces at the individual or subordinate level. These findings indicate initial exposure to multiple exemplars facilitates infant processing of other-species faces, and infants are efficient at subordinate-level categorization at 9 months of age.
Introduction
Being able to recognize a familiar face based on its unique features and categorize groups of novel faces based on their shared features is fundamental for human social interaction. However, humans are not born face experts, and mastering the ability to categorize and individuate faces is an important task of social development. Categorization is the process by which individual exemplars are classified into groups based on shared characteristics (e.g., classifying an animal as a cat), whereas individuation is the process of discriminating among exemplars of a category at the individual level (e.g., recognizing your family cat among a group of cats; Hugenberg et al., 2010). Infants show gains in processing faces at the individual (Mondloch, LeGrand, & Maurer, 2010) and categorical (Madole & Oakes, 1999) levels during infancy. For example, infants begin to categorize faces based on gender (e.g., Rennels, Kayl, Langlois, Davis, & Orlewicz, 2016), attractiveness (e.g., Ramsey, Langlois, Hoss, Rubenstein, & Griffin, 2004), and race (e.g., Anzures, Quinn, Pascalis, Slater, & Lee, 2010).
The development of categorization moves from general to specific in the first year of life. Superordinate (or global) categorization is the broadest level and has been seen in 2-month-olds when forming a category for animate objects that excludes inanimate objects (mammals vs. furniture; Quinn & Johnson, 2000). The next level, basic, is seen at 3–4 months of age, but not in younger infants. Three- to four-month-olds can form categories for different types of animals; for example, a category that includes cats but excludes horses (Eimas & Quinn, 1994). Subordinate-level representations are formed around 6–7 months of age (Eimas & Quinn, 1994). For example, 6- to 7-month-olds demonstrate evidence of forming categorical representations for Saint Bernards that excludes Beagles, which is a subordinate-level distinction among dog breeds (Quinn & Tanaka, 2007).
Several factors affect infants’ ability to form categories including initial exposure conditions and prior experience. For example, Oakes and Ribar (2005) tested 4-month-old infants’ ability to distinguish between cats and dogs using different familiarization procedures. One group was familiarized with paired presentations of two stimuli at the same time, and another group was familiarized with sequential presentations of a single stimulus. Infants given paired presentations of multiple stimuli were able to demonstrate basic-level categorization of cats and dogs. In contrast, infants given sequential presentations of a single stimulus during familiarization did not demonstrate basic-level categorization. Thus, exposure to multiple exemplars during familiarization may lead infants to engage in a process of comparison which likely facilitates infant categorization (also see: Cassasola & Park, 2013; Oakes, Kovack-Lesh, & Horst, 2009; Rose et al., 1982; Vukatana, Graham, Curtin, & Zepeda, 2015). Kovack-Lesh, Oakes, and McMurray (2012) examined the impact of prior experience and visual scanning on categorization for 4-month-olds. They found that infants were more likely to demonstrate basic-level categorization of cats versus dogs if they had cats at home and exhibited high switch rates in their looking behavior during testing, whereas infants were less likely to demonstrate basic-level categorization if they did not have cats at home and exhibited low switch rates. This finding indicates that regular exposure to individual members of other species (i.e., family pets) facilitates infants’ ability to form a basic-level category for that species that excludes other species.
There has been a great deal of research documenting perceptual narrowing in infant face processing, (for reviews, see Mauer & Werker, 2014; Scott, Pascalis, & Nelson, 2007). Perceptual narrowing occurs as infants transition from having perceptual sensitivities that are broadly tuned to a wide range of faces to being more narrowly focused and specialized for processing faces encountered regularly in the native environment. The other-species effect (OSE) is an example of perceptual narrowing in face processing. The body of behavioral research on this effect suggests that there is maintenance of perceptual sensitivity for own-species faces and a decrease in perceptual sensitivity for other-species faces (e.g, Pascalis et al., 2005; Simpson, Varga, Frick, & Fragaszy, 2011).
In one of the earliest studies on the OSE in infancy, Pascalis, de Haan, and Nelson (2002) tested infants at 6 and 9 months of age using a visual paired-comparison (VPC) task. The infants were familiarized with either a single monkey or a single human face for 20s of accumulated looking, and were then tested in VPC trials with the familiarized face paired with a novel face of the same species. At 6 months of age, infants demonstrated evidence of discrimination of the familiar face from the novel face in both the human and monkey conditions. In contrast, at 9 months of age, infants only demonstrated discrimination of the familiar face from the novel face in the human face condition. This finding indicates that from 6 to 9 months of age, infants maintain the ability to individuate own-species faces but show a decline in their ability to individuate other-species faces. Simpson and colleagues (2014) have proposed a learned attention model that states that with age, infants’ sensitivity increases for facial dimensions useful for identification, and this effect is strongest for own-species faces due to learned attention that occurs with experience. Consistent with this proposal, research has shown that infants trained with picture books of individually-labeled monkey faces between 6 to 9 months of age (Pascalis et al., 2005; Scott & Monesson, 2009, 2010) maintain the ability to individuate other-species faces at 9 months of age.
The event-related potential (ERP) has been widely used in research on face processing in both infants and adults. The N290 and P400 have been identified as ERP components related to face processing in infancy (de Haan, 2007; de Haan, Johnson, & Halit, 2003), and these components are believed to be precursors to the adult N170 face processing component (Halit, de Haan, & Johnson, 2003; Scott & Monesson, 2010). The N290 is a negatively polarized component that occurs over midline and posterior electrodes with peak latency between 290 and 350 ms after stimulus onset (Halit et al., 2003). By 3 months of age, the N290 is greater in amplitude to faces than noise (Halit, Csibra, Volein, & Johnson, 2004). The P400 is a positive-going component that occurs over midline posterior electrodes and reaches peak amplitude between 390 and 450 ms after stimulus onset. By 6 months of age, the P400 has a shorter latency to peak in response to faces than objects (de Haan & Nelson, 1999), and by 12 months of age, the P400 is shorter in latency to upright versus inverted human faces (Halit et al., 2003).
Scott, Shannon, and Nelson (2006) conducted one of the first ERP studies examining the OSE in infancy. Two groups of 9-month-old infants were tested using both own- and other-species faces. In the first condition, infants were familiarized with a human face in a frontal orientation and then tested for recognition of the familiar face in a different orientation in comparison a novel face in similar (frontal) and dissimilar (profile) orientations. The second condition was identical except that monkey faces were used instead of human faces. In contrast to the behavioral findings (Pascalis et al., 2002), 9-month-olds demonstrated recognition of previously viewed human and monkey faces. Infants demonstrated greater amplitude N290 to familiar compared to novel faces regardless of species. Furthermore, the P400 component was greater in amplitude to novel compared to familiar monkey faces. However, infants demonstrated differential amplitude of the P400 to human faces based on both familiarity and orientation. The authors concluded that although the infants demonstrated evidence of successful discrimination of novel and familiar monkey faces, the orientation effects found only in the P400 response to human faces indicated that the 9-month-olds were processing human faces at a more specific and specialized level (Scott et al., 2006).
Stimulus inversion has also been used in research to examine depth of processing of faces. The face-inversion effect (FIE) in which processing of inverted faces is impaired or delayed compared to upright faces is believed to be a marker for configural processing of face stimuli for infants and adults (Rossion & Curran, 2010; Yovel & Kanwisher, 2005). Although 9-month-old infants do not typically show inversion effects in ERP responding to monkey faces, Scott and Monesson (2010) found that 9-month-olds given three months of training with pictures of monkey faces labeled at the individual level do show inversion effects on the averaged peak-peak amplitude of the N290 and P400 components. This finding, coupled with the finding that 9-month-old infants given similar training can demonstrate behavioral evidence of discriminating monkey faces in the VPC task (Pascalis et al., 2005; Scott & Monesson, 2009), indicates that regular exposure to monkey faces labeled at the individual level in infancy can lead to a maintenance of face processing abilities at 9 months of age. However, these studies tested for evidence of individuation of monkey faces, but did not test for evidence of categorization.
Grossman and colleagues (Grossman, Gliga, Johnson, & Mareschal, 2009) conducted one of the first ERP studies examining infant categorization (see also, Leppänen, Richmond, Vogel-Farley, Moulson, & Nelson, 2009). Six-month-old infants were shown a series of repeated and non-repeated bird and object images. The authors found that differential amplitude of P1, an early latency posterior component involved in basic visual processing, was associated with repetition of an individual exemplar. The Negative central (Nc), a mid-latency anterior component associated with attentional engagement (Courchesne, Ganz, & Norcia, 1981; de Haan & Nelson, 1997, 1999; Reynolds 2016; Reynolds & Richards, 2005; Reynolds, Courage, & Richards, 2010; Reynolds & Romano, 2016) was found to be greater in amplitude to exemplars from a novel category. The authors concluded that the 6-month-olds had formed basic-level categories for the familiar objects or vehicles, and the Nc effects reflected greater attention to stimuli from novel categories.
Quinn, Doran, Reiss, & Hoffman (2010) recorded ERP data while 6- to 7-month-old infants were familiarized with multiple exemplars of Saint Bernard images, and then shown a series of novel Saint Bernard images interspersed with novel Beagle images. In addition to analyzing the Nc and P400 components, the authors analyzed the positive slow wave (PSW). Differential amplitude of PSW for familiar compared to novel stimuli has been utilized as an electrophysiological index of recognition memory in infancy (de Haan & Nelson, 1999; Guy, Reynolds, & Zhang; 2013; Reynolds, Guy, & Zhang, 2011; Nelson & Collins, 1991, 1992; Snyder, Webb, & Nelson, 2002; Snyder et al., 2010; Wiebe et al., 2006). Quinn and colleagues (2010) found that Nc amplitude was associated with novel category preference at the basic level. Novel category preference at the subordinate level was associated with differential amplitude of both the Nc and P400 components. It was concluded that the mechanisms involved in subordinate-level categorization are supplementary to the mechanisms involved in basic-level categorization, and that the P400 component is related to subordinate-level categorization. Infants also demonstrated differential amplitude of the PSW based on basic- and subordinate-level processing. The differences in PSW amplitude based on basic-level processing occurred at an earlier latency than differences in PSW amplitude based on subordinate-level processing. The authors proposed that these latency differences reflect a course-to-fine sequence of category formation in 6- to 7-month-olds.
In a more recent study, Peykarjou, Pauen, and Hoehl (2014) used a rapid repetition paradigm to examine 9-month-old infants’ categorization of human and ape faces at three different categorical levels–superordinate, basic, and individual. They found that infants first identified the stimuli as “faces” (the superordinate level) as shown by a greater amplitude P1 to faces than houses. They then categorized the faces as either ape or human as shown by differential amplitude of the N290. Surprisingly, no individual-level responses were observed for the human or ape faces. The authors concluded that the rapid repetition procedure may have interfered with the 9-month-olds’ ability to individuate human faces.
The current study
Taken together, the findings from the extant literature indicate that several factors, including familiarization procedure, influence individuation and categorization of other-species faces in infancy. The goal of the current study was to examine the effects of familiarization condition on infants’ individuation and subordinate-level categorization of other-species faces at 9 months of age. Although familiarization condition has been systematically manipulated in behavioral research of infant categorization (e.g., Oakes & Ribar, 2005) and ERP research on infant recogniton memory (Reynolds & Richards, 2005), no study to date has examined the impact of familiarization on neural correlates of subordinate-level categorization in infancy. Additionally, although many studies have tested 9-month-olds on individuation and basic-level categorization of monkey faces, their ability to categorize monkey faces at the subordinate level has yet to be examined. We thus utilized ERPs to examine neural correlates of individuation and subordinate-level categorization of other-species faces by 9-month-old infants. Infants were divided into two groups: a categorization group and an individuation group. The participants in the categorization group were familiarized with sequential presentations of multiple exemplars from the same monkey species (similar to Quinn et al., 2010), whereas the participants in the individuation group were familiarized with repeated presentations of the same individual monkey face. Following familiarization, both groups were presented with equal probability presentations of three different stimulus types: (1) novel faces from the same species as the familiar category (i.e., novel-same trials), (2) novel faces from a different species than the familiar category (i.e., novel-other trials), and (3) the face (or one of the faces) used during familiarization (i.e., familiar trials). We also collected ERP data during the initial familiarization trials (i.e., learning trials), and included this as the fourth stimulus type in our analysis.
Predictions
A summary of our hypotheses is presented in Table 1. Given Grossman and colleagues’ (2009) research indicating P1 represents early visual processing sensitive to basic stimulus repetition, we predicted that the individuation group would show reduced P1 amplitude for familiar trials in comparison to learning trials. Based on the finding that 9-month-olds demonstrate differential amplitude of the N290 based on familiarity regardless of species (Scott et al., 2006), we predicted that infants would demonstrate greater amplitude N290 on familiar trials in comparison to learning, novel-same, and novel-other trials. Because 6- to 7-month-olds demonstrate subordinate-level categorization of other-species through greater amplitude Nc and P400 to exemplars from novel categories (i.e., Beagles compared to Saint Bernards; Quinn et al., 2010), we predicted that the categorization group would show greater amplitude Nc and P400 to novel-other species monkey faces in comparison to novel-same species and familiar monkey faces representing greater attention to the novel category of faces. Findings have been mixed on 9-month-olds’ ability to individuate monkey faces with 20 s of exposure and most of the relevant ERP work using monkey faces has not focused on Nc. Because the bulk of the literature indicates relatively poor individuation of monkey faces for 9-month-olds without supplemental training (e.g., Pascalis et al., 2002, 2005; Scott & Monesson, 2009, 2010), we predicted that the individuation group would likely show equivalent Nc and P400 amplitude across all stimulus types. Our prediction that the categorization group would demonstrate differential Nc and P400 based on stimulus type and the individuation group would not was also based on research indicating that exposure to multiple exemplars during familiarization facilitates categorization (e.g., Cassosola & Park, 2013; Oakes & Ribar, 2005). Finally, based upon Quinn and colleagues’ (2010) finding that PSW activity may reflect both basic- and subordinate-level categorization processes, we predicted that infants in the categorization group would display reduced amplitude PSWs on familiar, novel-other, and novel-same trials in comparison to learn trials. Because the bulk of the extant literature indicates relatively poor individuation of monkey faces for 9-month-old infants and poor performance on recognition memory tasks for infants familiarized with a single stimulus (Oakes et al., 2009; Rose et al., 1982), we predicted no differences in PSW amplitude across stimulus types for the individuation group indicating a lack of recognition of the familiar face.
Table 1.
Hypotheses for ERP Components by Familiarization Condition
| Component | Categorization group | Individuation group |
|---|---|---|
| P1 | No differences | Greater amplitude on learning trials in comparison to familiar |
| N290 | Greater amplitude to familiar in comparison to all other trials | Greater amplitude to familiar in comparison to all other trials |
| Nc | Greater amplitude to novel-other in comparison to novel-same and familiar | No differences |
| P400 | Greater amplitude to novel-other in comparison to novel-same and familiar | No differences |
| PSW | Reduced amplitude on familiar, novel-other, and novel-same in comparison to learn trials | No differences |
Method
Participants
A sample of 29 infants (14 female, 15 male) were tested within 2 weeks of their 9-month birthdate. The mean age of infants at testing was 278 days (SD = 4.94, range = 266–288). Only infants who were born full-term (at least 38 weeks gestation) with no complications and a normal birth weight were recruited. Participants were drawn from a predominantly Caucasian and middle-class population. The racial distribution of infants included in the final dataset was: 26 Caucasian, 1 African American, 1 Asian, 1 Hispanic. An additional 28 infants were tested, but not included in the final sample due to distractibility/fussiness, insufficient number of artifact free ERP trials (i.e., minimum of 8 per condition), or technical problems. This attrition rate falls within a normal range for infant ERP studies (DeBoer et al., 2007).
Apparatus
Participants were seated in their parents’ lap in a sound-attenuated room, 55 cm away from a 27″ color LCD monitor (Dell 2707 WFP). To limit distraction during testing, the testing area was surrounded by black curtains on all sides except for the side located behind the participant. Room lighting was dimmed throughout testing. A digital camcorder (Sony DCR-HC28) was located just above the monitor for judging infant visual fixations. Fixations were judged online for the purpose of experimental control. The video was also recorded through use of Netstation software produced by Electrical Geodesics Incorporated (EGI; Eugene, Oregon). The Netstation was used to record EEG data and to synchronize this data with the video. The experimental procedure was controlled using E-Prime 2.0 software (Psychology Software Tools, Inc.; Sharpsburg, PA.). The E-Prime program sent experimental events to the Netstation and utilized a single-clock system to time-lock these experimental events with the EEG and video data.
Visual Stimuli
Other-species faces
Digital oval-shaped color photographs of adult capuchin and macaque faces were presented sequentially on the monitor (see Figure 1). The macaque images were drawn from the same database used in previous research in the area (Pascalis et al., 2005; Scott & Monesson, 2009, 2010). Images of capuchin faces were provided by Catherine F. Talbot with permission from the Georgia State University Language Research Center. Faces subtended a 20° square visual angle on the monitor from the position of the participant, and were centered on the screen against a white background.
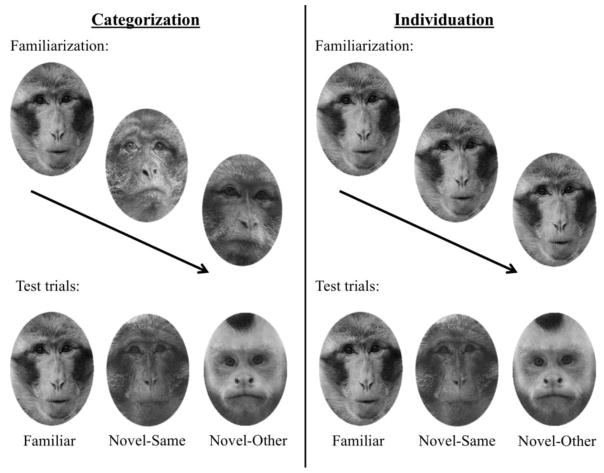
Figure 1.
Diagram of Experimental Procedure. A diagram of the experimental procedure including examples of the face stimuli used in the study is shown. The left panel provides a diagram of the categorization procedure, and the right panel presents a diagram of the individuation procedure. The face stimuli are presented in grayscale for publication purposes.
Attractor stimuli
During the familiarization phase, an orange star was used as an attractor to ensure that the infant was centrally fixated prior to every stimulus presentation. The orange star subtended a 15° square visual angle and was presented in the middle of the screen against a white background. Sesame Street clips: Video clips (with audio) of Sesame Street characters were used to redirect infants’ interest and attention to the monitor during the second phase of testing if they became distracted. The video covered a 15° square area centered on the monitor.
Procedure
After informed consent was obtained, infants were seated on a parent’s lap in the testing room. An appropriately sized EGI sensor net was then selected and placed on the infant. Net application took about 5–10 minutes. During the application of the net, a second experimenter interacted with the infant using infant-directed speech, silly faces, and/or rattles or other toys as a distraction to decrease the likelihood of fussiness.
The experiment had two stages (see Figure 1). The first stage was the familiarization phase of the experiment (i.e., learning trials). The individuation group (n = 15; 5 female/10 male) was familiarized through repeated presentations of a single monkey face presented twenty times. The image was presented centrally for 1000 ms following the presentation of an attractor stimulus used to ensure central fixation. When the infant was judged to be centrally fixated on the attractor stimulus, the experimenter initiated a stimulus presentation through a button press. The 1000 ms stimulus presentation was preceded by a 200 ms blank white screen (used for prestimulus ERP baselines) and followed by a blank white screen that varied randomly in duration from 1000–1500 ms. The categorization group (n = 14; 9 female/5 male) was shown 10 different monkey faces during familiarization, each face was presented twice for a total of 20 stimulus presentations. The timing of each stimulus presentation was identical to the timing of presentations in the individuation condition. The 10 monkey faces used for each categorization participant were drawn from the same species (either capuchin or macaque). For both the individuation and categorization conditions, the use of either capuchin or macaque faces for the familiarization condition was counter-balanced across participants to control for potential species effects. The second stage began immediately after the completion of the familiarization stage. The second stage consisted of ERP test trials consisting of presentations of three stimulus types: novel individual faces from the same species as the familiar faces (novel-same), novel faces from the other species than the familiar faces were from (novel-other), and faces used in the familiarization phase (familiar). The presentation of faces from these stimulus conditions was done in pseudo-random order, with equal probability. If an infant became distracted, dynamic Sesame Street video clips with accompanying sound tracks were played to regain the infant’s attention. Looking behavior was analyzed offline to ensure infants were looking during each trial. Trials in which infants were not looking or looked away during stimulus presentation were excluded from analyses. Testing continued until the infant was no longer on task, and typically lasted about 10 minutes. On average, infants completed 126 trials for both the 1st and 2nd stage. (SD = 28).
EEG Recording
The Electrical Geodesics Incorporated (EGI) Geodesic EEG System 300 (GES 300) 128 channel EEG recording system was used. This system consisted of infant-sized HydroCel Geodesic Sensor (HCGSN) nets, a GES 300 amplifier, and the Netstation recording program. The HCGSN sensor nets are arranged in a configuration of electrode pedestals on which 124 electrodes are mounted within electrolytic sponges. The net was soaked in an electrolyte solution before use. The elasticity of the net maintains the spacing between electrodes once it is placed, using the mastoid, nasion, and vertex locations to find the correct position on the participant. The average interelectrode distance of the electrodes on the scalp is 21 mm. Electrode impedances were measured prior to testing, and generally ranged from 10 to 50 kΩ. If during sensor net application, the impedance of an electrode was deemed as high (>100 kΩ), the electrode was repositioned until impedance values reached an acceptable level. A Dell Workstation was used to control the experimental protocol and to send time-locked experimental events to the EEG acquisition computer. The delay between the timing of the stimulus presentation initiated by the E-Prime computer and the actual display of the stimulus on the presentation monitor was determined using a photoreceptor and verified to have no drift across testing sessions. This stimulus display offset was corrected for during ERP segmenting. The EEG was sampled at 250 Hz with 20K amplification. The EEG recordings were band-pass filtered from 0.3 to 30 Hz.
The EEG was edited using the Netstation software to identify saccades, blinks, movement artifact, and poor recording. Artifact was defined as Δ > 250μV/250ms. EEG channels were marked bad if artifact was found. Trials in which over 10% of the channels were marked bad due to excessive artifact or noise were excluded from the analysis. For trials that were retained for the ERP analysis, individual channels marked bad were replaced using a spherical spline interpolation (Perrin, Pernier, Bertrand, Giard, & Echallier, 1987; Srinivasan, Tucker, & Murias, 1998). Only those participants who contributed a minimum of 8 artifact-free ERP trials per condition for stable ERP averages were included in the final dataset (Farroni, Johnson, & Csibra, 2004; Reynolds, Guy, & Zhang, 2011; Reynolds & Richards, in press). The average number of trials contributed per condition were: 15.28 trials (SD = 3.40) in the learning condition, 16.00 trials (SD = 4.44) in the familiar condition, 15.38 trials (SD = 3.80) in the novel-other condition, and 16.07 trials (SD =3.94) in the novel-same condition.
ERP averages were calculated from 200 ms before stimulus onset to 1500 ms after stimulus onset. The latency, duration, and electrode locations used to analyze each component of interest were determined primarily based on previous findings (Nc and PSW components: Guy, Reynolds, Mosteller, & Dixon, 2017; Quinn et al., 2010; Reynolds & Richards, 2005; N290 and P400 components: Peykarjou et al., 2014; Quinn et al., 2010; Reynolds, Bahrick, Lickliter, & Guy, 2014; Scott et al., 2006) as well as inspection of the grand average waveforms (averaged across experimental groups) to determine the electrode locations where each component was most prominent (DeBoer, Scott, & Nelson, 2007). The P1 was analyzed as the mean amplitude of the ERP from 75 – 175 ms following stimulus onset at midline occipital electrodes (81, 74, 82, 75, 73, and 88). The N290 was analyzed as the mean amplitude of the ERP from 225–325 ms following stimulus onset at the same midline occipital electrodes as the P1, and the P400 was also analyzed at these electrodes as the mean amplitude of the ERP from 350–600 ms following stimulus onset. Nc was analyzed as the mean amplitude of the ERP from 350–650 ms following stimulus onset at midline central electrodes (7, 106, 13, 6, and 112). The PSW was analyzed as the mean amplitude of the ERP from 600–1500 ms following stimulus onset at left frontal electrodes (24, 27, 28, and 20), and right frontal electrodes (124, 117, 118, and 123).
Design for Statistical Analysis
The ERP component averages were analyzed for experimental effects using 2 × 2 × 4 multivariate analysis of variance (MANOVA) with familiarization condition (2: categorization, individuation) and sex (2: female, male) as between-subjects factors, and stimulus type (4: learning, familiar, novel-other, novel-same) as a within-subjects factor. We also analyzed hemisphere (Guy et al., 2013, 2017; Reynolds et al., 2014) as a within-subjects factor for the PSW (2: left frontal, right frontal). For significant interaction effects, follow-up analyses were done using ANOVAs and the least significant differences test (LSD) as recommended by Saville (1990). The Greenhouse-Geisser correction was used in cases of violations of the assumption of sphericity. Effect sizes (ηp2) are reported on all significant effects, and all significant tests are reported at p < .05.
Results
The P1 component
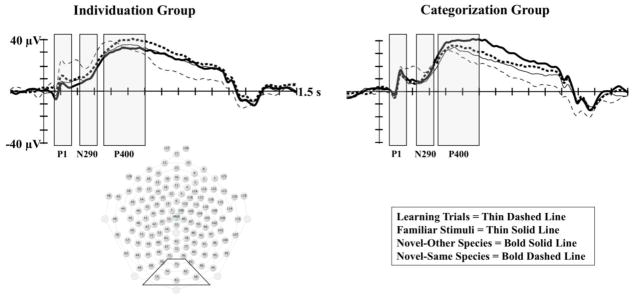
Table 2 presents a summary of the significant findings from our analyses. The results of the MANOVA done on P1 amplitude at midline occipital electrodes (Oz area) revealed a significant main effect of stimulus type, F(3, 25) 10.42; p < .01; Wilks Δ = .444; ηp2=.556). Infants demonstrated significantly greater amplitude P1 during learning trials (M = 17., SE = 1.92) in comparison to the familiar (M = 3.34, SE = 1.55), novel-other (M=5.31, SE = 1.85), and novel-same (M = 6.05, SE = 1.17) trials. This main effect was qualified by a significant interaction of stimulus type and familiarization condition, F(3, 25) 3.09; p < .05; Wilks Δ = .729; ηp2=.271. Follow up tests indicated there was a significant effect of stimulus type for the individuation group, F(1, 14) 29.50; p < .01; ηp2=.678. As can be seen in Figure 2, infants in the individuation group showed significantly greater amplitude P1 on learning trials (M = 19.83, SE = 2.66) in comparison to familiar (M = 5.98, SE = 1.38), novel-other (M = 2.46, SE = 1.87), and novel-same trials (M = 4.37, SE = 1.71). In contrast, the effect of stimulus type was not significant for the categorization group (p > .05 for all comparisons).
Table 2.
Analysis Results for ERP Components by Familiarization Condition
| Component | Categorization group | Individuation group |
|---|---|---|
| P1 | No differences | Greater amplitude to learning trials as compared to familiar |
| N290 | No differences | No differences |
| Nc | Greater amplitude to novel-other in comparison to novel-same and familiar | No differences |
| P400 | Greater amplitude to novel-other in comparison to novel-same and familiar | No differences |
| PSW | At left frontal electrodes:
|
No differences |
Figure 2.
The P1, N290, and P400 Components by Familiarization Group and Stimulus Type. The P1, N290, and P400 components are presented by familiarization group and stimulus type. The left panel shows ERP waveforms for the individuation group at Oz (75), and the right panel shows ERP waveforms for the categorization group at Oz. The midline occipital electrode cluster used in the analyses is indicated in the sensor net layout shown to the bottom left. The open rectangles indicate the time window of the analyses for each component.
The N290 component
There were no significant main or interaction effects found from the MANOVA on mean amplitude of the N290 at midline occipital electrodes (Oz area). However, given the significant main and interaction effects described above on the amplitude of the P1 component, which preceded the N290 at occipital electrodes (see Figure 2), we carried out a supplementary peak-to-trough analysis on the change in amplitude of the ERP from the time window of the analysis of the P1 to the time window of the analysis of the N290. Similar to the standard analysis of mean amplitude reported above, no significant main or interaction effects were found in the MANOVA for the peak-to-trough analysis. The interested reader is referred to Picton and colleagues (2000) for points regarding the advantages of baseline-to-peak analyses over peak-to-peak and peak-to-trough analyses.
The P400 component
The results of the MANOVA run on P400 amplitude at midline occipital electrodes (Oz area) revealed a main effect of stimulus type, F(3,23) 3.98; p < .05; Wilks Δ = .658; ηp2 =.342. This main effect was qualified by a significant interaction of familiarization condition and stimulus type, F(3,23) 3.95; p = .05; Wilks Δ = .660; ηp2 =.340. Follow-up ANOVAs run separately for each familiarization condition indicated no differences based on stimulus type for the individuation group (p = .97). In contrast, as illustrated in Figure 2, infants tested in the categorization condition demonstrated significantly greater amplitude P400 (all ps < .05) on novel-other trials (M = 35.35, SE = 4.98) in comparison to learning trials (M = 20.42, SE = 4.44), familiar trials (M = 21.07, SE = 4.89), and novel-same trials (M = 27.90, SE = 3.05).
The Nc component
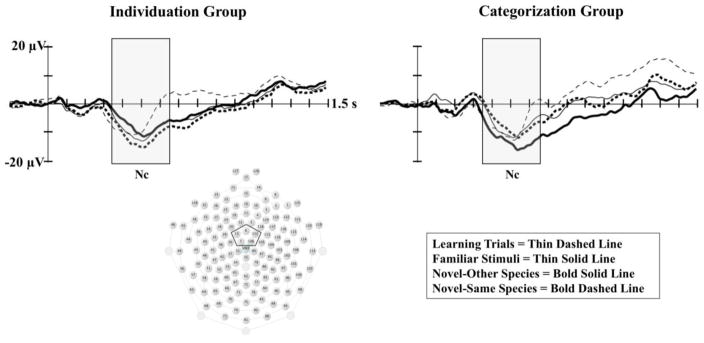
A MANOVA run at midline central electrodes (Cz area) indicated a significant interaction of familiarization condition and stimulus type on Nc amplitude, F(3,25) 3.81; p =.02; Wilks Δ = .686; ηp2=.314. Similar to the analysis of the P400 component, follow-up ANOVAs run separately for each familiarization condition indicated no differences based on stimulus type for the individuation group (p = .69). In contrast, as can be seen in Figure 3, the categorization group demonstrated significantly greater amplitude Nc (all ps < .05) on novel-other trials (M = −12.73, SE = 2.02) in comparison to learning trials (M = −6.64, SE = 1.70), familiar trials (M = −7.75, SE = 1.90), and novel-same trials (M = −8.43, SE = 1.48).
Figure 3.
The Nc Component by Familiarization Group and Stimulus Type. The Nc component is presented by familiarization group and stimulus type. The left panel shows ERP waveforms for the individuation group at lead 6, and the right panel shows ERP waveforms for the categorization group at lead 6. The midline central electrode cluster used in the analyses is indicated in the sensor net layout shown to the bottom left. The open rectangles indicate the time window for the analysis of Nc.
The PSW component
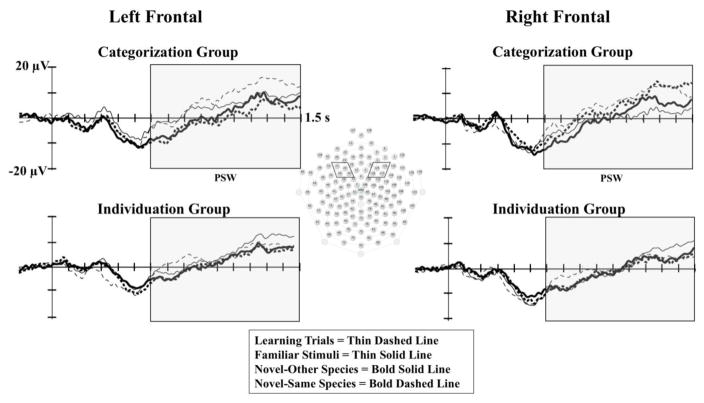
For the MANOVA conducted on PSW amplitude, we included hemisphere as a factor, using left frontal (F3 area) and right frontal (F4 area) electrodes. The MANOVA revealed a main effect of stimulus type, F(3,23) 3.27; p =.04; Wilks Δ = .701; ηp2=.299. This main effect was qualified by a significant three-way interaction of familiarization condition, stimulus type, and hemisphere, F(3,23) 4.54; p =.012; Wilks Δ = .628; ηp2=.372 (see Figure 4).
Figure 4.
The PSW Component by Familiarization Group, Stimulus Type, and Hemisphere. The PSW is presented by familiarization group, stimulus type, and hemisphere. The left panel shows ERP waveforms by group at F3 (24), and the right panel shows ERP waveforms by group at F4 (124). The left and right frontal electrode clusters used in the analyses are indicated in the sensor net layout shown in the center. The open rectangles indicate the time window of the analyses of the PSW.
To follow-up on this interaction, two-way ANOVAs were run separately for each familiarization group. For the individuation group, there were no differences in PSW amplitude based on stimulus type (p = .78), and no PSW amplitude differences based on the interaction of stimulus type and hemisphere (p = .93). For the categorization group, there was a significant main effect of stimulus type, F(3, 39) 4.98; p < .01; ηp2=.277. This main effect was qualified by a significant interaction of stimulus type by hemisphere, F(3,39) 5.78; p = .03; ηp2=.308. At left frontal electrodes, infants in the categorization group demonstrated significantly greater amplitude PSW on learning trials (M = 7.68, SE = 1.46) in comparison to novel-other trials (M = 0.39, SE = 2.22) and novel-same trials (M = 1.49, SE = 2.54). Additionally, infants in the categorization group demonstrated significantly greater amplitude PSW on familiar trials (M = 6.79, SE = 1.67) in comparison to novel-other trials. At right frontal electrodes, infants in the categorization demonstrated significant greater amplitude PSW on learning trials (M = 7.24, SE = 1.69) in comparison to familiar trials (M = −0.71, SE = 1.28) and novel-other trials (M = −1.41, SE = 1.99). Additionally, infants demonstrated greater amplitude PSW on novel-same trials (M = 3.52, SE = 2.93) in comparison to novel-other trials.
Discussion
The present study examined 9-month-old infants’ ability to individuate and categorize monkey faces when familiarized to either an individual face or a selection of exemplar faces from the same monkey species. We expected exposure to multiple exemplars to facilitate infants’ processing of faces, and thus predicted that infants in the categorization group would show evidence of subordinate-level categorization. In contrast, we predicted that infants in the individuation group would only demonstrate evidence of basic repetition effects on early visual processing, but that they would show no evidence of recognition memory in later latency ERP components associated with attention and recognition memory.
The P1 component
The P1 is an early latency component associated with early visual processing. Grossman and colleagues (2009) examined 6-month-old infants’ basic-level categorization of birds and vehicles and found that the P1 is sensitive to stimulus repetition with greater amplitude P1 to repeated stimuli in comparison to non-repeated stimuli during familiarization trials, and greater amplitude to non-repeated stimuli in comparison to repeated stimuli during test trials. This led the authors to conclude that the P1 is associated with basic repetition effects during early visual processing. Peykarjou and colleagues (2014) examined superordinate- and basic-level categorization of human and monkey faces for 9-month-old infants. They found that P1 was reduced in amplitude to primed stimuli that followed a prime from the same category. This was interpreted as representing categorical repetition effects early in the visual processing stream.
In the current study, we predicted that infants in the individuation group would demonstrate greater amplitude P1 on learning trials in comparison to familiar trials indicative of repetition effects (Grossman et al., 2009; Peykarjou et al., 2014). We did not expect this effect for the categorization group since infants in that group were shown multiple exemplars during familiarization instead of a single exemplar. The results supported our prediction for the P1. Infants demonstrated greater P1 amplitude on learning trials in comparison to all other trials. There was also a significant interaction of familiarization group and stimulus type on P1 amplitude. Infants in the individuation group showed greater amplitude P1 on learning trials in comparison to all other stimulus type trials.
The finding that the learning trials were significantly greater in P1 amplitude than both the novel-same and novel-other trials indicates this effect was not simply due to the novelty of the monkey faces at the start of testing. Instead, this general reduction in P1 amplitude from the first 20 (learning) trials to the subsequent test trials is most likely an effect of trial order. Past research has shown similar reductions in amplitude across testing sessions for the Nc component (Wiebe et al., 2006) and the PSW (Snyder, Webb, & Nelson, 2002). It is worthwhile to note that this effect was only significant for the P1 component. We did not find general amplitude reductions from learning trials to test trials for the later latency components. The Nc, P400, and PSW were affected by more specific effects driven by interactions of stimulus type and familiarization condition which indicates that the P1 may represent a more basic response associated with early visual processing and perhaps super-ordinate level categorization (Pekarjou et al., 2014) whereas the mid-latency (Nc and P400) components and PSW are most likely involved in higher-level processing related to attention, subordinate-level categorization, and recognition.
The N290 Component
The N290 is a face-sensitive ERP component that may be a precursor to the adult N170 component (de Haan et al., 2002, 2003, 2007; Halit et al., 2003; Reynolds et al., 2014). The N290 increases in specificity with age, and shows inversion effects for both human and monkey faces by 12 months (de Haan, Pascalis, & Johnson, 2002; Halit et al., 2003). Furthermore, infants demonstrate differential amplitude N290 to monkey faces in comparison to human faces (de Haan et al., 2002; Peykarjou et al., 2014). These findings indicate that the N290 may be related to basic-level category distinctions (inverted versus upright; human versus monkey).
For the N290, we predicted that infants would demonstrate greater amplitude N290 on familiar trials in comparison to all other trials. The results of the current study did not support our prediction. No significant experimental effects were found in our analysis of the N290. The lack of a significant effect of stimulus type on N290 in the current study was unexpected given Scott and colleagues’ (2006) finding that 9-month-old infants demonstrate greater amplitude of the N290 for familiar compared to novel monkey faces. The contrasting findings may have been due to procedural differences across studies. Scott and colleagues (2006) presented infants with repeated presentations of the familiar monkey in multiple orientations, which may have facilitated the infants’ individuation of the familiar from novel faces. We also found no N290 effects for our categorization group. In contrast, Peykarjou and colleagues (2014) found differential amplitude of the N290 for 9-month-olds in response to human compared to monkey faces which indicates that the N290 may be associated with basic- or superordinate-level categorization as opposed to the subordinate-level our categorization procedure was focused on. Previous infant ERP work on subordinate-level categorization (e.g., Quinn et al., 2006) has focused on the Nc, P400, and PSW components. Therefore, further research is needed to determine exactly what level(s) of categorization the N290 may be associated with throughout infancy.
The Nc and P400 components
Past research has found the Nc and P400 to be associated with attention and categorization (e.g., Hoehl, 2016; Grossman et al., 2009; Quinn et al., 2010). For the Nc and P400, we predicted that the categorization group in the current study would demonstrate greater amplitude Nc and P400 to novel-other faces in comparison to novel-same and familiar faces indicative of subordinate-level categorization. We also predicted that the individuation group would demonstrate equivalent Nc and P400 amplitude across conditions indicating an inability to fully process monkey faces at the individual level when only given 20 s of familiarization. The current findings were consistent with these hypotheses. The categorization group demonstrated greater amplitude Nc and P400 to novel-other species faces in comparison to all other stimulus types. This indicates greater attention to monkey faces categorized at the subordinate-level. Importantly, the individuation group showed no differences in Nc or P400 amplitude based on stimulus type indicating that this group did not discriminate novel monkey faces from the familiar face at either the individual level or the species level. The null findings for the individuation group indicate the categorization group’s greater amplitude Nc and P400 to novel-other faces in comparison to the familiar and novel-same faces was not simply due to the distinctiveness of cross-species comparisons. Thus, 9-month-old infants are quite efficient at categorizing other-species faces at the subordinate level when given exposure to multiple exemplars during category learning, whereas, they show no evidence of individuating monkey faces when exposed to repeated presentations of a single exemplar during learning.
The Positive Slow Wave
Based on Quinn and colleagues (2010) findings indicating the PSW may reflect both basic- and subordinate-level categorization processes, we predicted that infants in the categorization group would show significant differences in PSW amplitude on learning trials in comparison to the familiar, novel-other, and novel-same trials. We predicted no differences in PSW amplitude based on stimulus type for the individuation group. The results partially supported our predictions. For the individuation group, there were no differences in PSW amplitude based on stimulus type, again indicating the infants were unable to individuate monkey faces in this procedure. However, for the categorization group, there was an interaction of stimulus type and hemisphere. At left frontal electrodes, the PSW was significantly greater in amplitude on learning and familiar trials in comparison to novel-other trials. Additionally, the PSW was greater in amplitude on learning trials than novel-same trials. At right frontal electrodes, the categorization group had greater amplitude PSW on learning trials in comparison to familiar and novel-other trials, and greater amplitude PSW on novel-same trials in comparison to novel-other trials. Although this three-way interaction is difficult to interpret, these findings could reflect both a reduction in amplitude of the PSW from early (learning) trials to subsequent test trials as well as categorization effects (familiar compared to novel-other).
Conclusion
It is interesting to note that experimental effects became increasingly complex with later latency components in the processing stream. The results of the analyses of the Nc and P400 were remarkably similar, with infants in the categorization group demonstrating greater amplitude Nc and PSW on novel-other trials in comparison to all other stimulus types. The consistency in the effects of stimulus type, coupled with the similar latency of these two components, indicates that Nc and P400 may reflect similar processes related to attention (Guy, Zieber, & Richards, 2016; Scott et al., 2006). We believe these findings clearly demonstrate that attention plays a fundamental role in subordinate-level processing of other-species faces. Finally, the effects of familiarization condition and stimulus type on the PSW were complex and potentially driven by both repetition suppression (Snyder et al., 2010) and categorization processes. Quinn and colleagues’ (2010) proposal that infant ERPs reveal a course-to-fine processing sequence during subordinate-level categorization is consistent with the overall pattern of effects found across ERP components for the categorization group in the current study.
The individuation group in the current study failed to demonstrate differential responding based on familiarity and novelty of monkey faces in the current study. Daily experience likely serves to maintain infants’ ability to individuate own-species faces. For example, Sugden, Mohamed-Ali, and Moulson (2013) utilized head mounted cameras on 1- and 3-month-old infants in their home environments, and found that 25% of the infants’ waking time was spent exposed to faces. Thus, by 9 months of age, the typical human infants have had extensive experience with human faces, and the maintenance of the ability to individuate human faces is likely driven by extensive daily interaction with conspecifics. Given that we only tested 9-month-old infants in the current study, our results do not provide insight into potential effects related to perceptual narrowing in infant face processing. However, our findings do indicate that initial exposure conditions play an important role in 9-month-old infants’ performance on face processing tasks. The finding that exposure to multiple exemplars facilitates infant face processing and categorization is consistent with previous behavioral work showing that comparison fosters both categorization and recognition memory (Cassosola and Park, 2013; Oakes & Ribar, 2005; Oakes et al., 2009; Rose et al., 1982). Future studies should be aimed at examining the effects of familiarization condition on processing of both own- and other-species faces across a broad age range in early development.
Highlights.
The results indicate that human infants are efficient at categorizing other-species faces at 9 months of age.
Exposure to multiple exemplars facilitated 9-month-olds subordinate-level categorization of other-species faces.
The Nc and P400 ERP components are associated with subordinate-level categorization of other species faces.
Acknowledgments
We would like to extend our sincerest gratitude to Dr. Lisa Scott for providing us with digital images of the macaque monkeys. Research reported in this article and the writing of this article were supported by the National Institute of Child Health and Human Development Grant R21-HD065042, and the National Science Foundation Developmental and Learning Sciences Division Grant 1226646 to Greg D. Reynolds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anzures G, Quinn PC, Pascalis O, Slater AM, Lee K. Categorization, categorical perception, and asymmetry in infants’ representation of face race. Developmental Science. 2010;13(4):553–564. doi: 10.1111/j.1467-7687.2009.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casasola M, Park Y. Developmental changes in infant spatial categorization: When more is best and when less is enough. Child Development. 2013;84(3):1004–1019. doi: 10.1111/cdev.12010. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981:804–811. [PubMed] [Google Scholar]
- Dahl CD, Rasch MJ, Chen CC. The other-race and other-species effects in face perception–a subordinate-level analysis. Frontiers in Psychology. 2014;5:1068. doi: 10.3389/fpsyg.2014.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer T, Scott LS, Nelson CA. Methods for acquiring and analyzing infant event-related potentials. Infant EEG and event-related potentials. 2007:5–37. [Google Scholar]
- De Haan M. Visual attention and recognition memory in infancy. In: de Haan M, editor. Infant EEG and Event-Related Potentials. New York: Psychology Press; 2007. pp. 101–144. [Google Scholar]
- De Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: A review. International Journal of Psychophysiology. 2003;51(1):45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- De Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68(2):187–210. [PubMed] [Google Scholar]
- De Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- De Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. Journal of Cognitive Neuroscience. 2002;14(2):199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Eimas PD, Quinn PC. Studies on the formation of perceptually based basic-level categories in young infants. Child Development. 1994;65(3):903–917. [PubMed] [Google Scholar]
- Fair J, Flom R, Jones J, Martin J. Perceptual learning: 12- month- olds’ discrimination of monkey faces. Child Development. 2012;83(6):1996–2006. doi: 10.1111/j.1467-8624.2012.01814.x. [DOI] [PubMed] [Google Scholar]
- Farroni T, Johnson MH, Csibra G. Mechanisms of eye gaze perception during infancy. Journal of Cognitive Neuroscience. 2004;16:1320–1326. doi: 10.1162/0898929042304787. [DOI] [PubMed] [Google Scholar]
- Grossmann T, Gliga T, Johnson MH, Mareschal D. The neural basis of perceptual category learning in human infants. Journal of Cognitive Neuroscience. 2009;21(12):2276–2286. doi: 10.1162/jocn.2009.21188. [DOI] [PubMed] [Google Scholar]
- Guy MW, Reynolds GD, Mosteller SM, Dixon KC. The effects of stimulus symmetry on hierarchical processing in infancy. Developmental Psychobiology. 2017;59:279–290. doi: 10.1002/dev.21486. [DOI] [PubMed] [Google Scholar]
- Guy MW, Reynolds GD, Zhang D. Visual attention to global and local stimulus properties in six-month-old infants: Individual differences and event-related potentials. Child Development. 2013;84:1392–1406. doi: 10.1111/cdev.12053. [DOI] [PubMed] [Google Scholar]
- Guy MW, Zieber N, Richards JE. The cortical development of specialized face processing in infancy. Child Development. 2016;87(5):1581–1600. doi: 10.1111/cdev.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halit H, De Haan M, Johnson MH. Cortical specialisation for face processing: face-sensitive event-related potential components in 3-and 12-month-old infants. Neuroimage. 2003;19(3):1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Hannon EE, Trehub SE. Tuning in to musical rhythms: Infants learn more readily than adults. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(35):12639–12643. doi: 10.1073/pnas.0504254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Delaney M, Anzures G, Herbert JS, Quinn PC, Slater AM, Tanaka JW, … Pascalis O. Perceptual training prevents the emergence of the other race effect during infancy. PLoS One. 2011;6(5):e19858. doi: 10.1371/journal.pone.0019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl S. The development of category specificity in infancy–What can we learn from electrophysiology? Neuropsychologia. 2016;83:114–122. doi: 10.1016/j.neuropsychologia.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Hugenberg K, Young SG, Bernstein MJ, Sacco DF. The categorization-individuation model: An integrative account of the other-race recognition deficit. Psychological Review. 2010;117(4):1168. doi: 10.1037/a0020463. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18(12):1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovack-Lesh KA, Oakes LM, McMurray B. Contributions of attentional style and previous experience to 4-month-old infants’ categorization. Infancy. 2012;17(3):324–338. doi: 10.1111/j.1532-7078.2011.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, Richmond J, Vogel-Farley VK, Moulson MC, Nelson CA. Categorical representation of facial expressions in the infant brain. Infancy. 2009;14(3):346–362. doi: 10.1080/15250000902839393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Xiao WS, Xiao NG, Quinn PC, Zhang Y, Chen H, … Lee K. Development of visual preference for own- versus other-race faces in infancy. Developmental Psychology. 2015;51(4):500–511. doi: 10.1037/a0038835. [DOI] [PubMed] [Google Scholar]
- Madole KL, Oakes LM. Making sense of infant categorization: Stable processes and changing representations. Developmental Review. 1999;19(2):263–296. [Google Scholar]
- Maurer D, Werker JF. Perceptual narrowing during infancy: A comparison of language and faces. Developmental Psychobiology. 2014;56(2):154–178. doi: 10.1002/dev.21177. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Le Grand R, Maurer D. Development of expertise in face recognition. In: Gauthier I, Tarr M, Bub D, editors. Perceptual Expertise: Bridging Brain and Behavior. New York, NY: Oxford University Press; 2010. pp. 67–106. [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant and Child Development. 2001;10(1–2):3–18. [Google Scholar]
- Nelson CA, Collins PF. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: Implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain & Cognition. 1992;19:105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Oakes LM, Kovack-Lesh KA, Horst JS. Two are better than one: Comparison influences infants’ visual recognition memory. Journal of Experimental Child Psychology. 2009;104(1):124–131. doi: 10.1016/j.jecp.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes LM, Ribar RJ. A comparison of infants’ categorization in paired and successive presentation familiarization tasks. Infancy. 2005;7(1):85–98. doi: 10.1207/s15327078in0701_7. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296(5571):1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, Nelson CA. Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(14):5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neuropsychology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Peykarjou S, Pauen S, Hoehl S. How do 9-month-old infants categorize human and ape faces? A rapid repetition ERP study. Psychophysiology. 2014;51(9):866–878. doi: 10.1111/psyp.12238. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, … Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pons F, Lewkowicz DJ, Soto-Faraco S, Sebastián-Gallés N. Narrowing of intersensory speech perception in infancy. Proceedings of the National Academy of Sciences. 2009;106(26):10598–10602. doi: 10.1073/pnas.0904134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PC, Doran MM, Reiss JE, Hoffman JE. Neural markers of subordinate-level categorization in 6-to 7-month-old infants. Developmental Science. 2010;13(3):499–507. doi: 10.1111/j.1467-7687.2009.00903.x. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Johnson MH. Global-before-basic object categorization in connectionist networks and 2-month-old infants. Infancy. 2000;1(1):31–46. doi: 10.1207/S15327078IN0101_04. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Lee K, Pascalis O, Tanaka JW. Narrowing in categorical responding to other-race face classes by infants. Developmental Science. 2016;19(3):362–371. doi: 10.1111/desc.12301. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Tanaka JW. Early development of perceptual expertise: Within-basic-level categorization experience facilitates the formation of subordinate-level category representations in 6-to 7-month-old infants. Memory & Cognition. 2007;35(6):1422–1431. doi: 10.3758/bf03193612. [DOI] [PubMed] [Google Scholar]
- Ramsey JL, Langlois JH, Hoss RA, Rubenstein AJ, Griffin AM. Origins of a stereotype: categorization of facial attractiveness by 6-month-old infants. Developmental Science. 2004;7(2):201–211. doi: 10.1111/j.1467-7687.2004.00339.x. [DOI] [PubMed] [Google Scholar]
- Rennels JL, Kayl AJ, Langlois JH, Davis RE, Orlewicz M. Asymmetries in infants’ attention toward and categorization of male faces: The potential role of experience. Journal of Experimental Child Psychology. 2016;142:137–157. doi: 10.1016/j.jecp.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD. Infant visual attention and object recognition. Behavioural Brain Research. 2015;285:34–43. doi: 10.1016/j.bbr.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Bahrick LE, Lickliter R, Guy MW. Neural correlates of intersensory processing in 5-month-old infants. Developmental Psychobiology. 2014;56:355–372. doi: 10.1002/dev.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Courage ML, Richards JE. Infant attention and visual preferences: Converging evidence from behavior, event-related potentials, and cortical source localization. Developmental Psychology. 2010;46(4):886–904. doi: 10.1037/a0019670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Courage ML, Richards JE. The development of attention. In: Reisberg D, editor. Oxford Handbook of Cognitive Psychology. Oxford University Press; New York, NY: 2013. pp. 1000–1013. [Google Scholar]
- Reynolds GD, Guy MW, Zhang D. Neural correlates of individual differences in infant visual attention and recognition memory. Infancy. 2011;16(4):368–391. doi: 10.1111/j.1532-7078.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An ERP and cortical source localization study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Infant visual attention and stimulus repetition effects on object recognition. Child Development. doi: 10.1111/cdev.12982. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Romano AC. The development of attention systems and working memory in infancy. Frontiers in Systems Neuroscience. 2016;10:1–12. doi: 10.3389/fnsys.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Gottfried AW, Melloy-Carminar PM, Bridger WH. Familiarity and novelty preferences in infant recognition memory: Implications for information processing. Developmental Psychology. 1982;18:704–713. [Google Scholar]
- Sangrigoli S, De Schonen S. Recognition of own-race and other-race faces by three- month-old infants. Journal of Child Psychology and Psychiatry. 2004;45(7):1219–1227. doi: 10.1111/j.1469-7610.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- Saville DJ. Multiple comparison procedures: the practical solution. The American Statistician. 1990;44:174–180. [Google Scholar]
- Scott LS, Shannon RW, Nelson CA. Neural correlates of human and monkey face processing in 9-month-old infants. Infancy. 2006;10(2):171–186. doi: 10.1207/s15327078in1002_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LS, Monesson A. The Origin of Biases in Face Perception. Psychological Science. 2009;20(6):676–680. doi: 10.1111/j.1467-9280.2009.02348.x. [DOI] [PubMed] [Google Scholar]
- Scott LS, Pascalis O, Nelson CA. A domain-general theory of the development of perceptual discrimination. Current Directions in Psychological Science. 2007;16(4):197–201. doi: 10.1111/j.1467-8721.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Jakobsen KV, Fragaszy DM, Okada K, Frick JE. The development of facial identity discrimination through learned attention. Developmental Psychobiology. 2014;56(5):1083–1101. doi: 10.1002/dev.21194. [DOI] [PubMed] [Google Scholar]
- Simpson EA, Varga K, Frick JE, Fragaszy D. Infants experience perceptual narrowing for nonprimate faces. Infancy. 2011;16(3):318–328. doi: 10.1111/j.1532-7078.2010.00052.x. [DOI] [PubMed] [Google Scholar]
- Snyder K, Garza J, Zolot L, Kresse A. Electrophysiological signals of familiarity and recency in the infant brain. Infancy. 2010;15:270–299. doi: 10.1111/j.1532-7078.2009.00021.x. [DOI] [PubMed] [Google Scholar]
- Snyder K, Webb SJ, Nelson CA. Theoretical and methodological implications of variability in infant brain response during a recognition memory paradigm. Infant Behavior and Development. 2002;25(4):466–494. [Google Scholar]
- Srinivasan R, Tucker DM, Murias M. Estimating the spatial nyquist of the human EEG. Behavioral Research Methods, Instruments, & Computers. 1998;30:8–19. [Google Scholar]
- Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Developmental Science. 2005;8:605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development. 1984;7(1):49–63. [Google Scholar]
- Wiebe SA, Cheatham CL, Lukowski AF, Haight JC, Muehleck AJ, Bauer PJ. Infants’ ERP responses to novel and familiar stimuli change over time: Implications for novelty detection and memory. Infancy. 2006;9(1):21–44. [Google Scholar]
- Xiao WS, Quinn PC, Pascalis O, Lee K. Own- and other-race face scanning in infants: Implications for perceptual narrowing. Developmental Psychobiology. 2014;56(2):262–273. doi: 10.1002/dev.21196. [DOI] [PubMed] [Google Scholar]