Abstract
Stearoyl lysophosphatidylcholine (LPC) exerts protective effect during endotoxemia and in experimental sepsis, but the underlying mechanism is unclear. Here, we demonstrated that stearoyl LPC could block caspase-11 mediated macrophage pyroptosis. In vitro, stearoyl LPC significantly decreased caspase-11 activation and pyroptosis induced by LPS plus CTB independent of the receptor G2A. Stearoyl LPC did not affect LPS uptake by mouse peritoneal macrophages but, did significantly inhibit the interaction between LPS and caspase-11. Moreover, stearoyl LPC treatment conferred significant protection against lethal endotoxemia and significantly reduced the release of IL-1α and IL-1β. These findings identify stearoyl LPC as an inhibitor of LPS-mediated caspase-11 activation. This mechanism could explain the protective action of stearoyl LPC in experimental sepsis and endotoxemia.
Keywords: Innate immunity, non-canonical inflammasome, endotoxemia, pyroptosis
Introduction
Endotoxin (lipopolysaccharide: LPS), a major component of Gram-negative bacterial cell walls, is released during Gram-negative infection and can lead to septic shock and death (1). These lethal responses are mediated by the excessive activation of caspase-11, a newly identified cytosolic receptor for LPS (2). Upon LPS binding, caspase-11 oligomerizes to a complex, which cleaves pannexin-1 and gasdermin D into their active forms (3–10). These events trigger pyroptosis, a proinflammatory form of programmed lytic cell death (8–10). Genetic deletion of caspase-11 or its downstream targets prevents pyroptosis and promotes host survival during lethal endotoxemia (3–10). Whereas LPS induced activation of caspase-11 induces pyroptosis other known caspase-11 ligands, such as oxidized phospholipids (oxPAPC), induce caspase-11–dependent interleukin-1 release, but not pyroptosis (11). No specific inhibitor of LPS-induces caspase-11 activation has been reported.
Lysophosphatidylcholine (LPC), a major component of oxidized low-density lipoproteins, can be classified according to acyl chain length into several groups, including stearoyl (18:0), lauroyl (12:0), and caproyl LPC (6:0). Stearoyl LPC protects against experimental sepsis and the mechanism was described as dependent on LPS binding to receptor G2A and triggering production of H2O2, which stimulates neutrophils to eliminate invading pathogens (12). The same interaction between stearoyl LPC and receptor G2A appears to contribute to the inhibition of high mobility group box-1 protein (HMGB1) release which could also contribute to the protective actions of stearoyl LPC in experimental sepsis (13). The protective effects of stearoyl LPC in sepsis models and its structural similarity with oxPAPC led us to hypothesize that stearoyl LPC might inhibit caspase-11 activation and prevent lethality during endotoxemia.
Materials and Method
Antibodies and reagents
Stearoyl LPC (L-2131) was purchased from Sigma-Aldrich (USA). LPS was from Invivogen (tlrl-3blps) for in vitro and Sigma (L2630) for in vivo studies. Pam3CSK4 was obtained from Invivogen (tlrl-pms). Antibody against receptor G2A (sc-9692) and Anti-Gsdmd (sc-393581) were purchased from Santa Cruz Biotechnology (USA). Antibody against mouse caspase-11 (clone 17D9) was from Sigma (C1354), while antibody against E. coli LPS (clone2D7/1) came from Abcam (USA). FITC-labeled LPS was purchased from Sigma. The liposomal transfection reagent Dotap was obtained from Roche. Mouse IL-1α (MLA00) and IL-1β (MLB00C) Elisa Kit were from R&D.
Macrophage preparation and stimulation
Mouse peritoneal macrophages were isolated and cultured as described (14). Briefly, C57BL/6 mice (8–10weeks old) were injected intraperitoneally with 2 mL of sterile 4% thioglycollate broth to elicit peritoneal macrophages. At 48–72h later, cells were collected by lavage of the peritoneal cavity with 5 mL of sterile 11.6% sucrose. Harvested cells were washed, then re-suspended in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics (Gibco). Peritoneal macrophages were plated in 12-well plates (106/well) and primed for 6 h with ultra-pure LPS (100ng/ml) or Pam3CSK4 (1μg/ml). Primed cells were washed with phosphate-buffered saline (PBS) and treated for 2 h with stearoyl LPC, which had been prepared as before (13). Cells were washed again with PBS and treated with the combination of LPS (1μg/ml) and a mixture of cholera toxin subunit B (CTB, 20μg/ml) or Dotap, which had been prepared by mixing CTB and Dotap in Optim (Gibco) for 20 min at room temperature. At 16 h after treatment, cells were harvested and total lysates were prepared for analysis using Western blotting an assay for lactate dehydrogenase (LDH). In some experiments, macrophages were treated with LPC without priming. In other experiments, macrophages were treated with anti-G2A antibody (1μg/ml) alone or with the combination of LPC and anti-G2A antibody (1μg/ml).
Western blotting
Total cell lysates were prepared and micro-centrifuged. Proteins in the supernatant were extracted using methanol/chloroform, fractionated using 12% SDS-PAGE, and transferred onto PVDF membranes (Millipore). Membranes were blotted with antibodies against caspase-11 (1:500)or Gsdmd (1:500). Band density was normalized to levels of β-actin or GAPDH on the same blots (1:5000; Cell Signaling Technology, USA).
Cell death assay
Culture media were harvested and centrifuged to sediment cells; the resulting supernatant was subjected to the LDH Cytotoxicity Assay (Beyotime Biotechnology, China).
Limulus amebocyte lysate (LAL) assay
Isolation of cytosol fraction from mouse peritoneal macrophages was performed as previously described (15). Briefly, 5×106 cells were treated with Stearoyl LPC for 2h, and then for 2h or 6h with LPS (1μg/ml) plus CTB (20μg/ml) as indicated. After treatment, cells were washed with sterile Dulbecco’s Phosphate Buffered Saline (DPBS) then digested with 0.25% trypsin. Digestion was halted by addition of RPMI 1640 supplemented with 10% FBS. Cells were washed 3 times by DPBS then treated with 300μl of 0.005% digitonin extraction buffer for 20min on ice and the supernatant containing cytosol was collected after centrifugation and transferred to a new tube and stored at −80°C until analysis using a commercial LAL assay (Xiamen Bio Endo Technology, China).
Flow cytometry
Stearoyl LPC was labeled with an Alexa Fluor 488-conjugated antibody using the Alexa Fluor 488 Labeling Kit (Life Technologies, USA) according to the manufacturer’s instructions. Cells were incubated for 1 or 2 h with the resulting stearoyl LPC-488(20μM), washed three times with PBS, and then treated as previously (16) to quench extracellular fluorescence. After washing again with PBS, cells were digested with 0.25% trypsin and digestion was halted by adding RPMI 1640 supplemented with 10%FBS. Cell suspensions were centrifuged at 300g for 5min, the supernatant was removed. Cell pellets were re-suspended in PBS supplemented with 2% BSA. Re-suspended cells were then analyzed by flow cytometry on a BD FACS Cantoll.
Fluorescence intensity assay
Peritoneal macrophages were plated in 96-well fluorescence plates (105/well), primed and treated as described above. Cells were incubated for 1 or 2h with LPC-488 (20μM), washed three times with PBS, and treated as previously described to quench extracellular fluorescence. After washing again with PBS, the intracellular fluorescence intensity was measured using a fluorescence micro plate reader (BioTek SynergyTM2, USA). In separate experiments to assess intracellular LPS levels, cells were treated for 2h with unlabelled stearoyl LPC (20μM), washed and then incubated for 2h and 6h with FITC-LPS(1μg/ml) alone or FITC-LPS plus CTB(20μg/ml). Cells were washed three times with PBS, and intracellular fluorescence intensity was measured as described above.
Proximity-ligation assay(PLA)
Interaction between LPS and caspase-11 within mouse peritoneal macrophages was analyzed using a proximity-ligation assay kit (Sigma), which examines subcellular localization and protein-protein interactions in situ (17). Macrophages were cultured in a six-well glass dish in RPMI 1640, primed for 4 h with Pam3CSK4(1μg/ml), then treated for 2h with stearoyl LPC. Cells were washed, then treated with LPS (4μg/ml) alone or with LPS plus CTB (40μg/ml). Cells were fixed with 4% formaldehyde, permeabilized with Triton, and incubated overnight with a pair of primary antibodies from different species: mouse monoclonal 2D7/1 against LPS, and rat monoclonal 17D9 against caspase-11. Subsequently, the proximity-ligation assay was carried out according to the manufacturer’s instructions. Briefly, after incubation with primary antibodies, cells were incubated with a combination of oligonucleotide-conjugated probes (secondary antibodies) with mouse as “plus” and rat as “minus”. Then the ligase, polymerase and oligonucleotides were added to generate circular DNA strands by rolling circle amplification at sites where the probes were bound in close proximity. Fluorescently labeled probes were hybridized to complementary sequences in the rolling circle amplification product. Each protein pair generated a spot that could be visualized using fluorescence microscopy. Images were taken using a Leica confocal laser scanning microscope and quantified using Image-J software. Red spots were counted in five randomly selected fields (100X) and averaged.
Immunofluoresence microscopy
For immunofluorescence staining of spleen tissue, the whole animal was perfused with PBS and fixed in 2% paraformaldehyde. Tissue was then placed in 2% paraformaldehyde for 2 hour and then switched to 30% sucrose in distilled water solution for 12 h. Tissue sections (5 μm) were incubated with 2% bovine serum albumin (BSA) in PBS for 1 hour, followed by five washes with PBS containing 0.5% BSA (PBB). The samples were then incubated overnight with primary antibodies. TMR red-labeled In Situ Cell Death Detection Reagent kit (Roche) was used to detect cell death according to manufacturer’s instructions. Samples were washed with PBS prior to being cover slipped using Gelvatol (23 g polyvinyl alcohol 2000, 50mL glycerol, 0.1% sodium azide to 100 mL PBS). Imaging conditions were maintained at identical settings within each antibody-labeling experiment with original gating performed using the negative control. Imaging was performed using a Nikon A1 confocal microscope (purchased with 1S10OD019973-01 awarded to Dr. Simon C. Watkins).
Mouse model of endotoxic shock
We established the same mouse model of endotoxic shock as described(10); in this model, endotoxic shock results from activation of the caspase-11 dependent non-canonical inflammasome. Male or female C57BL/6 mice weighing 20–22g were primed by intraperitoneal injection with 400μg/kg LPS (E. coli 0111:B4), then challenged 7 h later with 10mg/kg LPS (E. coli O111:B4). At 30min before this second LPS injection, mice were subcutaneously injected with stearoyl LPC (10mg/kg) or PBS vehicle. On each of the following 3 days, stearoyl LPC (10mg/kg) or PBS was injected at 12-h intervals at different adjacent sites. Survival of the animals was followed for up to 7 days. For plasma IL-1α/β and TMR stain, mice were subcutaneously injected with two dozes stearoyl LPC or PBS at 30min before and 4 h after the second LPS injection.
Statistical analysis
All data were analyzed using GraphPad Prism (GraphPad Software). Differences between two groups were assessed for significance using Student’s t test. Survival data were compared using the log-rank test. The threshold of significance for all comparisons was P< 0.05. All values are reported as mean ± SD.
Result
Stearoyl LPC inhibits intracellular LPS-induced caspase-11 activation and pyroptosis
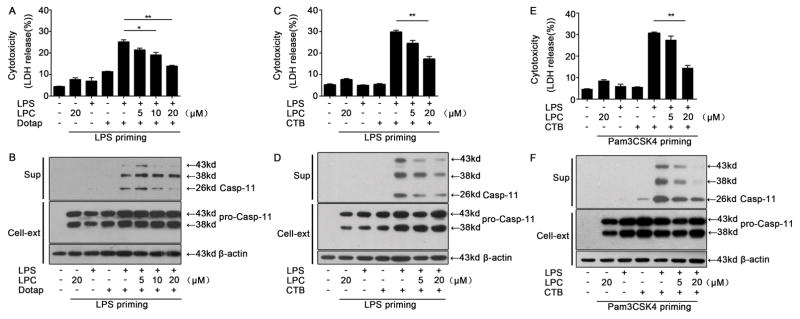
In order to ensure a uniform baseline level of caspase-11 expression before testing the effects of stearoyl LPC, we primed cells with the Toll-like receptor (TLR) agonists LPS or Pam3CSK4. This priming lead to an increase in intracellular caspase-11 expression as expected (Fig. 1B, D & F). LPS transfection using either Dotap or CTB after priming lead to cell death(Fig. 1A, C & E) associated with caspase-11 release(Fig. 1B, D & F) from cells. This was suppressed by stearoyl LPC in a concentration-dependent manner. These findings demonstrated that stearoyl LPC inhibits intracellular LPS-induced caspase-11 activation and pyroptosis.
Fig. 1. Stearoyl LPC inhibits intracellular LPS-induced caspase-11 activation and pyroptosis.
Peritoneal macrophages were primed for 6 h with LPS (100ng/ml) or Pam3CSK4 (1μg/ml), then treated as indicated with LPC for 2 h. Cells were then treated for 16 h with LPS(1μg/ml), CTB (20μg/ml) or Dotap as indicated. (A, C, E) LDH release assay for cell death. (B, D, F) Western blotting against caspase-11 in the culture supernatants (sup) of cell cytosol (Cell-ext). Graphs show mean ± SD. Data are representative of at least two independent experiments.*P<0.05,**P<0.01.
Stearoyl LPC inhibits intracellular LPS-induced cleavage of Gsdmd and release of IL-1α/β
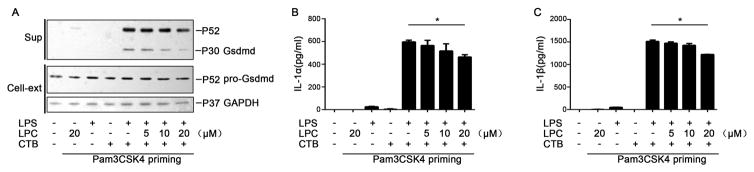
To further confirm that stearoyl LPC inhibits intracellular LPS-induced caspase-11 activation, we measured the Gsdmd cleavage and the IL-1α/β release, both of which are mediated by caspase-11 (9). Notably, stearoyl LPC inhibited the Gsdmd cleavage(Fig. 2A) and the IL-1α/β(Fig. 2B–C) release in a concentration-dependent manner.
Fig. 2. Stearoyl LPC inhibits intracellular LPS-induced cleavage of Gsdmd and release of IL-1α/β.
Mouse peritoneal macrophages were primed for 6 h with Pam3CSK4 (1μg/ml), then treated with indicated concentration of LPC for 2 h. Then the cells were simulated with LPS(1μg/ml) and CTB (20μg/ml) for 16h. (A)Western blotting to detect Gsdmd in the supernatants (sup) and cell lysates (Cell-ext). (B, C) ELISA to detect levels of IL-1α and IL-1β in supernatant. Graphs show mean ± SD. Data are representative of at least two independent experiments.*P<0.05.
Inhibitory effects of stearoyl LPC do not depend on receptor G2A
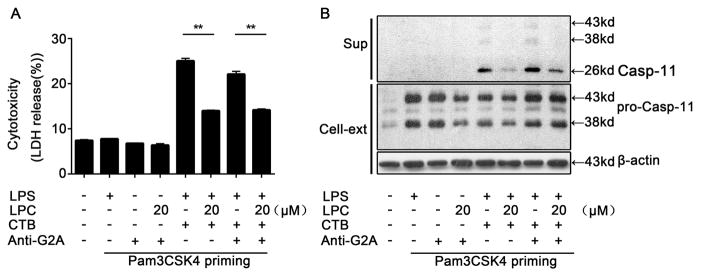
As previous studies showed that stearoyl LPC exerts its protective effect during endotoxemia through binding to the G2A receptor, we next determined whether stearoyl LPC inhibits caspase-11 activation through G2A. The inhibitory activity of stearoyl LPC on LPS-induced caspase-11 activation and pyroptosis was not affected by treatment with anti-receptor G2A antibody at the time of intracellular LPS delivery (Fig. 3A–B), indicating that the inhibitory effects of stearoyl LPC on caspase-11 activation do not depend on receptor G2A.
Fig. 3. Inhibitory effects of stearoyl LPC do not depend on receptor G2A.
Peritoneal macrophages were primed for 6 h with Pam3CSK4 (1μg/ml), then treated for 2 h with LPC or anti-G2A antibody (1μg/ml)as indicated. Finally, cells were treated for 16 h with LPS (1μg/ml) and CTB (20μg/ml) as indicated. (A) LDH release assay for cell death. (B) Western blotting against caspase-11 in the cell culture supernatants (sup) or cell cytosols (Cell-ext). Graphs show mean ± SD. Data are representative of at least two independent experiments.*P<0.05,**P<0.01.
Stearoyl LPC does not block LPS translocation into the cytosol
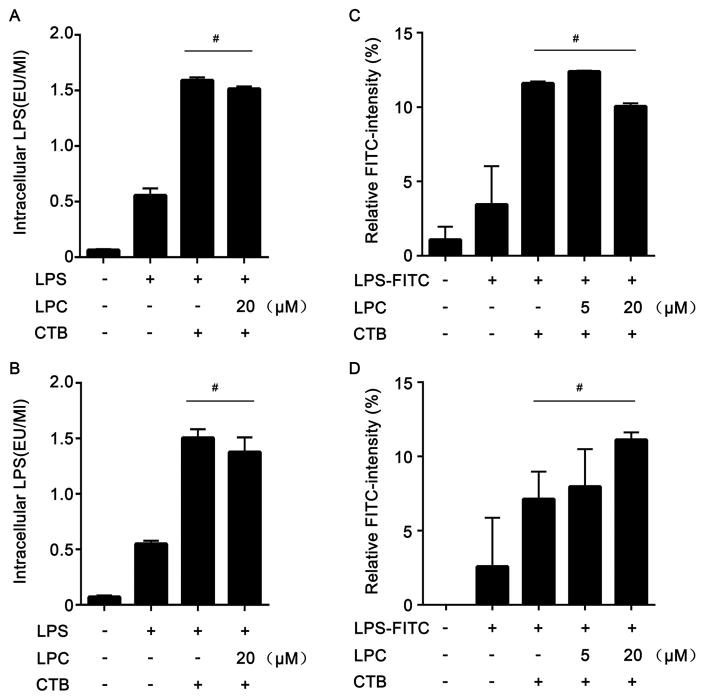
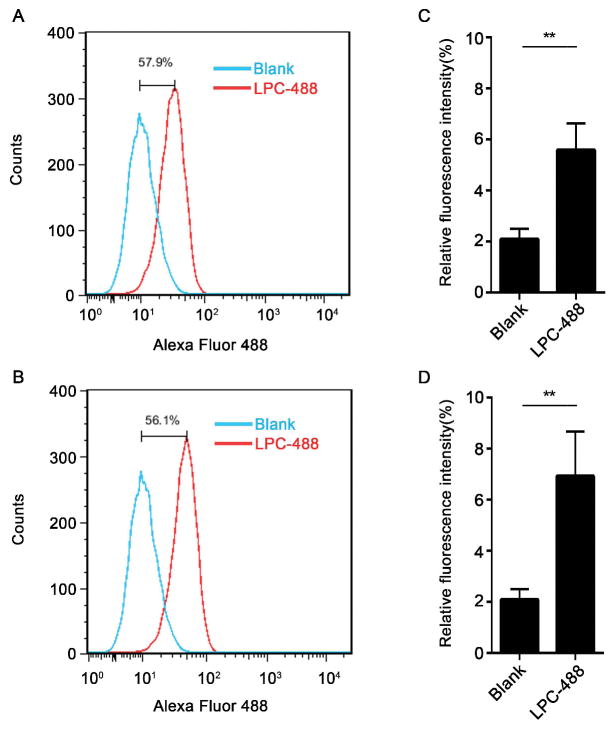
Since caspase-11 is a cytosolic LPS receptor and the translocation of LPS from extracellular space into the cytosol is required for caspase-11 activation (2), we investigated whether stearoyl LPC affects the translocation of LPS into the cytosol. To test this end, we isolated the cytosolic fraction of stimulated mouse peritoneal macrophages void of cytoplasmic membranes, endosomes, lysosomes, and endoplasmic reticulum, and the measured LPS levels in the cytosolic fraction using the LAL assay. Notably, stearoyl LPC treatment did not alter the cytosolic LPS levels in macrophages exposed to LPS and CTB (Fig. 4A–D). Interestingly, using fluorescently labeled stearoyl LPC and flow cytometry we observed that macrophages quickly uptake the fluorophore-labeled stearoyl LPC (Fig. 5A–D). These findings indicated that the mechanism of stearoyl LPC suppression of LPS-induced caspase-11 activation does not involve the prevention from LPS translocation into the cytosol, and suggested that stearoyl LPC might prevent caspase-11 activation by acting on an intracellular target.
Fig. 4. Inhibitory effects of stearoyl LPC do not depend on inhibiting LPS translocation into the cytosol.
Peritoneal macrophages were treated with LPC for 2 h, and then for 2 h or 6 h with LPS (1μg/ml) plus CTB (20μg/ml) as indicated. Intracellular levels of unlabeled LPS were measured using an LAL assay, and intracellular levels of FITC-labeled LPS were measured using a fluorescence intensity microplate reader. Cells were treated with LPS for 2 h (A,C) or 6 h (B,D). Graphs show mean ± SD. Data are representative of at least two independent experiments. #: no significant difference.
Fig. 5. Macrophages uptake the stearoyl LPC.
Flow cytometry and fluorescence intensity measurement of peritoneal macrophages stained with LPC-488 (A,C) for 1 h or (B,D) for 2 h. Graphs show mean ± SD. Data are representative of at least two independent experiments.*P<0.05,**P<0.01.
Stearoyl LPC inhibits the binding of LPS to caspase-11
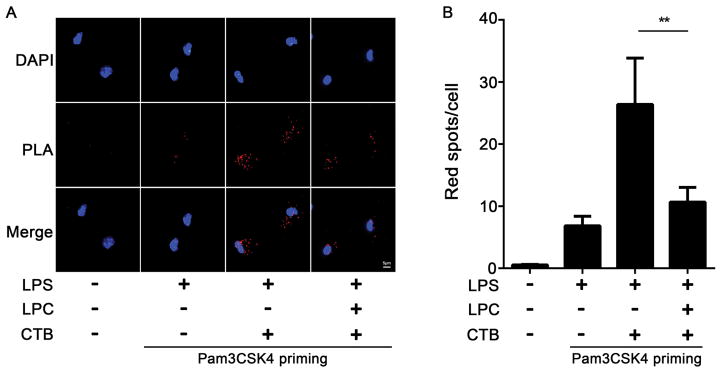
Based on the structural similarity between the LPC and LPS, we postulated that stearoyl LPC might act as a competitive antagonist of LPS interaction with caspase-11. To test this hypothesis, we assessed whether stearoyl LPC could directly inhibit LPS binding to caspase-11 using the proximity ligation assay. Notably, the interaction between caspase-11 and LPS was significantly reduced by stearoyl LPC treatment in mouse peritoneal macrophages primed with Pam3CSK4 and then stimulated with LPS plus CTB (Fig. 6A–B). These observations demonstrated that stearoyl LPC inhibits caspase-11 activation by preventing LPS binding to caspase-11.
Fig. 6. Stearoyl LPC inhibits the binding of LPS to caspase-11.
Peritoneal macrophages were primed for 6 h with Pam3CSK4 (1μg/ml), then for 2h with LPC (20μM), and finally for 2 h with LPS (4μg/ml) and CTB (40μg/ml) as indicated. (A) Interaction between caspase-11 and LPS was visualized as red spots under fluorescence microscopy using the proximity-ligation assay (Magnification, 100X). (B) Quantitation of red spots in five randomly selected 100X fields. Graphs show mean ± SD. Data are representative of at least two independent experiments.*P<0.05,**P<0.01.
Stearoyl LPC protects the mice against lethal endotoxemia
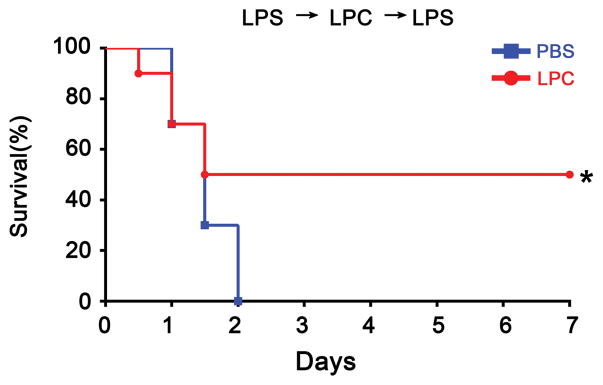
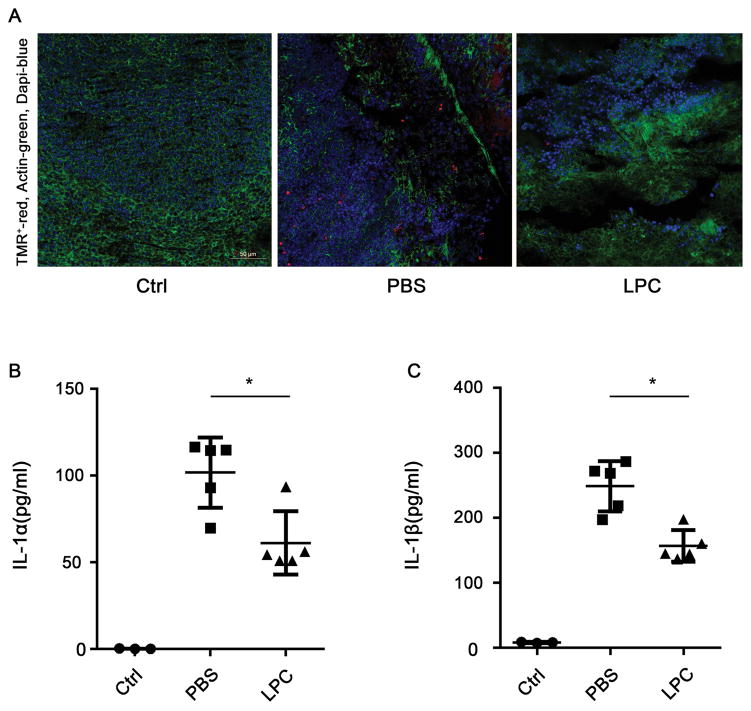
As it is established that caspase-11 mediates LPS-induced shock and lethality, we next determined whether stearoyl LPC exerts a protective effect during lethal endotoxemia. Using a mouse model of endotoxic shock, we found that stearoyl LPC treatment significantly promoted the survival during lethal endotoxemia (Fig. 7). Moreover, we observed that the cell death in spleen and the release of IL-1α and IL-1β in plasma were significantly attenuated by stearoyl LPC treatment during endotoxemia (Fig. 8.).
Fig. 7. Stearoyl LPC protects the mice against lethal endotoxemia.
C57BL/6 mice were primed by intraperitoneal injection with 400μg/kg LPS, then challenged 7 h later with 10mg/kg LPS. At 30min before the second LPS injection, mice were subcutaneously injected with stearoyl LPC (10 mg/kg, red line, n=10) or PBS vehicle (blue line, n=10). For each of the next 3 days, LPC and vehicle were injected at 12-h intervals at different adjacent sites. *P<0.05 based on the log-rank test. Data are representative of at least two independent experiments.
Fig. 8. Stearoyl LPC treatment decreased plasma IL-1α/β level and spleen TMR stain.
C57BL/6 mice were primed by intraperitoneal injection with 400μg/kg LPS, then challenged 7 h later with 10mg/kg LPS. At 30min before and 4 h after the second LPS injection, mice were subcutaneously injected with two dozes stearoyl LPC (10 mg/kg, n=5) or PBS vehicle (n=5), spleen and plasma were harvested at 8 h after second LPS injection. (A) TMR(red) stain in spleen. (B, C) ELISA of plasma IL-1α and IL-1β.*P<0.05.
Discussion
Elevated level of LPS during sepsis is associated with septic shock and LPS removal from the blood leads to improved outcomes in sepsis (18). Endotoxin lethality largely depends on the activation of caspase-11 and the subsequent pyroptosis that results in the release of excessive amount of eicosanoids (19). Upon binding to LPS, the caspase-11 oligomerizes into multi-protein complex and cleaves its substrate gasdermin D, leading to pyroptosis. Here we show that stearoyl LPC, a major component of oxidized low-density lipoprotein, can inhibit caspase-11-dependent pyroptosis through preventing LPS binding to caspase-11. We further show that this inhibition protects against the lethal endotoxemia.
LPC is involved in the pathogenesis of several chronic inflammatory diseases, such as atherosclerosis, psoriasis, asthma, rhinitis and systemic lupus erythematosus (20–25). However in sepsis, an acute systemic inflammatory syndrome, administration of stearoyl LPC improves survival of animals exposed to E. coli or cecal ligation puncture model (CLP)(12). Stearoyl LPC has also been shown to suppress the release of HMGB1 from immune cells exposed to LPS(13) and prevents organ dysfunction during experimental polymicrobial sepsis (26). Our findings indicate that an unrecognized action of LPC is the inhibition of caspase-11. Since it is well-established that caspase-11 drives lethality in the model of endotoxin hypersensitivity used in our studies (10), our results suggest that the inhibition of caspase-11 by LPC accounts, at least in part, for the protective effects of LPC in endotoxemia and sepsis.
To study more precisely how LPC inhibits the caspase-11 activation, we examined the steps leading to LPS-mediated activation of caspase-11. We ruled out an effect of LPC on LPS uptake into the cytosol of macrophages. LPC-induced G2A signaling was showed to promote bacterial clearance and reactive oxygen species production in experimental sepsis (12, 13). However blockade of receptor G2A did not reverse the LPC-mediated inhibition of cell death following LPS exposure.
In contrast to previous studies that concentrated on LPC action at the cell membrane, our work suggests that a mechanism of action for LPC lies within the cytosol, where LPC binds directly to caspase-11 and thereby blocks its activation and oligomerization in response to LPS. We cannot exclude the possibility that the stearoyl LPC might bind to another target protein, which then inhibits the caspase-11 oligomerization or activation. Together, the present study suggests that stearoyl LPC protects against lethal endotoxemia, at least in part, by inhibiting caspase-11 activation and subsequent pyroptosis.
Acknowledgments
This work was supported by National key scientific project 2015CB910700 (B.L.), National Natural Science Foundation of China (No. 81422027 (B.L.), No.81470345 (B.L.) and No. 81571879 (T.R.B.)) and NIH grant RO1 GM50441 (T.R.B.).
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Angus DC, Van der Poll T. Severe sepsis and septic shock. New England Journal of Medicine. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular lps. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 3.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 4.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic lps activates caspase-11: Implications in tlr4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular lps independent of tlr4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 7.Meunier E, Dick MS, Dreier RF, Schurmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, Yamamoto M, Broz P. Caspase-11 activation requires lysis of pathogen-containing vacuoles by ifn-induced gtpases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 8.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu JS, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang YF, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin d for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 9.Shi JJ, Zhao Y, Wang K, Shi XY, Wang Y, Huang HW, Zhuang YH, Cai T, Wang FC, Shao F. Cleavage of gsdmd by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 10.Yang DH, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 requires the pannexin-1 channel and the purinergic p2×7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, Kagan JC. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nature medicine. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 13.Chen GQ, Li JH, Qiang XL, Czura CJ, Ochani M, Ochani K, Ulloa L, Yang H, Tracey KJ, Wang P, Sama AE, Wang HC. Suppression of hmgb1 release by stearoyl lysophosphatidylcholine: An additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–627. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of pkr in inflammasome activation and hmgb1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VA. Bacterial outer membrane vesicles mediate cytosolic localization of lps and caspase-11 activation. Cell. 2016;165:1106–1119. doi: 10.1016/j.cell.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng M, Scott MJ, Loughran P, Gibson G, Sodhi C, Watkins S, Hackam D, Billiar TR. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of tlr4 during sepsis. Journal of immunology. 2013;190:5152–5160. doi: 10.4049/jimmunol.1300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 18.Davies B, Cohen J. Endotoxin removal devices for the treatment of sepsis and septic shock. The Lancet Infectious Diseases. 2011;11:65–71. doi: 10.1016/S1473-3099(10)70220-6. [DOI] [PubMed] [Google Scholar]
- 19.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta D, Gupta S, Gaur SN, Gangal SV, Agrawal KP. Increased leukocyte phospholipase a2 activity and plasma lysophosphatidylcholine levels in asthma and rhinitis and their relationship to airway sensitivity to histamine. The American review of respiratory disease. 1990;142:157–161. doi: 10.1164/ajrccm/142.1.157. [DOI] [PubMed] [Google Scholar]
- 21.Steinbrecher UP, Zhang HF, Lougheed M. Role of oxidatively modified ldl in atherosclerosis. Free radical biology & medicine. 1990;9:155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- 22.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: Basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 23.Ryborg AK, Gron B, Kragballe K. Increased lysophosphatidylcholine content in lesional psoriatic skin. The British journal of dermatology. 1995;133:398–402. doi: 10.1111/j.1365-2133.1995.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 24.George J, Harats D, Gilburd B, Levy Y, Langevitz P, Shoenfeld Y. Atherosclerosis-related markers in systemic lupus erythematosus patients: The role of humoral immunity in enhanced atherogenesis. Lupus. 1999;8:220–226. doi: 10.1191/096120399678847597. [DOI] [PubMed] [Google Scholar]
- 25.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: An overview. Free radical biology & medicine. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 26.Murch O, Collin M, Sepodes B, Foster SJ, Mota-Filipe H, Thiemermann C. Lysophosphatidylcholine reduces the organ injury and dysfunction in rodent models of gram-negative and gram-positive shock. British journal of pharmacology. 2006;148:769–777. doi: 10.1038/sj.bjp.0706788. [DOI] [PMC free article] [PubMed] [Google Scholar]