Abstract
The phylogenetic relationships of the recently described genus †Ticinolepis from the Middle Triassic of the Monte San Giorgio are explored through cladistic analyses of the so far largest morphological dataset for fossil actinopterygians, including representatives of the crown-neopterygian clades Halecomorphi, Ginglymodi and Teleostei, and merging the characters from previously published systematic studies together with newly proposed characters. †Ticinolepis is retrieved as the most basal Ginglymodi and our results support the monophyly of Teleostei and Holostei, as well as Halecomorphi and Ginglymodi within the latter clade. The patterns of relationships within these clades mostly agree with those of previous studies, although a few important differences require future research. According to our results, ionoscopiforms are not monophyletic, caturids are not amiiforms and leptolepids and luisiellids form a monophyletic clade. Our phylogenetic hypothesis confirms the rapid radiation of the holostean clades Halecomorphi and Ginglymodi during the Early and Middle Triassic and the radiation of pholidophoriform teleosts during the Late Triassic. Crown-group Halecomorphi have an enormous ghost lineage throughout half of the Mesozoic, but ginglymodians and teleosts show a second radiation during the Early Jurassic. The crown-groups of Halecomorphi, Ginglymodi and Teleostei originated within parallel events of radiation during the Late Jurassic.
Keywords: Mesozoic, Actinopterygii, Neopterygii, Holostei, systematics, phylogeny
1. Introduction
The Neopterygii is the largest group of living vertebrates, including ca 32 650 valid species [1], the vast majority of which are teleosts. The origin of this important clade goes back to the Palaeozoic [2,3], but its most important radiation occurred in the early Mesozoic [3]. Ginglymodi, Halecomorphi and Teleostei are the three major clades currently recognized among crown-neopterygians (Actinopterygii: Neopterygii). Whereas for much of the second half of the last century halecomorphs and teleosts were regarded as sister-groups, to the exclusion of ginglymodians, recent morphological (e.g. [4,5]), molecular [6–10] and combined [11] studies support the monophyly of the Holostei: a major clade including both Ginglymodi and Halecomorphi, which is, in turn, the sister-group of Teleostei. Although there is clearly a strong molecular signal supporting the Holostei clade, there is still uncertainty in the morphological data (see review by [2]) and the Holostei hypothesis is yet being questioned by challenging new studies (e.g. [12]). The main problem of molecular phylogenies is the lack of important information due to the exclusion of fossils and, thus, the concomitant historical information on the stem lineage of the clade under study (past morphological disparity, morphological evolution that led to the modern forms and those morphologies that did not succeed, and the reasons why they did not, past events of diversification and the understanding of the context in which they occurred etc.). The missing information is especially important when extinct lineages are excluded as they might represent a significant expansion of the currently expressed morphospace of a lineage. Only the combination of both molecular and morphological data leads to optimal phylogenetic results: a Total Evidence approach [13–15].
Including fossil taxa in Total Evidence analyses is necessary not only to achieve a solid pattern of neopterygian phylogenetic relationships, but also to fully understand the macroevolutionary processes that led to the near extinction of the holosteans along with the peerless radiation of teleosts (see Hunt & Slater [16] for a review of the advantages of including fossils in phylogenetic analyses). The main obstacle for the Total Evidence method is the usual large amount of missing data [17–19]. It is known that the amount of morphological data for fossil taxa depends on the quality of preservation (in particular, soft tissues are only exceptionally preserved), which, together with the lack of molecular information, leads to a very large proportion of missing data in Total Evidence matrices. However, the effects of missing data in these analyses have been investigated by Guillerme & Cooper [20,21], who demonstrated that the amount of missing data in fossil taxa is not problematic when there are enough morphological characters and enough morphological information of living taxa in the data matrix. Fortunately, this is the case in holosteans (see below).
Dramatically, in contrast to the ca 32 640 species of living teleosts, non-teleost neopterygians are currently represented by only seven species of gars (Ginglymodi: Lepisosteiformes) and the bowfin, Amia calva (Halecomorphi: Amiiformes). This tremendous asymmetry in the number of recent representatives of the crown-neopterygian lineages does, by far, not reflect the situation during the early Mesozoic. During the Triassic–Jurassic, the diversity of ginglymodians and halecomorphs probably equated or even exceeded that of teleosts [22]. Numerous studies have been dedicated to the morphology of living gars, Amia and their direct fossil relatives (see [5,23] and literature cited therein) and, although the morphology of many fossil holosteans is still poorly known, numerous fossil holostean and teleost taxa, several of them representing extinct lineages, have been studied in detail and included in cladistic analyses during the last decades (e.g. [24–40]). These studies represent important steps towards a Total Evidence analysis of the Neopterygii, in which missing information should not be problematic because the fossil taxa will be properly anchored in the tree thanks to the available molecular and morphological information on the living representatives [20,21]. However, more taxa and characters are needed and a lot of work remains to be done in this direction.
Triassic neopterygians are particularly interesting for several reasons. Most recent molecular phylogenies predict divergence dates during the Devonian (394–290 Ma) for the Neopterygii, and during the Carboniferous–Permian for the Holostei (312–245 Ma) and Teleostei (333–250 Ma) (data from Sallan [2]: table 1). On the other hand, the earliest certain representative of the lineage leading to crown-Neopterygii is known from the Permian, while the earliest members of the holostean and teleost total groups are Triassic [3]. Even if future palaeontological discoveries might fill the gap between the estimated divergence dates and the age of the currently oldest known fossils, the fossil record shows that the first important radiation of the Neopterygii occurred during the Triassic [3]. Triassic neopterygians further present conflicting combinations of morphological features that have been proposed as synapomorphies for one or the other of the three crown-neopterygian clades (e.g. [37,41,42]). Including Triassic taxa in phylogenetic studies of Neopterygii is thus necessary, even if they might increase the level of homoplasy [43].
Within this context, the present contribution is dedicated to explore the phylogenetic relationships of the recently described Middle Triassic neopterygian genus †Ticinolepis López-Arbarello, Bürgin, Furrer and Stockar, 2016 [37]. This is one of the taxa showing a mixture of morphological features typically ascribed to Ginglymodi or Halecomorphi, together with other features observed only in teleosts [37]. For this reason, to study the relationships of †Ticinolepis, we conducted a cladistic analysis based on the so far largest morphological dataset for fossil actinopterygians, including representatives of the three crown-neopterygian clades and merging the lists of characters from previously published systematic studies of neopterygians together with newly proposed characters. Our list of characters is not just the simple assemblage of characters taken from previous works. We have carefully revised previous lists of characters and the hypotheses of primary homology behind them. We have merged and modified the definition of most characters to avoid problematic coding as discussed by Jenner [44] and Brazeau [45]. Our list of 339 morphological characters is not yet complete, more characters will hopefully be added in the future, but it is certainly a solid base to start working on the endless task of completing information seeking for the neopterygian evolutionary tree.
2. Material and methods
2.1. Taxonomic sampling and nomenclature
The investigation of the phylogenetic relationships of †Ticinolepis was performed through a parsimony analysis of a matrix of 339 morphological characters scored for 99 species (92 extinct and 7 living taxa). All operational taxonomic units are species (electronic supplementary material, file S1).
According to previous phylogenetic hypotheses for crown-neopterygians (summarized by Friedman [3]) and considering the availability of published morphological information, the Early Triassic stem neopterygians †Australosomus kochi Stensiö, 1932 [46], †Boreosomus piveteaui Nielsen, 1942 [47], †Pteronisculus stensioi (Nielsen, 1942) [47] and †Plesiofuro mingshuica Su, 1993 [48], were chosen as out-group taxa. The in-group includes 36 ginglymodians (36%; two living species), 25 halecomorphs (25%; one living species), 29 teleosts (29%; four living species) and 5 taxa of uncertain relationships (the two species of †Ticinolepis and three species of †Dapedium).
Taxonomic names are used, proposed and/or defined according to the rules and recommendations of the International Code of Zoological Nomenclature (ICZN 2000) and in agreement with the PhyloCode [49]. Accordingly, the following clades are here recognized: total clade Neopterygii sensu Regan [50], crown-group Neopterygii sensu Patterson [51], Holostei sensu Grande [5], Ginglymodi sensu López-Arbarello [26], Halecomorphi sensu Patterson [52] and total clade Teleostei sensu de Pinna [53].
2.2. Character coding and scoring
López-Arbarello et al. [37] described the genus †Ticinolepis from the Ladinian (Middle Triassic) of the Monte San Giorgio and discussed the resemblance of this fish with ginglymodians, but also with halecomorphs and teleosts. Owing to this mixture of morphological features, none of the available data matrices would have been adequate to explore the phylogenetic relationships of this genus because each of them was designed for cladistic analyses of the relationships within one or the other of the three main crown-neopterygian groups, and solving the systematic position of †Ticinolepis required the inclusion of all of them. Compiling such a comprehensive data matrix needed the revision of all hypotheses of homology and the consequent redefinition of many characters and character states. The resulting data matrix includes a total of 339 morphological characters (see complete list of characters in electronic supplementary material, file S2). Among them, 76 are newly defined and the remaining characters are the result of merging and modifying characters from most previous cladistic analyses of crown-neopterygians (see complete list below). Emended definitions of characters are based on a thorough revision of primary homology hypotheses, taking special care to avoid those definitions that imply the use of unspecified absence states [44] as well as repeated absences, pseudo-ordering and compound characters [45].
Whenever possible, character scoring was based on direct examination of specimens or on descriptions in the literature if the material was not available to us (see list of examined material and literature in electronic supplementary material, file S1). The data matrix was prepared with Mesquite v. 3.31 [54]. There are 127 multistate characters with an average of 3 and a maximum of 9 character states (ch. 124: Largest infraorbital bone). The matrix contains average proportions of 37% and 35% missing data and 7% and 10% inapplicable scorings for the taxa and characters, respectively. The maximal amount of missing data is 75% for the ginglymodian †Cammerichthys lunae, which is only known from a partially complete skull, and 92% for character 211 ‘Infrapharyngobranchial tooth plates’, which is a feature rarely observed in fossils. The maximal amount of inapplicable scorings is 27% for †Australosomus kochi among the out-group taxa and 15% for †Pachycormus macropterus within the in-group and 92% for character 193 ‘anterior notch of preopercle’, which is a feature unique of a few pholidophoriform teleosts. Despite the high amount of missing data in certain characters (notably higher for endocranial features), all characters are parsimony informative.
Autapomorphic character states are not unusual in our data matrix. These states are not informative for the tree search, but are informative concerning the amount of homoplasy. The only valid alternative instead of scoring an autapomorphic character state would be to score the feature ‘inapplicable’ for the taxon in question. However, it is reasonable to keep the autapomorphic character states because they will probably be informative for other possible studies (e.g. disparity analyses), which might be based on these data in the future.
2.3. Cladistic methodology
The cladistic analyses were performed with TNT [55] under equal and implied weighting [56]. In contrast to the commonly used equal weighting analyses, where all characters are given the same weight, implied weighting analyses were designed to down-weight characters according to their level of homoplasy in order to obtain a hypothesis that maximizes the influence of the more reliable characters at the expense of the more homoplastic ones. In these procedures, the fit of each character is calculated with a concave function of its number of extra steps (i.e. the more homoplastic, the less fit) and the preferred tree(s) are those which maximize the total fit. The weighting strength (i.e. how strongly homoplastic characters are down-weighted) is determined by using different concavity constant (K-values). We have used several concavity constants to explore variations in the resulting pattern of relationships.
In both equal weighted and implied weighting analyses, tree search was performed with the traditional search option of TNT v. 1.1 [55,57] applying random addition sequence (RAS) and tree bisection reconnection (TBR) through 1000 replicates keeping 10 trees per replicate. TBR was applied to all the trees retained in memory and trees are rooted in †Pteronisculus stensioi. Most characters are unordered; three characters are ordered (chs. 32, 289 and 325). Branch support was evaluated also with TNT applying bootstrap expressed as values of GC (groups present/contradicted) through 10 000 replicates and calculating Bremer decay indexes for each node. Support measurements were calculated for implied (K = 8 and K = 3) and equal weighting analyses.
Considering that, within Teleostei, both Teleocephala (sensu de Pinna [53]) and Clupeocephala are well-corroborated groupings, demonstrated in several occasions by molecular and morphological phylogenetic analyses [4,10,11], but our dataset was not specifically designed to test the relationships among recent teleosts, we performed the analyses with constraints enforcing the monophyly of these two main clades of living teleosts. The phylogenetic data are freely available in the supplementary files, in Dryad Digital Repository (https://doi.org/10.5061/dryad.2tp53gr) [58] and in MorphoBank (www.morphobank.org; Project 2196).
The distribution of characters was analysed using the ‘trace character history’ option in Mesquite v. 3.31 [54] and both accelerated (ACCTRAN) and delayed transformation (DELTRAN) methods for character optimization were run with PAUP v.4.0a for Macintosh. Depending on the optimization, some character changes will be synapomorphic for a certain clade under ACCTRAN (opting for the earliest possible transformation and preferring reversal over convergence), but not if DELTRAN is assumed (opting for the latest possible transformation and preferring convergence over reversals), and vice versa [59,60]. These synapomorphies depend on the optimization and are thus ambiguous. Other character changes are unambiguous because they are synapomorphic under both delayed and accelerated transformation, i.e. both optimization methods will set the character change at the same node. Note that unambiguous character transformation only refers to the node in question and does not exclude that character transformations might be homoplastic at other nodes. Agnarsson & Miller [61] thoroughly discussed the advantages and disadvantages of ACCTRAN and DELTRAN optimizations and concluded that there are no theoretical reasons to prefer one or other method and both methods should be applied and considered to achieve the optimal understanding of character evolution. However, for this work, representing the first cladistic analyses of the three main crown-neopterygian lineages taken together, the resulted phylogenetic hypothesis is not robust enough and we do not want to hasten conclusions about character evolution at this early stage of research. Therefore, only the unambiguous synapomorphies were taken into account, discriminating between unique and non-unique synapomorphies.
3. Discussion of characters
All 339 characters are listed in the electronic supplementary material, file S2. Unless the definition of a character is self-explanatory or has been explained in some previous work, the characters are accompanied with additional explanatory paragraph and/or figure. Additionally, some characters need thorough discussions, which are included in this section.
3.1. Relative position of the dorsal fin
The relative position of the dorsal fin has been included in one or more characters and expressed in different ways by many authors. In many cases, attempts have been made to represent the position of the dorsal fin relative to the body in general (e.g. anterior, in the middle and posterior), which has important biomechanical implications. However, such characters are usually very vaguely defined and they should rather be expressed quantitatively, although this is also problematic. Alternatively, using the pelvic and anal fins as landmarks is a useful tool to represent the relative position of the dorsal fin in the body of actinopterygians, even in those fishes with extremely elongated bodies (e.g. †Saurichthys, †Aspidorhynchus, Belonidae). Therefore, we adopted the multistate character proposed by López-Arbarello ([26]: ch. 1), which was modified to encompass the variation included in our dataset.
A character used by several cladistic analyses ‘Dorsal and anal fins posteriorly placed’ (e.g. [62]: ch. 77, [63]: ch. 99, [64]: ch. 95, [65]: ch. 94, [66]: ch. 91), is an example of a vaguely defined character, and another character ‘Dorsal fin origin anterior to that of pelvic fin’ (e.g. [62]: ch. 78, [63]: ch. 100, [64]: ch. 96, [65]: ch. 95, [66]: ch. 92) would imply unspecified states [44] for our dataset, both in the presence, embracing the conditions described in our character states 2 and 3 (dorsal fin extending anterior to opposite of insertion of pelvic fins, or originating anterior to insertion of pelvic and extending opposite to anal fins), and the absence, embracing the conditions described in our character states 0 and 1 (dorsal fin originating posterior to insertion of pelvic and extending backwards not beyond middle of anal fin, or originating approximately at the level of the origin of the anal fin and extending opposite to it) and 4 and 5 (dorsal fin originating posterior to the origin of anal fin, or originating posterior to insertion of pelvic and extending backwards up to end of anal fin). Another binary character used in Arratia ([29]: ch. 118), ‘Dorsal fin placed posteriorly, closer to caudal fins than to pelvic fins’, or the reworded version of this character in Arratia ([39]: ch. 136) embraces the conditions described in our states 1 and 4 in one character state and the conditions in our states 0, 2, 3 and 5 in the other character state. Cavin ([67]: ch. 41) ‘Both dorsal and anal fins well developed, ending posteriorly close to the caudal peduncle’ is a compound character [45] involving unspecified absence of the two types discussed by Jenner [44].
3.2. Type of scales
Ideally one would like to distinguish the palaeoniscoid and lepisosteoid types of the ganoid scales because both lepisosteoid and elasmoid scales are homologous to palaeoniscoid scales, but it is possible that these two types evolved independently [68,69]. However, the transition from one type to the other is gradual and might occur within a single scale [70]. Even when histological information is available for some taxa, one would need to trace the presence/absence of dentine in scales from different regions of the body of each fish specimen, which would probably result in heterogeneous patterns. Therefore, pending a more rigorous and detailed analysis of the distribution of the different types of scales in Mesozoic actinopterygians, we apply here the simply morphological distinction between ganoid, amioid and cycloid scales (ch. 4).
Other authors use independent a/p characters for each lepisosteoid, amioid and cycloid scale types (e.g. [66]: chs. 140–142), which is methodologically incorrect because these characters are not mutually independent and involve unspecified absence states [44]. Alternatively, the lepisosteoid, amioid and cycloid scale types represent different states of single multistate characters in several recent phylogenetic analyses (e.g. [29]: ch. 156, [35]: ch. 175), which is a valid option, but the scoring of lepisosteoid type of scales for a certain taxon is questionable due to the lack of histological information (e.g. several Triassic teleosts in [29,39]).
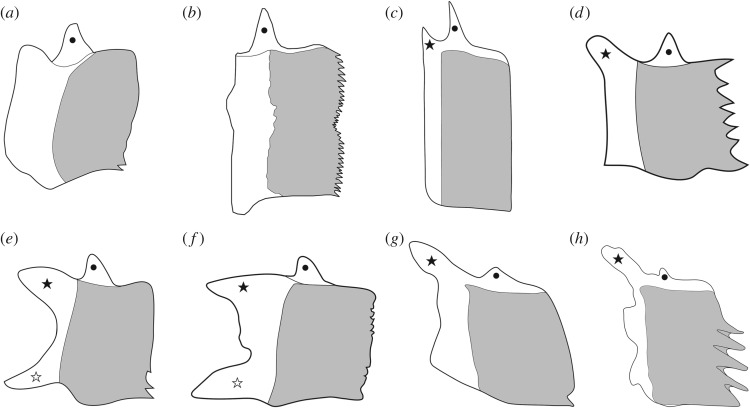
Among the ganoid scales, we distinguish different morphotypes (figure 1) according to the shape of the posterior margin of the scales (ch. 6) and the presence/absence of different articulatory processes (chs. 7–9). Most ganoid scales of actinopterygians are articulated through the so-called peg-and-socket articulation, consisting of a dorsal spine-like peg protruding from the dorsal border of the scale, which fits in a conical socket excavated in the medial surface of the scale (figure 1a–e). In some ginglymodians, the scales have only very reduced pegs and sockets, or this articulating structure is completely absent (figure 1f–h). The reduction to the complete absence of a peg-and-socket articulation occurs independently in the scales of several lepisosteiform taxa (†Lepidotes, †Scheenstia, †Dentilepisosteus, †Masillosteus janeae, Atractosteus spatula). In addition to the peg-and-socket articulation explained above, the scales of many ginglymodians (e.g. the species of †Lepidotes and †Scheenstia, the callipurbeckiids) also form a rostro-caudal or longitudinal articulation consisting of two anteriorly oriented processes protruding from the anteroventral and anterodorsal corners of the scale (figure 1e,f). These processes can be as strong as or stronger than the peg for the dorsoventral articulation, but they do not fit into sockets. In several taxa (e.g. †Semionotus, †Paralepidotus, †Pliodetes and the gars), the ventral anterior process is poorly developed and there is a strong dorsal anterior process (figure 1d,g,h). The scales of several other taxa (†Australosomus, †Archaeosemionotus, †Boreosomus, †Obaichthys, †Ophiopsis, †Panxianichths, †Plesiofuro) only have a small anterodorsal process (figure 1c). It is important to note that many authors (e.g. [71]: ch. 36, [72]: ch. 59, [73]: ch. 38) score the absence of peg-and-socket articulation for taxa with amioid or cycloid scales. However, the peg-and-socket articulation is a feature of the ganoid scales and the character is not applicable (–) for taxa with other scale types.
Figure 1.
Morphology of ganoid scales. (a) †Sangiorgioichthys sui, reconstruction based on GMPKU-P-1642; (b) †Siemensichthys macrocephalus reconstruction based on [60]: fig. 10B; (c) †Australosomus kochi reconstruction based on [45]: text-fig. 57C; (d) †Semionotus bergeri reconstruction based on NMC 15128a; (e) †Callipurbeckia minor reconstruction based on NHMUK PV P8047; (f) †Scheenstia mantelli reconstruction based on NHMUK PV 2397 and 4916; (g) Lepisosteus sp. reconstruction based on MB.f.18498; (h) †Dentilepisosteus laevis reconstruction based on MPSC 901 in [5]: fig. 109B. Black circle, dorsal peg for the peg-and-socket articulation of adjacent scales; black star, anterodorsal process and white star, anteroventral process, both for the longitudinal articulation of adjacent scales.
3.3. Endocranial fossae
Two main fossae have been identified in the otico-occipital region of the neopterygian endocranium: the post-temporal fossa and the fossa bridgei. However, according to Bjerring [74], the terms ‘post-temporal fossa’ and ‘fossa bridgei’ have been misunderstood and erroneously applied to different fossae in the braincase of the actinopterygians. After thorough discussions, Bjerring [74] proposes a basic scheme including five pairs of endocranial depressions and discusses hypotheses of homology for each of them. His arguments are sound and clearly presented and we thus adopt his ideas as hypotheses of primary homology for the definition of the following two characters. Understanding the homologies of the endocranial fossae in actinopterygians is difficult and we strongly recommend reading Bjerring [74] for a complete argumentation of his hypotheses of homology, which are being followed in this work.
It is important to note that Allis ([75]: p. 8) distinguishes the ‘temporal fossa of fishes is a hole formed by the more or less complete roofing, by dermal bones, of the temporal groove on the dorsal surface of the primordial cranium’. More correctly, Bjerring [74] and other authors (e.g. [76,77]) use the term fossa referring to the endocranial depressions.
As defined by Bjerring ([74]: p. 232), the fossa supra-auditiva ‘is a depression in the external surface of the otic region of the endocranium, situated dorsal to the lateral semicircular duct and lateral to the anterior and posterior semicircular ducts'. It has been labelled in different ways by different authors (e.g. in Amia: ‘post-temporal fossa’ by Patterson [51,78], or ‘fossa bridgei’ by Jarvik [79]). The fossa supra-auditiva is either present or the condition is unknown in the taxa included in our data matrix, so its presence is uninformative for our analysis.
3.4. Foramen for the glossopharyngeal nerve (IX)
The braincase in †Pteronisculus, †Australosomus and †Boreosomus ossifies in a few pieces, which are not directly comparable with the individual bones that form in neopterygians. In these fishes, the glossopharyngeal nerve exits the ventrolateral wall of the otic region within a groove for the vena jugularis, which is called the jugular depression. The IX-foramen in †Pachycormus macropterus is in the opisthotic, also in the anterior end of the jugular groove (Mainwaring AJ. 1978 Anatomical and systematic review of the Pachycormidae, a family of Mesozoic fossil fishes. Unpublished: Westfield College: p. 54), and this condition is autapomorphic for this taxon in our data matrix.
The passage of the glossopharyngeal nerve through the otic walls of the neurocranium of Amia is described in detail by Allis ([80]: p. 683): ‘It passes above the ramulus papillae lagenae acustici, under the ramulus ampullae posterioris acustici, between the sacculus and the sinus utriculi posterior … and issues from the cranium by its foramen …, which lies immediately behind the hind edge of the petrosal, in the angle between that edge and the ventral edge of the posterior process of the bone. The foramen lies entirely in the cartilage of the cranium, but its front and upper edges are formed by the petrosal.’ The shape of the prootic and intercalar bones in †Calamopleurus cylindricus is remarkably similar to the condition in Amia calva (compare [23]: figs 24 and 303) and there is no evidence for the IX-foramen in any of the bones at the lateroventral wall of the otic region, which is mostly unossified. Thus, we assume that the glossopharyngeal nerve in †Calamopleurus cylindricus exits the braincase in the same way as in Amia calva. Similarly, the same condition is interpreted for all those amiine halecomorphs, in which this region of the braincase is well preserved. In Amia calva and †Calamopleurus cylindricus, there is a foramen in the posterior region of the intercalar ([23]: figs 24C, 28 and 303), which, according to Allis ([80]: pl. 21) corresponds to the exit of the supratemporal branch of the glossopharyngeal nerve.
The foramen for the glossopharyngeal nerve (IX) pierces the prootic bone in ginglymodians, most non-amiinae halecomorphs and early Mesozoic teleosts such as aspidorhynchiforms, †Siemensichthys macrocephalus, †Dorsetichthys bechei and †Leptolepis coryphaenoides. Instead, in more derived teleosts, like †Tharsis dubius and teleocephalans, the IX-foramen is in the exoccipital. Among the taxa included in our analysis, †Ionoscopus cyprinoides is the only species in which the IX-foramen is enclosed at the anterior end of the intercalar [81].
Patterson & Rosen ([82]: ch. 26) defined an a/p character for the presence of the ‘foramen for the glossopharyngeal nerve (IX) in exoccipital rather than in prootic’, which was later used in Arratia ([62]: ch. 20 App. 3) and, with slightly rephrased definition in several cladistic analyses (e.g. [65]: ch. 20, [66]: ch. 19, [29]: ch. 32, [39]: ch. 37). All these examples include unspecified absences [44] with Amia scored equal to Lepisosteus and early Mesozoic teleosts with the absence character state.
3.5. Foramen for the vagus nerve (X)
The fissura otico-occipitales is open in the braincase of our out-group taxa †Pteronisculus, †Australosomus and †Boreosomus, and in †Watsonulus eugnathoides. Therefore, there is no foramen for the vagus nerve (X), which exits through the fissure in these fishes. The otico-occipital fissure is closed in more derived actinopterygians, and the vagus nerve exits the cranium through a foramen through one of the bones of the occipital region. In most halecomorphs and most basal teleosts, the X-foramen is surrounded by the intercalar and the exoccipital bones. In ginglymodians, the halecomorphs †Calamopleurus cylindricus and †Oshunia brevis, and in the teleosts †Catervariolus, †Varasichthys and the Teleocephala, the X-foramen is completely enclosed by the exoccipital. †Pachycormus macropterus presents a condition unique among the studied taxa, in which the vagus nerve exits the braincase through a foramen between the opisthotic and the intercalar.
The position of the foramen for the vagus nerve has been used for cladistic analyses in variably coded characters. Our coding (character 24) is taken from Gardiner etal. ([83]: ch. 5 App. 1) and Hurley etal. ([4]: ch. 9), who use a multistate character representing the position of the exit of the vagus nerve. Their character state 0, ‘anterior to exoccipital’ is equivalent to our character state 0 ‘through the fissura otico-occipitalis’, though we modified its formulation to make it more precise and descriptive. Their character state 1 ‘lateral outgrowths from intercalar form lateral margin’ is not represented in our data matrix. Their character state 2, expressed as ventral outgrowths from intercalar lateral margin ‘enclose ventral margin’ in [83], or ‘enclose dorsal margin’ in Hurley et al. [4] is equivalent to our character state 1 ‘between intercalar and exoccipital’, which is more flexible because the shapes of the intercalar outgrowths vary intra- and interspecifically and, thus, they contribute in different ways to the rim of the X-foramen. Finally, their character state 3 ‘enclosed by exoccipital’ is the same as our character state 2. The character state 1 of these authors is problematic because it was scored for †Pachycormus in Gardiner et al. ([83]: ch. 5 App. 1), which does not agree with Mainwaring (Mainwaring AJ. 1978 Anatomical and systematic review of the Pachycormidae, a family of Mesozoic fossil fishes. Unpublished: Westfield College), or for Polypterus in Hurley et al. ([4]: ch. 9), but the bone enclosing the X-foramen in this fish is not the intercalar but opisthotic according to Allis [84] and Claeson et al. [85].
Most other authors (e.g. [86]: ch. 3, [71]: ch. 11, [63]: ch. 25, [65]: ch. 21, [87]: ch. 2, [26]: ch. 3, [29]: ch. 33) only include an a/p character coding the presence of the X-foramen in the exoccipital (our state 2), thus producing an unspecified absence [44] when equally scoring Amia (here state 1), †Watsonulus (here state 0) and sometimes also †Pachycormus (here state 3) with the state ‘absence’.
3.6. Intercalar
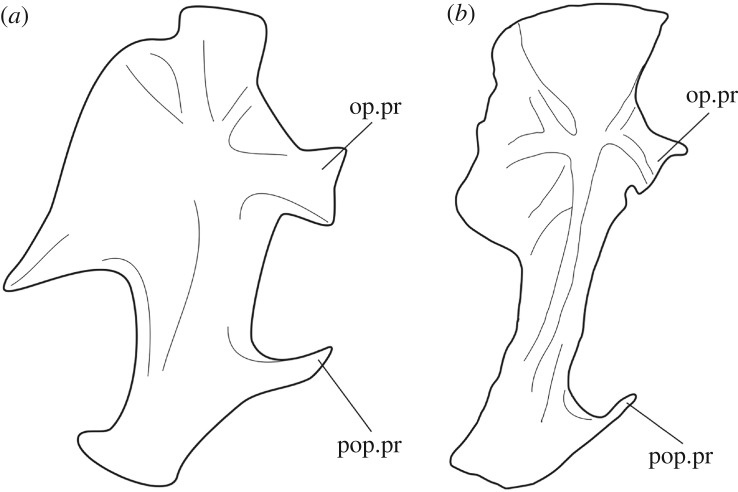
According to Patterson ([78]: p. 315), the intercalar bone is homologous to the cranio-spinal process present in the out-group taxa †Pteronisculus, †Australosomus and †Boreosomus [47,88], and in the halecomorph †Watsonulus eugnathoides [89] and the ginglymodian †Ticinolepis crassidens [37]. Patterson further interpreted that a dermal component develops from this endochondral core, which is homologous with the fully dermal intercalar in teleosts above the level of †Leptolepis coryphaenoides. Following Patterson's hypothesis of homology, we coded the a/p character 33 for the presence of an intercalar (chondral or dermal), and characters 34 and 35 representing different conditions observed regarding the development and relationships of the dermal component of the intercalar. In our character 33, we distinguish the intercalar without extensive dermal outgrowths (state 0; figure 2a) from the intercalar with outgrowths contacting the prootic (state 1; figure 2b) or with extensive outgrowths contacting the prootic and parasphenoid (state 2; figure 2c). We do not code the disappearance of the chondral component of the intercalar because it is almost impossible to evaluate this feature in fossils.
Figure 2.
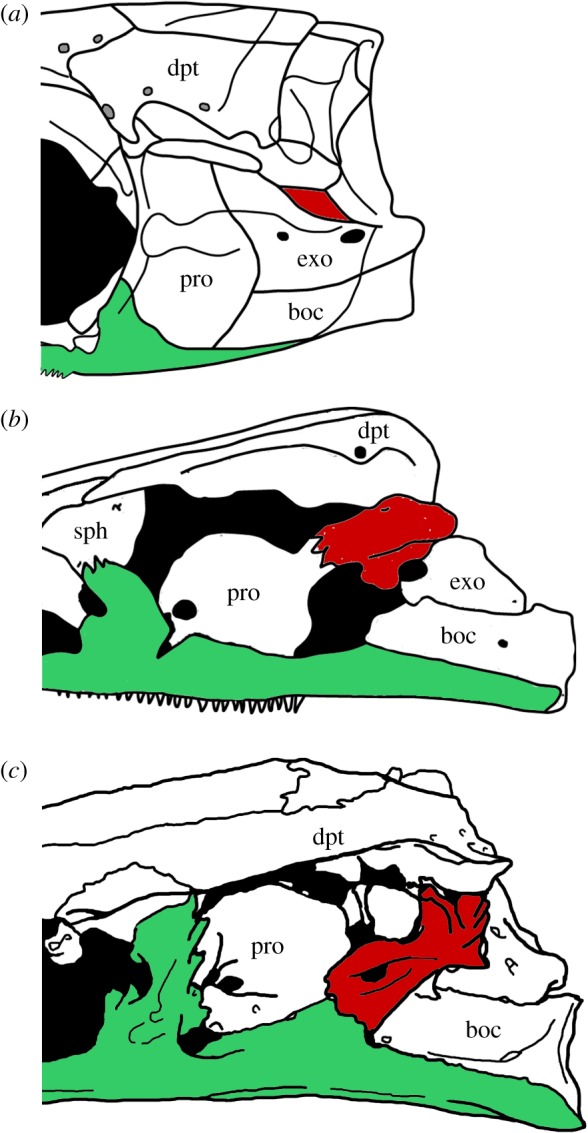
Extent of the dermal outgrowths of the intercalar bone. (a) small dermal component without extensive outgrowths in †Ichthyokentema purbeckensis, reconstruction based on [90]: fig. 2; (b) dermal outgrowths contacting the prootic in Amia calva, reconstruction based on AMNH 90970 SD in [23]: fig. 23B; (c) extensive outgrowths contacting the prootic and parasphenoid in †Ionoscopus cyprinoides, reconstruction based on NHMUK PV 37795a in [78]: fig. 1A. Intercalar bone painted in red, parasphenoid painted in green. Abbreviations: boc, basioccipital; dpt, dermopterotic; exo, exoccipital; pro, prootic; sph, sphenotic.
Olsen & McCune ([86]: chs. 9, 21) and Brito ([71]: chs. 9, 10) score the presence/absence of a chondral intercalar and the presence/absence of an intercalar with dermal outcrops in two independent characters. This way, the complete absence of an intercalar (e.g. Lepisosteus) is incorrectly scored the same as the presence of a dermal intercalar (e.g. Amia) in their state ‘absence of a chondral intercalar’.
3.7. Hyomandibular facet
There is much variation regarding the bones involved in the articulation of the hyomandibula with the neurocranium, so we have defined character 39, including six states summarizing the observed variation. In †Caturus furcatus, there is a single bone forming the lateral wall of the endocranium, which has been identified as a large prootic [91], but might be the result of the fusion between the prootic and opisthotic [92]. Owing to this uncertainty, this character is scored as ‘missing data’ (?) for †Caturus furcatus. In most other halecomorphs, the facet for articulation of the hyomandibula is formed in cartilage (ch. 39[0]) [23]. The cartilage is not preserved in fossils, but the condition can be inferred. Among the taxa studied, only in †Macrepistius the facet for the hyomandibula is completely included in the opisthotic (ch. 39[1]) (interpretation of this bone according to Maisey [81]). The condition in †Pachycormus macropterus, in which all the bones of the lateral wall of the neurocranium are involved in the formation of the hyomandibular facet (ch. 39[2]) (Mainwaring AJ. 1978 Anatomical and systematic review of the Pachycormidae, a family of Mesozoic fossil fishes. Unpublished: Westfield College), is unique among the studied taxa. Among teleosts, the facet is formed by the sphenotic, pterotic and prootic bones (ch. 39[3]) in the more basal forms and in Brycon and Hiodon, but the prootic is not involved in the facet in the other teleost taxa in our data matrix. The facet is formed by the pterotic and sphenotic (ch. 39[5]) in most of these species, or by the pterotic only in †Notelops and †Ebertichthys (ch. 39[4]).
Several authors have coded the inclination of the hyomandibular facet in variably defined morphological characters. For example, Gardiner & Schaeffer ([93]: ch. 20) coded the presence/absence of horizontal facets and Coates ([94]: ch. 27) coded posteroventrally versus ventrally oriented facets. The common problem with previous attempts to distinguish between different degrees of inclination of the hyomandibular facet is that there is no clear indication of the reference against which the measurement should be taken. To solve this problem, for our character 40 ‘Orientation of hyomandibular facet respect to the parasphenoid axis’, we have taken as reference the orientation of the orbital portion of the parasphenoid, which is the most constant structure reflecting the anteroposterior axis of the head in most actinopterygian braincases. On the contrary, the postorbital portion of the parasphenoid is very variable, not only in extension, but also in orientation.
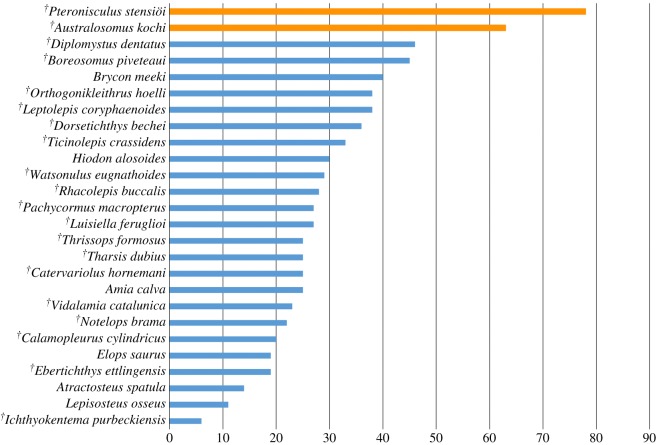
To define the character states, we have been able to measure the angle between the main axis of the hyomandibular facet and the longitudinal axes of the orbital portion of the parasphenoid in 26 taxa. This information is represented in figure 3, which shows a gradual change for angles below 50° followed by a significant gap between 50° and 60°. This pattern agrees with previous ideas concerning a significantly inclined versus an almost horizontal hyomandibular facet, the latter only present in neopterygians [93]. Our character states are thus defined around this gap. However, this hypothesis should be tested in a more comprehensive analysis of the variation of this feature among non-neopterygian actinopterygians, which might reveal facets oriented with angles larger than 50°, or they might refute our hypothesis with values filling the gap observed in this study. In such a case, this feature should best be treated as a continuous character.
Figure 3.
Bar chart representing the values of the angle between the main axis of the hyomandibular facet and the longitudinal axes of the orbital portion of the parasphenoid (horizontal axis) obtained for 26 studied taxa.
The hyomandibular facet is not directly observable in several taxa because it is formed in cartilage (see above), or it is hidden by other bones in fossils. In such cases, scoring the variation in the orientation of the hyomandibular facet is still possible through a rough estimation within the ranges proposed in the two character states when the parasphenoid and the head of the hyomandibula are well visible and preserved in situ.
An alternative coding proposed by Xu & Wu ([72]: ch. 36) is a character including three states based on ranges of angular values representing the orientation of the suspensorium. However, the orientation of the suspensorium is independent and does not directly reflect the inclination of the hyomandibular facet (see Gardiner & Schaeffer [93]: pp. 145–146). Such a variation would thus represent a separate character, but we think that the inclination of the suspensorium is not an independent feature but a direct consequence of the position of the lower jaw articulation (character 69).
3.8. Dermosphenotic and dermal component of the sphenotic
The presence of a dermal component of the sphenotic is discussed by Bartram [95] and this feature has been used in several cladistic analyses (e.g. [5]: ch. 23, [26]: ch. 7, [72]: ch. 16, [29]: ch. 10, [27]: ch. 34, [33]: ch. 75). The character is discussed by Grande ([5]: p. 760), who clearly distinguishes the condition in the †obaichthyids, in which the sphenotic is fused to the dermosphenotic, but scores the presence of a sphenotic dermal component for these taxa. However, the dermal component of the sphenotic in other neopterygians is an ossification independent from the dermosphenotic. The best example of the independence of these two ossifications is found in the living gars, in which the dermosphenotic and the exposed portion of the sphenotic are well separated by suborbital bones (see examples in Grande [5]). Therefore, the two conditions are not homologous and are thus here coded as different characters (chs. 41 and 42, respectively).
The fusion between dermosphenotic and sphenotic is also equated with the dermal component of the sphenotic and incorrectly scoring the presence of this latter feature in †Obaichthys and †Dentilepisosteus in Arratia ([29]: ch. 10), Cavin et al. ([27]: ch. 34), Bermúdez-Rochas & Poyato-Ariza ([96]: ch. 7), and other papers using those data matrices.
3.9. Lateral dermethmoids
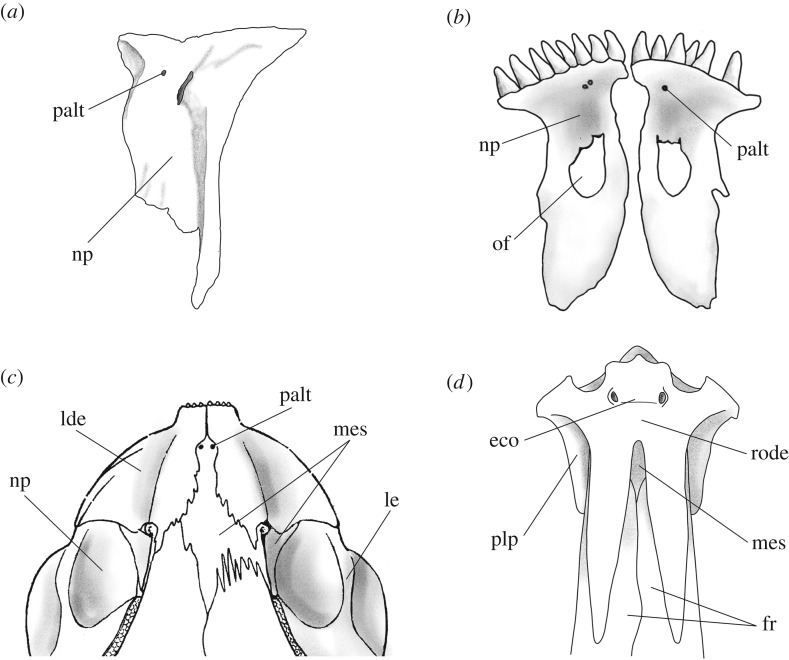
Characters 47 and 48 are based on the hypotheses of homology between the rhinal bone, the nasal process of the premaxilla in halecomorphs and ginglymodians (figure 4a,b), and the lateral dermethmoids of teleosts, which are discussed and summarized in López-Arbarello ([26]: p. 38) and thoroughly discussed in Patterson [78]. According to Patterson ([51]; [78]: p. 510) ‘the pholidophorid premaxilla and lateral dermethmoid are together the exact topographic homologues of the premaxilla and its nasal process’ and the lateral dermethmoids in †Siemensichthys macrocephalus (figure 4c) or †Dorsetichthys bechei are homologous of the lateral dermethmoid component of the compound mesethmoid of †Leptolepis coryphaenoides (figure 4d) and more derived teleosts.
Figure 4.
Lateral dermethmoids: (a) forming small nasal processes of the premaxilla, only partially surrounding the olfactory foramen, in †Ticinolepis longaeva, line drawing of the premaxilla of MCSN 8007; (b) forming large nasal processes of the premaxilla, enclosing the olfactory foramen, in Amia calva, reconstruction based on AMNH 90970 SD in [23]: fig. 42A; (c) forming toothed dermethmoids in †Siemensichthys macrocephalus, reconstruction based on [74]: fig. 145; (d) forming part of a compound mesethmoid with chondral and dermal components in †Tharsis dubius, reconstruction based on [74]: fig. 130a. Abbreviations: eco, ethmoidal commissure; fr, frontal bone; lde, lateral dermethmoid; le, lateral ethmoid; mes, mesethmoid; np, nasal process of the premaxilla; of, olfactory foramen; palt, foramen for terminal branch of palatine nerve; plp, postero-lateral process of lateral dermethmoid; rode, rostrodermethmoid.
According to this hypothesis, among the taxa included in this analysis, the lateral dermethmoids might be present or absent (ch. 47). If present, they might be forming the nasal process of a single premaxilla (figure 4a,b) or they might be toothed and separated from a pair of small premaxillae (figure 4c), or they might be forming part of a compound mesethmoid ossification including chondral and dermal components (figure 4d) (ch. 48).
3.10. Parasphenoid processes
Two ascending processes are identified in early actinopterygians: processus ascendens anterior and posterior (e.g. [46,47,77,79,97–99]). The processus ascendens posterior is more widely present among actinopterygians and it is usually referred to as the ascending process (e.g. [23,52,78,100–102]). The processus ascendens anterior is related to the processus basypterigius of the neurocranium ([46]: p. 273; [47]: pp. 105, 324), and it is usually referred to as the basipterygoid process (e.g. [100]), especially in neopterygians (e.g. [5,23,78]).
The basipterygoid process of the parasphenoid (processus ascendens anterior) is poorly developed in several taxa, including †Boreosomus [47,103], and Amia and other halecomorphs [23,79], and it is absent in several actinopterygians, including the chondrosteans †Birgeria [88], †Condorlepis [104], †Chondrosteus [105] and †Acipenser [106], but also in †Australosomus [88] and †Perleidus [46,103]. The absence of this process is probably related to the poor development or complete absence of a processus basypterigius in the neurocranium in these taxa.
3.11. Quadratojugal
The homologies of the quadratojugal bone of actinopterygians, which is probably not homologous with the quadratojugal bone of sarcopterygians [107], have been debated by several authors. Among non-neopterygian actinopterygians, the quadratojugal is a small dermal bone attached to the palatoquadrate and it contains a pit-line (e.g. see detailed descriptions and illustrations of the quadratojugal in †Mimipiscis toombsi and †Moythomasia durgaringa in Gardiner [100]). In Ginglymodi, the bone identified as the quadratojugal is a splint-like dermal ossification lying along the dorsal margin of the preopercle, with an anterior head that buttresses the articular process of the quadrate and a posterior spine-like portion (see López-Arbarello [26]: fig. 3). The symplectic articulates between the quadrate and the posterior spine-like portion of the quadratojugal. The homology between the splint-like quadratojugal of ginglymodians and the plate-like quadratojugal of other actinopterygians has been supported by several authors (e.g. [51,108,109]).
In teleosts and in most halecomorphs, there is no independent quadratojugal, which is considered absent in halecomorphs, but fused to the quadrate in teleosts. Among these fishes, no ossification resembling the quadratojugal has been found in halecomorphs (except for those with a distinct plate-like quadratojugal), but the strengthened posteroventral margin of the teleost quadrate has been considered homologous to the quadratojugal (e.g. [26,51,52,75,78,109–116]).
Our characters 70 to 72 are coded based on the hypotheses of homology between the plate-like quadratojugal of non-neopterygian actinopterygians and a few basal neopterygians, the splint-like quadratojugal of ginglymodians and the posteroventral border of the teleost quadrate. Character 70 refers to the complete absence of a quadratojugal, which only occurs in halecomorphs and †Boreosomus piveteaui among the taxa included in our analysis. The fusion of the quadratojugal with the quadrate is coded in our character 71, and character 72 distinguishes the plate-like from the splint-like quadratojugal. Therefore, characters 71 and 72 are inapplicable for most halecomorphs because they do not have a quadratojugal (70[0]), and character 72 is also inapplicable for teleosts because the shape of the quadratojugal cannot be established due to its complete fusion with the quadrate.
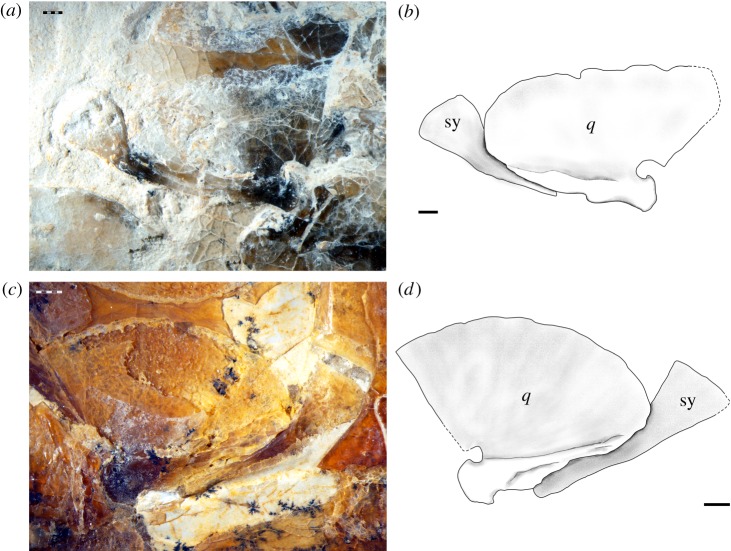
After a thorough revision of the osteology of †pachycormiforms and †aspidorhynchiforms, Gouiric-Cavalli [117] concluded that in these fishes the quadratojugal is absent and the symplectic participates in a double-jaw articulation resembling the condition in Amia calva. We agree with this author in the presence of a double jaw articulation in the specimen SNSB-BSPG AS.VII.1069 of †Belonostomus speciosus and the same condition has been reported in †Vinctifer comptoni [71]. However, the symplectic does not reach the jaw in JME 1997.III.6 (figure 5a,b) or other examined specimens of †Aspidorhynchus acutirostris (e.g. SNSB-BSPG 1964.XXIII.542; figure 5c,d), in which these bones are well preserved and exposed. Both in †Belonostomus speciosus (SNSB-BSPG AS.VII.1069) and in †Aspidorhynchus acutirostris (JME 1997.III.6, SNSB-BSPG 1964.XXIII.542), the bone tissue at the posteroventral border of the quadrate is different and partially separated from the rest of the quadrate, and it is here considered as a quadratojugal (figure 5).
Figure 5.
Symplectic-quadrate complex in †Aspidorhynchus acutirostris. (a) Photograph and (b) line drawing of JME 1997.III.6. (c) Photograph and (d) line drawing of SNSB-BSPG 1964.XXIII.542. Abbreviations: q, quadrate; sy, symplectic. Scale bars, 1 mm.
3.12. Infraorbital bones
López-Arbarello ([26]: p. 11) discusses the homologies between the bones carrying the infraorbital sensory canal. Without exception, these bones develop in connection with one or more neuromasts [108,113,118–120], but the association of each of these bones with particular neuromasts of the infraorbital sensory line is not possible because the number of neuromasts in this sensory line is highly variable [113], even between the left and right sides of the same specimen of an individual [108]. Nonetheless, the rostral, antorbital and dermosphenotic have a different developmental timing with respect to the rest of the series and can be distinguished because of their association with particular segments of the cephalic sensory canals ([26]; figure 6).
Figure 6.
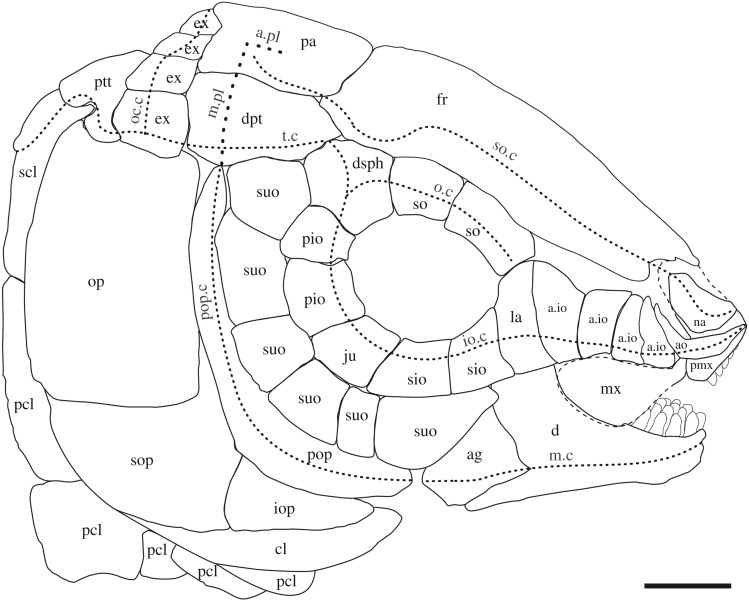
Reconstruction of the skull in †Scheenstia zappi, modified from [121]. Abbreviations: a.io, anterior infraorbital bone; ag, angular bone; ao, antorbital bone; a.pl, anterior pit line; cl, cleithrum; d, dentary; dpt, dermopterotic bone; dsph, dermosphenotic bone; ex, extrascapular bone; fr, frontal bone; io, infraorbital bone; io.c, infraorbital sensory canal; iop, interoperculum; ju, jugal bone; la, lachrymal; m.c, mandibular sensory canal; m.pl, middle pit line; mx, maxilla; na, nasal bone; o.c, orbital sensory canal; oc.c, occipital sensory canal; op, operculum; pa, parietal bone; pcl, postcleithrum; pio, posterior infraorbital bone; pmx, premaxilla; pop, preoperculum; pop.c, preopercular sensory canal; ptt, post-temporal bone; scl, supracleithrum; sio, subinfraorbital bone; so.c, supraorbital sensory canal; so, supraorbital bone; sop, suboperculum; suo, suborbital bone; t.c, temporal sensory canal. Scale bar, 1 cm.
The rostral bone is associated with the ethmoidal commissure, and the presence of the ethmoidal commissure in potentially compound bones (states 0, 2 and 3) indicates fusion of the rostral with other ossifications ([122,123]; e.g. †Leptolepis coryphaenoides, figure 4d). The complete absence of any trace of the ethmoidal commissure or a median ossification separate from the compound bones indicates that the neuromasts involved in the ethmoidal commissure or in the ethmoidal pit line do not induce any ossification and, thus, there is a real absence of a rostral bone.
The antorbital bone is associated with the junction between the infraorbital and supraorbital canals and it is adjacent to the rostral in holosteans and basal teleosts. In more derived teleosts, however, there are no separate bones anterior to the lachrymal or there is only a small bone in the position of the antorbital, but it has no sensory canals or pit-lines. This latter bone is considered homologous to the antorbital bone [62].
The dermosphenotic carries the last portion of the infraorbital sensory canal and it is placed at the posterodorsal corner of the orbit. In most neopterygians, the dermosphenotic is loosely attached to the frontal and dermopterotic, lying on the sphenotic, but it is tightly sutured and incorporated into the skull roof in several halecomorphs. The dermosphenotic might be fused to or separate and distant from the sphenotic in some taxa (see above).
The series of infraorbital bones between the antorbital and the dermosphenotic should be treated as a whole [26]. Among them, four regions can be distinguished for the sake of comparison: the anteroventral, ventral, posteroventral and posterior margins of the orbit. The number and/or shape of the infraorbital bone or bones involved in each of these regions have long been used in actinopterygian systematics and they have received different names according to their position (e.g. [5,23]). To facilitate comparisons, we adopt this terminology, but without direct implication of taxic primary homology for the individual bones. Accordingly, the terms lachrymal, subinfraorbitals, jugal and postinfraorbitals are used in reference to the bones involved in each of the regions previously indicated (figure 6).
3.13. Vertebral centra
The composition of the vertebral centrum is still very poorly understood in fossil neopterygians, except for some teleost taxa [29,63,124–126]. Three main tissues, product of different mineralization processes, might be involved in the ossification of the vertebral centrum: arcocentrum, chordacentrum and autocentrum. Other authors (e.g. op. cit.) have attempted to define different vertebral types according to the different combination of tissues involved in the ossified centrum. Here, we proposed a completely different approach based on the general hypothesis that each of these three tissues develops independently from each other, i.e. the presence of one tissue does not imply the presence or absence of any of the other tissues. Consequently, we propose three independent characters (chs. 225–227) scoring the presence/absence of each of these tissues.
The vertebrae in our out-group taxa †Pteronisculus and †Boreosomus, but also in the basal halecomorph †Watsonulus and, as far as know, in most ginglymodians outside Lepisosteoidea (†Semionotus elegans, †Paralepidotus ornatus, †Isanichthys lertboosi, †Thaiichthys buddhabutrensis, †Araripelepidotes temnurus) are only formed by the dorsal and ventral arcual elements, and the centrum does not ossify. Therefore, none of these tissues is present in the vertebral centra of these taxa.
The arcocentrum is an endochondral ossification derived from the dorsal and ventral arcualia. The crescent-shaped hemicentra of the vertebrae of the halecomorph †Caturus has been interpreted as hemichordacentra [23,127,128]. However, after close examination of specimens, we agree with Laerm ([125]: p. 195) that the hemicentra of caturids (clearly visible in the specimen NHMUK PV 20578 of †Caturus furcatus) are endochondral ossifications (225[1]). Similarly, the ring-like centra of †Ophiopsiella (previously †Ophiopsis, see [129]) have been thought to be formed by a cylinder of chordacentrum surrounded by autocentrum [95]. Laerm [125] questioned this hypothesis because the inner ring of the vertebral centra of †Ophiopsiella is ossified instead of being mineralized as is the case of the chordacentra in other actinopterygians. Gardiner et al. [83] consider the drum-like centra of †Ophiopsiella to be homologous with the centra in Amia. We, therefore, follow the latter authors and scored state (225[2]) for this taxon.
The vertebral centra of Amia, state (225[2]), is formed by endochondral replacement of cartilaginous arch base and intercalated anlagen [125,130,131]. In the abdominal region, the centra are monospondylous and result in perichordal ossification of the interdorsal and basiventral arcualia. The diplospondylous centra of the caudal region are formed by the fusion of the interdorsal and interventral, and basidorsal and basiventral arcocentra.
Among gars, the development of the holospondylous vertebrae has been studied in Lepisosteus [90,125,132–134], in which the centrum is mainly formed by endochondral replacement of a continuous perichordal tube of cartilage derived from the dorsal and ventral arch anlagen [125,134]. This condition corresponds to our character state (225[2]). The solidly ossified opisthocoelous centra of other fossil and living gars resemble the centra of Lepisosteus and there is no reason to suspect a different composition. The hemicentra of †Scheenstia mantelli are expanded arcocentra, according to Patterson ([51]: p. 294). Interestingly, the vertebral centra of †Macrosemius rostratus are perichordally ossified and continuous with the parapophysis, but not with the neural arches (SNSB-BSPG AS I 770, [95]: p. 157), thus resembling ontogenetic stages of Amia.
The vertebral centra of aspidorhynchids and some Jurassic teleosts like †Siemensichthys, are formed by annular chordacentra surrounded by the dorsal and ventral arcocentra [128,135].
The chordacentrum is the result of mineralization within the fibrous sheath of the notochord and it is the only component of the vertebral centra in several basal teleosts (226[1]). The chordacentrum in basal teleosts (e.g. †Annaichthys, †Pholidophoretes; [29]) might form complete rings (†Ichthyokentema, †Catervariolus; [136,137]) or might be represented by dorsal and/or ventral hemichordacentra (†Eurycormus, †Parapholidophorus, †Pholidoctenus, †Pholidorhynchodon; [29,138]). The hemichordacentra, however, usually fused to form complete rings (†Pholidophorus; [29]), so we consider this variation to be ontogenetic. Apart from these basal teleosts, the only other taxon with vertebral centra formed by mineralized chordacentrum in our data matrix is †Australosomus kochi [46,88].
The centra of living teleosts are mainly formed by direct membranous ossification of the sclerotomal perichordal tube (autocentrum), although chordacentral and arcocentral remnants are variably present [125,128,139,140].
3.14. Epurals
The term epurals refers to the series of median rod-like bones placed posterior to the last fully developed neural spine in the caudal skeleton of actinopterygians. Epurals have been interpreted as homologous to detached neural arches [141–144], homologous with radials [145–147], or homologous with the supraneurals [148,149]. Comparing with the series of epurals in the Triassic †Pteronisculus, †Australosomus and other actinopterygians, and considering the one-to-one relationship between these bones and the neural arches in these fishes, Patterson ([150]: pp. 220–221) concluded that the epurals are detached neural spines, which he considered homologous to the anterior series of supraneurals. However, the homology between supraneurals and neural spines has been questioned[151,152].
Schultze & Arratia [135,140] and Arratia & Schultze [152] restricted the term epurals to the median bones placed dorsal to the neural arches of preural and ural centra in teleosts, interpreting them as detached neural spines, which they did not consider homologous to supraneurals. These authors further distinguished the ‘epurals’ in Amia and other neopterygians, rejecting the homology between these elements and the epurals of teleosts.
In Amia and other halecomorphs (e.g. †Ionoscopus cyprinoides; see examples in Grande & Bemis [23]), the most anterior ‘epurals’ intercalate with the complete neural spines of the last preural vertebrae, but the more posterior epurals (only one in Amia, two in †Ionoscopus cyprinoides) are placed directly dorsal to short ural neural spines and/or ural neural arches, as is the case of the epurals in teleosts. Both ‘epurals’ and epurals are also present in non-neopterygian actinopterygians like Polyodontidae [153] and Polypteridae [5] and the question of homology between these elements and between them and the supraneurals remains open.
For the present study, we follow Schultze & Arratia [135,140] and code two independent a/p characters for the epurals (ch. 265) corresponding to the median bones posterior to the completely developed neural spine and interpreted as detached neural spines, and ‘epurals’ (ch. 267) corresponding to the median bones intercalating between the complete neural spines, which are attached to their corresponding neural arches. Accordingly, the median elements in the caudal skeleton of †Australosomus and †Pteronisculus are epurals [47,88]. Among the taxa included in our analysis, ‘epurals’ are only present in halecomorphs and pachycormiforms.
The ‘epurals’ of pachycormiforms need a special discussion. These elements are placed dorsal to a series of median bones named ‘uroneural-like elements’ by Lambers [154], which are interpreted as modified neural arches and spines of several preural and ural vertebrae [155]. Accordingly, because the neural spine is involved in the formation of the ‘uroneural-like elements’, the dorsal series is interpreted as ‘epurals’ in pachycormiforms [155].
3.15. Uroneurals, ‘posterior uroneurals’ and urodermals
The name uroneural was given by Regan ([145]: p. 355) to the modified ural neural arches in Elops and Megalops, which are elongated paired bones spreading along the dorso-lateral surface of the last preural and ural centra. The term was later brought to some confusion with the term urodermal (see below), but Patterson ([150]: pp. 221–231) clarified the use of both terms, fixing the name uroneurals to the series of paired elongated bones flanking the dorsolateral surfaces of preural and/or ural vertebral centra, which represent modified ural neural arches.
A series of uroneural bones is present in †Eurycormus. Arratia & Schultze ([138]: p. 32; also in [135]: table 3) distinguished the first element in this series as an uroneural-like bone, assuming that it represents the modified neural arch of the last preural centrum. Although these authors did not explain the reasons for this interpretation, it might be related to the absence of a neural arch on the last preural centrum in this fish. However, although possible, this absence does not necessarily imply that the first element in the series of uroneurals truly corresponds to the first preural centrum, and we thus do not make this distinction.
The uroneurals in †Tharsis dubius are described as forming two series (figure 7). The bones forming the anterior series are clearly uroneurals as described in character 273. The second series is formed by three bone splints, which have a different orientation and are placed more laterally than the anterior uroneurals. All or some of these bones are overlapping the bases of the most dorsal principal caudal fin rays in some specimens and, thus, their homology with uroneurals or urodermals (see discussion of character 294) is unclear. Pending a more detailed study to clarify the homology of these bones, we here code an independent character to analyse the presence of such ‘posterior uroneurals’ among the taxa studied.
Figure 7.
Caudal skeleton in †Tharsis dubius (SNSB-BSPG 1964.VIII.280). Detailed photograph showing the uroneurals (un) and ‘posterior uroneurals’ (p.un). Detailed photograph showing the broad neural spines, with a median groove (gr), on the upper right corner.
The homology between the series of reduced rhomboid scales flanking the bases of the uppermost principal caudal fin rays in Jurassic teleosts and the rhomboid scales in the body lobe of the heterocercal tail of some non-neopterygian actinopterygians was first proposed by Nybelin [142]. Nybelin, however, did not distinguish between these elements and the series of modified ural neural arches (i.e. uroneurals), which he also interpreted as derived from rhomboid scales that became (phylogenetically) absorbed and associated to the axial skeleton. Nybelin thus proposed the name urodermalia for the two series. Patterson [150] restricted the term urodermals to the posterior series, which represent modified rhomboid scales, and, following Regan [145], reaffirmed the term uroneurals for the anterior series, which represent modified ural neural arches.
Patterson [150] further discussed the homology between the urodermals and the patches of rhomboid scales in the reduced body lobe of Early Jurassic teleosts and accepted the homology between them and the scales in the body lobe of the heterocercal tail as proposed by Nybelin [142]. Following this hypothesis, we accept the homology between the few modified scales, with or without ganoine layer, which are flanking the base of the most dorsal principal caudal fin rays in several teleosts and halecomorphs, the bodies of which are naked or covered with elasmoid scales, and the scales in the complete body lobe of the heterocercal and abbreviated heterocercal tails of other actinopterygians.
3.16. Serrated appendages and clavicles
The term ‘serrated appendages’ was coined by Wilder [156] for the thin, toothed ossifications placed on each side of the isthmus, anterolateral to the cleithrum in Amia calva. These appendages develop independently and ‘have no connection with either cranial or postcranial bone or muscles’ ([157]: p. 522). Only the posterior serrated appendage abuts against the cleithrum, which has a similar microscopic composition of cellular bone bearing oblique denticle-bearing ridges ([157]: p. 528). At least, the posterior serrated appendage has been considered homologous with the clavicle of other actinopterygians [76,157–159].
Erroneously, the term ‘serrated appendage’ has been used by Arratia ([29]: p. 125) for the ornamented lateral surface of the cleithrum in several Triassic and other teleosts, although she clearly distinguished this structure from the condition in amiiforms. We agree with Arratia on the equivalence of the condition in the Triassic teleosts and toothed ridges on the cleithrum of †Watsonulus and †Atacamichthys, but there is no evidence for these structures developing as separate appendages. On the contrary, and in agreement with the observations of Liem & Woods [157], these toothed ridges are part of the cleithrum (our character 325).
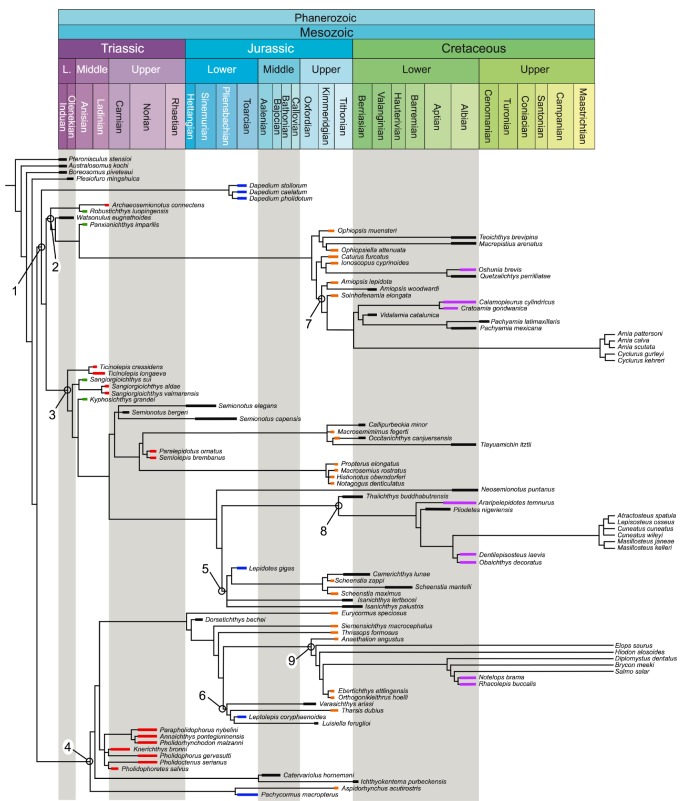
4. Phylogenetic analyses
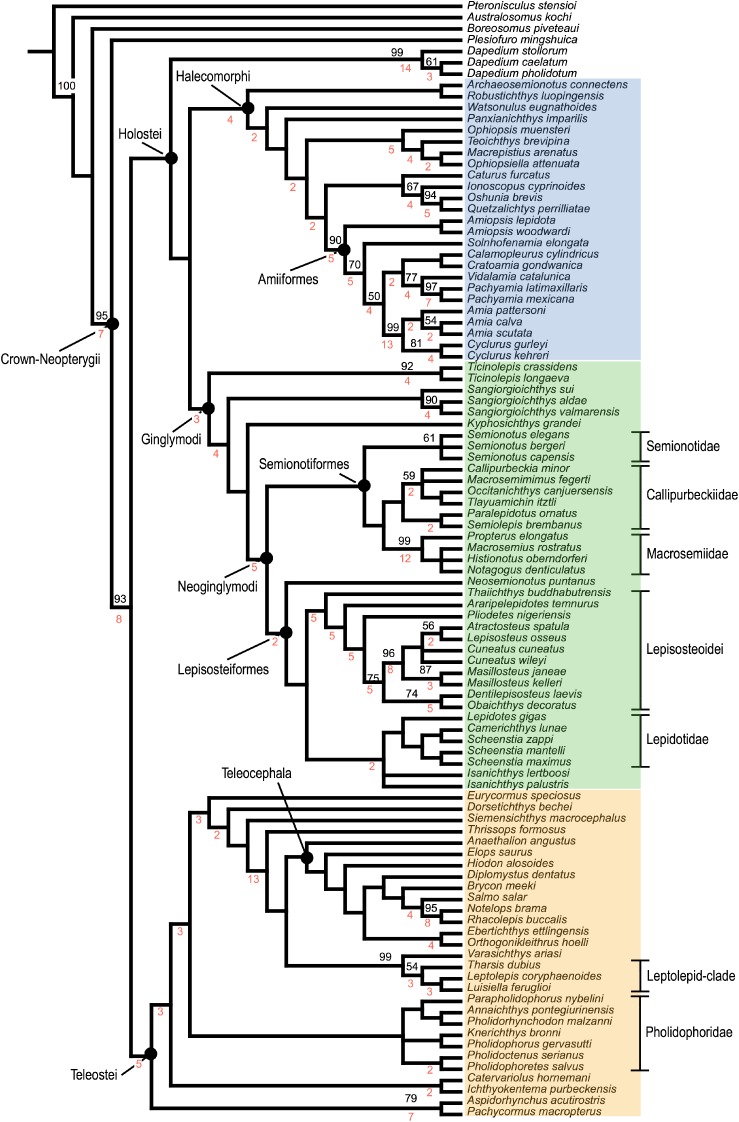
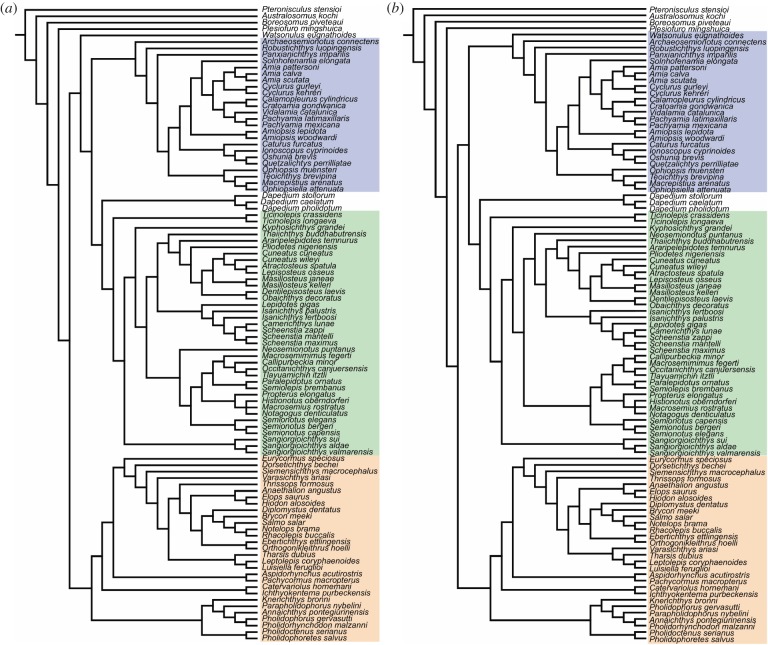
The equal weighted cladistic analysis resulted in 24 most parsimonious trees (MPTs) of 2175 steps, with a consistency index (CI) of 0.268 and a retention index (RI) of 0.678. The strict consensus of the 24 most parsimonious trees is represented in figure 8. The consensus shows a generally well-resolved phylogenetic hypothesis with a monophyletic crown-Neopterygii including monophyletic Teleostei and Holostei. Bremer decay indexes and bootstrap supports for all nodes are indicated in figure 8. In this phylogenetic hypothesis, several taxa (e.g. †Thrissops formosus, †Caturus furcatus) took an unexpected position (see discussions below). We thus run implied weighting analyses to explore the effect of homoplasies in our hypothesis. These analyses were performed with a moderate down-weighting K-value of 8 and strong K-value of 3 and the corresponding strict consensus trees are represented in figure 9 (Bremer and bootstrap values for these cladograms are included in the electronic supplementary material, file S3). The following discussions are mainly based on the results of the equal weighted analysis (topology of and character optimization on the strict consensus of the 24 MPTs). Different relationships obtained in one or the other of the implied weighted analyses are discussed whenever appropriate, but our conclusions are only based on the equal weighted analysis because recent simulation analysis using equal and applied weighting came to the conclusion that implied weighting performs worse than equal weighting in most cases (e.g. [160]).
Figure 8.
Strict consensus tree of 24 most parsimonious trees, equal weights analysis with constraints. Tree length = 2175 steps, consistency index = 0.268 and retention index = 0.678. Bremer indexes and bootstrap values larger than 50 are indicated with red and black numbers, respectively, at the corresponding nodes. Halecomorphi is highlighted in blue, Ginglymodi in green and Teleostei in orange.
Figure 9.
Single most parsimonious trees of the analyses with implied weights with constraints. (a) Strong K-value of 3; (b) moderate down-weighting K-value of 8. Halecomorphi is highlighted in blue, Ginglymodi in green and Teleostei in orange. Bremer and bootstrap values for these trees are available in electronic supplementary material, file S3.
4.1. Systematic position of †Ticinolepis
The monophyly of †Ticinolepis, including two species, †T. longaeva and †T. crassidens, is confirmed with one unique and four homoplastic synapomorphies (electronic supplementary material, file S4) and the genus is recovered as the sister-taxon of all other ginglymodians (figure 8). This phylogenetic position is stable, although in the hypotheses given by implied weighting analyses †Dapedium is included at the base of Ginglymodi as the sister-group of †Ticinolepis plus all other ginglymodians. The monophyly of Ginglymodi is supported by two unique synapomorphies: the presence of a splint-like quadratojugal (ch. 72[1]) and anterior infraorbital bones (ch. 130[1]). The node is further supported by four homoplastic characters. The ectopterygoid is crescent shaped (ch. 62[1]) in Ginglymodi except lepisosteoids, but also in †Pachycormus macropterus and in some teleosts (†Ebertichthys, †Tharsis dubius, †Luisiella feruglioi). The antorbital bone being excluded from the margin of the orbit (ch. 139[0]) is the condition shown by all studied ginglymodians, but also several halecomorphs and some teleosts. The subopercle is shallow (ch. 203[1]) in all ginglymodians except †Pliodetes and more derived gars, but it is also shallow in most halecomorphs and teleosts. The median gular is absent in all ginglymodians except †Kyphosichthys (ch. 208[0]).
López-Arbarello et al. [37] discussed several morphological features shared by †Ticinolepis and the halecomorphs. Some of these features, such as the relatively large broad nasal bones (ch. 106[0]) or the presence of a well-developed medial wing of the cleithrum (ch. 324[1]), are plesiomorphic conditions also present in other neopterygians and in the out-group taxa, but the presence of a supramaxillary notch (ch. 162[3]) and a branchiopercle (ch. 206[1]) is uniquely derived within Holostei (see below). The series of very shallow scales covering the ventrum in †Ticinolepis (ch. 10[1]) is, as indicated by López-Arbarello et al. [37] also present in some halecomorphs (†Archaeosemionotus, †Ophiopsis, †Ophiopsiella), but it also occurs in †Aspidorhynchus [161] and the out-group taxon †Australosomus [88].
The resemblances discussed by López-Arbarello et al. [37] between the braincase of †Ticinolepis and several halecomorphs are mostly plesiomorphic (e.g. the presence of well-ossified lateral ethmoids; ch. 46[0]). Similarly, a large, vertically oriented ascending process of the parasphenoid, which articulates with the sphenotic (ch. 54[0]) is the condition present in all studied halecomorphs, but also in the basal teleost †Catervariolus and the out-group taxa †Australosomos and †Pteronisculus. The facet for the articulation of the hyomandibula in †Ticinolepis is formed between the sphenotic, prootic and pterotic bones (ch. 39[3]), which is also the case in the halecomorph †Caturus furcatus (NHMUK 20578), but also in the basal teleosts †Catervariolus [137], †Ichthyokentema [136] and †Dorsetichthys [78]. The participation of bones in this facet (ch. 39) is highly variable among the taxa studied, but the state in †Ticinopelis is probably the plesiomorphic condition. The condition in the out-group taxa †Australosomos, †Boreosomus and †Pteronisculus, and in †Watsonulus is not comparable because there are no separate bones in the braincase of these fishes. Unfortunately, the braincase of other basal taxa like †Plesiofuro and the most basal halecomorphs †Archaeosemionotus, †Robustichthys and †Panxianichthys is unknown.
Resembling the amiids, the frontals of †Ticinolepis cover the orbital and large part of the temporal regions of the skull, with more than one-third of the length extending behind the orbit (ch. 103[1]). According to our cladistic analysis, this pattern is acquired independently in these two groups and in the halecomorphs †Caturus furcatus and †Watsonulus eugnathoides and in the teleost †Rhacolepis buccalis among the studied taxa. The same state is present in †Australosomus, †Pteronisculus and †Plesiofuro, suggesting these are reversals to a plesiomorphic condition.
López-Arbarello et al. [37] noted the presence of a descending lamina in the dermopterotics (ch. 96[1]) of †Ticinolepis and discussed the distribution of this feature in other actinopterygians. Although this trait is common among halecomorphs, the condition in most taxa is unknown and the known distribution among the taxa included in our analysis is rather patchy.
4.2. Holostei monophyly
Differing from all previous studies, our phylogenetic analysis includes numerous representatives of the main crown-neopterygian clades: Ginglymodians (36 taxa), Halecomorphi (25 taxa) and Teleostei (29 taxa). This more comprehensive dataset was necessary to explore the relationships of †Ticinolepis due to its uncertain systematic assessment caused by the mixture of morphological features discussed by López-Arbarello et al. [37]. The resulting phylogenetic hypothesis (figure 8) shows a monophyletic Holostei including Halecomorphi, Ginglymodi and †Dapedium, which is supported by two homoplastic synapomorphies: the presence of four or more suborbital bones (ch. 144[1]), which is also found in †Knerichthys browni; and the presence of a presupracleithrum (ch. 320[1]), which is also present in †Pteronisculus and might be a reversal because presupracleithra are known in several Palaeozoic and Triassic non-neopterygians (reversed also in †Pachycormus macrocephalus).
The clade formed by Halecomorphi and Ginglymodi, which we could call the crown-Holostei, is well supported by seven synapomorphies in the equal weighted analysis. Among them, the presence of a tapering canal bearing anterior arm of antorbital bone (ch. 137[1]) is uniquely derived in Holostei, which was previously proposed as a synapomorphy of this clade by Hurley et al. ([4]: ch. 32[1]), who described it as the shape of the antorbital ‘tapering towards slender anterior process; tri-radiate canal within broader, posterior, portion’, and Grande ([5]: ch. 12[1]). Two other synapomorphies are also uniquely derived in Holostei: a supramaxillary notch (ch. 162[3]) and a branchiopercle (ch. 206[1]). The supramaxillary notch, which was first noted and described by Grande & Bemis [23], first occurs at the base of the crown-Holostei, it is lost in most ginglymodians (present in †Ticinolepis, †Kyphosichthys) and halecomorphs, but occurs again in and is a synapomorphy of Amiinae. Similarly, the branchiopercle is present in all the studied halecomorph taxa except †Quetzalichthys, in the most basal ginglymodians †Ticinolepis and †Sangiorgioichthys, but it is lost in most ginglymodians and derived again in macrosemiids and the callipurbeckiids †Occitanichthys and †Tlayuamichin.
The remaining four synapomorphies supporting the crown-Holostei are also homoplastic and independently derived outside Holostei: a relatively long dermopterotic (ch. 93[1]), nasal bones that are not excavated by the posterior nostril (ch. 109[3]), two supraorbital bones (ch. 115[2]), and a maxilla with a straight ventral margin (ch. 159[0]). The shape of the pelvic bones in †Ticinolepis, with flat proximal end and widened anteriorly (ch. 336[0]), which is different in the out-group taxa and all studied teleosts except †Eurycormus, is another potential synapomorphy of Holostei.
Among previous morphological phylogenetic studies, Hurley et al. [4] proposed the monophyly of Holostei based on three synapomorphies. Among them, the shape of the antorbital was already discussed and it is here confirmed. The second synapomorphy relates to the shape of the rostral bone and would be equivalent to our state 0 of character 141 (relatively small approximately rectangular to tube-like), which is not given as a synapomorphy by the algorithm because the ancestral condition cannot be resolved in our data matrix. The third synapomorphy refers to the shape of the brain ‘optic tectum larger than telencephalon’ ([4]: ch. 66), which is not included in our dataset.
Grande [5] proposes 13 synapomorphies for Holostei, including the already discussed features of the antorbital and rostral bones (chs. 137 and 141). Most of the other features mentioned as synapomorphies of Holostei in Grande's analysis are found in more derived positions in our analysis and might have supported this clade in his analysis due to the more restricted sample of taxa. Among these other characters, the lack of a pterotic bone (ch. 37) derives independently in gars and the Triassic teleosts, and the presence of a small dermal component of the sphenotic (ch. 41[1]) derived independently in halecomorphs and some ginglymodians; the distribution of this feature is patchy within this latter clade. Similarly, the presence of large premaxillary nasal processes enclosing the olfactory foramen (ch. 152[1]) and the suture of the nasal processes of the premaxillae with the frontal bones (ch. 153[1,2]) are independently derived in amiiforms and ginglymodians above the level of †Ticinolepis. The presence of paired vomers (ch. 59[0]), a compound coronoid process (ch. 169), and the presence of fringing fulcra on the caudal fin (ch. 298[0]) are plesiomorphic for Neopterygii. The presence of serrated appendages (ch. 328[1]) is difficult to evaluate in fossils, and in many cases it is impossible to be certain about their absence. As far as we have been able to evaluate, this feature has a rather patchy distribution, but only within Holostei. Serrated appendages are known in Amia calva and †Caturus furcatus among halecomorphs, and in living gars, †Semionotus elegans and †Propterus elongatus among ginglymodians.
Other features proposed by Grande [5] are potential synapomorphies of Holostei, but more data are needed to understand the evolution of these characters because the ancestral condition cannot be resolved in our analysis. Among these, four hypobranchials (ch. 211[1]) are known only in †Pachycormus macrocephalus outside Holostei, and the lateral dermethmoids forming the nasal processes of the premaxilla (ch. 48[0]) is apparently unique of holosteans, but the condition of the lateral ethmoids is so far unknown outside Holostei or Teleostei.
The phylogenetic relationships of †Dapedium remain controversial: the genus is placed outside the crown-Holostei in the strict consensus tree of the equal weighted analysis (figure 8), but it is the most basal Ginglymodi in the implied weighted analyses (figure 9). These latter results are in agreement with the hypothesis proposed by Gibson [162] in the most recent and comprehensive cladistic analysis of dapediiforms, including four species of †Dapedium and seven other dapediiform genera. Gibson's analysis is largely based on the data matrix of López-Arbarello [26], with the addition of several taxa, including three teleosts and three halecomorphs, but little additions to the set of characters (originally meant for ginglymodians only). Consequently, solving the relationships of dapediiforms requires a cladistic analysis including the complete set of dapediiform taxa, as done by Gibson, and a complete set of neopterygian taxa and characters as the one provided in this work.
4.3. Relationships among crown-neopterygians
Within Holostei, the patterns of relationships of ginglymodians and halecomorphs mostly agree with previous hypotheses. Above the series of Triassic stem-taxa, the Ginglymodi split in the orders Lepisosteiformes and †Semionotiformes, as proposed by López-Arbarello [26]. Differing from this and the more recent study by López-Arbarello & Wencker [36], and in agreement with Sun & Ni [42], the Middle Triassic †Kyphosichthys and †Sangiorgioichthys are not semionotiforms but join the tree at the stem to the node (Lepisosteiformes, †Semionotiformes). In our analysis, †Ticinolepis also joins the tree at this stem as the most basal Ginglymodi and, thus, we now find it useful to distinguish the clade (Lepisosteiformes, †Semionotiformes) as the Neoginglymodi, defined as the clade including Lepisosteus and †Semionotus, and all descendants of their most recent common ancestor. Pending clarification of the relationships of †Dapedium, the Ginglymodi remain defined as proposed by López-Arbarello ([26]: p. 34): the clade including all taxa more closely related to Lepisosteus than to †Dapedium, Amia or †Pholidophorus. This definition might need to be changed if future analyses show that dapediids are nested among more derived ginglymodians.
In the hypothesis of Sun & Ni [42], the stem-Neoginglymodi †Kyphosichthys and †Sangiorgioichthys form a clade they called Kyphosichthyidae, which is not retrieved in our analysis. Apart from the addition of †Ticinolepis in our study, the taxonomic sample is different in the two cladistic analyses. In addition to †Kyphosichthys and †Sangiorgioichthys, Sun & Ni [42] include in their analysis †Luoxiongichthys hyperdorsalis Wen et al. [163], a still poorly understood taxon also from the Anisian of South China, which is not included in our dataset. On the other hand, those authors include only two species of †Sangiorgioichthys, †S. aldae from the Ladinian of the Monte San Giorgio (Swiss and Italian Alps) and †S. sui from the Anisian of South China, but we also include †S. valmarensis also from the Ladinian of the Monte San Giorgio (Swiss Alps). A fourth species of this genus, †S. yanjuanensis, also from the Anisian of South China, is incompletely known and not yet included in any cladistic analysis. Although these taxonomic differences could explain the different results, the putative synapomorphies supporting the Kyphosichthyidae are questionable according to the distribution of characters in our hypothesis. The putative synapomorphies of Kyphosichthyidae are the following: ‘triangular suborbital lateral to quadrate’ ([42]: p. 83) is uniquely derived in †Sangiorgioichthys (ch. 150[1]) and absent in †Kyphosichthys; ‘infraorbital bones forming the ventral border of the orbit subtriangular, broader ventrally, about 2 times deeper than long’ ([42]: p. 83) is the condition found in †Kyphosichthys and many other ginglymodians (ch. 127[0]), but not in the species of †Sangiorgioichthys (127[2,3]); ‘nasal process of the premaxilla not pierced by a large foramen for the olfactory nerve’ ([42]: p. 83) is a plesiomorphic holostean condition (ch. 152[0]). This latter feature is further unknown in †Kyphosichthys and the species of †Sangiorgioichthys, except for †S. sui, where the nasal process of the premaxilla encloses a complete olfactory foramen (GMPKU-P-1707), a trait that is a synapomorphy of ginglymodians above the level of †Ticinolepis (ch. 152[0]).
Sun & Ni [42] further argue that the genus †Sangiorgioichthys sensu López-Arbarello et al. [164] is not monophyletic because the sister-taxon of †S. sui is †Kyphosichthys grandei and not †S. aldae. In our analysis, however, the clade formed by the three species of †Sangiorgioichthys is well supported by one uniquely derived character, the quadrate being laterally covered by one or two suborbitals forming a triangular plate (ch. 150[1]), and three homoplastic synapomorphies: a single anterior infraorbital bone (ch. 131[0]), otherwise only present in †Araripelepidotes temnurus; the dorsal margin of the maxilla being gently concave and allocating supramaxilla (ch. 162[2]), a condition unique to this genus among the studied ginglymodians, but present in most teleosts and a few halecomorphs; and the coronoid process of the lower jaw being completely formed by the surangular (ch. 169[2]), which is a rather rare condition otherwise known in the gars and the Triassic teleost †Pholidoctenus serianus. In our hypothesis, †Kyphosichthys grandei is the sister-taxon to Neoginglymodi, and this relationship is supported by four synapomorphies: the presence of the longitudinal articulation of the scales of the body (ch. 8[1]), the above discussed shape of the infraorbital bones forming the ventral margin of the orbit (ch. 127[0]), the absence of a branchiopercle (ch. 206[0]), and the relatively low post-temporal bones, which are approximately as high as the dermopterotic (ch. 317[1]).
The monophyly of Neoginglymodi is supported by one uniquely derived synapomorphy: the presence of increasingly large and stout basal fulcra in the dorsal fin (see discussion of chs. 312 and 313 in electronic supplementary material, file S2) and six homoplastic synapomorphies (electronic supplementary material, file S4), and the relationships within this clade generally agree with López-Arbarello & Wencker [36], showing monophyletic Lepisosteidae and †Lepidotidae within Lepisosteiformes, and †Callipurbeckiidae, †Macrosemiidae and †Semionotidae within †Semionotiformes. However, the relationship between some of these clades, or some taxa within these clades, is variable, indicating that more taxa and characters still need to be added to achieve a more robust phylogenetic hypothesis. For example, whereas the relationships of the genus †Isanichthys from the Late Jurassic of Thailand remained unresolved in López-Arbarello [26], Cavin et al. [27] and Deesri et al. [31,32], the species †Isanichthys palustris is the most basal lepisosteoid in López-Arbarello and Wencker [36], but the two species of this genus are included in †Lepidotidae in the hypotheses presented here (figures 8 and 9), although the relationships of the taxa within †Lepidotidae are variable in the implied weighting analyses. Similarly, according to Cavin et al. [27] and Deesri et al. [31,32], the Early Cretaceous †Thaiichthys buddhabutrensis, also from Thailand, is the sister-taxon of a clade formed by the Early Cretaceous †Pliodetes from Africa and †Araripelepidotes from Brazil, which is the sister-group of the superfamily Lepisosteoidea sensu López-Arbarello [26]. However, this clade is not resolved in our analysis or in López-Arbarello & Wencker [36], and all those taxa are stem-Lepisosteoidea (figure 8). In this case, the relative position of these stem-taxa remains the same in both the equal and implied weighting analyses (figures 8 and 9). Very interestingly, the Early Cretaceous †Neosemionotus puntanus from western Argentina is here retrieved as the most basal lepisosteiform (figure 8) as indicated in 89% of the most parsimonious trees in López-Arbarello [26]. On the contrary, the implied weighting analysis with the strongest constant value of 3 suggests that this taxon is the most basal †semionotiform (figure 9a). In any case, as López-Arbarello [26] already stated, the basal position of †Neosemionotus, with a ghost lineage going back to the Triassic, indicates that the history of ginglymodians in South America is much longer than currently known. Ginglymodians are well represented in the Late Jurassic–Early Cretaceous of Brazil and Argentina [165–167], but no reliable evidence of their presence has been found before that time [168]. This missing information certainly plays a role regarding the uncertainties about the phylogenetic relationships of †Neosemionotus.
Among †semionotiforms, although the clade †Macrosemiidae is very well supported in all cladistic analyses, the relationships of this family vary from a position more basal than †Semionotidae or †Callipurbeckiidae in the earlier studies [26,27,31,32,72,87,96,169], via macrosemiids being the sister-group of †Semionotidae in a clade that is the sister-group of †Callipurbeckiidae in López-Arbarello & Wencker [36], to being the sister-group of †Callipurbeckiidae in our current hypotheses (figures 8 and 9).
In Halecomorphi, the relationships within Amiidae, as well as the basal position of the Triassic taxa generally, agree with previous cladistic analyses. On the contrary, the order †Ionoscopiformes is not monophyletic in our hypotheses, including the cladograms produced by the implied weighting analyses (figures 8 and 9). The name Ionoscopiformes was coined by Grande & Bemis [23] to name a clade including the families †Ionoscopidae, †Ophiopsidae and †Oshunidae. Later authors did not recognize the monotypic family †Oshunidae and placed †Oshunia brevis within †Ionoscopidae, together with †Ionoscopus cyprinoides and †Quetzalichthys perilliatae [24,41,170]. In the hypotheses proposed by these authors [33,171], this clade †Ionoscopidae is the sister-group of †Ophiopsidae, including the genera †Ophiopsis (previously †Furo) muensteri, †Ophiopsiella (previously †Ophiopsis), †Macrepistius and †Teoichthys, forming a monophyletic †Ionoscopiformes. Xu et al. [33] described the Triassic †Robustichthys, retrieved as the oldest †ionoscopiform in a polytomy with †Ionoscopidae and †Ophiopsidae, and Xu & Shen [170] described the slightly younger †Panxianichthys, retrieved as the sister-taxon to all other †ionoscopiforms. After adding the Triassic †Allolepidotus, †Asialepidotus and †Eoeugnathus to the analysis, Sun et al. [41] proposed very different relationships for †Panxianichthys and proposed a clade †Panxianichthyiformes including all these Triassic taxa (except †Robustichthys, which is not included in their analysis). On the other hand, López-Arbarello et al. [34] redescribed the Triassic †Archaeosemionotus connectens and proposed another different phylogenetic hypothesis. In this hypothesis, the †Ionoscopiformes were monophyletic, but the family †Ionoscopidae was not monophyletic and the sister-group of †Ophiopsidae was the clade †Furidae including †Ophiopsis (previously †Furo) muensteri, †Archaeosemionotus and †Robustichthys. Therefore, in the hypothesis of López-Arbarello et al. [34], the latter taxon was retrieved in a significantly more derived position compared to the other studies mentioned before. The disparities between all these studies indicate that the different sets of taxa and characters produce incompatible results for the relationships of the taxa classified in the order †Ionoscopiformes, and the results obtained in our cladistic analysis might be understood as the result of merging those previous datasets. In our hypotheses, the families †Ionoscopidae and †Ophiopsidae are monophyletic, but they are not sister-groups, and the Triassic †Archaeosemionotus, †Robustichthys and †Panxianichthys are retrieved at the base of the Halecomorphi, as well as the Early Triassic †Watsonulus (figures 8 and 9). However, the other Triassic taxa †Allolepidotus, †Asialepidotus and †Eoeugnathus are not included in our analysis and, thus, nothing can be said about the monophyly of †Panxianichthyformes sensu Sun et al. [41], which will be explored in a future study.
Another unexpected result of our analysis is the phylogenetic position of †Caturus furcatus outside the Amiiformes and within the †ionoscopid clade (figures 8 and 9). This sister-group relationship between †Caturus and †ionoscopids is supported by six homoplastic synapomorphies, and not even in the implied weighted analyses is †Caturus retrieved within the amiiform clade, as suggested by previous studies (e.g. [23,34,172]). Including other taxa like †Heterolepidotes, †Osteorachis, †Ainia (=†Callopterus), which are potentially closely related to †Caturus [23,51], in future, cladistic analyses will certainly solve the phylogenetic position of this taxon.
In agreement with the most recent phylogenetic hypothesis for basal teleosts [39], according to our results, the genera †Aspidorhynchus and †Pachycormus are sister-taxa and together represent the sister-group of all other teleosts (figure 8). Similarly, all Triassic teleosts form a monophyletic clade †Pholidophoridae sensu Arratia [29]. The Jurassic †Eurycormus, †Dorsetichthys and †Siemensichthys join the stem Teleocephala, but in different positions relative to each other compared to previous hypotheses [29,35], or †Eurycormus is not on this stem, but is the sister-group of †Pholidophoridae in Arratia [39]. The phylogenetic position of these basal teleost taxa is very different when implied weighting is applied (figure 9). In the cladograms obtained from both implied weighted analyses, †Pholidophoridae is the sister-group of all other teleosts, including the clade formed by †Aspidorhynchus and †Pachycormus. Furthermore, and contrary to previous hypotheses [29,35], this latter clade is well nested within Teleostei, placed above the level of a clade formed by †Ichthyokentema and †Catervariolus in both implied weighted analyses.
Recalling the family †Leptolepidae sensu Nybelin [173], †Leptolepis coryphaenoides and †Tharsis dubius are more closely related to each other than to any other teleost in our analysis (figure 8). The clade including these taxa also includes †Luisiella feruglioi, from the Late Jurassic of Argentina, and is supported with three uniquely and four homoplastic synapomorphies and it is stable also in the hypotheses given by the implied weighting analyses (figure 9). The characters uniquely derived in this Leptolepid-clade include the presence of a preopercular process of the hyomandibula (figure 10) and broad neural and haemal spines with a median groove (figure 7). The hyomandibula with a preopercular process was already recognized as a diagnostic of his Leptolepidae s. str. (†Leptolepis, †Proleptolepis and †Tharsis) by Nybelin [173], and at least some of these features are present in the other members of the family †Luisiellidae (†Cavenderichthys [176] and †Waldmanichthys [177]). Although several Jurassic teleost taxa are missing in our data matrix compared to previous studies, in particular the other members of the Gondwanan family †Luisiellidae [35], the close phylogenetic relationships between these taxa might be the result of expanded character sampling and a new understanding of character change evolution on the teleost lineage due to the inclusion of numerous non-teleost fossil taxa in our analysis.
Figure 10.
Preopercular process of the hyomandibula in (a), †Tharsis dubius, reconstruction based on [174]: pl. 12, fig. 7; (b) †Luisiella feruglioi, reconstruction based on [175]: fig. 7C. Abbreviations: op.pr, opercular process; pop.pro, preopercular process.
Contrary to the hypothesis of Arratia [65], and in agreement with that of Sferco et al. [35], the †Varasichthyidae (here represented by †Varasichthys) and †Crossognathiformes sensu Taverne [178] (here represented by †Notelops and †Rhacolepis) are not closely related to each other in our analyses. This result supports the proposal of Taverne [178], also supported by Patterson [121] and Cavin [67], that the †Crossognathiformes are teleocephalans. †Varasichthys is here instead retrieved outside the crown-group, on the stem Teleocephala. An extended discussion of the relationships between †Varasichthyidae and †Crossognathiformes is provided in Sferco et al. [35], where the family †Varasichthydae is represented by all its included taxa.
Further challenging previous ideas (e.g. [35,65]), †Varasichthys is retrieved as the sister-taxon of the †Leptolepid-clade in two of the three phylogenetic hypotheses obtained here (equal weighted analysis and implied weighted analysis with K = 8, figures 8 and 9b). †Varasichthys shares four synapomorphies with the Leptolepid-clade: presence of a leptolepid notch in the anterodorsal ascending margin of the dentary (ch 167: 0 → 1); epipleural bones in abdominal and anterior caudal region (ch 242: 0 → 1); absence of hypaxial basal fulcra (ch 292: 0 → 1) and the first anal pterygiophore posterior to first haemal spine or infrahaemal (ch 308: 1 → 0). However, these are all homoplastic characters, which are widely distributed among teleosts, especially among the taxa on the stem-Teleocephala (see for example the distribution of the presence of a leptolepid notch in the anterodorsal ascending margin of the dentary in Sferco et al. [35]: fig. 4a). Furthermore, the grouping of †Varasichthys with the †Leptolepid-clade has a low bootstrap value (below 50). Therefore, although it is an interesting hypothesis, the sister-group relationship between †Varasichthys and the †Leptolepid-clade should be taken with caution, considering that the other three †varasichthyid taxa, as well as the †luisiellids †Cavenderichthys and †Waldmanichthys and other potential members of the †Leptolepid-clade (e.g. several species of †Leptolepis [173] are not included in our present analysis.
Among the extinct lineages of Mesozoic teleosts, the †Ichthyodectiformes have been generally accepted as the sister-group of Teleocephala (e.g. [35,66]). †Ichthyodectiformes are only represented by †Thrissops formosus in our analysis, and this species is the sister-taxon of Teleocephala only in the strongest implied weighted analysis (k = 3; figure 9a). In the other two hypotheses, †Thrissops is retrieved at a slightly more basal position, and the sister-group of Teleocephala is the clade formed by †Varasichthys and the †Leptolepid-clade (see above). Although only one step further from Teleocephala, this is a significant difference because of the broad taxonomic, stratigraphic and geographical range of this potentially alternative sister-group of Teleocephala, which encompasses two so far exclusively Gondwanan clades, the families †Varasichthyidae and †Luisiellidae.
4.4. Chronostratigraphic distribution
The pattern of phylogenetic relationships and the chronostratigraphic distribution of the studied taxa indicate a rapid radiation of the holostean clades Halecomorphi (figure 11: Node 2) and Ginglymodi (figure 11: Node 3) during the approximately 15 Myr encompassed by the Early and Middle Triassic, immediately followed by a first rapid radiation of pholidophoriform teleosts (figure 11: Node 4) during the late Middle and Late Triassic. A second important radiation of ginglymodians and teleosts took place independently during the Early Jurassic. The lepidotids within Ginglymodi and the leptolepid-clade within Teleostei represent offshoots of their respective main stems during that period of time (figure 11: Nodes 5 and 6, respectively). In contrast, the crown-group Halecomorphi (figure 11: Node 7) present an enormous ghost lineage throughout half of the Mesozoic from the Middle Triassic to the early Late Jurassic. All three main clades Halecomorphi, Ginglymodi and Teleostei experienced a third important radiation during the Late Jurassic giving rise to their respective crown-groups (figure 11: Nodes 7–9).
Figure 11.
(Overleaf.) Strict consensus tree of 24 most parsimonious trees resulted from the equal weights analysis with constraints, calibrated on a chronostratigraphic chart. Numbers indicate: 1, Holostei; 2, Halecomorphi; 3, Ginglymodi; 4, Teleostei; 5, Lepidotidae; 6, †Varasichthys + Leptolepid-clade; 7, crown-Halecomorphi (Amiiformes); 8, crown-Ginglymodi (Lepisosteoidei); 9, crown-Teleostei (Teleocephala). Coloured bars highlight the taxa from the Middle Triassic of South China (green), Middle Triassic of the Alps (red), German Posidonienschiefer (blue), Late Jurassic of Southern Germany (orange) and the Aptian/Albian Araripe Basin of Brazil (purple). Chronostratigraphic provenance of the Mesozoic taxa: †Pteronisculus stensioi, †Boreosomus piveteaui and †Australosomus kochi, Fish Zone 2–5 of Kap Stosch, Early Triassic (Induan) of East Greenland [46,84,179]; †Plesiofuro mingshuica, Hongyanjing Formation of Gansu Province, China, Early Triassic (Olenekian) [69,180]; †Watsonulus eugnathoides, middle Sakamena Formation of Madagascar, Early Triassic [85,181]; †Kiphosichthys grandei, †Robustichthys luopingensis, †Sangiorgioichthys sui, Luoping biota, Member II of the Guanling Formation, Guizhou, China, Anisian (mid-Pelsonian), Middle Triassic [33,164,182]; †Panxianichthys imparilis, Panxian biota, Member II of the Guanling Formation, Guizhou, China, Anisian (mid-Pelsonian), Middle Triassic [164,182]; †Ticinolepis crassidens, uppermost Besano Formation, Monte San Giorgio, Switzerland, early Ladinian, Middle Triassic [37]; †Ticinolepis longaeva, uppermost Besano Formation and most of the Meride Limestone, Monte San Giorgio, Switzerland, Ladinian, Middle Triassic [37]; †Sangiorgioichthys aldae and †S. valmarensis, Kalkschieferzone, uppermost Meride Limestone, Monte San Giorgio, Switzerland, latest Ladinian, Middle Triassic [37,183,184]; †Archaeosemionotus connectens, Perledo Member of the Perledo-Varenna Formation, Italy, late Ladinian, Middle Triassic [34]; †Pholidophoretes salvus, Reingraben beds, Austria, early Carnian (Julian), early Late Triassic [29]; †Knerichthys bronni, Raild (=Cave del Predil), Friaul, Udine, Italy, Carnian, early Late Triassic [29]; †Semionotus bergeri, Hassberge Formation (Coburger Sandstein), Coburg, Germany, late Carnian, early Late Triassic [185]; †Paralepidotus ornatus, middle and late Norian localities of Italy and Austria, middle Late Triassic [186]; †Semiolepis brembanus, vertebrate level between the Calcare di Zorzino and the Argillite di Riva di Solto at the boundary between middle and late Norian, Italy, middle Late Triassic [187]; †Annaichthys pontegiurinensis and †Pholidophorus gervasuttii, Ponte Giurino, †Parapholidophorus nybelini, †Pholidoctenus serianus and †Pholidorhynchodon malzannii, Cene, Italy, Norian, middle Late Triassic [29]; †Semionotus elegans, Towaco and Boonton formations (Hettangian and Sinemurian), Newark basin, New Jersey, Portland Formation (Sinemurian), Connecticut and Waterfall Formation (Hettangian), Virginia, USA, early Early Jurassic [82]; †Dorsetichthys bechei, Lyme Regis, Dorset, England, early Sinemurian, early Early Jurassic [29,188]; †Semionotus capensis, Clarens Formation, South Africa, Sinemurian–Pliensbachian, Early Jurassic [189]; †Dapedium caelatum, †D. pholidotum and †D. stollorum, Early Toarcian localities of southern Germany, Luxembourg and northern France, Early Jurassic [190–192]; †Leptolepis coryphaenoides, several early Toarcian localities in the UK, France and Germany, late Early Jurassic [33,193]; †Pachycormus macropterus, several localities in the UK, France and Germany, Toarcian, late Early Jurassic [194]; †Catervariolus hornemani, Stanleyville Formation, Democratic Republic of Congo, Aalenian–Bathonian, Middle Jurassic [133,195]; †Varasichthys ariasi, Cordillera de Domeyko, Chile, Oxfordian, Late Jurassic [196]; †Luisiella feruglioi, ‘estratos de Almada’, Cañadón Calcáreo Formation, Argentina, late Oxfordian, Late Jurassic [175,197]; †Ebertichthys ettlingensis, †Histionotus oberndorferi, †Macrosemimimus fegerti, †Notagogus denticulatus, †Ophiopsis muensteri, †Orthogonikleithrus hoelli, †Scheenstia zappi, several late Kimmeridgian localities in southern Germany, Late Jurassic [38,121,193,198–200]; †Amiopsis lepidota, †Caturus furcatus, †Eurycormus speciosus, †Ionoscopus cyprinoides, †Ophiopsiella attenuata, †Scheenstia maximus, †Siemensichthys macrocephalus, †Solnhofenamia elongata, †Tharsis dubius, †Thrissops formosus, several late Kimmeridgian and early Tithonian localities in southern Germany, Late Jurassic [26,125,194]; †Anaethalion angustus, †Aspidorhynchus acutirostris, †Macrosemius rostratus, †Propterus elongatus, several early Tithonian localities in southern Germany, Late Jurassic [57,156,198,201]; †Occitanichthys canjuerensis, Canjuers, France, Early Tithonian, Late Jurassic and Dorset, UK, Middle Purbeck Beds, middle Berriasian, Early Cretaceous [36,202]; †Thaiichthys buddhabutrensis, †Isanichthys palustris, †I. lertboosi, Phu Kradung Formation, Thailand, Late Jurassic–Early Cretaceous [31,203]; †Ichthyokentema purbeckensis, Dorset, UK, Lower Purbeck Beds, early Berriasian, Early Cretaceous [90,202]; †Callipurbeckia minor, Dorset, UK, Middle Purbeck Beds, middle Berriasian, Early Cretaceous [202,204]; †Amiopsis woodwardi, †Vidalamia catalunica, Montsec Formation, Spain, Berriasian-Valanginian, Early Cretaceous [23]; †Pliodetes nigeriensis, Elrhaz Formation, Niger Republic, Aptian, Early Cretaceous [205], †Pachyamia mexicana, †Quetzalichthys perillatae, †Teoichthys brevipina, †Tlayuamichin itztli, Tlayúa Formation, Mexico, Albian, Early Cretaceous [23,24,30,161]; †Neosemionotus puntanus, Lower Member of the Lagarcito Formation, Argentina, Albian, Early Cretaceous [161]; †Araripelepidotes temnurus, †Calamopleurus cylindricus, Crato and Santana Formations, Brazil, late Aptian–Albian, Early Cretaceous [206]; †Cratoamia gondwanica, Crato Formation, Brazil, late Aptian–early Albian, Early Cretaceous [206]; †Dentilepisosteus laevis, †Notelops brama, †Obaichthys decoratus, †Oshunia brevis, †Rhacolepis buccalis, Santana Formation, Albian, Early Cretaceous [206]; †Macrepistius arenatus, Glen Rose Formation, Texas, USA, Albian, Early Cretaceous [207]; †Pachyamia latimaxillaris, Bet-Meir Formation, Ein-Yabrud near Ramallah, Middle East, early Cenomanian, early Late Cretaceous [23].
Figure 11.
(Caption overleaf.)
The Early–Middle Triassic radiation of neopterygians has been thoroughly explored and documented by Romano et al. [174] and it is part of what they called the Triassic actinopterygian revolution. This first important radiation of the crown-Neopterygii was apparently facilitated by the demise of several chondrichthyan clades and driven by the successful acquisition of several feeding, locomotory and reproductory innovations in the early members of the Holostei and Teleostei [174,175,179]. Several of these key innovations were also acquired by the so-called subholosteans, allegedly as a consequence of convergent evolution [175,179]. However, the systematic position of the taxa regarded as subholosteans and their phylogenetic relationships with the crown-neopterygians are still unclear, and at least some subholostean groups might be on the stem lineage to crown-Neopterygii [93,180–182]. Therefore, the rapid diversification of the early Mesozoic subholosteans and crown-neopterygians might be part of a single radiation event and should thus not be treated as separate clades, as in most recent diversity studies [174,183]. The hypothesis of a single radiation of Neopterygii including subholosteans is more consistent with published diversity analyses [174], but it should be tested in a phylogenetic framework. Based on the information provided by Romano et al. [174] in their table S3 and after the addition of more recently described taxa, we counted 262 species of crown-neopterygian and subholostean taxa in the Early and Middle Triassic only (figure 12). Most of these taxa, however, have never been included in a cladistic analysis and, thus, pending elucidation of their phylogenetic relationships, any hypothesis about the evolution of these groups is largely speculative.
Figure 12.
Graphic representation of the number of nominal species from Early and Middle Triassic freshwater (orange) and brackish and marine (green) sediments. Data from Romano et al. ([174]: table S3) with the addition of the following recently described taxa: †Ticinolepis longaeva and †T. crassidens from the Ladinian of the Monte San Giorgio [37]; †Frodoichthys luopingensis and †Gimlichthys dawaziensis from the Anisian of Yunnan Province [41]; †Mailingichthys nimaiguensis from the Ladinian of Guizhou Province [208]; †Panxianichthys imparilis from the Anisian of Paxian biota [164]; †Robustichthys luopingensis from the Anisian of Luoping Biota [33]; †Habroichthys dolomiticus from the Anisian of Monte Prà della Vacca [209]; †Altisolepis sinensis from the middle–late Anisian of Luoping [210]; †Calaichthys tehul from the Anisian of Cuyo Basin [211]; †Venusichthys comptus from the Pelsonian–Anisian of Luoping [212]; †Wushaichthys exquisitus [69]; †Peltopleurus nitidus from the Anisian of Luoping biota [177]; †Plesiofuro mingshuica from the Olenekian of Gansu Province [69].
The second radiation of ginglymodians and teleosts indicated by our study during the Early Jurassic might be seen as a part of a faunal recovery after the end-Triassic mass extinction event [184]. However, there is no clear evidence for a significant extinction of actinopterygians around the Triassic–Jurassic boundary [185,186] and these radiations of ginglymodians and teleosts might have started earlier, as is the case of dapediiforms [162], or they might be part of the initial radiation of these groups, which started at the early Triassic. Exploring this topic requires a more complete study of the phylogenetic relationships of dapediiforms (see discussion above) together with the numerous Early Jurassic neopterygians, most of which are in need of revision. It is likewise necessary to complete the apparent long ghost lineage of the crown-Halecomorphi, with several halecomorph taxa from the Late Triassic to Middle Jurassic that have not yet been included in cladistic analyses (e.g. species of the genera †Caturus, †Furo, †Heterolepidotus and †Osteorhachis [187]).
Incorporating more Late Triassic to Middle Jurassic neopterygians might also have consequences for the apparent parallel radiation of the three main crown-neopterygian lineages during the Late Jurassic. In our data matrix, these Late Jurassic radiations might be an artefact due to the proportionally high amount of taxa from European Lagerstätten of that age (figure 13).
Figure 13.
Provenance of the studied taxa highlighting the biases due to the much higher representation of the main Mesozoic Lagerstätte. Table including simplified references to the stratigraphic and geographical provenance of the taxa (left). Pie charts representing the proportions of taxa represented in our data matrix according to their provenance. (a) Triassic; (b) Jurassic; (c) Cretaceous.
5. Conclusion
The present study was designed and conducted to solve the phylogenetic relationships of the genus †Ticinolepis, including two species, †T. longaeva and †T. crassidens, which is retrieved as the most basal Ginglymodi. †Ticinolepis is known from the earliest to late Ladinian, while other more derived taxa are known from the Anisian, thus indicating a ghost lineage for this genus of at least 5 Ma at the base of Ginglymodi (figure 11: Node 3).
Owing to the morphological similarities shared by †Ticinolepis with ginglymodians, halecomorphs and teleosts, all crown-neopterygian groups were included in the data matrix, and the cladistic study presented here led to several significant and, partially, novel results. Several cases of homology have been revised and discussed thoroughly.
Some hypotheses of phylogenetic relationships are now confirmed in a more comprehensive taxonomic framework. Among them are the monophyly of Teleostei and Holostei, and its included clades Halecomorphi and Ginglymodi. Within Ginglymodi, the monophyly of Lepisosteiformes and †Semionotiformes is also confirmed, and we propose the name Neoginglymodi for the clade formed by these two orders. At a lower taxonomic level, our analyses retrieve monophyletic Lepisosteoidei and †Lepidotidae within Lepisosteiformes, and monophyletic †Semionotidae, †Callipurbeckiidae and †Macrosemiidae within †Semionotiformes. The sister-group relationship between †Callipurbeckiidae and †Macrosemiidae is here proposed for the first time. Among halecomorphs, the Amiiformes are confirmed to be monophyletic in our hypotheses, but the results of our analyses reject the hypothesis of the monophyly of †Ionoscopiformes. The monophyly of the teleost family †Pholidophoridae is also confirmed, but its systematic position remains unclear. This clade might be more derived than †aspidorhynchiforms, †pachycormiforms and the Jurassic genera †Catervarioulus and †Ichthyokentema, or it might be the sister-group of all other teleosts.
The relationships of some taxa within the monophyletic clades vary when implied weighting is applied. Most notable is that †Dapedium changes from a basal position as the sister-group of all other holosteans, to a position as the sister-group of Ginglymodi. Similarly, the ginglymodian †Neosemionotus might be the sister-taxon of Lepisosteiformes or the sister-taxon of †Semionotiformes. The phylogenetic relationships of the Triassic halecomorphs at the base of Halecomorphi are still not clear.
Furthermore, we obtained some challenging results within the Teleostei. The most strikingly of them is probably the †Leptolepid-clade, which is well supported and reiterates the hypothesis of the family Leptolepidae of Nybelin [173]. The controversial results obtained for the phylogenetic relationships of the well-known Jurassic genera †Varasichthys and †Thrissops should stimulate further research on the corresponding lineages.
Our data matrix, like most previous evolutionary studies of Mesozoic actinopterygians, is strongly biased because most of the included taxa come from a few main Mesozoic Lagerstätten, which might be responsible for the hypothetical pattern of radiations described above. More taxa of other geographical and stratigraphic provenance should be incorporated into the analysis to achieve more robust hypotheses about the evolution of neopterygians during the Mesozoic. One aim of this work has also been to provide a comprehensive dataset to facilitate those utterly needed phylogenetic studies. We have merged previously existing lists of characters, proposed several new characters, discussed several cases of conflicts of homology and have scored ourselves each entry in our data matrix. This has been an enormous effort and is certainly not free of failure, so we hope future scholars will contribute with a critical approach, discussing and improving this dataset with more than the addition of new taxa.
Data accessibility
Dryad Digital Repository: https://doi.org/10.5061/dryad.2tp53gr [58].
Phylogenetic data: MorphoBank, Project 2196 (https://morphobank.org/index.php/MyProjects/List/select/project_id/2196).
The datasets supporting this article have been uploaded as part of the electronic supplementary material:
Electronic supplementary material file S1: Material examined and data matrix; file S2: List of phenotypic characters; file S3: Branch support values obtained for the cladistic analyses; file S4: List of synapomorphies.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Completing the data matrix used for this phylogenetic analysis has been arduous work and took several years and the detailed study of hundreds of fossils in many different collections (electronic supplementary material, file S1). Accordingly, we specially thank the following people for access to the collections under their care: O. Rauhut (SNSB-BSPG), M. Kölbl-Ebert and M. Ebert (JME); R. Stockar (MCSN); H. Furrer and C. Klug (PIMUZ); E. Bernard, M. Richter, and Z. Johanson (NHM); Jiang D-Y. (GMPKU); M. Veran and G. Clement (MNHN); E. Maxwell and R. Böttcher (SMNS); M. Reiche and A. Gehler (GZG); F. Witzman (NM.f); E. Ruigómez (MEF), A. Kramarz (MACN), M. Reguero (MLP); J. Alvarado-Ortega (IGM, UNAM); M. Röper (BMMS); R. Brocke (SMF); E. Mönnig (NMC); J. Maisey (AMNH) and the private collections of H. Tischlinger and R. Albersdorfer. Special thanks are due to O. Rauhut for enlightening discussions during the long-term process of this work and for generously proof-reading the manuscript, and to Lionel Cavin and an anonymous reviewer for their constructive criticisms and suggestions that help improving the quality of our work.
Authors' contributions
A.L.-A. contributed to the conception and design, supervision of the project and drafting the article and supplements. A.L.-A. and E.S. contributed to the acquisition, analysis and interpretation of data, critical revision of the whole article including supplements, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
We declare we have no competing interests.
Funding
The work of A.L.-A. was mainly financed by the German Research Foundation (DFG LO 1405/3-1 to 3-3), the Staatlichen Naturwissenschaftlichen Sammlungen Bayerns (SNSB), Munich, Germany, and the Canton Ticino through the Museo Cantonale di Storia Naturale of Lugano (Risoluzione 722-16/21), with additional financial support from Mr Max Kuhn (Uster). E.S. was granted a DAAD scholarship (91557804: Re-invitation Programme for Former Scholarship Holders) for visiting collections. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Species by Family/Subfamily. 2016. See http://www.calacademy.org/scientists/projects/catalog-of-fishes (accessed 6 October 2016).
- 2.Sallan LC. 2014. Major issues in the origins of ray-finned fish (Actinopterygii) biodiversity. Biol. Rev. 89, 950–971. (doi:10.1111/brv.12086) [DOI] [PubMed] [Google Scholar]
- 3.Friedman M. 2015. The early evolution of ray-finned fishes. Palaeontology 58, 213–228. (doi:10.1111/pala.12150) [Google Scholar]
- 4.Hurley IA, Lockridge Mueller R, Dunn KA, Schmidt EJ, Friedman M, Ho RK, Prince VE, Yang Z, Thomas MG, Coates MI. 2007. A new time-scale for ray-finned fish evolution. Proc. R. Soc. B 274, 489–498. (doi:10.1098/rspb.2006.3749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grande L. 2011. An empirical synthetic pattern study of gars (Lepisosteiformes) and closely related species, based mostly on skeletal anatomy: the resurrection of Holostei. Copeia 2011, 612–613. [Google Scholar]
- 6.Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL. 2012. Resolution of ray-finned fish phylogeny and timing of diversification. Proc. Natl Acad. Sci. USA 109, 13 698–13 703. (doi:10.1073/pnas.1206625109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faircloth BC, Sorenson L, Santini F, Alfaro ME. 2013. A phylogenomic perspective on the radiation of ray-finned fishes based upon targeted sequencing of ultraconserved elements (UCEs). Plos ONE 8, e65923 (doi:10.1371/journal.pone.0065923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braasch I, et al. 2016. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 48, 427–437. (doi:10.1038/ng0616-700c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquier J, et al. 2016. Gene evolution and gene expression after whole genome duplication in fish: the PhyloFish database. BMC Genomics. 17, 10 (doi:10.1186/s12864-016-2709-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. 2017. Phylogenetic classification of bony fishes. BMC Evol. Biol. 17, 40 (doi:10.1186/s12862-017-0958-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirande JM. 2017. Combined phylogeny of ray-finned fishes (Actinopterygii) and the use of morphological characters in large-scale analyses. Cladistics 33, 333–350. (doi:10.1111/cla.12171) [DOI] [PubMed] [Google Scholar]
- 12.Majtanova Z, Symonova R, Arias-Rodriguez L, Sallan L, Rab P. 2017. ‘Holostei versus Halecostomi’ problem: insight from cytogenetics of ancient nonteleost actinopterygian fish, bowfin Amia calva. J. Exp. Zool. B 328, 620–628. (doi:10.1002/jez.b.22720) [DOI] [PubMed] [Google Scholar]
- 13.Kluge AG. 1989. A concern for evidence and a phylogenetic hypothesis of relationships among epicrates (Boidae, Serpentes). Syst. Zool. 38, 7–25. (doi:10.2307/2992432) [Google Scholar]
- 14.Eernisse DJ, Kluge AG. 1993. Taxonomic congruence versus total evidence, and amniote phylogeny inferred from fossils, molecules, and morphology. Mol. Biol. Evol. 10, 1170–1195. [DOI] [PubMed] [Google Scholar]
- 15.Kluge AG. 2004. On total evidence: for the record. Cladistics 20, 205–207. (doi:10.1111/j.1096-0031.2004.00020.x) [DOI] [PubMed] [Google Scholar]
- 16.Hunt G, Slater G. 2016. Integrating paleontological and phylogenetic approaches to macroevolution. In Annual review of ecology, evolution, and systematics, vol. 47 (ed. Futuyma DJ.), pp. 189–213. Palo Alto, CA: Annual Reviews. [Google Scholar]
- 17.Wiens JJ. 2003. Incomplete taxa, incomplete characters, and phylogenetic accuracy: is there a missing data problem? J. Vertebr. Paleontol. 23, 297–310. (doi:10.1671/0272-4634(2003)023[0297:ITICAP]2.0.CO;2) [Google Scholar]
- 18.Norell MA, Wheeler W. 2003. Missing entry replacement data analysis: a replacement approach to dealing with missing data in paleontological and total evidence data sets. J. Vertebr. Paleontol. 23, 275–283. (doi:10.1671/0272-4634(2003)023[0275:MERDAA]2.0.CO;2) [Google Scholar]
- 19.Simmons MP. 2014. Limitations of locally sampled characters in phylogenetic analyses of sparse supermatrices. Mol. Phylogenet. Evol. 74, 1–14. (doi:10.1016/j.ympev.2014.01.030) [DOI] [PubMed] [Google Scholar]
- 20.Guillerme T, Cooper N. 2016. Effects of missing data on topological inference using a Total Evidence approach. Mol. Phylogenet. Evol. 94, 146–158. (doi:10.1016/j.ympev.2015.08.023) [DOI] [PubMed] [Google Scholar]
- 21.Guillerme T, Cooper N. 2016. Assessment of available anatomical characters for linking living mammals to fossil taxa in phylogenetic analyses. Biol. Lett. 12, 20151003 (doi:10.1098/rsbl.2015.1003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke JT, Lloyd GT, Friedman M. 2016. Little evidence for enhanced phenotypic evolution in early teleosts relative to their living fossil sister group. Proc. Natl Acad. Sci. USA 113, 11 531–11 536. (doi:10.1073/pnas.1607237113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grande L, Bemis WE. 1998. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy: an empirical search for interconnected patterns of natural history. Mem. Soc. Vertebr. Paleontol. 4, 1–690. [Google Scholar]
- 24.Alvarado-Ortega J, Espinosa-Arrubarrena L. 2008. A new genus of ionoscopiform fish (Halecomorphi) from the Lower Cretaceous (Albian) lithographic limestones of the Tlayua quarry, Puebla, Mexico. J. Paleontol. 82, 163–175. (doi:10.1666/04-152.1) [Google Scholar]
- 25.Cavin L, Giner S. 2012. A large halecomorph fish (Actinopterygii: Holostei) from the Valanginian (Early Cretaceous) of southeast France. Cretaceous Res. 37, 201–208. (doi:10.1016/j.cretres.2012.03.020) [Google Scholar]
- 26.López-Arbarello A. 2012. Phylogenetic interrelationships of ginglymodian fishes (Actinopterygii: Neopterygii). PLoS ONE 7, e39370 (doi:10.1371/journal.pone.0039370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavin L, Deesri U, Suteethorn V. 2013. Osteology and relationships of Thaiichthys nov gen.: a Ginglymodi from the Late Jurassic - Early Cretaceous of Thailand. Palaeontology 56, 183–208. (doi:10.1111/j.1475-4983.2012.01184.x) [Google Scholar]
- 28.Cavin L, Forey PL, Giersch S. 2013. Osteology of Eubiodectes libanicus (Pictet & Humbert, 1866) and some other ichthyodectiformes (Teleostei): phylogenetic implications. J. Syst. Paleontol. 11, 115–177. (doi:10.1080/14772019.2012.691559) [Google Scholar]
- 29.Arratia G. 2013. Morphology, taxonomy, and phylogeny of Triassic pholidophorid fishes (Actinopterygii, Teleostei). J. Vertebr. Paleontol. 33, 1–138. (doi:10.1080/02724634.2013.835642) [Google Scholar]
- 30.Machado GP, Alvarado-Ortega J, Machado LP, Brito PM. 2013. Teoichthys brevipina, sp nov., a new ophiopsid fish (Halecomorphi, Ionoscopiformes) from the Lower Cretaceous Tlayua Formation, Central Mexico. J. Vertebr. Paleontol. 33, 482–487. (doi:10.1080/02724634.2013.729962) [Google Scholar]
- 31.Deesri U, Lauprasert K, Suteethorn V, Wongko K, Cavin L. 2014. A new species of the ginglymodian fish Isanichthys from the Late Jurassic Phu Kradung Formation, northeastern Thailand. Acta Palaeontol. Pol. 59, 313–331. [Google Scholar]
- 32.Deesri U, Jintasakul P, Cavin L. 2016. A new Ginglymodi (Actinopterygii, Holostei) from the Late Jurassic-Early Cretaceous of Thailand, with comments on the early diversification of Lepisosteiformes in Southeast Asia. J. Vertebr. Paleontol. 36, e1225747 (doi:10.1080/02724634.2016.1225747) [Google Scholar]
- 33.Xu G-H, Zhao L-J, Coates MI. 2014. The oldest ionoscopiform from China sheds new light on the early evolution of halecomorph fishes. Biol. Lett. 10, 20140204 (doi:10.1098/rsbl.2014.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-Arbarello A, Stockar R, Bürgin T. 2014. Phylogenetic relationships of the Triassic Archaeosemionotus Deecke (Halecomorphi, Ionoscopiformes) from the ‘Perledo Fauna’. PLoS ONE 9, e108665 (doi:10.1371/journal.pone.0108665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sferco E, López-Arbarello A, Báez AM. 2015. Phylogenetic relationships of †Luisiella feruglioi (Bordas) and the recognition of a new clade of freshwater teleosts from the Jurassic of Gondwana. BMC Evol. Biol. 15, 268 (doi:10.1186/s12862-015-0551-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.López-Arbarello A, Wencker LCM. 2016. New callipurbeckiid genus (Ginglymodi: Semionotiformes) from the Tithonian (Late Jurassic) of Canjuers, France. Palaeontol. Z. 90, 543–560. (doi:10.1007/s12542-016-0312-x) [Google Scholar]
- 37.López-Arbarello A, Burgin T, Furrer H, Stockar R. 2016. New holostean fishes (Actinopterygii: Neopterygii) from the Middle Triassic of the Monte San Giorgio (Canton Ticino, Switzerland). Peerj 4, 61 (doi:10.7717/peerj.2234). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arratia G. 2016. New remarkable Late Jurassic teleosts from southern Germany: Ascalaboidae n. fam., its content, morphology, and phylogenetic relationships. Fossil Rec. 19, 31–59. (doi:10.5194/fr-19-31-2016) [Google Scholar]
- 39.Arratia G. 2017. New Triassic teleosts (Actinopterygii, Teleosteomorpha) from northern Italy and their phylogenetic relationships among the most basal teleosts. J. Vertebr. Paleontol. 37, 24 (doi:10.1080/02724634.2017.1312690) [Google Scholar]
- 40.Yabumoto Y. 2017. A revision of the amiiform fish genus Sinamia with phylogeny of Sinamiidae. Paleontol. Res. 21, 76–92. (doi:10.2517/2016PR008) [Google Scholar]
- 41.Sun ZY, Tintori A, Xu YZ, Lombardo C, Ni PG, Jiang DY. 2017. A new non-parasemionotiform order of the Halecomorphi (Neopterygii, Actinopterygii) from the Middle Triassic of Tethys. J. Syst. Paleontol. 15, 223–240. (doi:10.1080/14772019.2016.1181679) [Google Scholar]
- 42.Sun Z, Ni P. 2018. Revision of Kyphosichthys grandei Xu & Wu, 2012 from the Middle Triassic of Yunnan Province, South China: implications for phylogenetic interrelationships of ginglymodian fishes. J. Syst. Paleontol. 16, 67–85. (doi:10.1080/14772019.2016.1269049) [Google Scholar]
- 43.Puttick MN, et al. 2017. Uncertain-tree: discriminating among competing approaches to the phylogenetic analysis of phenotype data. Proc. R. Soc. B 284, 20162290 (doi:10.1098/rspb.2016.2290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenner RA. 2002. Boolean logic and character state identity: pitfalls of character coding in metazoan cladistics. Contribut. Zool. 71, 67–91. [Google Scholar]
- 45.Brazeau MD. 2011. Problematic character coding methods in morphology and their effects. Biol. J. Linn. Soc. 104, 489–498. (doi:10.1111/j.1095-8312.2011.01755.x) [Google Scholar]
- 46.Stensiö E. 1932. Triassic fishes from east Greenland collected by the Danish expeditions in 1929–1931. Meddelelser om Grønland. 83, 1–305. [Google Scholar]
- 47.Nielsen E. 1942. Studies on Triassic fishes I: Glaucolepis and Boreosomus. Meddelelser om Grønland. 138, 1–394. [Google Scholar]
- 48.Su D. 1993. New Jurassic ganoid fishes from northwestern Gansu, China. Vertebr. Palasiatica. 31, 1–14. [Google Scholar]
- 49.Cantino PD, de Queiroz K. 2010. International code of phylogenetic nomenclature. Version 4c (accessed 1 January 2011).
- 50.Regan CT. 1923. The skeleton of Lepidosteus, with remarks on the origin and evolution of the lower neopterygian fishes. Proc. Zool. Soc. Lond. 1923, 445–461. (doi:10.1111/j.1096-3642.1923.tb02191.x) [Google Scholar]
- 51.Patterson C. 1973. Interrelationships of holosteans. In Interrelationships of fishes (eds Greenwood PH, Miles RS, Patterson C), pp. 233–305. London, UK: Academic Press. [Google Scholar]
- 52.Patterson C. 1982. Morphology and interrelationships of primitive actinopterygian fishes. Am. Zool. 22, 241–259. (doi:10.1093/icb/22.2.241) [Google Scholar]
- 53.de Pinna MCC. 1996. Teleostean monophyly. In Interrelationships of fishes (eds Stiassny MLJ, Parenti LR, Johnson GD), pp. 147–162. London, UK: Academic Press. [Google Scholar]
- 54.Maddison WP, Maddison DR.2017. Mesquite: a modular system for evolutionary analysis. Version 3.31. See http://mesquiteproject.org .
- 55.Goloboff P, Farris J.2003. Tree analysis using new technology. Program and documentation, available from the authors, and at www.zmuc.dk/public/phylogeny .
- 56.Goloboff PA. 1993. Estimating character weights during tree-search. Cladistics Int. J. Willi Hennig Soc. 9, 83–91. (doi:10.1111/j.1096-0031.1993.tb00209.x) [DOI] [PubMed] [Google Scholar]
- 57.Goloboff PA, Farris JS, Nixon KC. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786. (doi:10.1111/j.1096-0031.2008.00217.x) [Google Scholar]
- 58.López-Arbarello A, Sferco E.2018. Data from: Neopterygian phylogeny: the merger assay. Dryad Digital Repository. (https://doi.org/10.5061/dryad.2tp53gr. ) [DOI] [PMC free article] [PubMed]
- 59.Farris JS. 1970. Methods for computing Wagner trees. System. Zool. 19, 83–92. (doi:10.2307/2412028) [Google Scholar]
- 60.Swofford DL, Maddison WP. 1987. Reconstructing ancestral character states under Wagner parsimony. Math. Biosci. 87, 199–229. (doi:10.1016/0025-5564(87)90074-5) [Google Scholar]
- 61.Agnarsson I, Miller JA. 2008. Is ACCTRAN better than DELTRAN? Cladistics 24, 1032–1038. (doi:10.1111/j.1096-0031.2008.00229.x) [DOI] [PubMed] [Google Scholar]
- 62.Arratia G. 1997. Basal teleosts and teleostean phylogeny. Palaeo Ichthyol. 7, 5–168. [Google Scholar]
- 63.Arratia G. 1999. The monophyly of Teleostei and stem-group teleosts: consensus and disagreements. In Mesozoic fishes 2: systematics and fossil record (eds Arratia G, Schultze H-P), pp. 265–334. Munich, Germany: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- 64.Arratia G. 2000. New teleostean fishes from the Jurassic of southern Germany and the systematic problems concerning the ‘pholidophoriforms’. Paläontol. Z. 74, 113–143. (doi:10.1007/BF02987957) [Google Scholar]
- 65.Arratia G. 2008. The varasichthyid and other crossognathiform fishes, and the break-up of Pangaea. In Fishes and the break-up of pangaea. 295 (eds Longbottom C, Richter LM), pp. 71–92. Geological Society Special Publications. [Google Scholar]
- 66.Arratia G, Tischlinger H. 2010. The first record of Late Jurassic crossognathiform fishes from Europe and their phylogenetic importance for teleostean phylogeny. Fossil Rec. 13, 317–341. (doi:10.1002/mmng.201000005) [Google Scholar]
- 67.Cavin L. 2001. Osteology and phylogenetic relationships of the teleost Goulmimichthys arambourgi Cavin, 1995, from the Upper Cretaceous of Goulmima, Morocco. Eclogae geolHelv. 94, 509–535. [Google Scholar]
- 68.Sire JY, Huysseune A. 2003. Formation of dermal skeletal and dental tissues in fish: a comparative and evolutionary approach. Biol. Rev. 78, 219–249. (doi:10.1017/S1464793102006073) [DOI] [PubMed] [Google Scholar]
- 69.Huysseune A, Sire J-Y, Witten PE. 2009. Evolutionary and developmental origins of the vertebrate dentition. J. Anat. 214, 465–476. (doi:10.1111/j.1469-7580.2009.01053.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schultze H-P. 1996. The scales of Mesozoic actinopterygians. In Mesozoic fishes: systematics and paleoecology (eds Arratia G, Viohl G), pp. 83–93. Munich, Germany: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- 71.Brito PM. 1997. Révision des Aspidorhynchidae (Pisces, Actinopterygii) du Mésozoïque: ostéologie, relations phylogénétiques, données environnmentales et biogéographiques. Geodiversitas. 19, 681–772. [Google Scholar]
- 72.Xu G, Wu F. 2012. A deep-bodied ginglymodian fish from the Middle Triassic of eastern Yunnan Province, China, and the phylogeny of lower neopterygians. Chin. Sci. Bull. 57, 111–118. (doi:10.1007/s11434-011-4719-1) [Google Scholar]
- 73.Xu GH, Gao KQ, Coates MI. 2015. Taxonomic revision of Plesiofuro mingshuica from the Lower Triassic of Northern Gansu, China, and the relationships of early neopterygian clades. J. Vertebr. Paleontol. 35, 14. [Google Scholar]
- 74.Bjerring HC. 1984. The term ‘Fossa Bridgei’ and five endocranial fossae in teleostome fishes. Zool. Script. 13, 231–238. (doi:10.1111/j.1463-6409.1984.tb00040.x) [Google Scholar]
- 75.Allis EP. 1909. The cranial anatomy of the mail-cheeked fishes. Zoologica 57, 96. [Google Scholar]
- 76.Sagemehl M. 1884. Beitrage zur vergleichenden Anatomie der Fische. 1. Das cranium von Amia calva L. Morphologisches Jahrbuch. 9, 177–228. [Google Scholar]
- 77.Stensiö E. 1925. Triassic fishes from Spitzbergen, pp. 1–261. Stockholm, Sweden: Almqvist & Wiksells Boktryckeri-A-B. [Google Scholar]
- 78.Patterson C. 1975. The braincase of pholidophorid and leptolepid fishes, with a review of the actinopterygian braincase. Phil. Trans. R. Soc. Lond B 269, 275–579. (doi:10.1098/rstb.1975.0001) [DOI] [PubMed] [Google Scholar]
- 79.Jarvik E. 1980. Basic structure and evolution of vertebrates. London, UK: Academic Press. [Google Scholar]
- 80.Allis EP. 1897. The cranial muscles and cranial and first spinal nerves in Amia calva. J. Morphol. 12, 487–772. (doi:10.1002/jmor.1050120302) [Google Scholar]
- 81.Maisey JG. 1999. The supraotic bone in neopterygian fishes (Osteichthyes, Actinopterygii). Am. Mus. Novit. 3267, 1–52. [Google Scholar]
- 82.Patterson C, Rosen DE. 1977. Review of ichthyodectiform and other Mesozoic teleost fishes and the theory and practice of classifying fossils. Bull. Am. Mus. Nat. Hist. 158, 81–172. [Google Scholar]
- 83.Gardiner BG, Maisey JG, Littlewood DTJ. 1996. Interrelationships of basal neopterygians. In Interrelationships of fishes, Ch. 6 (eds Gardiner BG, Maisey JG, Littlewood DTJ), pp. 117–146. [Google Scholar]
- 84.Allis EP. 1922. The cranial anatomy of Polypterus, with special reference to Polypterus bichir. J. Anat. 56, 189–294. [PMC free article] [PubMed] [Google Scholar]
- 85.Claeson KM, Bemis WE, Hagadorn JW. 2007. New interpretations of the skull of a primitive bony fish Erpetoichthys calabaricus (Actinopterygii: Cladistia). J. Morphol. 268, 1021–1039. (doi:10.1002/jmor.10567) [DOI] [PubMed] [Google Scholar]
- 86.Olsen PE, McCune AR. 1991. Morphology of the Semionotus elegans species group from the Early Jurassic part of the Newark Supergroup of eastern North America with comments on the family Semionotidae (Neopterygii). J. Vertebr. Paleontol. 11, 269–292. (doi:10.1080/02724634.1991.10011398) [Google Scholar]
- 87.Cavin L. 2010. Diversity of Mesozoic semionotiform fishes and the origin of gars (Lepisosteidae). Naturwissenschaften 97, 1035–1040. (doi:10.1007/s00114-010-0722-7) [DOI] [PubMed] [Google Scholar]
- 88.Nielsen E. 1949. Studies on Triassic fishes II: Australosomus and Birgeria. Meddelelser om Grønland. 146, 1–309. [Google Scholar]
- 89.Olsen PE. 1984. The skull and pectoral girdle of the parasemionotid fish Watsonulus eugnathoides from the Early Triassic Sakamena Group of Madagascar, with comments on the relationships of the holostean fishes. J. Vertebr. Paleontol. 4, 481–499. (doi:10.1080/02724634.1984.10012024) [Google Scholar]
- 90.Balfour FM, Parker WN. 1882. On the structure and development of Lepidosteus. Phil. Trans. R. Soc. Lond B 173, 359–442. (doi:10.1098/rstl.1882.0008) [Google Scholar]
- 91.Rayner DH. 1948. The structure of certain Jurassic holostean fishes with special reference to their neurocrania. Phil. Trans. R. Soc. Lond. B 233, 287–345. (doi:10.1098/rstb.1948.0006) [Google Scholar]
- 92.Beltan L. 1957. Etude d'un neurocrane de Lepidotes du Bathonien du Maroc. B. Soc. Géol. Fr. 6, 1091–1106. [Google Scholar]
- 93.Gardiner BG, Schaeffer B. 1989. Interrelationships of lower actinopterygian fishes. Zool. J. Linn. Soc. 97, 135–187. (doi:10.1111/j.1096-3642.1989.tb00550.x) [Google Scholar]
- 94.Coates MI. 1999. Endocranial preservation of a Carboniferous actinopterygian from Lancashire, UK, and the interrelationships of primitive actinopterygians. Phil. Trans. R. Soc. Lond B 345, 435–462. (doi:10.1098/rstb.1999.0396) [Google Scholar]
- 95.Bartram AWH. 1975. The holostean fish genus Ophiopsis Agassiz. Zool. J. Linn. Soc. 56, 183–205. (doi:10.1111/j.1096-3642.1975.tb00263.x) [Google Scholar]
- 96.Bermudez-Rochas DD, Poyato-Ariza FJ. 2015. A new semionotiform actinopterygian fish from the Mesozoic of Spain and its phylogenetic implications. J. Syst. Paleontol. 13, 265–285. (doi:10.1080/14772019.2014.881928) [Google Scholar]
- 97.Aldinger H. 1937. Permische Ganoidfische aus Ostgrönland. Meddelelser om Grønland. 102, 1–392. [Google Scholar]
- 98.Bjerring HC. 1977. A contribution to structural analysis of the head of the craniate animals. Zool. Script. 6, 127–183. (doi:10.1111/j.1463-6409.1978.tb00792.x) [Google Scholar]
- 99.Štamberg S, Zajíc J. 2000. New data on the osteology of actinopterygian fish Sphaerolepis kounoviensis. Vestník Ceského geolického ústavu. 75, 455–458. [Google Scholar]
- 100.Gardiner BG. 1984. The relationships of the palaeoniscid fishes, a review based on new specimens of Mimia and Moythomasia from the Upper Devonian of Western Australia. Bull. Br. Mus. Geol. 37, 173–428. [Google Scholar]
- 101.Choo B. 2011. Revision of the actinopterygian genus Mimipiscis (= Mimia) from the Upper Devonian Gogo Formation of Western Australia and the interrelationships of the early Actinopterygii. Earth Environ. Sci. Trans. R. Soc. Edinburgh. 102, 77–104. (doi:10.1017/S1755691011011029) [Google Scholar]
- 102.Giles S, Coates MI, Garwood RJ, Brazeau MD, Atwood R, Johanson Z, Friedman M. 2015. Endoskeletal structure in Cheirolepis (Osteichthyes, Actinopterygii), an early ray-finned fish. Palaeontology 58, 849–870. (doi:10.1111/pala.12182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lehman JP. 1952. Etude complémentaire des poissons de l'Eotrias de Madagascar. Kungl Svenska Vetenskapsakademiens Handlingar. Band 2, 1–201. [Google Scholar]
- 104.López-Arbarello A, Sferco E, Rauhut OWM. 2013. A new genus of coccolepidid fishes (Actinopterygii, Chondrostei) from the continental Jurassic of Patagonia. Palaeontol. Electron. 16, 23. [Google Scholar]
- 105.Hilton EJ, Forey PL. 2009. Redescription of Chondrosteus acipenseroides Egerton, 1858 (Acipenseriformes, Chondrosteidae) from the lower Lias of Lyme Regis (Dorset, England), with comments on the early evolution of sturgeons and paddlefishes. J. Syst. Paleontol. 7, 427–453. (doi:10.1017/S1477201909002740) [Google Scholar]
- 106.Hilton EJ, Grande L, Bemis WE. 2011. Skeletal anatomy of the shortnose sturgeon, Acipenser brevirostrum Lesueur, 1818, and the systematics of sturgeons (Acipenseriformes, Acipenseridae). Field. Life Earth Sci. 3, 1–168. (doi:10.3158/2158-5520-3.1.1) [Google Scholar]
- 107.Schultze H-P. 2008. Nomenclature and homologization of cranial bones in actinopterygians. In Mesozoic fishes 4 - homology and phylogeny (eds Arratia G, Schultze H-P, Wilson MVH), pp. 23–48. Munich, Germany: Verlag Dr. F. Pfeil. [Google Scholar]
- 108.Hammarberg F. 1937. Zur Kenntnis der ontogenetischen Entwicklung des Schädels von Lepidosteus playstomus. Acta Zool. 18, 209–337. (doi:10.1111/j.1463-6395.1937.tb00680.x) [Google Scholar]
- 109.Jollie M. 1986. A primer of bone names for the understanding of the actinopterygian head and pectoral girdle skeletons. Can. J. Zool. 64, 365–379. (doi:10.1139/z86-058) [Google Scholar]
- 110.Holmgren S, Stensiö E. 1936. Kranium und Visceralskelett der Akranier, Cyclostomen und Fische. In Handbuch der vergleichenden anatomie der wirbeltiere. 4 (eds Bolk L, Göppert E, Kallius E, Lubosch W), pp. 233–500. Berlin, Germany: Urban und Schwrzenberg. [Google Scholar]
- 111.Andrews SM, Gardiner BG, Miles RS, Patterson C. 1967. Chapter 26 pisces. In The fossil record, pp. 637–683. London, UK: Geological Society; Special Publications. 2. [Google Scholar]
- 112.Jollie M. 1975. Development of the head skeleton and pectoral girdle in Esox. J. Morphol. 147, 61–88. (doi:10.1002/jmor.1051470106) [DOI] [PubMed] [Google Scholar]
- 113.Jollie M. 1984. Development of cranial and pectoral girdle bones of Lepisosteus with a note on scales. Copeia 1984, 476–502. (doi:10.2307/1445204) [Google Scholar]
- 114.Wiley EO. 1978. The phylogeny and biogeography of fossil and recent gars (Actinopterygii: Lepisosteidae). Syst. Biol. 27, 134–136. [Google Scholar]
- 115.Bartram AWH. 1977. The Macrosemiidae, a Mesozoic family of holostean fishes. Bull. Br. Mus. Geol. 29, 137–234. [Google Scholar]
- 116.Sanford CPJ. 2000. Salmonoid fish osteology and phylogeny (Teleostei: Salmonoidei). Theses Zool. 33, 264. [Google Scholar]
- 117.Gouiric-Cavalli S. 2013. Sistemática y relaciones biogeográficas de los peces del titoniano (jurásico tardío) de la cuenca neuquina de Argentina. PhD thesis, Universidad Nacional de La Plata, Argentina. [Google Scholar]
- 118.Pehrson T. 1940. The development of dermal bones in the skull of Amia calva. Acta Zool. Stockholm 21, 1–50. (doi:10.1111/j.1463-6395.1940.tb00338.x) [Google Scholar]
- 119.Aumonier FJ. 1941. Development of the dermal bones in the skull of Lepidosteus osseus. Quart. J. Microsc. Sci. 83, 1–33. [Google Scholar]
- 120.Chang CT, Franz-Odendaal TA. 2014. Perturbing the developing skull: using laser ablation to investigate the robustness of the infraorbital bones in zebrafish (Danio rerio). BMC Dev. Biol. 14, 13 (doi:10.1186/s12861-014-0044-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patterson C. 1993. Osteichthyes: Teleostei. In Fossils record 2 (ed. Benton MJ.), pp. 621–656. London, UK: Chapman & Hall. [Google Scholar]
- 122.Poplin C, Lund R. 1995. Fates of the rostral, postrostral and premaxillary in the early history of actinopterygians. Geobios. M.S. 19, 225–230. (doi:10.1016/S0016-6995(95)80119-7) [Google Scholar]
- 123.Mickle KE. 2015. Identification of the bones of the snout in fossil lower actinopterygians: a new nomenclature scheme based on characters. Copeia 103, 838–857. (doi:10.1643/CG-14-110) [Google Scholar]
- 124.Schaeffer B. 1967. Late Triassic fishes from the Western United States. Bull. Am. Mus. Nat. Hist. 135, 286–342. [Google Scholar]
- 125.Laerm J. 1982. The origin and homology of the neopterygian vertebral centrum. J. Palaeontol. 56, 191–202. [Google Scholar]
- 126.Schultze H-P, Arratia G. 1988. Reevaluation of the caudal skeleton of some actinopterygian fishes: II. Hiodon, Elops, and Albula. J. Morphol. 195, 257–303. (doi:10.1002/jmor.1051950304) [DOI] [PubMed] [Google Scholar]
- 127.Woodward AS. 1895. The fossil fishes of the Talbragar Beds (Jurassic?). Mem. Geol. Surv. New South Wales Palaeontol. 9, 1–27. [Google Scholar]
- 128.Arratia G, Schultze H-P, Casciotta J. 2001. Vertebral column and associated elements in dipnoans and comparison with other fishes: development and homology. J. Morphol. 250, 101–172. (doi:10.1002/jmor.1062) [DOI] [PubMed] [Google Scholar]
- 129.Lane JA, Ebert M. 2015. A taxonomic reassessment of Ophiopsis (Halecomorphi, Ionoscopiformes), with a revision of Upper Jurassic species from the Solnhofen Archipelago, and a new genus of Ophiopsidae. J. Vertebr. Paleontol. 35, 23 (doi:10.1080/02724634.2014.883238) [Google Scholar]
- 130.Hay OP. 1895. On the structure and development of the vertebral column of Amia. Publication V, Zoological series 1, pp. 1–55. Chicago, Il: Field Columbian Museum. [Google Scholar]
- 131.Goodrich ES. 1930. Studies on the structure and development of vertebrates. J. Hist. Biol. 21, 355–356. (doi:10.5962/bhl.title.82144) [Google Scholar]
- 132.Gegenbauer C. 1867. Über die Entwickelung der Wirbelsäule des Lepidotsteus mit vergelichend anatomischen Bemerkungen. Jenaische Zeitschrift für Medizin und Naturwissenschaft. 3, 359–419. [Google Scholar]
- 133.Gadow H, Abbott EC. 1895. On the evolution of the vertebral column of fishes. Phil. Trans. R. Soc. Lond. B 186, 163–221. (doi:10.1098/rstb.1895.0004) [Google Scholar]
- 134.Schultze H-P, Arratia G. 1986. Reevaluation of the caudal skeleton of actinopterygian fishes: I. Lepisosteus and Amia. J. Morphol. 190, 215–241. (doi:10.1002/jmor.1051900206) [DOI] [PubMed] [Google Scholar]
- 135.Schultze H-P, Arratia G. 2013. The caudal skeleton of basal teleosts, its conventions, and some of its major evolutionary novelties in a temporal dimension. In Mesozoic fishes 5 - global diversity and evolution (eds Arratia G, Schultze H-P, Wilson MVH), pp. 187–246. Munich, Germany: Verlag Dr Friederich Pfeil. [Google Scholar]
- 136.Griffith J, Patterson C. 1963. The structure and relationships of the Jurassic fish Ichthyokentema purbeckensis. Bull. Br. Mus. Geol. 8, 3–43. [Google Scholar]
- 137.Taverne L. 2011. Ostéologie et relations de Catervariolus (Teleostei, ‘Pholidophoriformes’) du Jurassique moyen de Kisangani (Formation de Stanleyville) en République Démocratique du Congo. Bull. Institut. R. Sci. Nat. Belgique Sci. Terre. 81, 175–212. [Google Scholar]
- 138.Arratia G, Schultze H-P. 2007. Eurycormus – Eurypoma, two Jurassic actinopterygian genera with mixed identity. Fossil Rec. 10, 17–37. (doi:10.5194/fr-10-17-2007) [Google Scholar]
- 139.Laerm J. 1976. Development, function and design of amphicoelous vertebrae in teleost fishes. Zool. J. Linn. Soc. 58, 237–254. (doi:10.1111/j.1096-3642.1976.tb00830.x) [Google Scholar]
- 140.Schultze H-P, Arratia G. 1989. The composition of the caudal skeleton of teleosts (Actinopterygii: Osteichthyes). Zool. J. Linn. Soc. 97, 189–231. (doi:10.1111/j.1096-3642.1989.tb00547.x) [Google Scholar]
- 141.Gosline WA. 1960. Contributions toward a classification of modern isospondylous fishes. Bull. Br. Mus. Nat. Hist. 6, 325–365. [Google Scholar]
- 142.Nybelin O. 1963. Zur Morphologie und Terminologie des Schwanzskelettes der Actinopterygier. Arkiv Zool. Ser. 2. 15, 485–516. [Google Scholar]
- 143.Monod T. 1968. Le complexe urophore des poissons téléostéens. Mémoires de l'Institut français d'Afrique noire. 81, 1–705. [Google Scholar]
- 144.Greenwood PH, Patterson C. 1967. A fossil osteoglossoid fish from Tanzania (E. Africa). J. Linn. Soc. 47, 211–223. (doi:10.1111/j.1096-3642.1967.tb01404.x) [Google Scholar]
- 145.Regan CT. 1910. XLVIII.—The caudal fin of the Elopidæ and of some other Teleostean fishes. Ann. Mag. Nat. Hist. 5, 354–358. (doi:10.1080/00222931008692779) [Google Scholar]
- 146.Regan CT. 1910. LIV.—The origin and evolution of the Teleostean fishes of the order Heterosomata. Ann. Mag. Nat. Hist. 6, 484–496. (doi:10.1080/00222931008692879) [Google Scholar]
- 147.Whitehouse RH. 1910. The caudal fin of the Teleostomi. Proc. Zool. Soc. Lond. 80, 590–627. (doi:10.1111/j.1096-3642.1910.tb01905.x) [Google Scholar]
- 148.Boreske J. 1974. A review of the North American fossil amiid fishes. Bull. Mus. Comp. Zool. 146, 1–87. [Google Scholar]
- 149.Bartsch P. 1988. Funktionelle morphologie und evolution des Axialskelettes und der Caudalis ursprünglicher Knochenfische. Palaeontogr. Abt. A. 204, 117–226. [Google Scholar]
- 150.Patterson C. 1968. The caudal skeleton in Lower Liassic Pholidophorid fishes. Bull. Br. Mus. Geol. 16, 203–239. [Google Scholar]
- 151.Mabee PM. 1988. Supraneural and predorsal bones in fishes: development and homologies. Copeia 1988, 827–838. (doi:10.2307/1445705) [Google Scholar]
- 152.Arratia G, Schultze H-P. 1992. Reevaluation of the caudal skeleton of certain actinopterygian fishes: III. Salmonidae. Homologization of caudal skeletal structures. J. Morphol. 214, 187–249. (doi:10.1002/jmor.1052140209) [DOI] [PubMed] [Google Scholar]
- 153.Grande L, Bemis WE. 1991. Osteology and phylogenetic relationships of fossil and Recent paddlefishes (Polyodontidae) with comments on the interrelationships of Acipenseriformes. Soc. Vertebr. Paleontol. 11, 1–121. (doi:10.1080/02724634.1991.10011424) [Google Scholar]
- 154.Lambers P. 1992. On the ichthyofauna of the Solnhofen Lithographic Limestone (Upper Jurassic, Germany). PhD thesis, Rijkuniv, Groningen, The Netherlands. [Google Scholar]
- 155.Arratia G, Lambers P. 1996. The caudal skeleton of pachycormiforms: Parallel evolution? In Mesozoic fishes: systematics and paleoecology (eds Arratia G, Viohl G), pp. 191–218. Munich, Germany: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- 156.Wilder B. (ed.). 1876. On the serrated appendages of the throat of amia. Proc. American Association for the Advancement of Science 25, 259–263. [Google Scholar]
- 157.Liem KF, Woods LP. 1973. A probable homologue of the clavicle in the holostean fish Amia calva. J. Zool. 170, 521–531. (doi:10.1111/j.1469-7998.1973.tb05067.x) [Google Scholar]
- 158.Wright RR. 1884. On the function of the serrated appendages of the throat of Amia. Science 4, 511 (doi:10.1126/science.ns-4.96.511-c) [DOI] [PubMed] [Google Scholar]
- 159.Jarvik E. 1944. On the exoskeletal shoulder-girdle of teleostomian fishes, with special reference to Eusthenopteron goordi Whiteaves. K svenska VetenskAkad Handl. 21, 1–32. [Google Scholar]
- 160.O'Reilly JE, Puttick MN, Parry L, Tanner AR, Tarver JE, Fleming J, Pisani D, Donoghue PCJ. 2016. Bayesian methods outperform parsimony but at the expense of precision in the estimation of phylogeny from discrete morphological data. Biol. Lett. 12, 5 (doi:10.1098/rsbl.2016.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.López-Arbarello A, Schröder KM. 2014. The species of Aspidorhynchus Agassiz, 1833 (Neopterygii, Aspidorhynchiformes) from the Jurassic plattenkalks of Southern Germany. Palaeontol. Z. 88, 167–185. (doi:10.1007/s12542-013-0187-z) [Google Scholar]
- 162.Gibson SZ. 2016. Redescription and phylogenetic placement of dagger Hemicalypterus weiri Schaeffer, 1967 (Actinopterygii, Neopterygii) from the Triassic Chinle Formation, southwestern United States: new insights into morphology, ecological niche, and phylogeny. PLoS ONE 11, e0163657 (doi:10.1371/journal.pone.0163657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wen W, Zhang Q-Y, Hu S-X, Zhou C-Y, Xe T, Huang J-Y, Chen ZQ, Benton MJ. 2012. A new basal actinopterygian fish from the Anisian (Middle Triassic) of Luoping, Yunnan Province, Southwest China. Acta Palaeontol. Pol. 57, 149–160. (doi:10.4202/app.2010.0089) [Google Scholar]
- 164.López-Arbarello A, Sun Z-Y, Sferco E, Tintori A, Xu G-H, Sun Y-L, Wu F, Jiang Y. 2011. New species of Sangiorgioichthys Tintori and Lombardo, 2007 (Neopterygii, Semionotiformes) from the Anisian of Luoping (Yunnan Province, South China). Zootaxa 2011, 25–39. [Google Scholar]
- 165.Gallo V, Brito PM. 2004. An overview of Brazilian semionotids. In Mesozoic fishes 3 – systematics, paleoenvironments and biodiversity (eds Arratia G, Tintori A), pp. 253–264. Munich, Germany: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- 166.López-Arbarello A. 2004. The record of Mesozoic fishes from Gondwana (excluding India and Madagascar). In Mesozoic fishes 3 - systematics, paleoenvironments and biodiversity (eds Arratia G, Tintori A), pp. 597–624. Munich, Germany: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- 167.López-Arbarello A, Codorniú L. 2007. Semionotids (Neopterygii, Semionotiformes) from the Lower Cretaceous Lagarcito Formation, San Luis Province, Argentina. J. Vertebr. Paleontol. 27, 811–826. (doi:10.1671/0272-4634(2007)27[811:SNSFTL]2.0.CO;2) [Google Scholar]
- 168.López-Arbarello A, Rauhut OWM, Cerdeno E. 2010. The Triassic fish faunas of the Cuyana Basin, Western Argentina. Palaeontology 53, 249–276. (doi:10.1111/j.1475-4983.2010.00931.x) [Google Scholar]
- 169.Gibson SZ. 2013. Biodiversity and evolutionary history of dagger Lophionotus (Neopterygii: dagger Semionotiformes) from the western United States. Copeia 2013, 582–603. (doi:10.1643/CI-12-028) [Google Scholar]
- 170.Xu G-H, Shen C-C. 2015. Panxianichthys imparilis gen. et sp. nov., a new ionoscopiform (Halecomorphi) from the Middle Triassic of Guizhou, China. Vertebr. Palasiatica. 53, 1–15. [Google Scholar]
- 171.Ma X-Y, Xu G-H. 2017. A new ionoscopiform fish (Holostei: Halecomorphi) from the Middle Triassic (Anisian) of Yunnan, China. Vertebr. Palasiatica. 55, 162–176. [Google Scholar]
- 172.Lambers PH. 1994. The Halecomorph fishes Caturus and Amblysemius in the lithographic limestone of Solnhofen (Tithonian), Bavaria. Geobios 16, 91–99. (doi:10.1016/S0016-6995(94)80024-3) [Google Scholar]
- 173.Nybelin O. 1974. A revision of the leptolepid fishes. Acta Reg. Soc. Sci. Litterarum Gothoburgensis Zool. 9, 1–202. [Google Scholar]
- 174.Romano C, Koot MB, Kogan I, Brayard A, Minikh AV, Brinkmann W, Bucher H, Kriwet J. 2016. Permian-Triassic Osteichthyes (bony fishes): diversity dynamics and body size evolution. Biol. Rev. 91, 106–147. (doi:10.1111/brv.12161) [DOI] [PubMed] [Google Scholar]
- 175.Brough J. 1936. On the evolution of bony fishes during the Triassic period. Biol. Rev. 25, 385–405. (doi:10.1111/j.1469-185X.1936.tb00912.x) [Google Scholar]
- 176.Bean LB. 2006. The leptolepid fish Cavenderichthys talbragarensis (Woodward, 1895) from the Talbragar Fish Bed (Late Jurassic) near Gulgong, New South Wales. Rec. West. Aust. Mus. 23, 43–76. (doi:10.18195/issn.0312-3162.23(1).2006.043-076) [Google Scholar]
- 177.Waldman M. 1971. Fish from the freshwater Lower Cretaceous of Victoria, Australia, with comments on the palaeo-environment, pp. 1–124. Special Papers in Palaeontology; London, UK: Palaeontological Association. [Google Scholar]
- 178.Taverne L. 1989. Crossognathus Pictet, 1858 du Crétacé inférieur de l'Europe et systématique, paléozoogéographie et biologie des Crossognathiformes nov. ord. (Téléostéens) du Crétacé et du Tertiaire. Palaeontogr. Abt. A. 1989, 79–105. [Google Scholar]
- 179.Schaeffer B. 1956. Evolution of the subholostean fishes. Evolution 10, 201–212. (doi:10.1111/j.1558-5646.1956.tb02845.x) [Google Scholar]
- 180.Gardiner BG, Schaeffer B, Masserie JA. 2005. A review of the lower actinopterygian phylogeny. Zool. J. Linn. Soc. 144, 511–525. (doi:10.1111/j.1096-3642.2005.00181.x) [Google Scholar]
- 181.Tintori A, Sassi D. 1987. Flying fish of the genus Thoracopterus in the Lombardian Norian Italy preliminary note. Riv. Ital. Paleontol. S. 93, 337–346. [Google Scholar]
- 182.Xu GH, Ma XY. 2016. A Middle Triassic stem-neopterygian fish from China sheds new light on the peltopleuriform phylogeny and internal fertilization. Sci. Bull. 61, 1766–1774. (doi:10.1007/S11434-016-1189-5) [Google Scholar]
- 183.Tintori A, Hitij T, Jiang D, Lombardo C, Sun Z. 2014. Triassic actinopterygian fishes: the recovery after the end-Permian crisis. Integr. Zool. 9, 394–411. (doi:10.1111/1749-4877.12077) [DOI] [PubMed] [Google Scholar]
- 184.Raup DM, Sepkoski JJ. 1982. Mass extinctions in the marine fossil record. Science 215, 1501–1503. (doi:10.1126/science.215.4539.1501) [DOI] [PubMed] [Google Scholar]
- 185.McCune AR, Schaeffer B. 1986. Triassic and Jurassic fishes: patterns of diversity. In The beginning of the age of dinosaurs (ed. Padian K.), pp. 171–181. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 186.Benton MJ. 1993. The fossil record 2. London, UK: Chapman & Hall. [Google Scholar]
- 187.Woodward AS. 1895. Catalogue of the fossil fishes in the British Museum. London, UK: British Museum (Natural History). [Google Scholar]
- 188.Smith DG. 1989. Stratigraphic correlation of presumed Milankovitch cycles in the Blue Lias (Hettangian to earliest Sinemurian), England. Terra Nova. 1, 457–460. (doi:10.1111/j.1365-3121.1989.tb00410.x) [Google Scholar]
- 189.Sciscio L, de Kock M, Bordy E, Knoll F. 2017. Magnetostratigraphy across the Triassic-Jurassic boundary in the main Karoo Basin. Gondwana Res. 51(Suppl. C), 177–192. (doi:10.1016/j.gr.2017.07.009) [Google Scholar]
- 190.Thies D, Waschkewitz J. 2016. Redescription of Dapedium pholidotum (Agassiz, 1832) (Actinopterygii, Neopterygii) from the Lower Jurassic Posidonia Shale, with comments on the phylogenetic position of Dapedium Leach, 1822. J. Syst. Paleontol. 14, 339–364. (doi:10.1080/14772019.2015.1043361) [Google Scholar]
- 191.Thies D, Hauff R. 2011. A new species of Dapedium Leach, 1822 (Actinopterygii, Neopterygii, Semionotiformes) from the Early Jurassic of South Germany. Palaeodiversity. 4, 185–221. [Google Scholar]
- 192.Thies D, Hauff RB. 2008. A neotype for Dapedium caelatum Quenstedt, 1858 (Actinpoterygii, Neopterygii, Semionotiformes) from the Early Jurassic (Early Toarcian) of South Germany. Geologica et Palaeontologica. 42, 23–38. [Google Scholar]
- 193.Arratia G. 1991. The caudal skeleton of Jurassic teleosts: a phylogenetic analysis. In Early vertebrates and related problems in evolutionary biology (eds Chang M-M, Liu H, Zhang G), pp. 249–340. Beijing, China: Science Press. [Google Scholar]
- 194.GBIF Home Page [Internet]. 2017. See https://www.gbif.org/.
- 195.Colin JP. 1994. Mesozoic–Cenozoic lacustrine sediments of the Zaire Interior Basin. In Global geological record of lake basins, IGCP Project 324. 4 (eds Gierlowski-Kordeschand E, Keltz K), pp. 31–36. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 196.Arratia G. 1981. Varasichthys ariasi n. gen. et sp. from the Upper Jurassic of Chile (Pisces, Teleostei, Varasichthyidae n. fam.). Palaeontogr. Abteil. A. 175, 107–139. [Google Scholar]
- 197.Cúneo R, et al. 2013. High-precision U–Pb geochronology and a new chronostratigraphy for the Cañadón Asfalto Basin, Chubut, central Patagonia: Implications for terrestrial faunal and floral evolution in Jurassic. Gondwana Res. 24, 1267–1275. (doi:10.1016/j.gr.2013.01.010) [Google Scholar]
- 198.Ebert M. 2012. Histionotus (Actinopterygii, Macrosemiidae) – Eine Gattung mit vielen Fragezeichen. Archaeopteryx. 30, 5–15. [Google Scholar]
- 199.Schröder KM, López-Arbarello A, Ebert M. 2012. Macrosemimimus, gen. nov. (Actinopterygii, Semionotiformes), from the Late Jurassic of Germany, England, and France. J. Vertebr. Paleontol. 32, 512–529. (doi:10.1080/02724634.2012.649626) [Google Scholar]
- 200.Lane JA, Ebert M. 2012. Revision of Furo muensteri (Halecomorphi, Ophiopsidae) from the Upper Jurassic of Western Europe, with comments on the genus. J. Vertebr. Paleontol. 32, 799–819. (doi:10.1080/02724634.2012.680325) [Google Scholar]
- 201.Ebert M, Lane JA, Kolbl-Ebert M. 2016. Palaeomacrosemius thiollieri, gen. et sp. nov., a new Macrosemiidae (Neopterygii) from the Upper Jurassic of the Solnhofen Archipelago (Germany) and Cerin (France), with a revision of the genus Macrosemius. J. Vertebr. Paleontol. 36, 22 (doi:10.1080/02724634.2016.1196081) [Google Scholar]
- 202.Allen P, Wimbledon WA. 1991. Correlation of NW European Purbeck-Wealden (nonmarine Lower Cretaceous) as seen from the English type-areas. Cretaceous Res. 12, 511–526. (doi:10.1016/0195-6671(91)90005-W) [Google Scholar]
- 203.Cavin L, Deesri U, Suteethorn V. 2014. Ginglymodian fishes (Actinopterygii, Holostei) from Thailand: an overview. J. Sci. Technol. Mahasarakham Univers. 33, 348–356. [Google Scholar]
- 204.Woodward AS. 1919. The fossil fishes of the English Wealden and Purbeck formations. Palaeontogr. Soc. 1916-Part II, 49–104. [Google Scholar]
- 205.Wenz S. 1999. †Pliodetes nigeriensis, gen. nov. et. sp. nov., a new semionotid fish from the Lower Cretaceous of Gadoufaoua (Niger Republic): phylogenetic comments. In Mesozoic fishes 2 – systematics and fossil record (eds Arratia G, Schultze H-P), pp. 107–120. Munich, Germany: Verlag Dr. Friederich Pfeil. [Google Scholar]
- 206.Brito PM, Yabumoto Y. 2011. An updated review of the fish faunas from the Crato and Santana formations in Brazil, a close relationship to the Tethys fauna. Bull. Kitakyushu Mus. Nat. Hist. Serie A. 9, 107–136. [Google Scholar]
- 207.Schaeffer B. 1971. The braincase of the Holostean fish Macrepistius, with comments on neurocranial ossification in the actinopterygii. Amer. Mus. Novit. 2459, 1–34. [Google Scholar]
- 208.Tintori A, Sun Z, Ni P, Lombardo C, Jiang D, Motani R. 2015. Oldest stem teleost from the late Ladinian (Middle Triassic) of Southern China. Rev. Ital. Paleontol. S. 121, 285–296. [Google Scholar]
- 209.Tintori A, Lombardo C, Kustatscher E. 2016. The Pelsonian (Anisian, Middle Triassic) fish assemblage from Monte Prà della Vacca/Kühwiesenkopf (Braies Dolomites, Italy). Neues Jahrb. Geol. Paläontol. A. 282, 181–200. (doi:10.1127/njgpa/2016/0612) [Google Scholar]
- 210.Sun Z-Y, Lombardo C, Tintori A, Jiang D-Y. 2015. A new species of Altisolepis (Peltopleuriformes, Actinopterygii) from the Middle Triassic of Southern China. J. Vertebr. Paleontol. 35, e909819 (doi:10.1080/02724634.2014.909819) [Google Scholar]
- 211.Gouiric-Cavalli S, Zavattieri AM, Gutierrez PR, Cariglino B, Balarino L. 2017. Increasing the fish diversity of the Triassic faunas of Gondwana: a new redfieldiiform (Actinopterygii) from the Middle Triassic of Argentina and its palaeobiogeographical implications. Pap. Palaeontol. 3, 559–581. (doi:10.1002/spp2.1089) [Google Scholar]
- 212.Xu G-H, Zhao L-J. 2016. A Middle Triassic stem-neopterygian fish from China shows remarkable secondary sexual characteristics. Sci. Bull. 61, 338–344. (doi:10.1007/s11434-016-1007-0) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- López-Arbarello A, Sferco E.2018. Data from: Neopterygian phylogeny: the merger assay. Dryad Digital Repository. (https://doi.org/10.5061/dryad.2tp53gr. ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Dryad Digital Repository: https://doi.org/10.5061/dryad.2tp53gr [58].
Phylogenetic data: MorphoBank, Project 2196 (https://morphobank.org/index.php/MyProjects/List/select/project_id/2196).
The datasets supporting this article have been uploaded as part of the electronic supplementary material:
Electronic supplementary material file S1: Material examined and data matrix; file S2: List of phenotypic characters; file S3: Branch support values obtained for the cladistic analyses; file S4: List of synapomorphies.