Abstract
Purpose of Review
Acute HIV infection is characterized by high-level viral replication throughout the body’s lymphoid system, particularly in gut-associated lymphoid tissues resulting in damage to structural components of gut tissue. This damage is irreversible and believed to contribute to the development of immune deficiencies. Antiretroviral therapy (ART) does not restore gut structure and function. Studies in macaques point to an alternative treatment strategy that may ameliorate gut damage. Integrin α4β7 mediates the homing of lymphocytes to gut tissues. Vedolizumab, a monoclonal antibody (mAb) antagonist of α4β7, has demonstrated efficacy and has been approved for the treatment of inflammatory bowel disease in humans. Here, we describe our current knowledge, and the gaps in our understanding, of the role of α4β7 in HIV pathogenesis and treatment.
Recent Findings
When administered to macaques prior to infection, a nonhuman primate analogue of vedolizumab prevents transmission of SIV. In combination with ART, this mAb facilitates durable virologic control following treatment interruption.
Summary
Targeting α4β7 represents a novel therapeutic approach to prevent and treat HIV infection.
Keywords: HIV/SIV, GALT, Integrin α4β7, Inflammatory bowel disease, Mucosal transmission, Antiretroviral therapy
Introduction
A defining feature of acute HIV infection is high-level viral replication in gut-associated lymphoid tissue (GALT). The propensity of HIV to replicate in GALT was first recognized over 25 years ago [1]. The association of GALT with acute infection came from two independent studies carried out in an SIV/Rhesus macaque model [2, 3]. From these studies, it was noted that high-level viral replication in GALT is accompanied by a profound depletion of gut CD4+ T cells. Subsequently, it was demonstrated that HIV infection in humans leads to a similar loss of gut CD4+ T cells in the very early stages of infection [4–6]. This gut-tropic aspect of acute HIV infection is believed to play a central role in the development of immune deficiencies that define HIV disease. The rapid loss of CD4+ T cells is accompanied by damage to the structural integrity of the gut, which has been linked to chronic systemic immune activation [7, 8]. Thus, there is considerable evidence to suggest that events that occur in gut tissues in the first weeks of infection play a central role in AIDS pathogenesis [9] The development of effective antiretroviral therapies (ART) have proven to be extraordinarily effective in suppressing viral replication in HIV-infected individuals. ART delays the onset of HIV-mediated immune deficiencies and significantly extends the life of individuals infected with HIV. However, ART is associated with varying degrees of toxicity, and once it is withdrawn plasma viremia typically rebounds [10–12]. Furthermore, ART does not fully reverse the early damage to the structural integrity of the gut, nor does it allow CD4+ T cells in GALT to fully recover [13]. Chronic immune activation persists in patients, even in individuals in whom ART is administered shortly after infection [14]. In one recent study, initiation of ART as early as ~ 2 weeks postinfection did not prevent long-term and apparently irreversible damage to the gut [15].
In addition to replicating in GALT, HIV replicates in the peripheral lymph nodes, spleen, and other tissues and organs, and this replication also contributes significantly to HIV pathogenesis. Yet, it is generally recognized that the early infection and irreversible destruction of CD4+ T cells in GALT is a key event in the eventual development of immune deficiencies. Understanding the specific events surrounding this damage to gut lymphoid tissues may point to new and improved ways to prevent and treat HIV infection.
Migration of immune cells into and out of GALT is tightly regulated by receptors that control cell trafficking. Prominent among gut homing receptors is integrin α4β7 (α4β7). This heterodimeric receptor is comprised of a 180-kDa α chain (α4) and a 130-kDa β chain (β7). α4β7 is expressed on subsets of CD4+ and CD8+ T cells, B cells, NK cells, and macrophages. Both α4 and β7 can pair with other integrin chains; however, the α4β7 heterodimer is distinct in promoting trafficking of lymphocytes to GALT. The mechanism by which α4β7-expressing cells home to GALT involves a specific interaction with the mucosal addressin cell adhesion molecule (MAdCAM). MAdCAM in healthy adults is expressed on the cell surface of high endothelial venules (HEVs) of gut inductive sites and in the gut lamina propria. It is also found on the surface of follicular dendritic cells (FDCs) in mesenteric lymph nodes [16]. A majority of naïve, and a subset of memory, CD4+ T cells express α4β7, and these cells circulate throughout the peripheral lymphoid system. Importantly, it is the tissue-specific expression of MAdCAM that defines α4β7 as a gut homing receptor.
Integrins following ligation, including α4β7, are capable of delivering intracellular signals (outside-in signaling). Signaling is precisely coordinated with other integrins (α4β1 and LFA-1) and chemokine receptors in a multistep adhesion cascade that results in extravasation of α4β7 positive cells through venules and into gut tissues [17, 18]. However, α4β7 signaling is not limited to processes involving cell trafficking. Similar to α4β1 and LFA-1, α4β7 can provide costimulatory signals to CD4+ T cells [19–21]. Little is known regarding the context in which α4β7-mediated costimulation contributes to CD4+ T cell activation in vivo, although it is presumably most relevant in GALT [22].
α4β7 and the Gut-Tropic Phenotype of HIV
A growing number of viruses have been shown to interact directly with integrins [23]. In some cases, the adaptive value of this interaction involves signal transduction that facilitates infection [23]. Certain rotaviruses as well as both HIV and SIV have been shown to engage α4β7 [21, 24–26]. For HIV and SIV, this interaction is mediated by the gp120 subunit of the envelope glycoprotein [24, 27, 28]. The identification of an interaction between HIV and α4β7 provided a new perspective to the earlier descriptions of the gut-tropic nature of HIV and SIV and the HIV/SIV-mediated pathologies that are associated with acute infection [29]. However, α4β7 is not required for entry into CD4+ T cells and its specific role in HIV pathogenesis remains under investigation.
HIV and SIV gp120 Bind to and Signal Through α4β7
The gp120 subunit of HIV and SIV envelope proteins have been described as a series of five constant domains interspersed with five variable domains (C1, V1, V2, C2, V3, C3, V4, C4, V5, C5). The V2 domain of gp120 (V2-loop) is a ~ 50–90 amino acid region bounded by cysteines. It is contiguous with the V1 domain. The principal α4β7 binding site lies within V2 [21, 28, 30–32] and involves a conserved tripeptide motif, LDV/I. This tripeptide appears to function in a similar manner as tripeptide motifs encoded in MAdCAM (LDT), VCAM (IDS), and fibronectin (LDV), the three natural ligands of α4β7 [33]. All share a core aspartic acid that coordinates a Mg++ ion that sits in the metal ion dependent adhesion site (MIDAS) of the α4 chain. Metal ion coordination is required for binding of these natural ligands to α4β7, as well as for the binding of HIV and SIV gp120.
Binding of a recombinant HIV gp120 protein to α4β7 on primary CD4+ T cells rapidly activates LFA-1 [21]. LFA-1 activation, and possibly other aspects of gp120-mediated signal transduction through α4β7, may facilitate the formation of virological synapses. The utilization of an Asp-containing tripeptide, along with the requirement of a metal ion in mediating gp120 binding to α4β7, represents a striking example of molecular mimicry and suggests that HIV-α4β7 interactions evolved to serve an important role in HIV pathogenesis.
α4β7high Memory CD4+ T Cells Are Early Targets of HIV Infection
HIV gp120 V2 encodes a variable number of potential N-linked glycosylation sites (PNGs). Viruses isolated in the first months after infection can be distinguished from chronic isolates by tending to have fewer PNGs [34]. The removal of V2 PNGs enhances gp120 binding to α4β7 [27, 28]. Based on this observation, we proposed that α4β7high memory CD4+ T cells are early targets of infection following mucosal transmission [35]. Subsequently, studies in the SIV/macaque model showed that α4β7high memory CD4+ T cells are rapidly depleted following mucosal transmission of virus. Among the cells bearing this phenotype are TH-17 cells, which have independently been implicated as early targets of infection. Recently, Sivro and colleagues reported that, in HIV-infected women, α4β7high memory CD4+ T cells are preferentially depleted from gut tissues as early as the first 2 weeks following infection [15]. That these cells might be among the first cells productively infected is further supported by the observation that they can be found in cervical cytobrush samples [36–38], and in both humans and macaques the frequency of α4β7high memory CD4+ T cells in blood and cervical tissues increases in the context of sexually transmitted diseases and is associated with increased susceptibility to acquisition [39, 40]. In addition, the frequency of α4β7high memory CD4+ T cells in mucosal tissues correlates with their frequency in blood. Blood frequencies range from ~ 7 to 25% and appear to be stable in healthy individuals over time. Interestingly, one study comparing matched groups of young, healthy African American and Caucasian men found that the frequencies of α4β7high memory CD4+ T cells were higher in the African Americans [41], and suggested that this difference might contribute at least in part to the relatively increased incidence of HIV in this group of Americans. Thus, in vitro and in vivo studies point to a role for α4β7 in the initial stages of infection, which set the stage for studies aimed at determining whether targeting α4β7 could reduce the efficiency of viral transmission (see below).
Development of an Anti-α4β7 Antagonist for Use in an SIV/Rhesus Macaque Model
Inflammatory bowel disease (IBD), which includes both Crohn’s disease and ulcerative colitis, afflicts over 1.3 million people in the USA. IBD is an autoimmune disease of unknown etiology localized primarily to the gut. One therapeutic strategy designed to suppress the inflammatory response associated with IBD involves administration of a humanized anti-α4β7 monoclonal antibody (mAb) [42–44]. This mAb, termed vedolizumab (Entyvio), is a derivative of a mouse mAb named Act-1 that was discovered more than 30 years ago [45, 46]. It was believed generally that Act-1 binds to an epitope shared between α4 and β7. However, based on crystallographic data [47], it has been proposed that Act-1 binds exclusively to the β7 chain of α4β7 in a way that specifically inhibits binding to MAdCAM.
Vedolizumab has proved to be effective in the treatment of IBD [42–44]. By blocking α4β7-MAdCAM interactions, it is thought that vedolizumab reduces trafficking of α4β7high memory CD4+ T cells into GALT, which in turn should reduce localized inflammatory responses. Data exist to support this mechanism of action; however, the effects of vedolizumab are likely to be pleiotropic and other mechanisms have not been ruled out [48, 49]. Because of the tissue-specific expression of MAdCAM, vedolizumab does not exhibit the more potent and diffuse immunosuppressive effects associated with natulizumab, an α4 antagonist that targets both α4β7 and α4β1.
Act-1 does not block HIV infection of primary CD4+ T cells. In vitro, it has been shown to mediate modest reductions in the replication of some isolates; however, it has no impact on others [21, 31, 50]. The underlying mechanism of these effects is not known. Act-1 does, however, block binding of gp120 to α4β7, which we believe may have an impact on HIV pathogenesis. Considering the preferential infection of α4β7high memory CD4+ T cells in the early stages of SIV/HIV infection, we reasoned that Act-1 might interfere with mucosal transmission. To test this hypothesis, we turned to an α4β7/SIV macaque model developed by Ansari and colleagues [51, 52].
In order to gain further insight into the role of GALT in HIV pathogenesis, Ansari and colleagues developed a primatized version of Act-1 [52]. The rationale underlying the development of this reagent was, in part, to determine whether reducing inflammatory responses in GALT would minimize HIV-mediated damage to gut tissues. This rationale is similar to the rationale for the use of vedolizumab in the treatment of IBD [53].
The primatized analogue of Act-1 has a serum half-life of 11.4 days. When administered intravenously at a dose of 50 mg/kg, it fully masked the expression of α4β7 expressed on the surface of lymphocytes harvested from colon biopsies [24, 51, 52]. Although the framework onto which the Act-1 complementary-determining regions (CDRs) were grafted was derived from a rhesus macaque IgG1 mAb, administration of the primatized anti-α4β7 (anti-α4β7 mAb) induces rhesus anti-rhesus antibodies (RARA), but at a low frequency (~ 20%). Our findings strongly suggest that RARA impacted treatment in an adverse way [54]. The frequency of anti-drug antibodies (ADA) in IBD patients treated with vedolizumab is low [53, 55–57].
Treatment of SIV Infected Macaques with a Primatized Anti-α4β7 mAb
The initial evaluation of anti-α4β7 mAb in SIV infected macaques involved pre-treatment of macaques with the mAb 3 days prior and 3 weeks following IV inoculation with a high dose of SIVmac239. Animals received a second dose of mAb 3 weeks post-virus inoculation. No other drugs were included in this study. The viral inoculum was prepared as a stock using day 3 PHA activated rhesus PBMCs [51]. In untreated control macaques, infection with this virus typically leads to AIDS within 18 months. Relative to control animals, macaques receiving anti-α4β7 mAb exhibited significantly delayed peak SIV RNA and ~ 8-fold lower plasma set-point SIV RNA levels [51]. Of note, peak levels of viral RNA copies in jejeunal and colorectal biopsies were reduced by ~ 2 logs in the mAb-treated animals, and copies of proviral DNA in colorectal biopsies were low to undetectable. Thus, anti-α4β7 mAb was effective in reducing the amount of virus in GALT. Treatment also led to the preservation of peripheral CD4+ CCR5+ T cells. The impact of anti-α4β7 mAb on disease course was significant. While control animals developed AIDS 60–80 weeks after infection, treated animals remained healthy for 5 years (Ansari, unpublished).
In a recent study [58], we evaluated the frequencies of CD4+ T cells in colorectal biopsies from these anti-α4β7 mAb-treated animals. After an initial loss in the acute phase of infection, CD4+ T cells gradually increased over time, such that 60+ months postinfection, levels were close to that observed in healthy uninfected controls. This is notable in two ways. First, the effects of anti-α4β7 mAb were durable. Two infusions during acute infection were sufficient to dampen damage to gut tissues and preserve CD4+ T cells over an extended period of time. This preservation persisted despite the presence of significant plasma viremia, suggesting that a key aspect of anti-α4β7 mAb therapy may involve protection and/or restoration of the gut ultrastructure. Second, the capacity of a brief period of anti-α4β7 mAb therapy to alter the course of disease indicates that, at least in this SIV model, early virus replication in GALT and the consequent inflammation of gut tissues plays an important role in the development of disease many months later. It is important to keep in mind that the immune cell composition is different in small and large intestines [17, 59, 60], and are associated with different infections and pathologies [59]. We found that anti-α4β7 mAb treatment reduced virus in the colon to a greater extent than the small intestine [58].
Anti-α4β7 mAb in the Prevention of Mucosal Transmission
McKinnon and colleagues isolated α4β7high memory CD4+ T cells from cervical cytobrush specimens and found that they co-express multiple HIV susceptibility markers [37]. Moreover, α4β7 expression was correlated with CCR5 expression. These features would seem to make these cells favorable targets for productive infection. It is reasonable then to suggest that virus infects α4β7high memory CD4+ T cells in the genital or rectal mucosa, and that these infected cells eventually migrate into GALT. An alternative way that virus might access gut tissues can be inferred from the recent description of the incorporation of α4β7 on the surface of free virus, and the capacity of virion-associated α4β7 to mediate virus adhesion to MAdCAM on HEVs [61]. For this to occur requires that virus first infects α4β7-expressing cells, which is consistent with the scenario outlined above.
With this scenario in mind, we asked whether anti-α4β7 mAb could reduce the efficiency of mucosal transmission of SIV. To address this question, we employed a stringent low-dose vaginal transmission model that utilized a highly infectious stock of SIVmac251. Animals were pretreated with anti-α4β7 mAb (50 mg/kg) or a control IgG mAb and then challenged weekly until 10 of 12 control animals were determined to be viremic. After 6 challenges, 6/12 anti-α4β7 mAb-treated animals remained uninfected while 10 of the 12 controls became infected. For those anti-α4β7 mAb-treated animals that did become infected, their infection was somewhat delayed since more total challenges were required than for animals in the control arm of the study [24].
The specific mechanisms by which animals were protected from infection remain unclear. Of note, anti-α4β7 mAb treatment did not alter the number of CD4+ T cells in the cervicovaginal compartment. However, it did mask > 99% of the α4β7 heterodimers present on the surface of cervicovaginal CD4+ T cells. One of the more intriguing results from this study was our finding that, in anti-α4β7 mAb-treated animals, but not in placebo controls, we could detect proviral DNA in cervical tissues 10 weeks after the last challenge. This would seem to indicate that anti-α4β7 mAb interfered with the egress of cells out of the vaginal tissue. MAdCAM is normally absent from the female genital tract. However, chlamydia infection of the female genital tract induces MAdCAM neo-expression [62–64], and one can speculate that SIV might promote a similar response. We proposed three mechanisms which alone or together may have reduced the efficiency of infection. Anti-α4β7 mAb may have interfered with: (1) trafficking, by blocking α4β7 binding to MAdCAM; (2) V2 peptide interacting with α4β7; and (3) signaling through α4β7 on CD4+ T cells, either by MAdCAM or V2.
There exist significant gaps in our understanding of the early events that lead to irreversible SIV/HIV infection. Our demonstration that anti-α4β7 mAb can prevent infection, at least in the context of vaginal transmission, argues that α4β7-expressing cells play an early role in establishing infection. Key questions remain, including whether α4β7 is involved in rectal transmission. Martinelli and colleagues report that the susceptibility of macaques to rectal SIV challenge correlates directly with the frequency of α4β7high memory CD4+ T cells in rectal tissue [65], suggesting that this might be the case. Finally, it remains to be determined whether α4β7-expressing cells contribute to long-lived viral reservoirs, and if treatment with α4β7 antagonists can alter the size and/or durability of those reservoirs.
α4β7 Antagonists as Adjunctive Therapies to ART
Current ART therapies are highly effective in allowing subjects to control viremia for an extended period of time. Yet, the persistence of a viral reservoir, despite ART, requires that the vast majority of HIV-infected individuals remain on therapy indefinitely. Moreover, ART does not fully resolve the chronic immune activation associated with infection. Given the demonstrated capacity of anti-α4β7 mAb to promote durable control of viremia in SIV-infected macaques in the absence of ART [24, 51], it is reasonable to ask whether α4β7 antagonists might prove useful as an adjunctive therapy to ART. Unlike many other proposed adjunct therapies, the clinical development of α4β7 antagonists is well underway. The demonstrated efficacy of vedolizumab in the treatment of IBD has catalyzed efforts aimed toward the development of new and hopefully more effective α4β7 antagonists [42].
To explore how an α4β7 antagonist might compliment ART, we employed a stock of SIVmac239 prepared as described by Derosiers et al. [66]. Considering the cumulative impact of infection on the immune system over time, we designed a protocol in which infection was established, but the immune system remained partially competent. Animals were placed on ART 5 weeks postinfection around the time that set-point viral load is typically established [54]. Four weeks later, the first of eight anti-α4β7 mAb infusions and, for purposes of control, normal IgG infusions, were administered to two groups of Rhesus macaques. Nine weeks later, ART was terminated. While 7/7 of the animals that received ART and a control IgG mAb showed significant plasma viral rebound, 8/8 of the animals that received ART + anti-α4β7 mAb controlled plasma viremia to either low or undetectable levels. Control of viremia in the anti-α4β7 mAb arm persisted in all animals for > 3 years, with some animals showing intermittent blips in plasma viremia. CD4+ T cells counts in blood were restored to near pre-infection levels, and gut CD4+ T cells also recovered. In brief, the addition of an anti-α4β7 mAb to a standard form of ART promoted durable control that has allowed these animals to control viremia for an extended period following withdrawal of all therapy. Although plasma aviremic, each of these animals remain infected, and studies are ongoing to identify the immune mechanisms responsible for viral control.
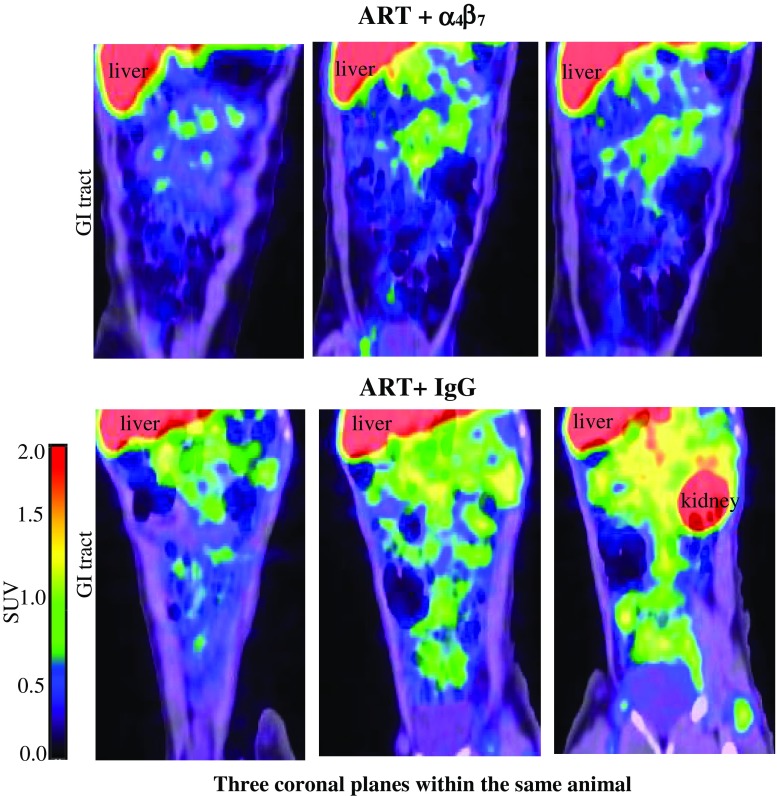
In attempts to identify the mechanisms that mediated viral control, we focused on the dual therapy period when both ART + IgG and ART+ anti-α4β7 mAb-treated animals were plasma aviremic, reasoning that the immune responses that would eventually control viral replication in the animals that received anti-α4β7 mAb likely developed during this period. Among the most notable differences between the two groups during the dual therapy phase of the study was a relative increase in CD4+ T cells in the animals receiving anti-α4β7 mAb. These increases were observed in both blood and colorectal tissues, and included Th17/22 cells. Loss of Th17/22 cells is noteworthy because it is correlated with increased damage to GALT [67]. Plasma I-FABP levels, which also correlate directly with gut damage, began to decrease during the dual therapy phase of the study in the animals receiving anti-α4β7 mAb. In a follow-up study, that employed PET/CT image analysis, we determined that the addition of anti-α4β7 mAb reduced the number of infected cells in gut tissues relative to ART alone during the dual therapy period [58]. Given that ART alone efficiently suppresses plasma viremia, it is unclear how anti-α4β7 mAb was able to reduce the number of infected cells in gut tissues beyond that achieved by ART; however, this reduction is likely relevant to the durable control that we observed in these animals. Interestingly, following interruption of both ART and anti-α4β7 mAb the reduction of infected cells in gut tissues persisted (Fig. 1). Other differences that appeared during the dual therapy period included increases in colonic NKp44+ ILCs [54, 68, 69], and levels of plasma retinoic acid (ATRA) in the anti-α4β7 mAb-treated group. Interestingly, plasma TGF-β levels were significantly lower in the ART+ anti-α4β7 mAb-treated animals compared to the ART + control IgG group during the dual therapy phase of treatment.
Fig. 1.
Immuno-PET/CT analysis of SIV gp120 in animals following treatment with ART+ anti α4β7 mAb. Comparison of gp120 signals in gut tissues of an ART + α4β7 mAb-treated animal (RLN12, upper) and an ART-only animal (RuS14, lower) at 34 and 39 weeks postinfection, respectively. Three coronal planes of the GI tract from each animal are shown. Immuno-PET images acquired as described in Santangelo et al. [58]. Further details in Byrareddy et al. [54]
Regarding adaptive immune responses, we could find no evidence of CD8+ T cell activity that distinguished the two treatment groups. None of the animals generated neutralizing antibody responses. However, all eight of the animals receiving anti-α4β7 mAb developed measurable antibody responses to a 15 amino acid region within the V2 domain, while only 3/7 ART+ IgG animals generated a similar response. Interestingly these V2 antibodies block SIV gp120 binding to α4β7 (submitted).
Elucidating the mechanisms of control in ART+ anti-α4β7 mAb-treated macaques remains a work in progress. It will be important to better understand which features of the experimental design were critical to achieving control and whether the protocol we employed can be further optimized. Is the control these animals achieved restricted to the stock of SIVmac239 or can it be extended to other SIV isolates? Would control be achieved if the animals were left untreated with anti-α4β7 mAb for a longer period of time? It is possible that if infection was allowed to go untreated into the chronic phase of infection that the immune cells providing control would be disabled. Conversely, how much viral antigenic experience is required for control? Would control be achieved if monkeys were placed on ART earlier than 5 weeks postinfection, when the viral antigenic load was lower? Does control require the anti-α4β7 mAb we employed, or would other α4β7 antagonists also promote durable control? Contrary to vedolizumab, whose Fc component was altered and disabled for use in humans, the Rhesus mAb we employed retains Fc effector functions. Were those functions relevant to control? Lastly, will similar results be obtained in humans treated with an α4β7 antagonist, whether they start ART in acute or chronic HIV infection? These and other questions await further investigation.
Conclusion
HIV and SIV are frequently viewed as gut-tropic viruses, at least in the early stages of infection. This concept stems from observations made over 15 years ago that, soon after infection, high levels of viral replication occur in GALT and a large fraction of gut CD4+ T cells are consequently lost. It is now possible to refine this concept. Studies from human cohorts and nonhuman primates have shown, in a convincing way, that α4β7high memory CD4+ T cells are highly susceptible to infection. It is therefore more accurate to view HIV and SIV as viruses that exhibit a tropism for this subset of CD4+ T cells, and as a consequence of this cellular tropism viral replication is rapidly established in GALT. Understanding HIV in this way provides a potential target for both prevention of infection, and treatment after infection. Nonhuman primate studies support α4β7 as a target for both. To fully understand the role of α4β7 in pathogenesis and take full advantage of it as a therapeutic target, it is important to consider several points. First, α4β7 is a structurally dynamic receptor that mediates outside-in signaling to cells. Second, the HIV envelope protein gp120 binds to and signals through α4β7. Finally, the development of α4β7 antagonists for the treatment of IBD is well underway, with one drug already approved. To determine if and how these antagonists can be used in the treatment of HIV infection, it is critical that we increase our basic understanding of how α4β7 contributes to HIV pathogenesis.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on HIV Pathogenesis and Treatment
References
- 1.Nelson JA, Wiley CA, Reynolds-Kohler C, Reese CE, Margaretten W, Levy JA. Human immunodeficiency virus detected in bowel epithelium from patients with gastrointestinal symptoms. Lancet. 1988;1(8580):259–262. doi: 10.1016/S0140-6736(88)90348-0. [DOI] [PubMed] [Google Scholar]
- 2.Heise C, Miller CJ, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169(5):1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 3.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 4.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehandru S, Dandekar S. Role of the gastrointestinal tract in establishing infection in primates and humans. Curr Opin HIV AIDS. 2008;3(1):22–27. doi: 10.1097/COH.0b013e3282f331b0. [DOI] [PubMed] [Google Scholar]
- 6.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3(3):356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breed MW, Elser SE, Torben W, Jordan AP, Aye PP, Midkiff C, et al. Elite control, gut CD4 T cell sparing, and enhanced mucosal T cell responses in Macaca nemestrina infected by a simian immunodeficiency virus lacking a gp41 trafficking motif. J Virol. 2015;89(20):10156–10175. doi: 10.1128/JVI.01134-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett L, Fowke KR, Grant MD. Cytomegalovirus, aging, and HIV: a perfect storm. AIDS Rev. 2012;14(3):159–167. [PubMed] [Google Scholar]
- 11.Saison J, Cotte L, Chidiac C, Ferry T. Fatal cumulative toxicities of HAART in a stable, AIDS-free, HIV-infected patient. BMJ Case Rep. 2012;2012. [DOI] [PMC free article] [PubMed]
- 12.Warriner AH, Burkholder GA, Overton ET. HIV-related metabolic comorbidities in the current ART era. Infect Dis Clin N Am. 2014;28(3):457–476. doi: 10.1016/j.idc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3(12):e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs SJ, Ananworanich J. Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr Opin HIV AIDS. 2016;11(2):163–172. doi: 10.1097/COH.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 15.Sivro ASA, Sheward D, Joag V, Yegorov S, Liebenberg L, Balgobin A, Yende N, Garrett N, Samsunder N, Nawaz F, Cicala C, Arthos J, Fauci A, Anzala O, Kimani J, Bagaya B, Kiwanuka N, Williamson C, Kaul R, Passmore J, Phanuphak N, Ananworanich J, Ansari A, Karim Q, Karim S, McKinnon L. Integrin α4β7 expression on systemic CD4+ T cells predicts higher rates of HIV acquisition and disease progression. Sci Transl Med. 2018. [DOI] [PMC free article] [PubMed]
- 16.Szabo MC, Butcher EC, McEvoy LM. Specialization of mucosal follicular dendritic cells revealed by mucosal addressin-cell adhesion molecule-1 display. J Immunol. 1997;158(12):5584–5588. [PubMed] [Google Scholar]
- 17.Habtezion A, Nguyen LP, Hadeiba H, Butcher EC. Leukocyte trafficking to the small intestine and colon. Gastroenterology. 2016;150(2):340–354. doi: 10.1053/j.gastro.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6(12):1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 19.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 20.Shamri R, Grabovsky V, Feigelson SW, Dwir O, Van Kooyk Y, Alon R. Chemokine stimulation of lymphocyte alpha 4 integrin avidity but not of leukocyte function-associated antigen-1 avidity to endothelial ligands under shear flow requires cholesterol membrane rafts. J Biol Chem. 2002;277(42):40027–40035. doi: 10.1074/jbc.M206806200. [DOI] [PubMed] [Google Scholar]
- 21.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9(3):301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 22.Ansari AA, Byrareddy SN. The role of integrin expressing cells in modulating disease susceptibility and progression (January 2016) Int Trends Immun. 2016;4(1):11–27. [PMC free article] [PubMed] [Google Scholar]
- 23.Hussein HA, Walker LR, Abdel-Raouf UM, Desouky SA, Montasser AK, Akula SM. Beyond RGD: virus interactions with integrins. Arch Virol. 2015;160(11):2669–2681. doi: 10.1007/s00705-015-2579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, Kersh EN, McNicholl JM, Hanson D, Reimann KA, Brameier M, Walter L, Rogers K, Mayne AE, Dunbar P, Villinger T, Little D, Parslow TG, Santangelo PJ, Villinger F, Fauci AS, Ansari AA. Targeting alpha4beta7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med. 2014;20(12):1397–1400. doi: 10.1038/nm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham KL, Fleming FE, Halasz P, Hewish MJ, Nagesha HS, Holmes IH, et al. Rotaviruses interact with alpha4beta7 and alpha4beta1 integrins by binding the same integrin domains as natural ligands. J Gen Virol. 2005;86(Pt 12):3397–3408. doi: 10.1099/vir.0.81102-0. [DOI] [PubMed] [Google Scholar]
- 26.Lopez S, Arias CF. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol. 2004;12(6):271–278. doi: 10.1016/j.tim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Chand S, Messina EL, AlSalmi W, Ananthaswamy N, Gao G, Uritskiy G, Padilla-Sanchez V, Mahalingam M, Peachman KK, Robb ML, Rao M, Rao VB. Glycosylation and oligomeric state of envelope protein might influence HIV-1 virion capture by alpha4beta7 integrin. Virology. 2017;508:199–212. doi: 10.1016/j.virol.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, et al. The genotype of early-transmitting HIV gp120s promotes alpha (4) beta(7)-reactivity, revealing alpha (4) beta(7) +/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011;7(2):e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattentau Q. HIV's gut feeling. Nat Immunol. 2008;9(3):225–227. doi: 10.1038/ni0308-225. [DOI] [PubMed] [Google Scholar]
- 30.Rao M, Peachman KK, Kim J, Gao G, Alving CR, Michael NL, Rao V. HIV-1 variable loop 2 and its importance in HIV-1 infection and vaccine development. Curr HIV Res. 2013;11(5):427–438. doi: 10.2174/1570162X113116660064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson SI, Gray ES, Mkhize NN, Sheward DJ, Lambson BE, Wibmer CK, Masson L, Werner L, Garrett N, Passmore JAS, Karim QA, Karim SSA, Williamson C, Moore PL, Morris L. South African HIV-1 subtype C transmitted variants with a specific V2 motif show higher dependence on alpha4beta7 for replication. Retrovirology. 2015;12(1):54. doi: 10.1186/s12977-015-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tassaneetrithep B, Tivon D, Swetnam J, Karasavvas N, Michael NL, Kim JH, Marovich M, Cardozo T. Cryptic determinant of alpha4beta7 binding in the V2 loop of HIV-1 gp120. PLoS One. 2014;9(9):e108446. doi: 10.1371/journal.pone.0108446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newham P, Craig SE, Seddon GN, Schofield NR, Rees A, Edwards RM, Jones EY, Humphries MJ. Alpha4 integrin binding interfaces on VCAM-1 and MAdCAM-1. Integrin binding footprints identify accessory binding sites that play a role in integrin specificity. J Biol Chem. 1997;272(31):19429–19440. doi: 10.1074/jbc.272.31.19429. [DOI] [PubMed] [Google Scholar]
- 34.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303(5666):2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 35.Cicala C, Arthos J, Fauci AS. HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV. J Transl Med. 2011;9(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A. 2009;106(49):20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187(11):6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 38.McKinnon LR, Nyanga B, Kim CJ, Izulla P, Kwatampora J, Kimani M, et al. Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. J Acquir Immune Defic Syndr. 2015;68(1):6–12. doi: 10.1097/QAI.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 39.Martinelli E, Tharinger H, Frank I, Arthos J, Piatak M, Jr, Lifson JD, et al. HSV-2 infection of dendritic cells amplifies a highly susceptible HIV-1 cell target. PLoS Pathog. 2011;7(6):e1002109. doi: 10.1371/journal.ppat.1002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shannon B, Yi TJ, Thomas-Pavanel J, Chieza L, Janakiram P, Saunders M, Tharao W, Huibner S, Remis R, Rebbapragada A, Kaul R. Impact of asymptomatic herpes simplex virus type 2 infection on mucosal homing and immune cell subsets in the blood and female genital tract. J Immunol. 2014;192(11):5074–5082. doi: 10.4049/jimmunol.1302916. [DOI] [PubMed] [Google Scholar]
- 41.Kelley CF, Lai L, Ibegbu C, Rosenberg ES, Kaur S, Patel K, Mulligan MJ, Marconi VC, Sullivan PS, Amara RR. Differences in expression of gut-homing receptors on CD4+ T cells in black and white HIV-negative men who have sex with men. AIDS. 2016;30(8):1305–1308. doi: 10.1097/QAD.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hindryckx P, Vande Casteele N, Novak G, Khanna R, D'Haens G, Sandborn WJ, et al. The expanding therapeutic armamentarium for inflammatory bowel disease: how to choose the right drug(s) for our patients? J Crohns Colitis. 2017. [DOI] [PubMed]
- 43.Jovani M, Danese S. Vedolizumab for the treatment of IBD: a selective therapeutic approach targeting pathogenic a4b7 cells. Curr Drug Targets. 2013;14(12):1433–1443. doi: 10.2174/13894501113146660206. [DOI] [PubMed] [Google Scholar]
- 44.Vedolizumab. FDA Advisory Committee recommends approval of vedolizumab. http://www.drugs.com/nda/vedolizumab_131209html. 2013.
- 45.Lazarovits AI, Moscicki RA, Kurnick JT, Camerini D, Bhan AK, Baird LG, et al. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J Immunol. 1984;133(4):1857–1862. [PubMed] [Google Scholar]
- 46.Schweighoffer T, Tanaka Y, Tidswell M, Erle DJ, Horgan KJ, Luce GE, et al. Selective expression of integrin alpha 4 beta 7 on a subset of human CD4+ memory T cells with hallmarks of gut-trophism. J Immunol. 1993;151(2):717–729. [PubMed] [Google Scholar]
- 47.Yu Y, Zhu J, Mi LZ, Walz T, Sun H, Chen J, Springer TA. Structural specializations of alpha(4)beta(7), an integrin that mediates rolling adhesion. J Cell Biol. 2012;196(1):131–146. doi: 10.1083/jcb.201110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novak G, Hindryckx P, Khanna R, Jairath V, Feagan BG. The safety of vedolizumab for the treatment of ulcerative colitis. Expert Opin Drug Saf. 2017;16(4):501–507. doi: 10.1080/14740338.2017.1300251. [DOI] [PubMed] [Google Scholar]
- 49.Wyant T, Leach T, Sankoh S, Wang Y, Paolino J, Pasetti MF, Feagan BG, Parikh A. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64(1):77–83. doi: 10.1136/gutjnl-2014-307127. [DOI] [PubMed] [Google Scholar]
- 50.Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, Hopper J, Hora B, Kumar A, Mahlokozera T, Yuan S, Coleman C, Vermeulen M, Ding H, Ochsenbauer C, Tilton JC, Permar SR, Kappes JC, Betts MR, Busch MP, Gao F, Montefiori D, Haynes BF, Shaw GM, Hahn BH, Doms RW. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin alpha4beta7. PLoS Pathog. 2012;8(5):e1002686. doi: 10.1371/journal.ppat.1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, Wang X, Li J, Price AA, Little DM, Zaidi M, Lyles R, Villinger F. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186(2):1044–1059. doi: 10.4049/jimmunol.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pereira LE, Onlamoon N, Wang X, Wang R, Li J, Reimann KA, Villinger F, Pattanapanyasat K, Mori K, Ansari AA. Preliminary in vivo efficacy studies of a recombinant rhesus anti-alpha(4)beta(7) monoclonal antibody. Cell Immunol. 2009;259(2):165–176. doi: 10.1016/j.cellimm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15(3):173–183. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, Sidell N, Kane MA, Yu J, Jones JW, Santangelo PJ, Zurla C, McKinnon LR, Arnold KB, Woody CE, Walter L, Roos C, Noll A, van Ryk D, Jelicic K, Cimbro R, Gumber S, Reid MD, Adsay V, Amancha PK, Mayne AE, Parslow TG, Fauci AS, Ansari AA. Sustained virologic control in SIV+ macaques after antiretroviral and alpha4beta7 antibody therapy. Science. 2016;354(6309):197–202. doi: 10.1126/science.aag1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, Ponich T, Fox I, Feagan BG. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18(8):1470–1479. doi: 10.1002/ibd.21896. [DOI] [PubMed] [Google Scholar]
- 56.Raine T. Vedolizumab for inflammatory bowel disease: changing the game, or more of the same? United European Gastroenterol J. 2014;2(5):333–344. doi: 10.1177/2050640614550672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosario M, Wyant T, Leach T, Sankoh S, Scholz C, Parikh A, Fox I, Feagan BG. Vedolizumab pharmacokinetics, pharmacodynamics, safety, and tolerability following administration of a single, ascending, intravenous dose to healthy volunteers. Clin Drug Investig. 2016;36(11):913–923. doi: 10.1007/s40261-016-0437-4. [DOI] [PubMed] [Google Scholar]
- 58.Santangelo PCC, Byrareddy S, Ortiz K, Little D, Lindsay K, Gumber S, Hong J, Jelicic K, Zurla C, Villinger F, Ansari A, Fauci A, Arthos J. Early treatment of SIV+ macaques with an α4β7 mAb alters virus distribution and preserves CD4+ T cells in later stages of infection. Mucosal Immunol. 2018. [DOI] [PMC free article] [PubMed]
- 59.Bowcutt R, Forman R, Glymenaki M, Carding SR, Else KJ, Cruickshank SM. Heterogeneity across the murine small and large intestine. World J Gastroenterol. 2014;20(41):15216–15232. doi: 10.3748/wjg.v20.i41.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dzutsev A, Hogg A, Sui Y, Solaymani-Mohammadi S, Yu H, Frey B, et al. Differential T cell homing to colon vs. small intestine is imprinted by local CD11c+ APCs that determine homing receptors. J Leukoc Biol. 2017. [DOI] [PMC free article] [PubMed]
- 61.Guzzo C, Ichikawa D, Park C, Phillips D, Liu Q, Zhang P, et al. Virion incorporation of integrin alpha4beta7 facilitates HIV-1 infection and intestinal homing. Sci Immunol. 2017;2(11). [DOI] [PMC free article] [PubMed]
- 62.Kelly KA, Natarajan S, Ruther P, Wisse A, Chang MH, Ault KA. Chlamydia trachomatis infection induces mucosal addressin cell adhesion molecule-1 and vascular cell adhesion molecule-1, providing an immunologic link between the fallopian tube and other mucosal tissues. J Infect Dis. 2001;184(7):885–891. doi: 10.1086/323341. [DOI] [PubMed] [Google Scholar]
- 63.Kelly KA, Rank RG. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65(12):5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly KA, Wiley D, Wiesmeier E, Briskin M, Butch A, Darville T. The combination of the gastrointestinal integrin (alpha4beta7) and selectin ligand enhances T-cell migration to the reproductive tract during infection with chlamydia trachomatis. Am J Reprod Immunol. 2009;61(6):446–452. doi: 10.1111/j.1600-0897.2009.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinelli E, Veglia F, Goode D, Guerra-Perez N, Aravantinou M, Arthos J, Piatak M, Jr, Lifson JD, Blanchard J, Gettie A, Robbiani M. The frequency of alpha(4)beta(7)(high) memory CD4(+) T cells correlates with susceptibility to rectal simian immunodeficiency virus infection. J Acquir Immune Defic Syndr. 2013;64(4):325–331. doi: 10.1097/QAI.0b013e31829f6e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65(4):651–662. doi: 10.1016/0092-8674(91)90097-I. [DOI] [PubMed] [Google Scholar]
- 67.Mudd JC, Brenchley JM. Gut mucosal barrier dysfunction, microbial dysbiosis, and their role in HIV-1 disease progression. J Infect Dis. 2016;214(Suppl 2):S58–S66. doi: 10.1093/infdis/jiw258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kloverpris HN, Kazer SW, Mjosberg J, Mabuka JM, Wellmann A, Ndhlovu Z, et al. Innate lymphoid cells are depleted irreversibly during acute HIV-1 infection in the absence of viral suppression. Immunity. 2016;44(2):391–405. doi: 10.1016/j.immuni.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mudd JC, Brenchley JM. ILC you later: early and irreparable loss of innate lymphocytes in HIV infection. Immunity. 2016;44(2):216–218. doi: 10.1016/j.immuni.2016.01.022. [DOI] [PubMed] [Google Scholar]