Abstract
Purpose of Review
This review will outline the multilevel effects of biological sex on HIV acquisition, pathogenesis, treatment response, and prospects for cure. Potential mechanisms will be discussed along with future research directions.
Recent Findings
HIV acquisition risk is modified by sex hormones and the vaginal microbiome, with the latter acting through both inflammation and local metabolism of pre-exposure prophylaxis drugs. Female sex associates with enhanced risk for non-AIDS morbidities including cardiovascular and cerebrovascular disease, suggesting different inflammatory profiles in men and women. Data from research on HIV cure points to sex differences in viral reservoir dynamics and a direct role for sex hormones in latency maintenance.
Summary
Biological sex remains an important variable in determining the risk of HIV infection and subsequent viral pathogenesis, and emerging data suggest sex differences relevant to curative interventions. Recruitment of women in HIV clinical research is a pathway to both optimize care for women and to identify novel therapeutics for use in both men and women.
Keywords: HIV, Sex, Inflammation, Prevention, Pathogenesis, Cure
Introduction
A combination of environmental factors, host genetics, and viral features determines the acquisition and pathogenesis of HIV infection. Some of these features, such as host HLA genotype, have been delineated, but the diversity of clinical manifestations of HIV suggests multiple sources of variation that are, as yet, undefined. Biological sex, with a distinct genetic complement, hormonal environment, and behavioral and social context, is a substantial contributor to heterogeneity in host responses. Research defining sex differences serves a dual purpose: first, defining sex-specific responses will insure that interventions have efficacy in both men and women, and second, differences may highlight pathways that can be modulated in both sexes to optimize treatment and prevention and curative interventions.
Clinical studies to isolate the effects of biological sex are challenging, but work to date has yielded important insights. This review will address sex-specific features of HIV prevention, pathogenesis, and cure research, and then outline potential biological mechanisms for these differences. Finally, barriers to research on sex differences and to enrolling women in clinical trials are discussed, along with the opportunities to circumvent these obstacles.
Prevention
Sex-Specific Acquisition Risks
The risk of HIV seroconversion per heterosexual act is estimated to be approximately twofold higher for the female compared to male partner [1], with multiple contributing factors. The unique characteristics of the female genital tract as compared with rectal and penile mucosal surfaces confer differences in transmission risk. Inflammation at the cervicovaginal mucosa lowers the barrier to HIV infection [2–5], and both the vaginal microbiome itself [6] and sexually transmitted infections [7–11] are important determinants of the levels of local inflammation. The association of depot medroxyprogesterone (DMPA) hormonal contraception with enhanced risk of infection (hazard ratio of 1.4) [12–14] underlines the sex-specific risk associated with hormone exposure, which also impacts the vaginal microbiome. Clearly, these factors have distinct manifestations in the male and female genital tracts and these basic differences have important implications for prevention interventions discussed below.
Vaccine Responses
Sex differences in both adverse effects and the efficacy of protective responses to vaccination are well described [15]. These differences are of clinical significance as seen in the higher rates of vaccine-associated severe viscerotropic yellow fever disease in women [16, 17] and the HSV glycoprotein vaccine that was protective only in women [18]. The mechanisms driving these differences are not totally clear; no specific immunologic correlate was reported for the sex differences in the HSV vaccine trial [18] although subsequent work suggested that specific epitopes may be preferentially recognized in women [19]. Systems biology analysis of gene expression profiles after yellow fever vaccine identified sex-specific programs of gene induction [20], highlighting the potential for studies of sex differences to identify correlates of successful protection. In HIV vaccine trials, there has not been clear evidence of sex differential effects. In the RV144 study, protective efficacy was estimated 25.8% in men (n = 4875) and 38.6% in women (n = 3085), with no statistical difference associated with sex [21]. In terms of immune correlates of protection, differences in humoral and cell-mediated immune responses have been seen in multiple vaccines [20]. Mechanistically, there is evidence for more potent induction of inflammatory pathways in cytotoxic T cells from women [22]; sex comparison of the magnitude and breadth of T cell responses induced by vaccines would be of interest. Likewise, there is data to suggest that somatic hypermutation is enhanced by estrogen [23] and that antibody glycosylation patterns are influenced by sex [24] suggesting that biological sex may influence both antibody affinity and non-neutralizing functions.
Moving forward, sex-specific analyses of both efficacy and immune correlates of protection should be leveraged to enhance responses. For example, sex-specific induction of type 1 interferons or the inflammasome might indicate a role for specific adjuvanting strategies in men versus women. Given the challenges of vaccine development, all avenues for optimization bear consideration.
Pre-Exposure Prophylaxis
In the absence of an effective vaccine, pharmacologic strategies have become a critical adjunct to the prevention of transmission. Notably, despite initial studies showing high levels of efficacy for PrEP in men who have sex with men [25] and in serodiscordant couples [26], studies of PrEP exclusively in women showed no efficacy, results that were attributed to very low adherence to study drug [27, 28]. Clinical pharmacology studies have highlighted differences in drug concentration at the rectal mucosal and cervicovaginal tissues [29] that may obligate different levels of adherence in women versus men to maximize effectiveness. To circumvent this, topical delivery designed for the vaginal microenvironment is another potential route to modulate risk of infection in women; the CAPRISA 004 study reported a 39% risk reduction with tenofovir gel [30], although the VOICE study, which was limited by low adherence, did not show efficacy in the vaginal gel arm [27]. The topical approach using a vaginal ring preparation of the novel antiretroviral dapivirine has recently demonstrated a significant but modest reduction in the risk of HIV acquisition (27–31%) [31, 32]. Importantly, recent work has shown that adherence is not the only challenge to the topical approach. Local metabolism of tenofovir itself by components of the vaginal microbiome is associated with reduced efficacy of protection [33]. As studies defining the effects of topical exposure at the rectal mucosa have suggested that tenofovir may increase certain inflammatory mediators [34], specific assessment of the in vivo cervicovaginal effects is also warranted. Further studies are necessary to define the optimal approach to risk reduction in both men and women; advantages of topical preparations must be considered in light of adherence challenges, and careful studies are necessary to fully define sex-specific modulators of efficacy at the sites of acquisition. Taken together, the data suggest that there are sex-specific features of risk perception and medication adherence, along with critical differences in pharmacologic properties and the microenvironment at sites of acquisition in men and women. Considering these differences will be critical in the design and implementation of chemoprophylaxis strategies.
Pathogenesis
Disease Progression
Sex is a clear contributor to disease pathogenesis in multiple infectious diseases [35], and HIV follows this paradigm. Across most studies, women have lower HIV viral loads early during infection but despite this difference, disease progression is comparable between the sexes [36–46]. Substantial differences in immune activation may underlie this apparent paradox; women have higher CD8+ T cell activation at a given level of HIV viremia, corresponding to activation seen in men at one log10 higher viral load [47]. Similarly, the expression of interferon-stimulated genes was higher in women when controlling for HIV viral load [48]. Given the role of immune activation in driving HIV disease progression [49, 50] and in comorbidities that emerge during effective ART [51, 52], the sex difference in immune setpoint likely has clinical consequences.
In selected individuals, HIV disease progression is attenuated, with either spontaneous control of viral replication in the absence of drug therapy [53–55] or sustained viral suppression after interruption of ART (post-treatment controllers; PTCs) [56]. The factors that allow natural control of HIV are not fully defined but include host genetics, highly efficient immune responses, and in select cases, viral fitness [53–55]. Cohort studies have reported that women are more likely to be categorized as spontaneous controllers of HIV [57, 58] although the determinants of this advantage have not been elucidated. Similarly, women are overrepresented in cohorts of post-treatment control: women were 36% of PTCs, 43% of low viremia patients (viral load between 50 and 500), and only 14% of post-treatment non-controllers in one study [59]. Again, sex-specific mechanisms of protection have not been defined within this group, and it should be noted that the total numbers evaluated are very low. Thus, although limited by biases in case finding, women more frequently demonstrate phenotypes of viral control. This suggests that identifying sex determinants of immune response and viral setpoint may shed light onto features of a successful host response.
Response to Treatment
Consistent with sex differences in pharmacokinetics/pharmacodynamics, drug metabolism, body composition, and drug distribution, the rates of adverse reactions with the early generation of antiretroviral therapies showed sex variation [60, 61]. Efforts to analyze these differences are hampered by the limited enrollment of women in trials of new therapeutics [62]. In response to this challenge, the GRACE (Gender, Race And Clinical Experience) trial specifically enrolled women to determine the sex-specific efficacy of a darunavir-based ART regimen [63] and yielded critical insights into the barriers to participation by women (discussed further below) [64]. Recent subgroup analyses of therapeutic trials have largely demonstrated similar efficacy in men and women, consistent with the improved therapeutic index of modern antiretrovirals [65–67]. However, unanticipated effects of antiretrovirals, such as the recently reported weight gain associated with integrase inhibitor regimens in a predominantly male cohort (14% women in integrase inhibitor subgroup) [68], should be carefully evaluated for sex effects. In addition, the response to treatment as measured by CD4+ T cell recovery has been reported to favor women, although with unclear implications for immune competence [69]. Complications of immune reconstitution such as the immune reconstitution inflammatory syndrome (IRIS) have not been reported to have a particular sex predilection. However, this is difficult to clearly establish given the heterogeneity in case definitions of IRIS, bias for women to be enrolled in resource-limited settings, and lack of disaggregation of data by sex in some larger studies.
Treatment-induced changes in biomarkers of inflammation also show discordance; in one cohort, women had higher baseline high-sensitivity C reactive protein (hsCRP) levels and less change with therapy, along with higher levels of soluble CD163, a marker of monocyte activation [70]. Other cohorts have reported similar differences in response to treatment, although inconsistent differences in baseline levels [71]. Further work will be necessary to dissect the direct contribution of HIV and ART as compared with concurrent inflammatory stimulators such as coinfections and smoking, and modulators such as sex hormones given the potential for direct effects of estrogen on some markers such as CRP [72]. Overall, women and men can both achieve viral suppression with ART but differences in residual immune activation and reconstitution may remain, with consequences for comorbid conditions.
Non-AIDS Morbidity and Mortality
With the advent of effective ART, morbidity and mortality among people living with HIV has shifted to non-AIDS events including cardiovascular disease, cancer, and neurocognitive dysfunction, many of which are driven by inflammatory consequences of HIV infection. Biological sex is one contributor to the multifactorial determinants of these comorbidities [52]. The excess risk of cardiovascular events in people living with HIV [73] is amplified in women [74] and linked to higher levels of circulating markers of monocyte activation [75]. Likewise, the increased risk of cerebrovascular events in HIV-infected individuals [76, 77] is exaggerated in women [78]. Of note, the epidemiology of these comorbid conditions varies significantly across different social and geographic contexts obligating thoughtful design of trials to assess for the contribution of sex [79]. The differences in risk profile between men and women highlight the potential for studies of sex differences to identify causal pathways or biomarkers of disease pathogenesis.
HIV Eradication and Functional Cure
The goal of HIV eradication or functional cure has become a focal point for HIV research. It is not known whether sex differences in viral and inflammatory set points in untreated infection translate into differences in ART-treated disease that have implications for HIV cure efforts. As women bear half the burden of the HIV epidemic, any intervention that will have a meaningful impact will need to be effective for both men and women. Importantly, several of the interventions in development for HIV cure are immunomodulatory [80]; this is an important divergence from the direct antiviral agents used in suppressive ART. Subtle immunologic differences between men and women may play a critical role in determining the safety and efficacy of curative interventions.
There are limited data defining sex differences in viral reservoir size and dynamics. Two cross-sectional studies with approximately 30% enrollment of women reported lower levels of HIV DNA in women [81, 82]. However, data from a prospectively enrolled cohort of ART-treated men and women did not show any significant difference in HIV DNA levels, but rather showed lower levels of residual viremia by single copy assay and lower levels of multiply-spliced cell associated HIV RNA from women (Scully et al., Abstract 281, CROI 2017). In general, conclusions are limited by the underrepresentation of women in studies relevant to cure [83]. Specifically, in seminal work comparing different methods of reservoir quantitation, there were no XX participants and only 2 of 30 are identified as transgender (male to female) without data about exogenous hormone exposure [84]. In studies assessing the role of HIV DNA in predicting viral rebound, cohorts have been 82–100% male [85–87]. Of participants in trials of the histone deacetylase (HDAC) inhibitor class of latency reversal agents, only 2 of 50 participants were women [88–91]. As mentioned above, curative interventions such as TLR agonists and exhaustion reversal with immune checkpoint inhibitors are primarily targeting host and not viral factors. Both the TLR7 agonist pathway [47] and the immune checkpoint inhibitor pathways [92, 93] have shown sex-specificity in other contexts that should be considered carefully in the development of clinical trials.
Potential Mechanisms
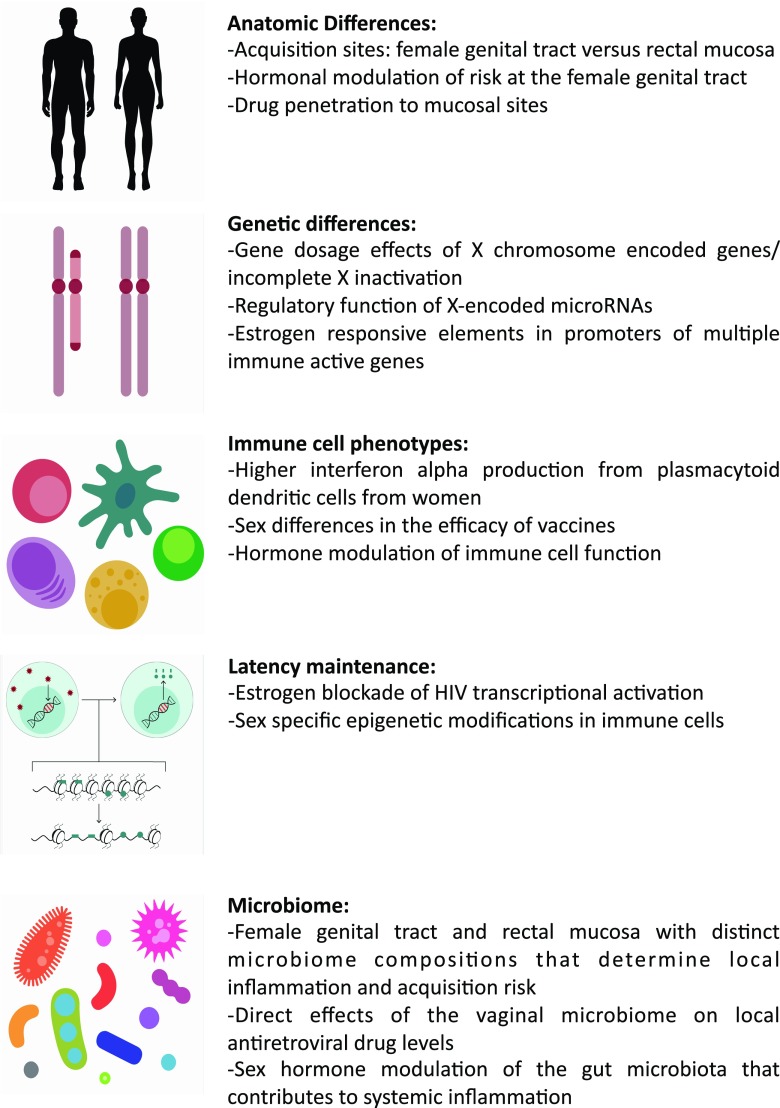
Outlined above are multiple features of HIV acquisition, prevention, pathogenesis, and persistence that show sex variation. Behavioral and social characteristics differ between men and women, and these factors play an important role in sexual agency, reproductive health, and access to education and medical care. Indeed, sex-specific behaviors around adherence to interventions proved to be critical modifiers of the efficacy of PrEP [94]. Aside from these factors, there are a few domains of biological sex-specificity that are likely contributing to differences and can be exploited to therapeutic benefit (Fig. 1).
Fig. 1.
Summary of five critical domains of sex differences with relevance for HIV infection and potential or demonstrated mechanisms for their effects
Sex Hormone Effects
As noted above, there is an association with DMPA contraceptive use and enhanced rates of infection. The precise mechanisms are unclear, as the progestin-associated thinning of the vaginal mucosal observed in non-human primate models [95–97] has not been seen in women [98–102]. Recent data identified endogenous and exogenously administered progesterone-induced variations in the frequency of cervical HIV-susceptible target cells [103]. There are additional intersections between sex hormone levels and inflammation induced by microbiome composition and concurrent infections [104]. Given the global need for effective family planning methods and widespread use of hormonal contraception, determining the mechanisms of hormonal contribution to risk of infection and potential pathways for modification is of critical importance.
Outside of acquisition, estrogen is also a direct modifier of HIV transcription. Previous work has demonstrated that the estrogen receptor can be indirectly recruited to the HIV-1 long terminal repeat (LTR) and act to repress transcriptional activity [105]. More recently, using an unbiased small hairpin RNA screening strategy, the estrogen receptor was identified as a potent inhibitor of HIV transcription in latency models and primary cells (Karn et al., IAS, 2015; Das et al., submitted). Ex vivo studies using primary cells from both men and women confirmed that estrogen is repressive to latency reversal, and that blockade of the estrogen receptor can enhance reactivation (Karn et al., IAS 2015; Das et al., submitted).
Sex hormones have also been reported to have a variety of direct effects on immune cell function. Both estrogen and progesterone have been reported to modulate plasmacytoid dendritic cell IFNα secretion [47, 106–109]. Cytotoxic T cells from women have higher expression of inflammatory/cytotoxic pathways after ex vivo restimulation, and multiple genes in these pathways have estrogen responsive elements in their promoters [22]. Of note, the presence of estrogenic compounds in standard cell culture media components [110, 111] and the difficulty in replicating the in vivo balance of hormones with in vitro studies obligates careful interpretation of these studies. However, hormonal pathways can be safely modulated in vivo and offer a potential adjunctive therapeutic pathway that may be of use in studies of HIV cure.
Microbiome
Sex-specificity of the microbiome composition in the genital tracts is one determinant of the local immune environment. Further, recent work identified novel features of this relationship, with specific microbiome components associated with alterations in wound healing [112] and direct microbial metabolism of tenofovir associated with reduced efficacy of PrEP in the female genital tract [33]. Aside from this direct role, animal studies have demonstrated that sex hormones impact microbiome composition in the gut, with implications for sex-specific susceptibility to autoimmunity [113, 114]. Studies have confirmed sex variation in gut microbial contents in humans [115–117] and further work will be necessary to determine if these differences have consequences for inflammation in HIV disease. Interventions to reshape the microbiome (e.g., with probiotics) may offer therapeutic benefits.
Genetic Differences
The sex-specific chromosomal complement is an additional pathway to biological differences. The X chromosome carries critical immune genes including TLR7, which encodes a pathogen sensor, FOXP3, a transcription factor critical for regulatory immune responses, and 10% of all microRNAs which have pleiotropic regulatory roles [118].
As some sex differences including lower viral loads in females are present prior to the onset of puberty, non-hormonal mechanisms including genetics are likely to play a role [119]. Gene dosage effects are attenuated by X chromosome inactivation, but the enhanced risk of female predominant diseases such as systemic lupus erythematosus in phenotypic males with XXY karyotype suggests that this is incomplete [120]. Growing evidence demonstrates that up to 20% of X chromosome genes escape inactivation [121]; this has clinical implications, with recent work suggesting that these genes may determine a portion of sex-specific susceptibility to cancer [122]. The role of sex chromosome-encoded genes in differential vaccine responses, HIV pathogenesis, and cure efforts is undefined; it is notable that the HIV controllers genome-wide association study to assess for genetic determinants of spontaneous control was restricted to autosomes [123]. Studies to identify polymorphisms in sex chromosomal genes should be pursued.
Of note, research has also demonstrated sex-specific transcriptional programs related to both chromosomal determinants and ongoing hormonal programming [124]. Analysis of the methylation patterns and transcriptome of immune cell subsets identifies differences between men and women, supporting a potential role for epigenetic regulation in sex differences in immune responses [125]. Given the potential use of epigenetic modifiers in latency reversal, sex-specific patterns of epigenetic regulation should be explored.
Immunological Differences
The combined effects of sex hormones, microbiome, and chromosomal complement contribute to distinct immune profiles. Preliminary work suggests that the relationship between residual virus activity and T cell activation and exhaustion phenotypes may be different between men and women, with men showing more activation and exhaustion and more correlations with measures of viral reservoir (Scully et al., Abstract 281,CROI 2017). Previous work has also demonstrated sex differences, partially mediated by estrogen, in antibody features including subclass, levels of hypermutation, and Fc glycan modifications [23, 24]. Sex-stratified comparisons of the humoral responses to vaccines may provide insight into the critical features of a successful response.
Also notable is the role of sex hormones in lipid metabolism that is in turn linked to innate cellular activation and risk of non-AIDS morbidity and mortality in HIV infection [126, 127]. Of note, recent data suggests that there may be sex-specific responses to lipid-lowering therapy, with women showing qualitatively greater reductions in sCD163 after treatment with pitavastatin [128]. In studies of soluble markers of inflammation, sex differences in baseline levels and in the changes after ART initiation have been reported; neopterin (marker of cellular activation associated with HIV-related neurocognitive disease) was higher in women with impaired cognition, a finding not observed in men alone, and TNF-RII was similarly elevated in cognitively impaired women but not in men [129]. In a heterogenous cohort of men and women from multiple global sites, women were reported to have lower baseline levels of CRP, lipopolysaccharide, and soluble CD14 (sCD14) but less decrease in CRP and sCD14 and more increase in TNFα after ART [71]. In contrast, in a more homogenous cohort comparison, women had lower CRP than men did at baseline but again showed limited change after initiation of ART [70]. In total, the data are far from definitive and the multiple determinants of inflammatory outcomes including coinfections, microbiome differences, sex hormones, and immune setpoints will need to be carefully parsed to guide interventions. What is clear is that sex is a modifier of immune responses and may also dictate which biomarkers are predictive of risk for a particular population.
Gaps in Knowledge and Opportunities
Historically, there has been limited enrollment of women in clinical trials of HIV therapy in the developed world, a problem that has extended to the field of cure research [62, 83, 130]. Given the multiple lines of evidence for sex-based differences in immune responses [131], HIV disease pathogenesis [132], and pharmacokinetics/pharmacodynamics [133], it is imperative that biological sex is considered in the development and implementation of new clinical interventions; successful innovations will need to have efficacy in both men and women. Further, as discussed above, sex differences offer a comparator point that may elucidate pathways critical for robust immune responses or curative strategies that can be leveraged to therapeutic success in both sexes.
Although not the focus of this review, the intersection between genetic complement and sex hormone exposure is particularly highlighted in transgender individuals. Given the burden of HIV in transgender individuals [134] and growing evidence for the feasibility of high-quality studies in this population [135], HIV cure research needs to include transgender participants. Thoughtful comparative analysis may point to mechanistic links between the genetic complement and hormonal exposure and virologic and immunologic outcomes and will be critical to verify the safety and efficacy of proposed interventions.
Given the importance of analyzing the role of sex [136], what are the barriers to implementation? From the perspective of the investigator, the cyclic variation in hormone levels and/or exogenous hormone administration and potential for pregnancy introduce variables and safety concerns that can require larger sample sizes and more intensive monitoring of interventions. These concerns notwithstanding, the global burden of HIV infection in women and the population of women and girls at risk obligates that research specifically address the optimal treatment, prevention, and curative interventions for women [137]. From the view of the potential study participants, engagement with research, education about risks and benefits, and addressing logistical challenges to enrollment are all feasible [138]. Prior work has established that women can be successfully recruited and retained in HIV research [139, 140], and these experiences should be used to guide recruitment efforts. In addition, exploratory basic and clinical studies should report data by sex; while not always sufficient for a powered analysis, this data can be helpful in aggregate to determine when sex differences bear more focused investigation.
Conclusion
Sex differences in HIV arise from the combinatorial effects of sex hormones, genetic differences, and sociobehavioral and environmental influences. These differences are clinically relevant, translating into enhanced risk for acquisition and non-AIDS morbidity in women, but also potentially for more efficacious immune responses to vaccination. The role of sex differences in cure interventions remains to be defined. Robust sex comparisons must be carefully controlled as enrollment of women tends to be preferentially in resource-limited settings introducing potentially confounding genetic and environmental differences when compared to predominantly male cohorts from the developed world. Despite these challenges, focused investigation of sex differences has uncovered important features of disease, highlighting pathogenic inflammatory pathways. The direct role of sex hormones in modulating immune subset distribution and HIV transcription exemplifies how this research can lead to therapeutic interventions with hormone receptor antagonists or specific selection of contraceptive preparations. Likewise, highlighting the immune pathways that differ between men and women may indicate mechanisms to optimize treatment responses with adjuvant or immunomodulatory interventions that target these pathways in the “weaker” sex, whichever that may be.
Acknowledgments
The author would like to thank Avery Normandin for expert assistance in figure design.
Funding Information
Dr. Scully is supported by K08AI116344.
Compliance with Ethical Standards
Conflict of Interest
The author declares that she has no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on HIV Pathogenesis and Treatment
References
- 1.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28(10):1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, Abou M, Westmacott GR, McCorrister S, Kwatampora J, Nyanga B, Kimani J, Masson L, Liebenberg LJ, Abdool Karim SS, Passmore JAS, Lauffenburger DA, Kaul R, McKinnon LR. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2016;9(1):194–205. doi: 10.1038/mi.2015.51. [DOI] [PubMed] [Google Scholar]
- 3.Masson L, Passmore JA, Liebenberg LJ, Werner L, Baxter C, Arnold KB, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis. 2015;61(2):260–269. doi: 10.1093/cid/civ298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naranbhai V, Abdool Karim SS, Altfeld M, Samsunder N, Durgiah R, Sibeko S, Abdool Karim Q, Carr WH, CAPRISA004 TRAPS team Innate immune activation enhances hiv acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis. 2012;206(7):993–1001. doi: 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selhorst P, Masson L, Ismail SD, Samsunder N, Garrett N, Mansoor LE, Abdool Karim Q, Abdool Karim SS, Passmore JAS, Williamson C. Cervicovaginal inflammation facilitates acquisition of less infectious HIV variants. Clin Infect Dis. 2016;64:79–82. doi: 10.1093/cid/ciw663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, Ghebremichael MS, Nusbaum C, Huttenhower C, Virgin HW, Ndung’u T, Dong KL, Walker BD, Fichorova RN, Kwon DS. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JM, Wald A, Hubbard A, Rungruengthanakit K, Chipato T, Rugpao S, Mmiro F, Celentano DD, Salata RS, Morrison CS, Richardson BA, Padian NS. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21(12):1515–1523. doi: 10.1097/QAD.0b013e3282004929. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 9.Masson L, Arnold KB, Little F, Mlisana K, Lewis DA, Mkhize N, Gamieldien H, Ngcapu S, Johnson L, Lauffenburger DA, Abdool Karim Q, Abdool Karim SS, Passmore JAS. Inflammatory cytokine biomarkers to identify women with asymptomatic sexually transmitted infections and bacterial vaginosis who are at high risk of HIV infection. Sex Transm Infect. 2016;92(3):186–193. doi: 10.1136/sextrans-2015-052072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, Gamieldien H, Williamson C, Mckinnon LR, Walzl G, Abdool Karim Q, Abdool Karim SS, Passmore JAS. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect. 2014;90(8):580–587. doi: 10.1136/sextrans-2014-051601. [DOI] [PubMed] [Google Scholar]
- 11.van de Wijgert JH, Morrison CS, Brown J, Kwok C, Van Der Pol B, Chipato T, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African women. Sex Transm Dis. 2009;36(6):357–364. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 12.Morrison CS, Chen PL, Kwok C, Baeten JM, Brown J, Crook AM, van Damme L, Delany-Moretlwe S, Francis SC, Friedland BA, Hayes RJ, Heffron R, Kapiga S, Karim QA, Karpoff S, Kaul R, McClelland RS, McCormack S, McGrath N, Myer L, Rees H, van der Straten A, Watson-Jones D, van de Wijgert JHHM, Stalter R, Low N. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med. 2015;12(1):e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polis CB, Curtis KM, Hannaford PC, Phillips SJ, Chipato T, Kiarie JN, Westreich DJ, Steyn PS. Update on hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence, 2016. AIDS. 2016;30:2665–2683. doi: 10.1097/QAD.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women’s risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15(2):181–189. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26(29–30):3551–3555. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 16.Seligman SJ. Yellow fever virus vaccine-associated deaths in young women. Emerg Infect Dis. 2011;17(10):1891–1893. doi: 10.3201/eid1710.101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seligman SJ. Risk groups for yellow fever vaccine-associated viscerotropic disease (YEL-AVD) Vaccine. 2014;32(44):5769–5775. doi: 10.1016/j.vaccine.2014.08.051. [DOI] [PubMed] [Google Scholar]
- 18.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G, GlaxoSmithKline Herpes Vaccine Efficacy Study Group Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol. 2008;15(9):1436–1449. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil J, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. New Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 22.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10(5):509–516. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9(1):56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Wang Y, Qiu L, Qin X, Liu H, Wang X, Wang Y, Song G, Li F, Guo Y, Li F, Guo S, Li Z. Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J Proteome. 2012;75(10):2824–2834. doi: 10.1016/j.jprot.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell J, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV, iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C, Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Mâsse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan I, Chirenje ZM, VOICE Study Team Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrix CW. The clinical pharmacology of antiretrovirals for HIV prevention. Curr Opin HIV AIDS. 2012;7(6):498–504. doi: 10.1097/COH.0b013e32835847ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdool Karim Q, Sibeko S, Baxter C. Preventing HIV infection in women: a global health imperative. Clin Infect Dis. 2010;50(Suppl 3):S122–S129. doi: 10.1086/651483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier S, MTN-020–ASPIRE Study Team Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, Kamali A, Kotze P, Louw C, Mabude Z, Miti N, Kusemererwa S, Tempelman H, Carstens H, Devlin B, Isaacs M, Malherbe M, Mans W, Nuttall J, Russell M, Ntshele S, Smit M, Solai L, Spence P, Steytler J, Windle K, Borremans M, Resseler S, van Roey J, Parys W, Vangeneugden T, van Baelen B, Rosenberg Z, Ring Study Team Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133–2143. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 33.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356(6341):938–945. doi: 10.1126/science.aai9383. [DOI] [PubMed] [Google Scholar]
- 34.Hladik F, Burgener A, Ballweber L, Gottardo R, Vojtech L, Fourati S, et al. Mucosal effects of tenofovir 1% gel. elife. 2015;4 10.7554/eLife.04525. [DOI] [PMC free article] [PubMed]
- 35.vom Steeg LG, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016;12(2):e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anastos K, Gange SJ, Lau B, Weiser B, Detels R, Giorgi JV, Margolick JB, Cohen M, Phair J, Melnick S, Rinaldo CR, Kovacs A, Levine A, Landesman S, Young M, Muñoz A, Greenblatt RM. Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr. 2000;24(3):218–226. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Bush CE, Donovan RM, Markowitz N, Baxa D, Kvale P, Saravolatz LD. Gender is not a factor in serum human immunodeficiency virus type 1 RNA levels in patients with viremia. J Clin Microbiol. 1996;34(4):970–972. doi: 10.1128/jcm.34.4.970-972.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans JS, Nims T, Cooley J, Bradley W, Jagodzinski L, Zhou S, Melcher GP, Burke DS, Vahey M. Serum levels of virus burden in early-stage human immunodeficiency virus type 1 disease in women. J Infect Dis. 1997;175(4):795–800. doi: 10.1086/513973. [DOI] [PubMed] [Google Scholar]
- 39.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, Quinn TC, Vlahov D. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352(9139):1510–1514. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35(3):313–322. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 41.Katzenstein DA, Hammer SM, Hughes MD, Gundacker H, Jackson JB, Fiscus S, Rasheed S, Elbeik T, Reichman R, Japour A, Merigan TC, Hirsch MS. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Group study 175 virology study team. N Engl J Med. 1996;335(15):1091–1098. doi: 10.1056/NEJM199610103351502. [DOI] [PubMed] [Google Scholar]
- 42.Lyles CM, Dorrucci M, Vlahov D, Pezzotti P, Angarano G, Sinicco A, Alberici F, Alcorn TM, Vella S, Rezza G. Longitudinal human immunodeficiency virus type 1 load in the italian seroconversion study: correlates and temporal trends of virus load. J Infect Dis. 1999;180(4):1018–1024. doi: 10.1086/314980. [DOI] [PubMed] [Google Scholar]
- 43.Moore RD, Cheever L, Keruly JC, Chaisson RE. Lack of sex difference in CD4 to HIV-1 RNA viral load ratio. Lancet. 1999;353(9151):463–464. doi: 10.1016/S0140-6736(98)05379-3. [DOI] [PubMed] [Google Scholar]
- 44.Napravnik S, Poole C, Thomas JC, Eron JJ., Jr Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr. 2002;31(1):11–19. doi: 10.1097/00126334-200209010-00002. [DOI] [PubMed] [Google Scholar]
- 45.Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180(3):666–672. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 46.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344(10):720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 47.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. Sex differences in the toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15(8):955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang JJ, Woods M, Lindsay RJ, Doyle EH, Griesbeck M, Chan ES, Robbins GK, Bosch RJ, Altfeld M. Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. J Infect Dis. 2013;208(5):830–838. doi: 10.1093/infdis/jit262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 50.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 51.Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis. 2016;214(Suppl 2):S44–S50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raghavan A, Rimmelin DE, Fitch KV, Zanni MV. Sex differences in select non-communicable HIV-associated comorbidities: exploring the role of systemic immune activation/inflammation. Curr HIV/AIDS Rep. 2017;14:220–228. doi: 10.1007/s11904-017-0366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blankson JN. Control of HIV-1 replication in elite suppressors. Discov Med. 2010;9(46):261–266. [PubMed] [Google Scholar]
- 54.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27(3):406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Saag M, Deeks SG. How do HIV elite controllers do what they do? Clin Infect Dis. 2010;51(2):239–241. doi: 10.1086/653678. [DOI] [PubMed] [Google Scholar]
- 56.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLoS Pathog. 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crowell TA, Gebo KA, Blankson JN, Korthuis PT, Yehia BR, Rutstein RM, Moore RD, Sharp V, Nijhawan AE, Mathews WC, Hanau LH, Corales RB, Beil R, Somboonwit C, Edelstein H, Allen SL, Berry SA, HIV Research Network Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis. 2015;211(11):1692–1702. doi: 10.1093/infdis/jiu809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madec Y, Boufassa F, Porter K, Meyer L, Collaboration C Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS. 2005;19(17):2001–2007. doi: 10.1097/01.aids.0000194134.28135.cd. [DOI] [PubMed] [Google Scholar]
- 59.Goujard C, Girault I, Rouzioux C, Lecuroux C, Deveau C, Chaix ML, et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther. 2012;17(6):1001–1009. doi: 10.3851/IMP2273. [DOI] [PubMed] [Google Scholar]
- 60.Matthews LT, Giddy J, Ghebremichael M, Hampton J, Guarino AJ, Ewusi A, Carver E, Axten K, Geary MC, Gandhi RT, Bangsberg DR. A risk-factor guided approach to reducing lactic acidosis and hyperlactatemia in patients on antiretroviral therapy. PLoS One. 2011;6(4):e18736. doi: 10.1371/journal.pone.0018736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ofotokun I, Pomeroy C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med. 2003;11(2):55–59. [PubMed] [Google Scholar]
- 62.Soon GG, Min M, Struble KA, Chan-Tack KM, Hammerstrom T, Qi K, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000-2008) AIDS Patient Care STDs. 2012;26(8):444–453. doi: 10.1089/apc.2011.0278. [DOI] [PubMed] [Google Scholar]
- 63.Currier J, Averitt Bridge D, Hagins D, Zorrilla CD, Feinberg J, Ryan R, Falcon R, Tennenberg A, Mrus J, Squires K, GRACE (Gender, Race, And Clinical Experience) Study Group Sex-based outcomes of darunavir-ritonavir therapy: a single-group trial. Ann Intern Med. 2010;153(6):349–357. doi: 10.7326/0003-4819-153-6-201009210-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Squires K, Feinberg J, Bridge DA, Currier J, Ryan R, Seyedkazemi S, Dayaram YK, Mrus J. Insights on GRACE (gender, race, and clinical experience) from the patient’s perspective: GRACE participant survey. AIDS Patient Care STDs. 2013;27(6):352–362. doi: 10.1089/apc.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Squires K, Bekker LG, Katlama C, Yazdanpanah Y, Zhou Y, Rodgers AJ, et al. Influence of sex/gender and race on responses to raltegravir combined with tenofovir-emtricitabine in treatment-naive human immunodeficiency virus-1 infected patients: pooled analyses of the STARTMRK and QDMRK studies. Open Forum Infect Dis. 2017;4(1):ofw047. doi: 10.1093/ofid/ofw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Squires K, Kityo C, Hodder S, Johnson M, Voronin E, Hagins D, Avihingsanon A, Koenig E, Jiang S, White K, Cheng A, Szwarcberg J, Cao H. Integrase inhibitor versus protease inhibitor based regimen for HIV-1 infected women (WAVES): a randomised, controlled, double-blind, phase 3 study. Lancet HIV. 2016;3(9):e410–ee20. doi: 10.1016/S2352-3018(16)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Squires KE, Young B, Santiago L, Dretler RH, Walmsley SL, Zhao HH, Pakes G, Ross L, Shaefer M. Response by gender of HIV-1-infected subjects treated with abacavir/lamivudine plus atazanavir, with or without ritonavir, for 144 weeks. HIV AIDS (Auckl) 2017;9:51–61. doi: 10.2147/HIV.S108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, Hulgan T, Raffanti S, Haas DW, Sterling TR, Koethe JR. Brief report: weight gain in persons with hiv switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–531. doi: 10.1097/QAI.0000000000001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gandhi RT, Spritzler J, Chan E, Asmuth DM, Rodriguez B, Merigan TC, Hirsch MS, Shafer RW, Robbins GK, Pollard RB, ACTG 384 Team Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42(4):426–434. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 70.Ticona E, Bull ME, Soria J, Tapia K, Legard J, Styrchak SM, Williams C, Mitchell C, la Rosa A, Rosa AL, Coombs RW, Frenkel LM. Biomarkers of inflammation in HIV-infected Peruvian men and women before and during suppressive antiretroviral therapy. AIDS. 2015;29(13):1617–1622. doi: 10.1097/QAD.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mathad JS, Gupte N, Balagopal A, Asmuth D, Hakim J, Santos B, Riviere C, Hosseinipour M, Sugandhavesa P, Infante R, Pillay S, Cardoso SW, Mwelase N, Pawar J, Berendes S, Kumarasamy N, Andrade BB, Campbell TB, Currier JS, Cohn SE, Gupta A, New Work Concept Sheet 319 and AIDS Clinical Trials Group A5175 (PEARLS) Study Teams Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr. 2016;73(2):123–129. doi: 10.1097/QAI.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lakoski SG, Herrington DM. Effects of hormone therapy on C-reactive protein and IL-6 in postmenopausal women: a review article. Climacteric. 2005;8(4):317–326. doi: 10.1080/13697130500345109. [DOI] [PubMed] [Google Scholar]
- 73.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, Lo J, Grinspoon SK. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208(11):1737–1746. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–358. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sico JJ, Chang CC, So-Armah K, Justice AC, Hylek E, Skanderson M, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84(19):1933–1940. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chow FC, Regan S, Zanni MV, Looby SE, Bushnell CD, Meigs JB, et al. Elevated ischemic stroke risk among women living with HIV infection. AIDS. 2017 10.1097/QAD.0000000000001650. [DOI] [PMC free article] [PubMed]
- 79.Stone L, Looby SE, Zanni MV. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr Opin HIV AIDS. 2017;12(6):585–593. doi: 10.1097/COH.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim Y, Anderson JL, Lewin SR. Getting the “kill” into “shock and kill”: strategies to eliminate latent HIV. Cell Host Microbe. 2018;23(1):14–26. doi: 10.1016/j.chom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fourati S, Flandre P, Calin R, Carcelain G, Soulie C, Lambert-Niclot S, Maiga A, Ait-Arkoub Z, Tubiana R, Valantin MA, Autran B, Katlama C, Calvez V, Marcelin AG. Factors associated with a low HIV reservoir in patients with prolonged suppressive antiretroviral therapy. J Antimicrob Chemother. 2014;69(3):753–756. doi: 10.1093/jac/dkt428. [DOI] [PubMed] [Google Scholar]
- 82.Cuzin L, Pugliese P, Saune K, Allavena C, Ghosn J, Cottalorda J, et al. Levels of intracellular HIV-DNA in patients with suppressive antiretroviral therapy. AIDS. 2015;29(13):1665–1671. doi: 10.1097/QAD.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 83.Johnston RE, Heitzeg MM. Sex, age, race and intervention type in clinical studies of HIV cure: a systematic review. AIDS Res Hum Retrovir. 2015;31(1):85–97. doi: 10.1089/aid.2014.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O'Doherty U, Palmer S, Deeks SG, Siliciano JD. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9(2):e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams JP, Hurst J, Stohr W, Robinson N, Brown H, Fisher M, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. elife. 2014;3:e03821. doi: 10.7554/eLife.03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katzenstein TL, Oliveri RS, Benfield T, Eugen-Olsen J, Nielsen C, Gerstoft J, et al. Cell-associated HIV DNA measured early during infection has prognostic value independent of serum HIV RNA measured concomitantly. Scand J Infect Dis. 2002;34(7):529–533. doi: 10.1080/00365540110080845. [DOI] [PubMed] [Google Scholar]
- 87.Goujard C, Bonarek M, Meyer L, Bonnet F, Chaix ML, Deveau C, Sinet M, Galimand J, Delfraissy JF, Venet A, Rouzioux C, Morlat P, Agence Nationale de Recherche sur le Sida PRIMO Study Group CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin Infect Dis. 2006;42(5):709–715. doi: 10.1086/500213. [DOI] [PubMed] [Google Scholar]
- 88.Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, et al. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 2015;11(9):e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1(1):e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 90.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sékaly RP, Lewin SR. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10(10):e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ, Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis. 2014;210(5):728–735. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dronca RS, Dong H. A gender factor in shaping T-cell immunity to melanoma. Front Oncol. 2015;5:8. doi: 10.3389/fonc.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nosrati A, Tsai KK, Goldinger SM, Tumeh P, Grimes B, Loo K, Algazi AP, Nguyen-Kim TDL, Levesque M, Dummer R, Hamid O, Daud A. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116(9):1141–1147. doi: 10.1038/bjc.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sheth AN, Rolle CP, Gandhi M. HIV pre-exposure prophylaxis for women. J Virus Erad. 2016;2(3):149–155. [PMC free article] [PubMed] [Google Scholar]
- 95.Butler K, Ritter JM, Ellis S, Morris MR, Hanson DL, McNicholl JM, et al. A depot medroxyprogesterone acetate dose that models human use and its effect on vaginal SHIV acquisition risk. J Acquir Immune Defic Syndr. 2016;72(4):363–371. doi: 10.1097/QAI.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hild-Petito S, Veazey RS, Larner JM, Reel JR, Blye RP. Effects of two progestin-only contraceptives, depo-provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. AIDS Res Hum Retrovir. 1998;14(Suppl 1):S125–S130. [PubMed] [Google Scholar]
- 97.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2(10):1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 98.Bahamondes MV, Castro S, Marchi NM, Marcovici M, Andrade LA, Fernandes A, et al. Human vaginal histology in long-term users of the injectable contraceptive depot-medroxyprogesterone acetate. Contraception. 2014;90(2):117–122. doi: 10.1016/j.contraception.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 99.Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, Doncel GF. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retrovir. 2013;29(3):592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, Pan Z, Morrison CS, Chen-Mok M, Archer DF, Gabelnick HL. The effect of one injection of depo-provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60(1):15–24. doi: 10.1016/S0010-7824(99)00058-X. [DOI] [PubMed] [Google Scholar]
- 101.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96(3):431–439. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 102.Mitchell CM, McLemore L, Westerberg K, Astronomo R, Smythe K, Gardella C, Mack M, Magaret A, Patton D, Agnew K, McElrath MJ, Hladik F, Eschenbach D. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis. 2014;210(4):651–655. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Byrne EH, Anahtar MN, Cohen KE, Moodley A, Padavattan N, Ismail N, Bowman BA, Olson GS, Mabhula A, Leslie A, Ndung'u T, Walker BD, Ghebremichael MS, Dong KL, Kwon DS. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in south African women: a prospective cohort study. Lancet Infect Dis. 2016;16(4):441–448. doi: 10.1016/S1473-3099(15)00429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fichorova RN, Chen PL, Morrison CS, Doncel GF, Mendonca K, Kwok C, Chipato T, Salata R, Mauck C. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221-15. doi: 10.1128/mBio.00221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Szotek EL, Narasipura SD, Al-Harthi L. 17beta-estradiol inhibits HIV-1 by inducing a complex formation between beta-catenin and estrogen receptor alpha on the HIV promoter to suppress HIV transcription. Virology. 2013;443(2):375–383. doi: 10.1016/j.virol.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180(4):2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 107.Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P, Sharei A, Kourjian G, Porichis F, Hart M, Palmer CD, Sirignano M, Beisel C, Hildebrandt H, Cenac C, Villani AC, Diefenbach TJ, le Gall S, Schwartz O, Herbeuval JP, Autran B, Guery JC, Chang JJ, Altfeld M. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-alpha production in women. J Immunol. 2015;195(11):5327–5336. doi: 10.4049/jimmunol.1501684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laffont S, Rouquie N, Azar P, Seillet C, Plumas J, Aspord C, et al. X-chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J Immunol. 2014;193(11):5444–5452. doi: 10.4049/jimmunol.1303400. [DOI] [PubMed] [Google Scholar]
- 109.Seillet C, Laffont S, Tremollieres F, Rouquie N, Ribot C, Arnal JF, Douin-Echinard V, Gourdy P, Guery JC. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119(2):454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 110.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cao Z, West C, Norton-Wenzel CS, Rej R, Davis FB, Davis PJ, Rej R. Effects of resin or charcoal treatment on fetal bovine serum and bovine calf serum. Endocr Res. 2009;34(4):101–108. doi: 10.3109/07435800903204082. [DOI] [PubMed] [Google Scholar]
- 112.Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, Novak RM, McCorrister S, McKinnon LR, Cohen CR, Mackelprang R, Lingappa J, Lauffenburger DA, Klatt NR, Burgener AD. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog. 2016;12(9):e1005889. doi: 10.1371/journal.ppat.1005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 114.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, Hayes RB, Ahn J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One. 2015;10(4):e0124599. doi: 10.1371/journal.pone.0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haro C, Rangel-Zuniga OA, Alcala-Diaz JF, Gomez-Delgado F, Perez-Martinez P, Delgado-Lista J, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11(5):e0154090. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJF, Dore J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72(2):1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghorai A, Ghosh U. miRNA gene counts in chromosomes vary widely in a species and biogenesis of miRNA largely depends on transcription or post-transcriptional processing of coding genes. Front Genet. 2014;5:100. doi: 10.3389/fgene.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruel TD, Zanoni BC, Ssewanyana I, Cao H, Havlir DV, Kamya M, Achan J, Charlebois ED, Feeney ME. Sex differences in HIV RNA level and CD4 cell percentage during childhood. Clin Infect Dis. 2011;53(6):592–599. doi: 10.1093/cid/cir484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dillon S, Aggarwal R, Harding JW, Li LJ, Weissman MH, Li S, Cavett JW, Sevier ST, Ojwang JW, D’Souza A, Harley JB, Hal Scofield R. Klinefelter’s syndrome (47,XXY) among men with systemic lupus erythematosus. Acta Paediatr. 2011;100(6):819–823. doi: 10.1111/j.1651-2227.2011.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carrel L, Brown CJ. When the Lyon(ized chromosome) roars: ongoing expression from an inactive X chromosome. Philos Trans R Soc Lond Ser B Biol Sci. 2017;372(1733):20160355. doi: 10.1098/rstb.2016.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dunford A, Weinstock DM, Savova V, Schumacher SE, Cleary JP, Yoda A, Sullivan TJ, Hess JM, Gimelbrant AA, Beroukhim R, Lawrence MS, Getz G, Lane AA. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat Genet. 2017;49(1):10–16. doi: 10.1038/ng.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.International HIVCS. Pereyra F, Jia X, PJ ML, Telenti A, de Bakker PI, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holterhus PM, Bebermeier JH, Werner R, Demeter J, Richter-Unruh A, Cario G, Appari M, Siebert R, Riepe F, Brooks JD, Hiort O. Disorders of sex development expose transcriptional autonomy of genetic sex and androgen-programmed hormonal sex in human blood leukocytes. BMC Genomics. 2009;10:292. doi: 10.1186/1471-2164-10-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mamrut S, Avidan N, Staun-Ram E, Ginzburg E, Truffault F, Berrih-Aknin S, Miller A. Integrative analysis of methylome and transcriptome in human blood identifies extensive sex- and immune cell-specific differentially methylated regions. Epigenetics. 2015;10(10):943–957. doi: 10.1080/15592294.2015.1084462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Distelmaier K, Schrutka L, Wurm R, Seidl V, Arfsten H, Cho A, Manjunatha S, Perkmann T, Strunk G, Lang IM, Adlbrecht C. Gender-related impact on outcomes of high density lipoprotein in acute ST-elevation myocardial infarction. Atherosclerosis. 2016;251:460–466. doi: 10.1016/j.atherosclerosis.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 127.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, McCurdy Pate M, Aberg JA, Zanni MV, Grinspoon SK. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS. 2017;31(6):797–806. doi: 10.1097/QAD.0000000000001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krebs SJ, Slike BM, Sithinamsuwan P, Allen IE, Chalermchai T, Tipsuk S, Phanuphak N, Jagodzinski L, Kim JH, Ananworanich J, Marovich MA, Valcour VG, SEARCH 011 study team Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV-infected individuals. AIDS. 2016;30(10):1533–1542. doi: 10.1097/QAD.0000000000001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr. 2016;71(2):181–188. doi: 10.1097/QAI.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 131.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 132.Griesbeck M, Scully E, Altfeld M. Sex and gender differences in HIV-1 infection. Clin Sci (Lond) 2016;130(16):1435–1451. doi: 10.1042/CS20160112. [DOI] [PubMed] [Google Scholar]
- 133.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 134.Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S210–S219. doi: 10.1097/QAI.0000000000001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Quinn VP, Nash R, Hunkeler E, Contreras R, Cromwell L, Becerra-Culqui TA, Getahun D, Giammattei S, Lash TL, Millman A, Robinson B, Roblin D, Silverberg MJ, Slovis J, Tangpricha V, Tolsma D, Valentine C, Ward K, Winter S, Goodman M. Cohort profile: study of transition, outcomes and gender (STRONG) to assess health status of transgender people. BMJ Open. 2017;7(12):e018121. doi: 10.1136/bmjopen-2017-018121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Geretti AM, Loutfy M, D'Arminio Monforte A, Latysheva I, Perez Elias MJ, Rymer J, et al. Out of focus: tailoring the cascade of care to the needs of women living with HIV. HIV Med. 2017;18(Suppl 2):3–17. doi: 10.1111/hiv.12533. [DOI] [PubMed] [Google Scholar]
- 138.Grewe ME, Ma Y, Gilbertson A, Rennie S, Tucker JD. Women in HIV cure research: multilevel interventions to improve sex equity in recruitment. J Virus Erad. 2016;2:49–51. [PMC free article] [PubMed] [Google Scholar]
- 139.Falcon R, Bridge DA, Currier J, Squires K, Hagins D, Schaible D, Ryan R, Mrus J, GRACE Study Group Recruitment and retention of diverse populations in antiretroviral clinical trials: practical applications from the gender, race and clinical experience study. J Women’s Health (Larchmt) 2011;20(7):1043–1050. doi: 10.1089/jwh.2010.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zanni MV, Fitch K, Rivard C, Sanchez L, Douglas PS, Grinspoon S, Smeaton L, Currier JS, Looby SE. Follow YOUR heart: development of an evidence-based campaign empowering older women with HIV to participate in a large-scale cardiovascular disease prevention trial. HIV Clin Trials. 2017;18(2):83–91. doi: 10.1080/15284336.2017.1297551. [DOI] [PMC free article] [PubMed] [Google Scholar]