Abstract
Purpose of Review
Parkinson’s disease (PD) is a complex neurodegenerative disorder, the aetiology of which is still largely unknown. Overwhelming evidence indicates that mitochondrial dysfunction is a central factor in PD pathophysiology. Here we review recent developments around mitochondrial dysfunction in familial and sporadic PD, with a brief overview of emerging therapies targeting mitochondrial dysfunction.
Recent Findings
Increasing evidence supports the critical role for mitochondrial dysfunction in the development of sporadic PD, while the involvement of familial PD-related genes in the regulation of mitochondrial biology has been expanded by the discovery of new mitochondria-associated disease loci and the identification of their novel functions.
Summary
Recent research has expanded knowledge on the mechanistic details underlying mitochondrial dysfunction in PD, with the discovery of new therapeutic targets providing invaluable insights into the essential role of mitochondria in PD pathogenesis and unique opportunities for drug development.
Keywords: Parkinson’s disease, Neurodegeneration, Mitochondria, Bioenergetics, Mitophagy, Mitochondrial biogenesis, Therapy
Introduction
The key manifestations associated with a clinical diagnosis of Parkinson’s disease (PD) are motor deficits resultant of focal dopaminergic nigral neurodegeneration. However, these manifestations appear late in the disease course, with mounting evidence indicating seminal pathogenic events occur a decade or more prior [1]. Prevailing theory holds that PD progression is largely mediated by pathological protein aggregation that is either the cause or corollary of dysfunction in multiple interrelated cellular pathways [2].
PD is now widely accepted as a complex, multifactorial disease that can have diverse genetic, biological and environmental influences [3]. Although sporadic PD patients, who lack evidential family history and a definitive genetic basis, account for > 90% of disease cases, the familial forms of PD have inferred cellular pathways central to PD pathophysiology [4]. With the majority of genetic PD loci directly associated with mitochondria, mitochondrial dysfunction has been implicated as an integral disease component [5]. This review focuses on recent advances in understanding of the role that mitochondrial dysfunction plays in the pathogenesis of both sporadic and familial PD.
Mitochondrial Dysfunction in Familial Parkinson’s Disease
To date, a handful of genes have been identified as monogenic causes of familial PD, with many of the pathogenic mutations in these genes directly linked to mitochondrial dysfunction (i.e. autosomal dominant SNCA and LRRK2 mutations and autosomal recessive Parkin, PINK1 and ATP13A2 mutations) [6]. More recently, new roles in the regulation of mitochondrial biology have been determined for these genes, and new PD genes associated with mitochondrial (dys)function, such as VPS35 and CHCHD2, have been identified, further underpinning the essential role of mitochondrial function to the aetiology of PD (Fig. 1).
Fig. 1.
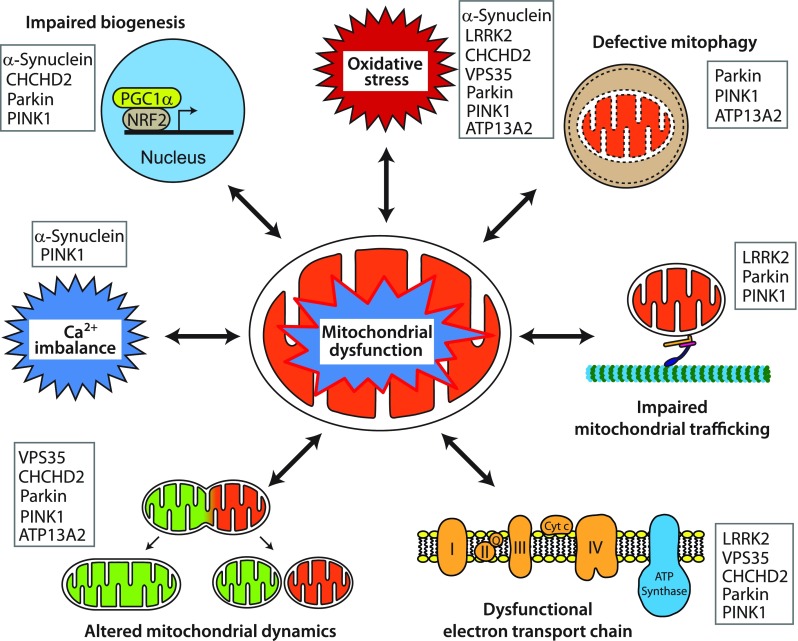
Representative pathways of mitochondrial dysfunction involved in Parkinson’s disease pathophysiology. Mitochondrial dysfunction associated with PD pathogenesis can result from impairment of mitochondrial biogenesis, increased reactive oxygen species production, defective mitophagy, compromised trafficking, electron transport chain dysfunction, variations to mitochondrial dynamics, calcium imbalance or combinations thereof. The potential complex interplay of the various functions leads to a vicious cycle of progressive cellular dysfunction that ultimately results in neurodegeneration that underlies PD pathogenesis and progression. Proteins mentioned in this review that contribute pathologically to the different pathways are listed
Autosomal Dominant PD
SNCA
α-Synuclein (α-Syn) is a small 140 amino acid polypeptide, encoded by SNCA. Although its function is still largely unknown, it has been reported to mediate neurotransmitter release at presynaptic terminals and interact with membranes of various organelles, including mitochondria. Indeed, α-Syn has a non-canonical mitochondrial targeting sequence, and has been localised to mitochondrial membranes and shown to influence mitochondrial structure and function [7].
α-Syn was initially linked to PD as the main component of Lewy bodies, with SNCA later identified as the first genetic familial PD gene [8•]. Increased levels of wild-type (WT) α-Syn and, to a greater extent, α-Syn with PD-linked mutations, such as A53T, E46K and H50Q, induce mitochondrial fragmentation and reactive oxygen species (ROS) production in vitro and in vivo [9]. Furthermore, α-Syn was recently localised to mitochondria-associated membranes (MAM), a specialised structure forming an interface between the endoplasmic reticulum (ER) and mitochondria that is important for regulating Ca2+ signalling and apoptosis. Pathogenic mutations in α-Syn were found to reduce binding to MAM and increased mitochondrial fragmentation, suggesting a role for α-Syn in regulating mitochondrial morphology [10]. For example, overexpressed WT or mutant α-Syn was found to cause dissociation of ER and mitochondria at MAM, thereby impairing Ca2+ exchange and reducing mitochondrial energy production [11].
In addition to direct effects on mitochondrial morphology, a recent study showed that α-Syn can influence mitochondrial biogenesis via regulation of peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α). In this study, treatment of human DA neurons carrying A53T with mitochondrial toxins induced S-nitrosylation of the transcription factor myocyte-specific enhancer factor 2C (MEF2C), leading to a reduction in mitochondrial biogenesis via downregulation of PGC1α [12].
LRRK2
Mutations in Leucine Rich Repeat Kinase 2 (LRRK2) cause a variably penetrant autosomal dominant form of PD and have been identified as the most common cause of familial PD [6]. LRRK2 is a multifunctional protein kinase and LRRK2 mutants are known to exert their pathogenic action via increased kinase activity. Various models overexpressing WT or PD-associated mutant LRRK2 have shown increased vulnerability to mitochondrial toxins, along with defects in mitochondrial dynamics and increased ROS production (reviewed in [9]). Consistently, physiological levels of the common LRRK2 G2019S mutant were found in association with mitochondrial abnormalities in patient-derived dopaminergic neurons [13], as well as knock-in mice [14].
Several proteins are known to interact with LRRK2 and mediate pathological effects on mitochondria. For instance, the mitochondrial fission protein, dynamin-related protein 1 (DRP1), has been shown to function as an effector of mitochondrial fragmentation through LRRK2-mediated phosphorylation at S616 [15] (Fig. 2). Moreover, LRRK2 appears to interact with other fission/fusion proteins, such as mitofusin (MFN) 1/2 and optic atrophy 1 (OPA1) [16]. LRRK2-mediated increased proton leak and loss of mitochondrial membrane potential (ΔΨm) are likely caused by upregulation of mitochondrial uncoupling protein (UCP) 2 and UCP4 [17]. In addition, mutant LRRK2 contributes to defective mitophagy by interfering with mitochondrial trafficking, as G2019S has been shown to impair proteasomal degradation of Miro, an outer mitochondrial membrane (OMM) protein that tethers mitochondria to microtubule motor proteins, and thereby mitophagy by disrupting the interaction between LRRK2 and Miro [18].
Fig. 2.
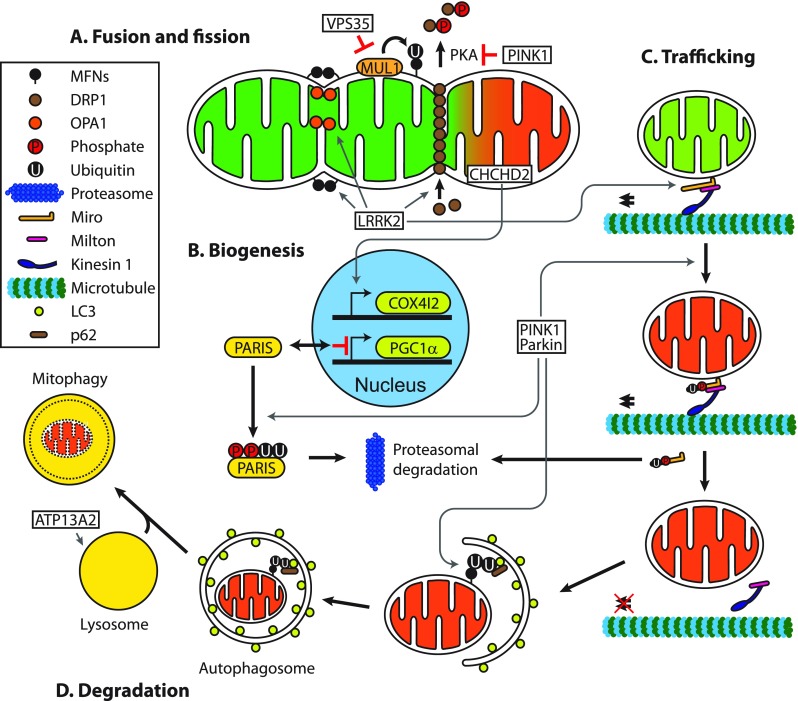
Mitochondrial function of Parkinson’s disease-related proteins. A. VPS35 mediates degradation of mitochondrial E3 ubiquitin ligase 1 (MUL1), which ubiquitinates mitofusins (MFNs), acting as a pro-fusion factor. Conversely, PINK1 inhibits protein kinase A (PKA) mediated release of dynamin-related protein 1 (DRP1) from mitochondria, promoting mitochondrial fission. Additionally, LRRK2 acts on several fission and fusion effectors, such as MFNs, optic atrophy 1 (OPA1) and DRP1, to variably alter the balance of mitochondrial dynamics. B. Parkin interacting substrate (PARIS) inhibits mitochondrial biogenesis by suppressing expression of the master regulator peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α). Under steady-state conditions, PINK1 and Parkin mediate the degradation of PARIS by phosphorylation and ubiquitination respectively, followed by proteasomal degradation, which maintains PGC1α levels and mitochondrial biogenesis. Under mitochondrial stress, CHCHD2 translocates to the nucleus and upregulates expression of mitochondrial complex IV subunit 4 isoform (COX4I2). C. Miro facilitates mitochondrial transportation with another adaptor protein Milton and the motor protein Kinesin-1. PINK1 and Parkin promote mitophagy of dysfunctional mitochondria by inducing proteasomal degradation of Miro and thereby halting mitochondrial transport. Similarly, LRRK2 has been shown to facilitate removal of Miro. D. Parkin, activated by PINK1, ubiquitinates outer mitochondrial membrane proteins, such as MFNs, to which the autophagosomal protein microtubule-associated protein light chain 3 (LC3) binds with p62, a polyubiquitin-binding protein, leading to engulfment of dysfunctional mitochondria by autophagosomes. Degradation of mitochondria occurs upon fusion with lysosomes. ATP13A2 ensures mitophagy by maintaining functional lysosomes
VPS35
The association between vacuolar protein sorting-associated protein 35 (VPS35) and PD was first observed in European PD cohorts with a family history suggestive of an autosomal dominant inheritance [19, 20]. VPS35 is a core component of the retromer complex that mediates retrograde delivery of cargo from endosomes to Golgi, as well as recycling cargo from endosomes to the cell surface [21]. Early studies reported that PD-associated mutations in VPS35 conferred vulnerability to the mitochondrial toxin 1-methyl-4-phenylpyridinium (MPP+) in vitro [22].
The main function of VPS35 in mitochondria seems to be in regulating mitochondrial dynamics through interaction with mitochondrial fission/fusion proteins. Recent studies have shown that mutant VPS35 can trigger mitochondrial fragmentation, which leads to neurodegeneration. This occurs through either a decrease in the degradation of mitochondrial E3 ubiquitin ligase 1 (MUL1), which in turn increases MFN degradation [23] (Fig. 2), or by enhancing the turnover of DRP1 complexes via mitochondrial-derived vesicle-dependent trafficking to lysosomes [24]. Also, increased mitochondrial fragmentation caused by the VPS35 mutation D620N was shown to impair mitochondrial complex I assembly and activity [25].
CHCHD2
Recently, mutations in coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2) have been identified as a cause of autosomal dominant, late-onset PD in three Japanese families [26•]. CHCHD2 is a mitochondrial intermembrane space protein with a dual function in the mitochondria and nucleus. Under normal conditions, CHCHD2 mainly exists in mitochondria bound to mitochondrial complex IV and reduced expression of CHCHD2 has consistently been shown to decrease mitochondrial complex IV activity, with resulting increases in ROS production and mitochondrial fragmentation [27]. Intriguingly, CHCHD2 was found to translocate into the nucleus and function as a transcription factor under stress conditions, regulating the expression of mitochondrial complex IV subunit 4 isoform (COX4I2) [27] (Fig. 2). Furthermore, Drosophila deficient of CHCHD2 [28] or expressing PD-associated mutants [29] also displayed structural and biochemical mitochondrial abnormalities leading to dopaminergic neurodegeneration and motor dysfunction. These findings strongly suggest that mutations in CHCHD2 lead to nigrostriatal neurodegeneration and PD by impairing mitochondrial function.
Autosomal Recessive PD
Parkin
Mutations in Parkin are the most frequent cause of autosomal recessive PD [30•], with over 120 pathogenic mutations identified so far [6]. Parkin is a cytosolic E3 ubiquitin ligase that ubiquitinates target proteins for signalling or proteasomal degradation. Parkin primarily functions in association with mitochondria, as Parkin-deficient models show profound defects in mitochondrial morphology and function [31]. Consistently, ubiquitylome analysis has revealed that the majority of Parkin targets are localised to mitochondria [32].
Parkin has diverse functions in maintaining healthy mitochondria by regulating their biogenesis and degradation via mitophagy (reviewed in [33]). In the early stages of mitochondrial degradation, Parkin is recruited to damaged or dysfunctional mitochondria and activated by PTEN-induced putative kinase 1 (PINK1), another PD-related protein (see below), leading to ubiquitination of OMM proteins and subsequent proteasomal degradation (Fig. 2). The process of mitophagy removes dysfunctional mitochondria from the healthy mitochondrial pool and facilitates their degradation via the autophagy-lysosomal pathway. Despite recent literature broadening the detailed mechanism by which Parkin mediates mitophagy in vitro, relevance to disease pathogenesis has been controversial given the lack of evidence that Parkin mediates mitophagy in vivo. However, recent studies have demonstrated endogenous Parkin-mediated mitophagy in the distal axons of rodent neurons [34] and in age-related dopaminergic neurodegeneration accompanying PD-linked motor symptoms in Parkin knockout mice with defective mitochondrial DNA replication [35•]. These findings further highlight the pathophysiological significance of Parkin-mediated mitophagy in PD over the insights obtained from in vitro models. In addition, newly developed transgenic mouse models expressing mitophagy reporters such as mt-Keima [36•] and mito-QC [37•] have finally made it possible to monitor mitophagy in mammalian brain, promising to unravel the long-standing mystery surrounding mitophagy in vivo.
Besides function in mitophagy, Parkin is known to maintain the functional mitochondrial pool by regulating mitochondrial biogenesis [31]. Under homeostatic conditions, Parkin mediates the degradation of parkin interacting substrate (PARIS), a repressor of PGC1α activity, leading to nuclear translocation of PGC1α and transcriptional activation of mitochondria-associated genes [38] (Fig. 2). Consequently, loss of Parkin function allows PARIS to accumulate and repress mitochondrial biogenesis, resulting in reduced mitochondrial mass and functional defects [39]. These findings highlight the pivotal role Parkin plays in modulating the balance of mitochondrial production and destruction.
PINK1
Mutations in PINK1 are the second most common cause of autosomal recessive early-onset PD [6, 40•]. PINK1 is a mitochondrial serine/threonine kinase that plays a crucial role in maintaining mitochondrial homeostasis. Loss of PINK1 impairs various aspects of mitochondrial biology, including degradation, morphology and trafficking. The most widely studied of these is the function of PINK1 in mitophagy; facilitating removal of damaged mitochondria by recruiting and activating Parkin [33, 41]. PINK1 activates Parkin by a twofold mechanism: (1) direct phosphorylation of Parkin at S65 [42] and (2) trans-activation by phosphorylation of ubiquitin at S65 and subsequent binding to Parkin [43•, 44•, 45•]. In addition, PINK1 can mediate mitophagy in a Parkin-independent manner by recruiting nuclear dot protein 52 kDa (NDP52) and optineurin (OPTN) [46]. Furthermore, in a similar manner to LRRK2, PINK1 has been shown to promote mitophagy by terminating mitochondrial trafficking through phosphorylation and Parkin-mediated proteasomal degradation of Miro [47] (Fig. 2).
Loss of PINK1 has been shown to induce a wide range of mitochondrial dysfunction in cell models, Drosophila and mice. This is largely a result of the loss of PINK1/Parkin-mediated mitophagy, but PINK1 also regulates mitochondrial homeostasis in a number of other ways [31]. For instance, PINK1 deficiency has been found to result in mitochondrial Ca2+ overload [48], and the specific reduction of mitochondrial complexes I and III [49]. On the other hand, PINK1 has been shown to enhance mitochondrial fission by increasing protein kinase A (PKA)-mediated DRP1 activation [50] and to modulate mitochondrial biogenesis via regulating Parkin-mediated degradation of PARIS [51] (Fig. 2).
ATP13A2
Mutations in ATP13A2 cause Kufor-Rakeb syndrome (KRS), a rare form of autosomal recessive juvenile-onset PD [52]. ATP13A2 encodes a type P5B ATPase, which mainly localises to the endo/lysosomal compartment. Although ATP13A2 is believed to transport cations across organellar membranes, its transporting activity is yet to be fully defined. Nonetheless, loss of ATP13A2 in patient-derived cells shows increased susceptibility to several cations including Zn2+ and Mn2+, indicating a role for ATP13A2 in regulating these metals [52]. The association of ATP13A2 with mitochondrial function was first implicated by observation of mitochondrial dysfunction in KRS patient-derived skin fibroblasts [53]. Consistently, several studies employing ATP13A2-deficient cell models have comprehensively shown underlying mitochondrial dysfunction, including reduced ATP production, increased mitochondrial fragmentation and increased ROS production [54, 55]. In addition, loss of ATP13A2 was also found to impair glycolysis, which aggravated mitochondrial dysfunction, suggesting a broader impact of ATP13A2 deficiency on cellular bioenergetics [56].
Existence of ATP13A2 outside mitochondria led to speculation that ATP13A2 may indirectly regulate mitochondrial function. Indeed, loss of ATP13A2 has been shown to cause Zn2+ dyshomeostasis by impairing vesicular sequestration, leading to mitochondrial dysfunction [55]. Also, dysregulated Zn2+ metabolism causes lysosomal dysfunction [57], which may contribute to defective mitophagy (Fig. 2), highlighting the complex interplay between closely associated cellular pathways known to be involved in the pathogenesis of PD.
Mitochondrial Dysfunction in Sporadic Parkinson’s Disease
Sporadic PD occurs as a seemingly random occurrence due to undetermined genetic or environmental bases in the absence of an obvious family history. It is well established that PD is a multifactorial disorder caused by impaired cellular functions that impact upon interrelated pathways and create complex feedback cycles leading to neurodegeneration [2]. Broadly, affected cellular pathways include proteostasis, oxidative stress and the multiple pathways relating to mitochondrial function (Fig. 1) [58], all of which are evident in sporadic PD.
Genetic and Environmental Influences on Sporadic PD
It is increasingly apparent that environmental and genetic aspects contribute to PD, with combinatorial insults being more pathological than either individually [3]. Phenotypes consistent with sporadic PD can be induced by a number of endogenous and exogenous inhibitors of mitochondrial function, including rotenone, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), paraquat, nitric oxide, the dopamine metabolite aminochrome and others [3, 9]. Consistently, an increased risk for developing idiopathic PD has been demonstrated in rural populations exposed to agricultural pesticides and herbicides [59] and a significantly younger onset of sporadic PD has been linked to chronic occupational exposure to pesticides and heavy metals [60]. From a genetic perspective, Genome-Wide Association Studies (GWAS) have provided evidence that polymorphisms in familial PD genes are risk factors for developing sporadic PD, linking the pathogenesis of familial and sporadic PD [61]. Sporadic PD risk has been attributed to a number of loci including regions of as yet unknown influence [62•, 63], as well as familial PD genes associated with mitochondrial dysfunction, e.g. Parkin, PINK1, ATP13A2, CHCHD2, SNCA, LRRK2 and GBA [64].
α-Synuclein in Sporadic PD
α-Syn has been found to bind to OMM proteins, such as voltage-dependent anion-selective channel 1 (VDAC1), translocase of outer membrane (TOM) 40 and TOM20, and mediate mitochondrial dysfunction [65]. Furthermore, VDAC1 levels were found to be reduced in sporadic PD patient nigral neurons in association with α-Syn aggregations and is therefore implicated as a component of overall mitochondrial dysfunction in sporadic PD [66]. This may be mediated via the α-Syn-induced activation of the mitochondrial permeability transition pore, which depolarises the mitochondrial membrane potential leading to mitochondrial fragmentation and degradation. In the setting of dysfunctional mitophagy and trafficking (as discussed above), this would be expected to enhance cellular dysfunction and death [66]. Furthermore, α-Syn pathogenicity is related to aggregation rather than a loss of intrinsic function, with the formation of Lewy bodies, chiefly comprised of α-Syn, a hallmark of neuronal degeneration in sporadic PD [67]. Aggregated α-Syn affects proteostasis by impairing the function and trafficking between ER, Golgi and the autophagy-lysosomal system, as well as impacting on mitochondrial functions including energy production, calcium and iron buffering and ROS production.
Iron Accumulation and Oxidative Stress
Oxidative stress is intimately linked to mitochondrial (dys)function, with mitochondria producing ~ 90% of cellular ROS [68]. It is apparent that synucleinopathy, oxidative stress and mitochondrial dysfunction are locked in a vicious interdependent feedback cycle in sporadic PD [58], with mitochondrial accumulation of α-Syn inhibiting complex I activity and driving ROS production via the consequent respiratory chain dysfunction [7]. In particular, iron accumulation observed in the substantia nigra of sporadic PD patient brain causes increased ROS production, transcriptional upregulation of SNCA and increased α-Syn aggregation [69, 70]. Mitochondria have active iron exchange with the cytoplasm, required for the synthesis of iron sulphur clusters, which are integral components of complex I and II and sensitive to oxidative stress. Consequently, inhibition of complex I by rotenone, MPTP and paraquat poisoning have been shown to result in iron accumulation in association with PD [71]. Inhibition of the ubiquitin proteasome system also causes cellular iron dyshomeostasis, further adding to the positive feedback on ROS generation and α-Syn aggregation [72]. In addition, neuronal iron accumulation impacts on mitochondrial reticular connectivity, as shown for calcineurin-dependent effects on DRP1 [73] and calcium release via Ryanodine receptors [74].
Mitochondrial Quality Control
The mitochondrial quality control mechanisms of dynamic complementation in concert with balanced mitophagy and biogenesis work to maintain a healthy cellular mitochondrial pool and bioenergetic function under steady-state conditions [33], reasoning why disruption of these pathways cause mitochondrial dysfunction that underlies PD pathogenesis.
An emerging area of interest is the influence that lipids and lipid pathways have on PD. For instance, the master regulator of lipogenesis, sterol regulatory element binding transcription factor 1 (SREBF1), was identified by GWAS as a risk locus for sporadic PD [75] and was subsequently validated by genome-wide RNAi screening as a regulator of Parkin-mediated mitophagy [76]. This was further endorsed by administration of genistein, an inhibitor of sterol regulatory element binding protein (SREBP) activation, which blocked Parkin recruitment to mitochondria and was partially rescued by exogenous lipid supplementation, thereby providing a mechanistic link between lipid synthesis and mitophagy and filling an evidence gap for the association of mitophagy with sporadic PD [77].
The importance of mitophagy in PD pathogenesis is evident from the prevalence of familial cases associated with PINK1 and Parkin mutations. However, cytoplasmic hybrid cells generated from sporadic PD patient platelets were also found to have fragmented mitochondrial networks [15]. This was the result of fusion impairment due to proteolysis of OPA1 and fission enhancement by phosphorylation of DRP1 S616. In a more recent study using neurotoxin models of sporadic PD, it was shown that increased nitric oxide levels caused the nitrosylation of Parkin, impairing its ubiquitin ligase activity and resulting in an upregulation of phosphorylated S616 DRP1 recruitment to mitochondria and consequential mitochondrial hyper-fragmentation [78]. This study contextualised the role of DRP1 in mitochondrial fragmentation and dysfunction that leads to neuronal cell death in sporadic PD and identified nitrosylated Parkin as a possible therapeutic angle.
Cargo trafficking along axonal microtubules is important for shuttling cellular components to and from the synaptic terminals. Of particular interest for the pathogenesis of sporadic PD is the axonal trafficking of α-Syn and mitochondria [79]. Dysfunctional trafficking has been linked to sporadic PD by a number of mechanisms, including a reduction in motor protein expression with consequent accumulation of α-Syn in the axons and soma [80•], as well as decreased degradation of the mitochondrial-molecular motor tether Miro and consequent impairment of mitochondrial motility (also a feature in familial PD; see above) [18]. Additionally, LRRK2 and Parkin recruitment to mitochondria was impaired upon CCCP treatment in sporadic PD patient-derived fibroblasts, highlighting that the LRRK2/DRP1 and PINK1/Parkin pathways act in parallel, converge on Miro and are impaired in sporadic PD [18].
It has been identified that expression of PGC1α is reduced in sporadic PD brain [81•] and can be reduced by direct binding of accumulated α-Syn to the PPARGC1A promoter in the setting of oxidative stress [82] or by methylation of the PPARGC1A promoter [83]. On the converse, PGC1α expression has been shown to mitigate α-Syn oligomerisation [81•] and protect DA neurons [84]. These findings indicate that PGC1α-mediated mitochondrial biogenesis imparts neuroprotection that becomes compromised in the setting of sporadic PD.
Dysfunctional Electron Transport Chain and Alterations to the Mitochondrial Genome
Since the original observation of MPTP causing mitochondrial dysfunction in PD, mitochondrial complex I has been considered central to the pathogenesis of PD. However, one question that arises when considering complex I in PD is, why do mitochondrial disease patients with complex I deficiency rarely develop PD. To date, no mitochondrial DNA (mtDNA) mutations have been found to cause PD, despite genes integral to complex I being encoded by mtDNA. Instead, Parkinsonism associated with mitochondrial diseases is largely restricted to mutations affecting the mtDNA maintenance genes POLG and TWINKLE (encoding the mtDNA polymerase and helicase, respectively), but is inconsistently observed [85, 86]. Some insight was provided by the exonuclease dysfunctional POLG mutator mouse, which alone did not recapitulate a PD phenotype due to compensatory mitochondrial biogenesis, but when crossed with a Parkin knockout mouse convincingly displayed a PD phenotype [35]. This suggests accumulation of somatic mtDNA mutations is insufficient to cause PD and other insults are required to elicit disease. Nevertheless, supporting the notion of increased mtDNA mutation in PD, rotenone treatment of rats was found to increase the rate of somatic mtDNA mutation, particularly in the substantia nigra [87].
Respiratory chain enzymology in single neurons from idiopathic PD patients showed complex I and II were typically affected [88]. In addition, mtDNA from these cells showed multiple deletions on the background of a common deletion. Consistently, neuronal mtDNA copy number was found to increase with age in controls, but not in PD patients [88]. In fact, the accumulation of deleted mtDNA in PD patients meant there was wild-type mtDNA depletion, which effectively raised the relative levels of somatic mutations, likely contributing to an underlying mitochondrial bioenergetic defect in sporadic PD neurons [89]. Supporting this, sporadic PD patients show an accumulation of mtDNA mutations in the setting of reduced mtDNA copy number, predominantly in the substantia nigra [90, 91]. On this basis, as age is the greatest risk factor for developing PD and ageing is associated with a decline in mitochondrial function (which results from accumulation of mtDNA mutations, reduction in respiratory chain activity and an increase in oxidative stress that ultimately causes reduced cellular bioenergetics and favours α-Syn aggregation), it appears that mtDNA and respiratory chain based mitochondrial dysfunction contributes to PD pathogenesis by lowering the threshold for susceptibility to other genetic and environmental insults.
Emerging Therapeutic Strategies
The common involvement of mitochondrial dysfunction in PD represents an attractive target for drug development. Accordingly, various strategies have been devised to improve mitochondrial function in both familial and sporadic PD. Enhancing mitophagy presents as an effective approach due to growing evidence for its general impairment in PD. Increasing Parkin activity by inhibiting c-Abl-mediated phosphorylation using nilotinib has been shown to be neuroprotective [92], while the ATP analog kinetin triphosphate increased mutant PINK1 activity, leading to enhanced Parkin recruitment [93]. Inhibition of deubiquitinating enzymes also increases Parkin-mediated mitophagy as ubiquitin specific peptidase (USP) 8, 15 and 30 antagonize the action of Parkin, whereas inhibition of these USPs increased mitochondrial degradation [94]. Additionally, activation of non-canonical mitophagy may provide an alternative avenue to restore mitochondrial function in PD as several proteins such as Fun14 domain-containing protein 1 (FUNDC1) and autophagy and beclin 1 regulator 1 (Ambra1) displayed an ability to modulate mitophagy in a PINK1/Parkin-independent manner [95]. In particular, Nip3-like protein X-mediated mitophagy [96] was recently found to restore mitochondrial function and prevent neurodegeneration in the setting of Parkin or PINK1 deficiency, highlighting this pathway as a potential target for therapeutic intervention.
Increasing mitochondrial biogenesis is another strategy to replenish neurons with healthy mitochondria. Dimethyl fumarate or BG-12 has been effective in phase III trials of relapsing multiple sclerosis [97] and approved for treating patients, highlighting a potential application in PD. A recent study showed that BG-12 exerts beneficial effect by increasing mitochondrial biogenesis in mice and humans via the transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF2) [98]. Another activator of the NRF2 pathway, synthetic triterpenoids, showed a protection of dopaminergic neurons against MPTP [99]. Likewise, PGC1α has been a popular target due to its potent role in inducing mitochondrial biogenesis. Bezafibrate [100] and quercetin [101] showed beneficial effects by increasing mitochondria in rodent models for neurodegeneration, proposing an opportunity for new drug development.
Mitochondrial-targeted antioxidants and flavonoids have shown promising results in animal models, and attempts to mitigate mitochondrial dysfunction using antioxidants have produced positive outcomes in preclinical settings [102]. However, recent clinical trials for creatine and coenzyme Q10 have not demonstrated disease-modifying benefit in patients with PD [103, 104], indicating that more targeted antioxidant approaches may be required or that oxidative stress is a downstream effect of mitochondrial dysfunction rather than a direct cause of PD-related neurodegeneration.
Summary
PD is a multifactorial disease caused by combinations of genetic and environmental factors in which the balance may vary from individual to individual. Among these factors, mitochondrial dysfunction plays an integral role in the pathogenesis of PD, with accumulated evidence supporting centrality in both sporadic and familial PD. Furthermore, the discovery of new mitochondria-associated genes as causes of PD continues to expand our understanding of the molecular mechanisms underlying mitochondrial dysfunction and consequential impact on neurodegeneration. Rapid advances in such knowledge have created an unprecedented opportunity for the development of effective PD therapies by targeting mitochondrial dysfunction. Although several drug candidates have failed in recent clinical trials, cohorts have not been stratified according to these risk factors potentially offering an explanation for their lack of success. Preclinical results of other drugs targeting newly identified molecules are promising, leaving hope for future effective PD therapies. Much work remains to define the mechanisms underlying mitochondrial dysfunction and its pathogenic influence in the development of both sporadic and familial PD.
Compliance with Ethical Standards
Conflict of Interest
Jin-Sung Park and Ryan L. Davis declare no conflict of interest.
Carolyn M. Sue has a patent No. PCT/AU2015/000194 pending.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Movement Disorders
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, Ziemssen T. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27(5):617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 2.Michel PP, Hirsch EC, Hunot S. Understanding dopaminergic cell death pathways in Parkinson disease. Neuron. 2016;90(4):675–691. doi: 10.1016/j.neuron.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 3.Polito L, Greco A, Seripa D. Environmental exposure and their interaction in Parkinson’s disease. Parkinsons Dis. 2016;2016:9. doi: 10.1155/2016/6465793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson’s disease: progress and therapeutic implications. Mov Disord. 2013;28(1):14–23. doi: 10.1002/mds.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser DN, Hastings TG. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol Dis. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lill CM. Genetics of Parkinson’s disease. Mol Cell Probes. 2016;30(6):386–396. doi: 10.1016/j.mcp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Mullin S, Schapira A. alpha-Synuclein and mitochondrial dysfunction in Parkinson’s disease. Mol Neurobiol. 2013;47(2):587–597. doi: 10.1007/s12035-013-8394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 9.Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200–210. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S. alpha-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci. 2014;34(1):249–259. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paillusson S, Gomez-Suaga P, Stoica R, Little D, Gissen P, Devine MJ, Noble W, Hanger DP, Miller CCJ. alpha-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017;134(1):129–149. doi: 10.1007/s00401-017-1704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, Lee B, Lopez K, Nutter A, Shan B, Molokanova E, Zhang Y, Han X, Nakamura T, Masliah E, Yates JR, III, Nakanishi N, Andreyev AY, Okamoto SI, Jaenisch R, Ambasudhan R, Lipton SA. Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1alpha transcription. Cell. 2013;155(6):1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinhardt P, Schmid B, Burbulla LF, Schondorf DC, Wagner L, Glatza M, et al. Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 2013;12(3):354–367. doi: 10.1016/j.stem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Yue M, Hinkle KM, Davies P, Trushina E, Fiesel FC, Christenson TA, Schroeder AS, Zhang L, Bowles E, Behrouz B, Lincoln SJ, Beevers JE, Milnerwood AJ, Kurti A, McLean PJ, Fryer JD, Springer W, Dickson DW, Farrer MJ, Melrose HL. Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol Dis. 2015;78:172–195. doi: 10.1016/j.nbd.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos D, Esteves AR, Silva DF, Januario C, Cardoso SM. The impact of mitochondrial fusion and fission modulation in sporadic Parkinson’s disease. Mol Neurobiol. 2015;52(1):573–586. doi: 10.1007/s12035-014-8893-4. [DOI] [PubMed] [Google Scholar]
- 16.Stafa K, Tsika E, Moser R, Musso A, Glauser L, Jones A, Biskup S, Xiong Y, Bandopadhyay R, Dawson VL, Dawson TM, Moore DJ. Functional interaction of Parkinson’s disease-associated LRRK2 with members of the dynamin GTPase superfamily. Hum Mol Genet. 2014;23(8):2055–2077. doi: 10.1093/hmg/ddt600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papkovskaia TD, Chau KY, Inesta-Vaquera F, Papkovsky DB, Healy DG, Nishio K, Staddon J, Duchen MR, Hardy J, Schapira AHV, Cooper JM. G2019S leucine-rich repeat kinase 2 causes uncoupling protein-mediated mitochondrial depolarization. Hum Mol Genet. 2012;21(19):4201–4213. doi: 10.1093/hmg/dds244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, et al. Functional impairment in miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell. 2016;19(6):709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89(1):162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small SA, Petsko GA. Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat Rev Neurosci. 2015;16(3):126–132. doi: 10.1038/nrn3896. [DOI] [PubMed] [Google Scholar]
- 22.Bi F, Li F, Huang C, Zhou H. Pathogenic mutation in VPS35 impairs its protection against MPP(+) cytotoxicity. Int J Biol Sci. 2013;9(2):149–155. doi: 10.7150/ijbs.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang FL, Liu W, Hu JX, Erion JR, Ye J, Mei L, Xiong WC. VPS35 deficiency or mutation causes dopaminergic neuronal loss by impairing mitochondrial fusion and function. Cell Rep. 2015;12(10):1631–1643. doi: 10.1016/j.celrep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Wang X, Fujioka H, Hoppel C, Whone AL, Caldwell MA, Cullen PJ, Liu J, Zhu X. Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat Med. 2016;22(1):54–63. doi: 10.1038/nm.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Wang W, Hoppel C, Liu J, Zhu X. Parkinson’s disease-associated pathogenic VPS35 mutation causes complex I deficits. Biochim Biophys Acta. 2017;1863(11):2791–2795. doi: 10.1016/j.bbadis.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Funayama M, Ohe K, Amo T, Furuya N, Yamaguchi J, Saiki S, et al. CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: a genome-wide linkage and sequencing study. Lancet Neurol. 2015;14(3):274–282. doi: 10.1016/S1474-4422(14)70266-2. [DOI] [PubMed] [Google Scholar]
- 27.Aras S, Bai M, Lee I, Springett R, Huttemann M, Grossman LI. MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism. Mitochondrion. 2015;20:43–51. doi: 10.1016/j.mito.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Meng H, Yamashita C, Shiba-Fukushima K, Inoshita T, Funayama M, Sato S, Hatta T, Natsume T, Umitsu M, Takagi J, Imai Y, Hattori N. Loss of Parkinson’s disease-associated protein CHCHD2 affects mitochondrial crista structure and destabilizes cytochrome c. Nat Commun. 2017;8:15500. doi: 10.1038/ncomms15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tio M, Wen R, Lim YL, Zukifli ZHB, Xie S, Ho P, Zhou Z, Koh TW, Zhao Y, Tan EK. Varied pathological and therapeutic response effects associated with CHCHD2 mutant and risk variants. Hum Mutat. 2017;38(8):978–987. doi: 10.1002/humu.23234. [DOI] [PubMed] [Google Scholar]
- 30.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 31.Scarffe LA, Stevens DA, Dawson VL, Dawson TM. Parkin and PINK1: much more than mitophagy. Trends Neurosci. 2014;37(6):315–324. doi: 10.1016/j.tins.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496(7445):372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85(2):257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206(5):655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickrell AM, Huang CH, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, et al. Endogenous Parkin preserves dopaminergic substantia nigral neurons following mitochondrial DNA mutagenic stress. Neuron. 2015;87(2):371–381. doi: 10.1016/j.neuron.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun N, Yun J, Liu J, Malide D, Liu C, Rovira II, et al. Measuring in vivo mitophagy. Mol Cell. 2015;60(4):685–696. doi: 10.1016/j.molcel.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McWilliams TG, Prescott AR, Allen GF, Tamjar J, Munson MJ, Thomson C, et al. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J Cell Biol. 2016;214(3):333–345. doi: 10.1083/jcb.201603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144(5):689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens DA, Lee Y, Kang HC, Lee BD, Lee YI, Bower A, Jiang H, Kang SU, Andrabi SA, Dawson VL, Shin JH, Dawson TM. Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc Natl Acad Sci U S A. 2015;112(37):11696–11701. doi: 10.1073/pnas.1500624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 41.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 42.Kondapalli C, Kazlauskaite A, Zhang N, Woodroof HI, Campbell DG, Gourlay R, Burchell L, Walden H, Macartney TJ, Deak M, Knebel A, Alessi DR, Muqit MMK. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012;2(5):120080. doi: 10.1098/rsob.120080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510(7503):162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 44.Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MMK. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014;460(1):127–139. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205(2):143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147(4):893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kostic M, Ludtmann MH, Bading H, Hershfinkel M, Steer E, Chu CT, et al. PKA phosphorylation of NCLX reverses mitochondrial calcium overload and depolarization, promoting survival of PINK1-deficient dopaminergic neurons. Cell Rep. 2015;13(2):376–386. doi: 10.1016/j.celrep.2015.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amo T, Saiki S, Sawayama T, Sato S, Hattori N. Detailed analysis of mitochondrial respiratory chain defects caused by loss of PINK1. Neurosci Lett. 2014;580:37–40. doi: 10.1016/j.neulet.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 50.Pryde KR, Smith HL, Chau KY, Schapira AH. PINK1 disables the anti-fission machinery to segregate damaged mitochondria for mitophagy. J Cell Biol. 2016;213(2):163–171. doi: 10.1083/jcb.201509003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee Y, Stevens DA, Kang SU, Jiang H, Lee YI, Ko HS, Scarffe LA, Umanah GE, Kang H, Ham S, Kam TI, Allen K, Brahmachari S, Kim JW, Neifert S, Yun SP, Fiesel FC, Springer W, Dawson VL, Shin JH, Dawson TM. PINK1 primes Parkin-mediated ubiquitination of PARIS in dopaminergic neuronal survival. Cell Rep. 2017;18(4):918–932. doi: 10.1016/j.celrep.2016.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park JS, Blair NF, Sue CM. The role of ATP13A2 in Parkinson’s disease: clinical phenotypes and molecular mechanisms. Mov Disord. 2015;30(6):770–779. doi: 10.1002/mds.26243. [DOI] [PubMed] [Google Scholar]
- 53.Grunewald A, Arns B, Seibler P, Rakovic A, Munchau A, Ramirez A, et al. ATP13A2 mutations impair mitochondrial function in fibroblasts from patients with Kufor-Rakeb syndrome. Neurobiol Aging 2012;33(8):1843.e1–7. [DOI] [PubMed]
- 54.Ramonet D, Podhajska A, Stafa K, Sonnay S, Trancikova A, Tsika E, Pletnikova O, Troncoso JC, Glauser L, Moore DJ. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum Mol Genet. 2012;21(8):1725–1743. doi: 10.1093/hmg/ddr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park JS, Koentjoro B, Veivers D, Mackay-Sim A, Sue CM. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum Mol Genet. 2014;23(11):2802–2815. doi: 10.1093/hmg/ddt623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park JS, Koentjoro B, Davis RL, Sue CM. Loss of ATP13A2 impairs glycolytic function in Kufor-Rakeb syndrome patient-derived cell models. Parkinsonism Relat Disord. 2016;27:67–73. doi: 10.1016/j.parkreldis.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 57.Tsunemi T, Krainc D. Zn(2)(+) dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum Mol Genet. 2014;23(11):2791–2801. doi: 10.1093/hmg/ddt572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganguly G, Chakrabarti S, Chatterjee U, Saso L. Proteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer’s disease and Parkinson’s disease. Drug Design, Development Therapy. 2017;11:797–810. doi: 10.2147/DDDT.S130514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pezzoli G, Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology. 2013;80(22):2035–2041. doi: 10.1212/WNL.0b013e318294b3c8. [DOI] [PubMed] [Google Scholar]
- 60.Ratner MH, Farb DH, Ozer J, Feldman RG, Durso R. Younger age at onset of sporadic Parkinson’s disease among subjects occupationally exposed to metals and pesticides. Interdiscip Toxicol. 2014;7(3):123–133. doi: 10.2478/intox-2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.International Parkinson Disease Genomics Consortium. Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377(9766):641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.International Parkinson’s Disease Genomics Consortium, Wellcome Trust Case Control Consortium 2 A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS Genet. 2011;7(6):e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beecham GW, Dickson DW, Scott WK, Martin ER, Schellenberg G, Nuytemans K, Larson EB, Buxbaum JD, Trojanowski JQ, van Deerlin VM, Hurtig HI, Mash DC, Beach TG, Troncoso JC, Pletnikova O, Frosch MP, Ghetti B, Foroud TM, Honig LS, Marder K, Vonsattel JP, Goldman SM, Vinters HV, Ross OA, Wszolek ZK, Wang L, Dykxhoorn DM, Pericak-Vance MA, Montine TJ, Leverenz JB, Dawson TM, Vance JM. PARK10 is a major locus for sporadic neuropathologically confirmed Parkinson disease. Neurology. 2015;84(10):972–980. doi: 10.1212/WNL.0000000000001332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lesage S, Brice A. Role of Mendelian genes in “sporadic” Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Supplement 1):S66–S70. doi: 10.1016/S1353-8020(11)70022-0. [DOI] [PubMed] [Google Scholar]
- 65.Pozo Devoto VM, Falzone TL. Mitochondrial dynamics in Parkinson’s disease: a role for alpha-synuclein? Dis Model Mech. 2017;10(9):1075–1087. doi: 10.1242/dmm.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu Y, Goldman JG, Kelly L, He Y, Waliczek T, Kordower JH. Abnormal alpha-synuclein reduces nigral voltage-dependent anion channel 1 in sporadic and experimental Parkinson’s disease. Neurobiol Dis. 2014;69(Supplement C):1–14. doi: 10.1016/j.nbd.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Yasuda T, Nakata Y, Mochizuki H. α-Synuclein and neuronal cell death. Mol Neurobiol. 2013;47(2):466–483. doi: 10.1007/s12035-012-8327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perfeito R, Cunha-Oliveira T, Rego AC. Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease—resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med. 2012;53(9):1791–1806. doi: 10.1016/j.freeradbiomed.2012.08.569. [DOI] [PubMed] [Google Scholar]
- 69.Febbraro F, Giorgi M, Caldarola S, Loreni F, Romero-Ramos M. α-Synuclein expression is modulated at the translational level by iron. Neuroreport. 2012;23(9):576–580. doi: 10.1097/WNR.0b013e328354a1f0. [DOI] [PubMed] [Google Scholar]
- 70.Carboni E, Lingor P. Insights on the interaction of alpha-synuclein and metals in the pathophysiology of Parkinson’s disease. Metallomics. 2015;7(3):395–404. doi: 10.1039/c4mt00339j. [DOI] [PubMed] [Google Scholar]
- 71.Munoz Y, Carrasco CM, Campos JD, Aguirre P, Nunez MT. Parkinson’s disease: the mitochondria-iron link. Parkinsons Dis. 2016;2016:7049108. doi: 10.1155/2016/7049108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le W. Role of iron in UPS impairment model of Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:S158–SS61. doi: 10.1016/S1353-8020(13)70038-5. [DOI] [PubMed] [Google Scholar]
- 73.Park J, Lee DG, Kim B, Park S-J, Kim J-H, Lee S-R, Chang KT, Lee HS, Lee DS. Iron overload triggers mitochondrial fragmentation via calcineurin-sensitive signals in HT-22 hippocampal neuron cells. Toxicology. 2015;337(Supplement C):39–46. doi: 10.1016/j.tox.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 74.SanMartín CD, Paula-Lima AC, García A, Barattini P, Hartel S, Núñez MT, et al. Ryanodine receptor-mediated Ca(2+) release underlies iron-induced mitochondrial fission and stimulates mitochondrial Ca(2+) uptake in primary hippocampal neurons. Front Mol Neurosci. 2014;7:13. doi: 10.3389/fnmol.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston JW, Wojcicki A, Eriksson N. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 2011;7(6):e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ivatt RM, Sanchez-Martinez A, Godena VK, Brown S, Ziviani E, Whitworth AJ. Genome-wide RNAi screen identifies the Parkinson disease GWAS risk locus SREBF1 as a regulator of mitophagy. Proc Natl Acad Sci U S A. 2014;111(23):8494–8499. doi: 10.1073/pnas.1321207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ivatt RM, Whitworth AJ. SREBF1 links lipogenesis to mitophagy and sporadic Parkinson disease. Autophagy. 2014;10(8):1476–1477. doi: 10.4161/auto.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Z, Liu L, Jiang X, Zhai S, Xing D. The essential role of Drp1 and its regulation by S-nitrosylation of Parkin in dopaminergic neurodegeneration: implications for Parkinson’s disease. Antioxid Redox Signal. 2016;25(11):609–622. doi: 10.1089/ars.2016.6634. [DOI] [PubMed] [Google Scholar]
- 79.Esteves AR, Gozes I, Cardoso SM. The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson’s disease. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2014;1842(1):7–21. doi: 10.1016/j.bbadis.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain. 2012;135(Pt 7):2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eschbach J, von Einem B, Müller K, Bayer H, Scheffold A, Morrison BE, et al. Mutual exacerbation of PGC-1α deregulation and α-synuclein oligomerization. Annals Neurology. 2015;77(1):15–32. doi: 10.1002/ana.24294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, Melov S, Andersen JK. Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson’s disease. Free Radic Biol Med. 2012;53(4):993–1003. doi: 10.1016/j.freeradbiomed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su X, Chu Y, Kordower JH, Li B, Cao H, Huang L, Nishida M, Song L, Wang D, Federoff HJ. PGC−1α promoter methylation in Parkinson’s disease. PLoS One. 2015;10(8):e0134087. doi: 10.1371/journal.pone.0134087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ciron C, Zheng L, Bobela W, Knott GW, Leone TC, Kelly DP, Schneider BL. PGC-1α activity in nigral dopamine neurons determines vulnerability to α-synuclein. Acta Neuropathologica Communications. 2015;3:16. doi: 10.1186/s40478-015-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reeve A, Meagher M, Lax N, Simcox E, Hepplewhite P, Jaros E, Turnbull D. The impact of pathogenic mitochondrial DNA mutations on substantia Nigra neurons. J Neurosci. 2013;33(26):10790–10801. doi: 10.1523/JNEUROSCI.3525-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tzoulis C, Schwarzlmüller T, Biermann M, Haugarvoll K, Bindoff LA. Mitochondrial DNA homeostasis is essential for nigrostriatal integrity. Mitochondrion. 2016;28(Supplement C):33–37. doi: 10.1016/j.mito.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Sanders LH, McCoy J, Hu X, Mastroberardino PG, Dickinson BC, Chang CJ, Chu CT, van Houten B, Greenamyre JT. Mitochondrial DNA damage: molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol Dis. 2014;70:214–223. doi: 10.1016/j.nbd.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grünewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, Turnbull DM. Mitochondrial DNA depletion in respiratory chain-deficient Parkinson disease neurons. Ann Neurol. 2016;79(3):366–378. doi: 10.1002/ana.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dölle C, Flønes I, Nido GS, Miletic H, Osuagwu N, Kristoffersen S, Lilleng PK, Larsen JP, Tysnes OB, Haugarvoll K, Bindoff LA, Tzoulis C. Defective mitochondrial DNA homeostasis in the substantia nigra in Parkinson disease. Nat Commun. 2016;7:13548. doi: 10.1038/ncomms13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coxhead J, Kurzawa-Akanbi M, Hussain R, Pyle A, Chinnery P, Hudson G. Somatic mtDNA variation is an important component of Parkinson’s disease. Neurobiology of Aging. 2016;38(217):e1–e6. doi: 10.1016/j.neurobiolaging.2015.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pyle A, Anugrha H, Kurzawa-Akanbi M, Yarnall A, Burn D, Hudson G. Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurobiology of Aging. 2016;38(216):e7–e10. doi: 10.1016/j.neurobiolaging.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karuppagounder SS, Brahmachari S, Lee Y, Dawson VL, Dawson TM, Ko HS. The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson’s disease. Sci Rep. 2014;4:4874. doi: 10.1038/srep04874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hertz NT, Berthet A, Sos ML, Thorn KS, Burlingame AL, Nakamura K, Shokat KM. A neo-substrate that amplifies catalytic activity of Parkinson’s-disease-related kinase PINK1. Cell. 2013;154(4):737–747. doi: 10.1016/j.cell.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dikic I, Bremm A. DUBs counteract parkin for efficient mitophagy. EMBO J. 2014;33(21):2442–2443. doi: 10.15252/embj.201490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hamacher-Brady A, Brady NR. Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci. 2016;73(4):775–795. doi: 10.1007/s00018-015-2087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koentjoro B, Park JS, Sue CM. Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson’s disease. Sci Rep. 2017;7:44373. doi: 10.1038/srep44373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linker RA, Gold R. Dimethyl fumarate for treatment of multiple sclerosis: mechanism of action, effectiveness, and side effects. Curr Neurol Neurosci Rep. 2013;13(11):394. doi: 10.1007/s11910-013-0394-8. [DOI] [PubMed] [Google Scholar]
- 98.Hayashi G, Jasoliya M, Sahdeo S, Sacca F, Pane C, Filla A, et al. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum Mol Genet. 2017;26(15):2864–2873. doi: 10.1093/hmg/ddx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, Liby KT, Williams CR, Yamamoto M, Kensler TW, Ratan RR, Sporn MB, Beal MF, Gazaryan IG, Thomas B. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxid Redox Signal. 2013;18(2):139–157. doi: 10.1089/ars.2011.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johri A, Calingasan NY, Hennessey TM, Sharma A, Yang L, Wille E, Chandra A, Beal MF. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Hum Mol Genet. 2012;21(5):1124–1137. doi: 10.1093/hmg/ddr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X, Wang H, Gao Y, Li L, Tang C, Wen G, Yang Y, Zhuang Z, Zhou M, Mao L, Fan Y. Quercetin induces mitochondrial biogenesis in experimental traumatic brain injury via the PGC-1alpha signaling pathway. Am J Transl Res. 2016;8(8):3558–3566. [PMC free article] [PubMed] [Google Scholar]
- 102.Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta. 2014;1842(8):1282–1294. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71(5):543–552. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 104.Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, Ross GW, et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA. 2015;313(6):584–593. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]


